Abstract
Humoral alloimmunity negatively impacts both short- and long-term cell and solid organ transplant survival. We previously reported that alloantibody-mediated rejection (AMR) of transplanted hepatocytes is critically dependent on host macrophages. However, the effector mechanism(s) of macrophage-mediated injury to allogeneic liver parenchymal cells is not known. We hypothesized that macrophage-mediated destruction of allogeneic hepatocytes occurs by cell-cell interactions requiring Fcγ receptors (FcγRs). To examine this, alloantibody-dependent hepatocyte rejection in CD8-depleted wild-type and Fcγ chain KO (lacking all functional Fcγ receptors) transplant recipients was evaluated. Alloantibody-mediated hepatocellular allograft rejection was abrogated in recipients lacking FcγR compared to wild-type recipients. We also investigated anti-FcγRI mAb, anti-FcγRIII mAb, and inhibitors of intracellular signaling [to block phagocytosis, cytokines, and reactive oxygen species (ROS)] in an in vitro alloantibody-dependent, macrophage-mediated hepatocytoxicity assay. Results showed that in vitro alloantibody-dependent, macrophage-mediated hepatocytotoxicity was critically dependent on FcγRs and ROS. The adoptive transfer of wild-type macrophages into CD8-depleted FcγR-deficient recipients was sufficient to induce AMR, while adoptive transfer of macrophages from Fcγ chain KO mice or ROS-deficient (p47 KO) macrophages was not. These results provide first evidence that alloantibody-dependent hepatocellular allograft rejection is mediated by host macrophages through FcγR signaling and ROS cytotoxic effector mechanisms. These results support the investigation of novel immunotherapeutic strategies targeting macrophages, FcγRs and/or downstream molecules, including ROS, to inhibit humoral immune damage of transplanted hepatocytes and perhaps other cell and solid organ transplants.
Introduction
Clinical and experimental studies highlight the barrier that acute and chronic antibody-mediated allograft damage poses to successful allograft survival [reviewed in (1)]. Antibody-mediated rejection (AMR) occurs despite the use of powerful maintenance immunosuppressive agents and is associated with worse graft outcome than T cell-mediated rejection (2). Theoretically, cellular transplants are more vulnerable to rejection and graft loss due to humoral immunity than solid organ transplants (SOT) due to their smaller tissue mass and increased exposure to circulating alloantibodies. Clinical experience implicates the role of humoral immunity in the progressive loss of cell transplant function, such as after initially successful pancreatic islet cell (3) or hepatocellular transplantation (4), despite immunosuppressive therapies.
The current understanding of alloantibody-mediated damage to solid organ transplants is limited, largely focusing on complement-dependent damage to the donor organ endothelium (5). Complement deposition is usually detected in a perivascular location, which supports the inference that antibodies and complement target graft endothelial cells with subsequent ischemic graft damage [reviewed in (1, 6)]. However, it is now recognized that AMR in the absence of complement deposition also occurs after renal transplantation and, accordingly, the Banff criteria for clinical diagnosis of AMR was revised in 2013 (7). Cellular transplantation is distinct from solid organ transplantation in that there is no donor endothelium separating the vasculature and the graft parenchymal cells, which uniquely focuses the investigation of alloantibody-mediated damage to allogeneic parenchymal cells. Published work by our laboratory has shown that alloantibody targets allogeneic liver parenchymal cells for immune damage and that this involves a macrophage-mediated, complement-independent mechanism (8, 9).
Experimental data and clinical data show that immune damage to transplanted organs and cells by cellular (10–19) and/or humoral rejection (4, 8, 9, 14–21) mechanisms can occur separately or concurrently (mixed cellular and humoral rejection) (22–25). When cell-mediated rejection is inhibited by depletion of CD8+ T cells, hepatocyte transplant recipients produce high titer alloantibody (26) and undergo rapid rejection that is dependent on alloantibody and macrophages (9). In our studies, alloantibody is sufficient to mediate acute humoral rejection and is dose-dependent, as increasing amounts of transferred allosera into immunodeficient SCID recipients accelerates the onset of rejection (8). However, when host macrophages are genetically-deficient or depleted [macrophage-deficient (MCSF KO) or liposomal clodronate treated recipients, respectively] in hosts with high alloantibody levels, AMR is abrogated. Furthermore, once alloantibody is formed, the effector mechanism(s) for AMR in this model is independent of complement, CD4+ T cells, neutrophils, and NK cells (9).
Macrophages are innate immune cells that have multiple functions including phagocytosis, antigen presentation, and cytotoxic effector functions (27, 28). Macrophages recognize antibody-coated cells through their Fc receptors; Fcγ receptors (FcγRs) specifically recognize the Fc domain of IgG antibody. At present, four types of FcγRs are known in mice, three are activating (FcγRI, FcγRIII, and FcγRIV) and one is inhibitory (FcγRIIb) (29). IgG antibody isotypes are selectively recognized by specific mouse FcγRs (30). The predominant isotype of alloantibody produced in our model of hepatocyte transplantation is IgG1 with a lesser amount of IgG3 [(26, 31)]. Since FcγRI is the only activating FcγR binding IgG3 and FcγRIII is the only FcγR binding IgG1 (32–34), in the current studies we tested the role of these specific activating receptors in alloantibody-dependent, macrophage-mediated hepatocytotoxicity. Upon recognition of target cells through Fcγ receptors, macrophages exert cytotoxic effector functions through phagocytosis, secretion of pro-inflammatory cytokines, and/or reactive oxygen species (ROS) (28, 35, 36). Consequently, we performed studies to further delineate the mechanism of macrophage-mediated hepatocytotoxicity and AMR after hepatocyte transplant.
Materials and Methods
Experimental animals.
FVB/N (H-2q MHC haplotype, Taconic), CD8 KO (H-2b, Jackson Labs, Cd8atm1Mak targeted mutation), C57BL/6 [wild-type (WT); H-2b, Jackson], and p47-deficient (H-2b, Jackson, Ncf1m1J spontaneous mutation) mouse strains (all 6–10 weeks of age) were used in this study. Fcγ chain KO mice (H-2b, Fcer1gtm1Rav targeted mutation), a generous gift from Dr. J. Ravetch (Rockefeller University), were also used in this study. Transgenic FVB/N mice expressing human α−1-antitrypsin (hA1AT) were the source of “donor” hepatocytes, as previously described (37). All animals were maintained in sterile housing at The Ohio State University and all experiments performed in compliance with the guidelines of the IACUC of The Ohio State University (Protocol 2008A0068-R2).
Hepatocyte isolation, purification, and transplantation.
Hepatocyte isolation and purification were performed, as previously described (37). Hepatocyte viability and purity were consistently >95%. Donor FVB/N hepatocytes (2×106) were transplanted by intrasplenic injection with rapid circulation (less than 24 hours) of donor hepatocytes to the host liver where they engraft. Donor hepatocytes can be detected by immunohistochemical staining for hA1AT throughout the parenchyma of the host liver (37). Graft survival was determined by detection of secreted hA1AT by ELISA in serial recipient serum samples (37, 38). Graft survival was reflected by stable and persistent serum hA1AT levels, whereas graft rejection was reflected by loss of serum hA1AT to undetectable levels (<0.5 μg/ml). The reporter protein hA1AT does not elicit a deleterious immune response to transplanted hepatocytes; consequently, syngeneic, hA1AT-expressing hepatocytes survive long-term in both WT and CD8-depleted transplant recipients (37).
CD8+ T cell depletion.
Mice underwent CD8+ T cell depletion by treatment with 100 μg (intraperitoneal injection) of anti-CD8 monoclonal antibody on day −3 and −1 prior to transplant and weekly post transplant (clone 53.6.72; National Cell Culture Center, Minneapolis, Minnesota). Depletion was confirmed through flow cytometric analysis of recipient peripheral blood lymphocytes (<1% CD8+ peripheral blood lymphocytes).
Donor-reactive antibody titer.
To measure alloantibody titer, we analyzed the recipient serum using published methods (21). Briefly, serum was serially diluted and incubated with allogeneic FVB/N target splenocytes. Splenocytes were then stained with FITC-conjugated goat anti-mouse IgG Fc (Organon Teknika, Durham, NC). The mean channel fluorescence intensity (MFI) was measured for each sample and the dilution that returned the MFI observed when the splenocytes were stained with the 1:4 dilution of naïve C57BL/6 serum was divided by two and recorded as the titer.
Isolation, culture and purity of Bone Marrow Macrophages.
Bone marrow macrophages (BMM) were isolated and cultured as previously described (39). Briefly, bone marrow cells were collected by flushing the femurs of mice with DMEM and cultured in BMM media (50% DMEM, 20% heat inactivated FBS, 30% L-cell conditioned media, 50 μM 2mercaptoethanol) for 7 days. The culture plates were washed to obtain only the adherent cells and subsequently scraped off the plate. BMM derived in this manner were >98% positive for macrophage markers, F4/80 and CD11b, as determined by flow cytometry.
In vitro alloantibody-dependent, macrophage-mediated hepatocytotoxicity assay.
Purified mouse FVB/N allogeneic hepatocytes (H-2q) were incubated for 30 min (37oC) with serum from naïve mice (control) or mice with high alloantibody titers (CD8 KO recipients, H-2b; collected 14 days post transplant). Allosera consisted of blood from hepatocyte transplant recipients that was collected and freshly centrifuged. Allosera was confirmed to be high alloantibody titer by flow cytometric assessment of binding to allogeneic targets, as previously described (21). Aliquots of control or alloantibody-incubated hepatocytes (1.5×105 cells/well) were then added to 12-well plates with hepatocyte media (RPMI 1640, 10% fetal bovine serum, 1% antibiotics, 10mM HEPES, 10mM β-mercaptoethanol, 2mM L-glutamine). RAW 264.7 macrophages (BALB/c derived; H-2d), a gift from Susheela Tridandapani (Ohio State University Medical Center, Columbus, Ohio) or BMM were pre-incubated with IFN-γ (2.5 ng/mL; 18 hours prior to co-culture) and then added to the co-cultures as effector cells.
In some experimental groups, macrophages were incubated for 30 minutes with anti-FcγRI (2 μg/million cells; clone N-19; Santa Cruz Biotechnologies, Santa Cruz, California), anti-FcγRIII (2 μg/million cells; R&D Microsystems, Minneapolis, MN), or both antibodies [2 μg/million cells of rat IgG (Sigma Aldrich) was used as a negative control]. In addition, anti-C5 mAb (clone BB5.1, Hycult Biotech Inc., Plymouth Meeting, PA) was added to co-cultures to investigate the role of complement. In other experimental groups, co-cultures were treated with UO126 (5–10 μM), LY294002 (20–80 μM), BAY11–7085 (5–20 μM; all inhibitors from Thermo Fisher Scientific, Waltham, MA), anti-TNF-α mAb (5–20 μg/mL; clone MP6-XT22MP6; National Cell Culture Center, Minneapolis, MN), cytochalasin D (1–10 μg/mL; Sigma Aldrich), superoxide dismutase (200–1,000 U/mL; Sigma Aldrich), or Apocynin (0.25–1 mM; Sigma Aldrich). Macrophages were added to the co-culture wells at a 10:1 effector to target (E:T) ratio. Macrophage-hepatocyte co-cultures were incubated for an additional 8 h at 37°C. Supernatants were then collected and analyzed for lactate dehydrogenase (LDH) release as an indicator of cellular cytotoxicity (CytoTox-ONE Homogenous Membrane Integrity Assay, Promega, Madison, WI). Baseline LDH was determined by PBS-treated hepatocytes and 100% LDH was determined by lysis buffer of hepatocytes alone (Promega). Color development was determined on a Spectramax Plus microplate reader (Molecular Devices) at a wavelength of 590 nm. Results were confirmed by trypan blue staining of hepatocytes and macrophages following co-culture.
Transwell assay.
Transwell macrophage:hepatocyte co-cultures were used to assess the importance of cell contact for in vitro macrophage-mediated hepatocytotoxicity. IFN-γ activated macrophages were FcγR-stimulated by incubation with formalin-fixed alloantibody-incubated hepatocytes and separated by a transwell membrane (3.0 μm pore Polyester Membrane, Corning Life Sciences, Lowell, MA) from viable (not formalin fixed) target alloantibody-incubated allogeneic hepatocytes in the bottom well, as described above. After 8 hours, supernatant was analyzed for LDH release to assess hepatocyte cytotoxicity. No LDH is released from formalin-fixed hepatocytes.
Statistical analysis.
Graft survival between experimental groups was compared using Kaplan-Meier survival curves and log-rank statistics (SPSS). Other Statistical calculations were performed using a one-tailed Student’s t test to analyze differences between experimental groups. P<0.05 was considered significant. To demonstrate the distribution of the data, results are listed as the mean plus or minus the standard error.
Results
In vitro alloantibody-dependent macrophage-mediated hepatocytotoxicity is contact-dependent.
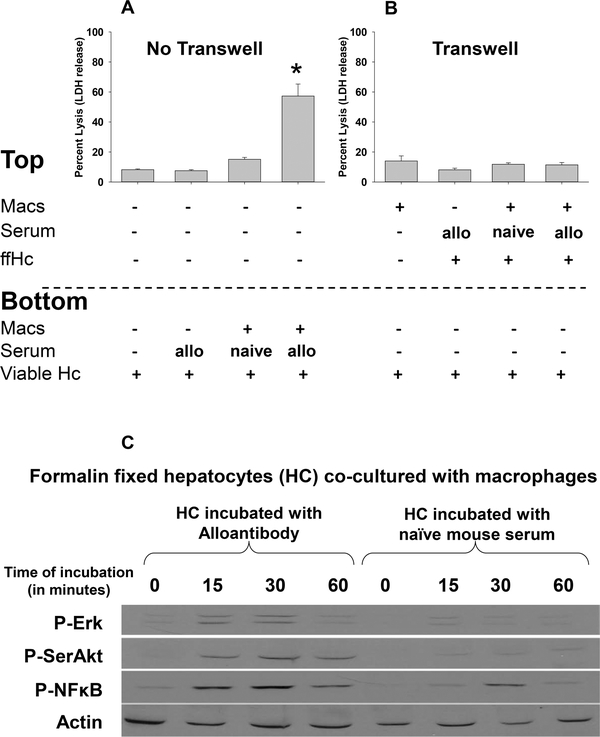
It has been reported that hepatocyte apoptosis can be mediated by a variety of mechanisms, including soluble factors such as macrophage-derived TNF-α [reviewed in (40)]. To determine if macrophages require direct cell contact to mediate alloantibody-dependent hepatocytotoxicity or whether this can occur by the macrophage-mediated release of soluble factors, we utilized a transwell co-culture system in which the semi-permeable membrane allows for passage of soluble factors but prevents contact between cells in chambers on either side of the membrane. Target FVB/N hepatocytes (1.5×105 cells) were plated in the bottom of all wells. In the transwell inserts, we added RAW 264.7 effector macrophages (10:1, 1.5×106 cells) pretreated with murine IFN-γ (2.5 ng/mL). To stimulate effector function of macrophages in the transwell, transwell macrophages were co-cultured with alloantibody-incubated, formalin-fixed allogeneic hepatocytes or media alone as a negative control. As a positive control, macrophages were added directly to the bottom well with alloantibody-incubated viable target hepatocytes. Following 8 hours of co-culture, supernatant was analyzed for lactate dehydrogenase (LDH) release. Significant hepatocyte cytotoxicity was only observed when cell-cell contact was intact (57.3±8.0%, p=0.001; Figure 1A). Cytotoxicity was confirmed to be specific to hepatocytes by Trypan Blue staining. In the absence of cellular contact, activated macrophages did not mediate cytotoxic damage of viable hepatocytes since cytotoxicity in transwell co-cultures (11.4±1.6%, p=ns) was similar to that of hepatocytes cultured alone (8.2±0.5%; Figure 1B) and significantly less than cytotoxicity in macrophage:hepatocyte co-cultures without transwells (57.3±8.0%, p<0.001; Figure 1A). Macrophages not activated with IFN-γ prior to co-culture did not mediate significant in vitro hepatocytotoxicity (not shown). Immunoblot analysis of IFN-γ-treated macrophages shows that only macrophages co-cultured with alloantibody-incubated, formalin-fixed hepatocytes substantially upregulate expression of phosphorylated or intracellular signaling proteins such as p-ERK, p-SerAkt and p-NFkB which is consistent with macrophage activation through FcγR (Figure 1C).
Figure 1. In vitro macrophage-mediated hepatocytotoxicity is contact-dependent.
FVB/N hepatocytes were isolated and cultured in vitro. Allogeneic hepatocytes were incubated with alloserum or control serum from naïve mice. RAW 264.7 macrophages were activated by pretreatment with IFN-γ (2.5 ng/mL; 18 hours). Following pretreatment, macrophages (Mac; 1.5×106 cells) were washed and co-incubated with hepatocytes (1.5×105 cells). A) In positive control co-cultures, activated macrophages and allogeneic hepatocytes were co-cultured in the same well. When activated macrophages and viable hepatocytes were co-cultured with alloantibody for 8 hours, significant cytotoxicity was observed [57.3±8.0%, p<0.0005 compared to hepatocytes cultured alone (8.2±0.5%), with serum from naïve mice (7.5±0.7%), or with alloserum (15.1±1.3%), as denoted by “*”; n=4 for all conditions] as reflected by LDH release in the culture supernatant. B) In other co-cultures, macrophages were separated from viable hepatocytes by a transwell membrane. To activate macrophages in the transwell group, cells were co-cultured with formalin-fixed alloantibody-incubated hepatocytes (ffHc) in the transwell and viable hepatocytes were in the bottom well. Following 8 hours of co-culture, supernatant was analyzed for LDH release. In the absence of activated macrophage cell contact with viable allogeneic hepatocytes (transwell), minimal cytotoxicity was detected in co-cultures (last bar: activated macrophages= 11.4±1.6%; p=ns compared to hepatocytes cultured alone and p=.0004 compared to positive control culture in (A); n=4 for all conditions). Data for co-cultures in (A) and (B) are representative from triplicate experiments. C) To confirm activation of macrophages by formalin-fixed alloantibody-incubated hepatocytes, macrophages were lysed at 15, 30, and 60 minutes after co-culture. Macrophages were tested for phosphorylated-Erk, SerAkt, and NFκB by western blot. Beta actin was used as a loading control.
In vitro macrophage-mediated hepatocytotoxicity is FcγRI- and FcγRIII-dependent.
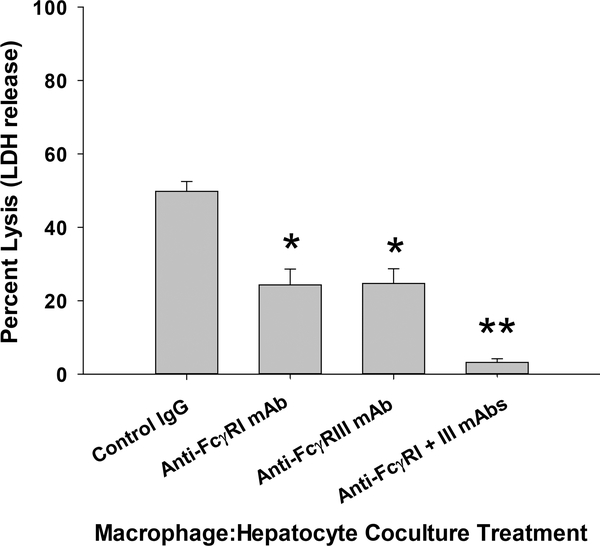
In order to determine if macrophages require FcγRI and/or FcγRIII to mediate hepatocytotoxicity, we blocked FcγR-mediated signaling using antibodies directed towards FcγRI and FcγRIII. Prior to co-culture, macrophages were incubated with control rat IgG, anti-FcγRI mAb, and/or anti-FcγRIII mAb (2 μg per 1×106 macrophages). The macrophages were washed with PBS and then added to a co-culture with alloantibody-incubated target hepatocytes for 8 hours. No macrophage-mediated cytotoxicity is observed for hepatocytes incubated with naïve control serum (9). Treatment with anti-FcγRI mAb (24.3±4.3%) or anti-FcγRIII mAb (24.7±4.0%, p<0.0001 for both) significantly reduced macrophage-mediated cytotoxicity against alloantibody-incubated hepatocytes in comparison to control IgG-treated macrophages (49.8±2.7%; Figure 2). When combination treatments were tested in co-cultures (blocking both FcγRI and FcγRIII), macrophage-mediated cytotoxicity was abrogated (3.2±1.0%, p<0.0001). The addition of anti-C5 mAb (45.6±6.9%, p=ns) to the co-cultures did not significantly alter macrophage-mediated hepatocytotoxicity indicating that alloantibody-dependent, macrophage-mediated hepatocytotoxicity is independent of complement. In contrast, our results show that macrophages require both FcγRI and FcγRIII to mediate in vitro alloantibody-dependent hepatocytotoxicity. Next, we investigated the role of FcγR-mediated signaling on in vivo alloantibody-dependent hepatocyte rejection.
Figure 2. In vitro macrophage-mediated hepatocytotoxicity is FcγRI- and FcγRIII-dependent.
Macrophage:hepatocyte co-cultures consisted of FVB/N hepatocytes and RAW 264.7 macrophages activated by pretreatment with IFN-γ (2.5 ng/mL; 18 hours). Prior to co-culture RAW macrophages were incubated with FcγRI and/or FcγRIII blocking antibody (2 μg per million macrophages) or control IgG. The macrophages were washed with PBS and then added to the hepatocyte co-culture for an 8 hour incubation (1.5×106 cells macrophages and 1.5×105 hepatocytes). Control IgG-treated macrophages mediated hepatocytotoxicity against alloantibody incubated target hepatocytes (49.8±2.7%; n=24). Anti-FcγRI mAb (24.3±4.3%; n=11) and anti-FcγRIII mAb (24.7±4.0%; n=9) treatment significantly blocked macrophage-mediated hepatocytotoxicity (p<0.0001 for both, as denoted by “*”). Combination treatment with both anti-FcγRI and anti-FcγRIII mAbs completely inhibited macrophage-mediated hepatocytotoxicity (3.2±1.0%; n=12, p<0.0001, as denoted by “**”). Data is combined from duplicate experiments.
Alloantibody-dependent hepatocyte rejection is FcγR-dependent.
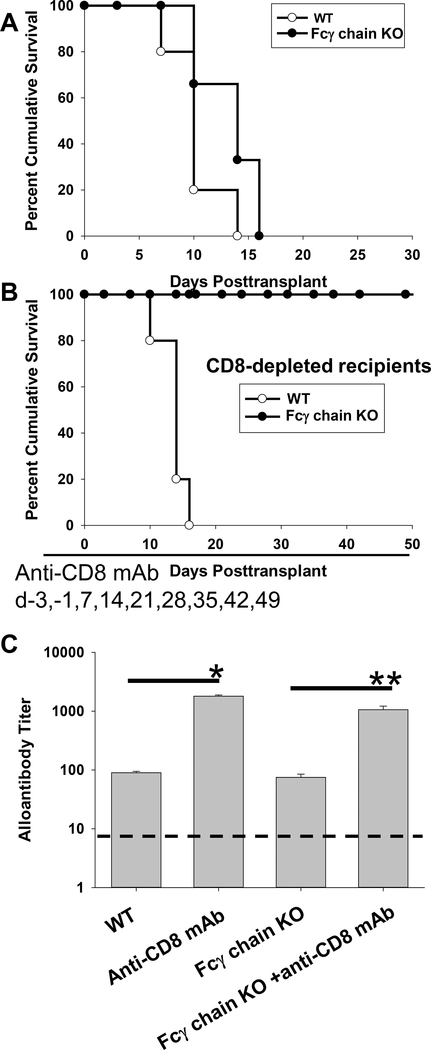
C57BL/6 (WT) and Fcγ chain KO mice (H-2b) were transplanted with allogeneic FVB/N hepatocytes (H-2q) on day 0. Both WT and Fcγ chain KO recipients rejected hepatocellular allografts with a median survival time of 10 days and 14 days, respectively (p=ns; Figure 3A), indicating that host adaptive immune responses are not impaired in Fcγ chain KO mice and consistent with published findings that humoral and CD8-mediated immune responses readily occur in Fcγ chain KO mice (41, 42)]. To focus on AMR without the influence of CD8+ T cellular-mediated rejection, cohorts of recipients were CD8-depleted by intraperitoneal administration of anti-CD8 mAb on days −3, −1, and weekly post transplant until day 49. As previously reported, CD8-depleted recipients are high alloantibody producers and rapidly reject hepatocellular allografts with a median survival time of 14 days. Furthermore, rejection in these CD8-depleted recipients is alloantibody-dependent and macrophage-mediated (8, 9). Similarly, in the current studies, positive control CD8-depleted WT mice (high alloantibody producers) rapidly rejected hepatocellular allografts with a median survival time of 14 days (Figure 3B). In contrast, CD8-depleted Fcγ chain KO recipients, also high alloantibody producers, exhibited significantly delayed rejection with ongoing hepatocellular survival to the study endpoint on day 50 in all recipients. Once anti-CD8 mAb treatment was stopped though, hepatocyte rejection occurred in all recipients by day 63 indicating that long-term engrafted hepatocytes remain vulnerable to (CD8-mediated) hepatocyte rejection. The occurrence of rejection 14 days after cessation of anti-CD8 mAb treatment was expected since this time-frame corresponds with reconstitution of CD8+ T cells in the periphery and transplanted hepatocytes are highly susceptible to CD8+ T cell mediated rejection (13, 43–45). Our data is consistent with the interpretation that, despite the presence of high alloantibody titers (Figure 3C), transplanted allogeneic hepatocytes are not rejected in CD8-depleted recipients with impaired FcγR signaling. However, when anti-CD8 mAb therapy is discontinued, allogeneic hepatocytes are rejected by CD8-dependent cellular rejection pathway (9). Based on our prior studies which showed that only depletion of host macrophages prevented alloantibody-dependent hepatocyte rejection in CD8-depleted recipients, we investigated potential molecular mechanisms which could play a role in alloantibody-dependent, macrophage-mediated hepatocytotoxicity.
Figure 3. Alloantibody-mediated hepatocellular allograft rejection is FcγR-dependent.
C57BL/6 (wild-type; WT) and Fcγ chain KO mice (H-2b) were transplanted with allogeneic FVB/N hepatocytes (H-2q) on day 0. A cohort of recipients was CD8-depleted (days −3, −1, and weekly post transplant until day +49) to suppress cell-mediated rejection. A) Allogeneic hepatocyte rejection occurred rapidly in both WT (n=5; MST=day 10) and Fcγ chain KO (n=6; MST=day 14) recipients. B) Alloantibody-mediated hepatocyte rejection in CD8-depleted WT mice also occurred rapidly (n=5; MST= day 14). In contrast alloantibody-mediated rejection was not observed in CD8-depleted Fcγ chain KO recipients (n=4) by the end of the study period (day 50). C) Alloantibody titer was measured on day 14 post transplant. WT (titer=90±5) and Fcγ chain KO (titer=75±10) recipients both produced similar alloantibody titers (control serum from naïve mice represented by the dashed line). CD8-depleted WT mice produced markedly increased alloantibody (titer=1800±89) compared to WT recipients (p<0.0001, as denoted by “*”). CD8-depleted Fcγ chain KO recipients also exhibited a significantly increased alloantibody titer (titer=1062±157) compared to Fcγ chain KO recipients (titer=75±10; p<0.0001, as denoted by “**”). Titers in CD8-depleted WT and Fcγ chain KO recipients were comparable. Data for all is combined from duplicate experiments.
In vitro alloantibody-dependent macrophage-mediated hepatocytotoxicity is reactive oxygen species-dependent.
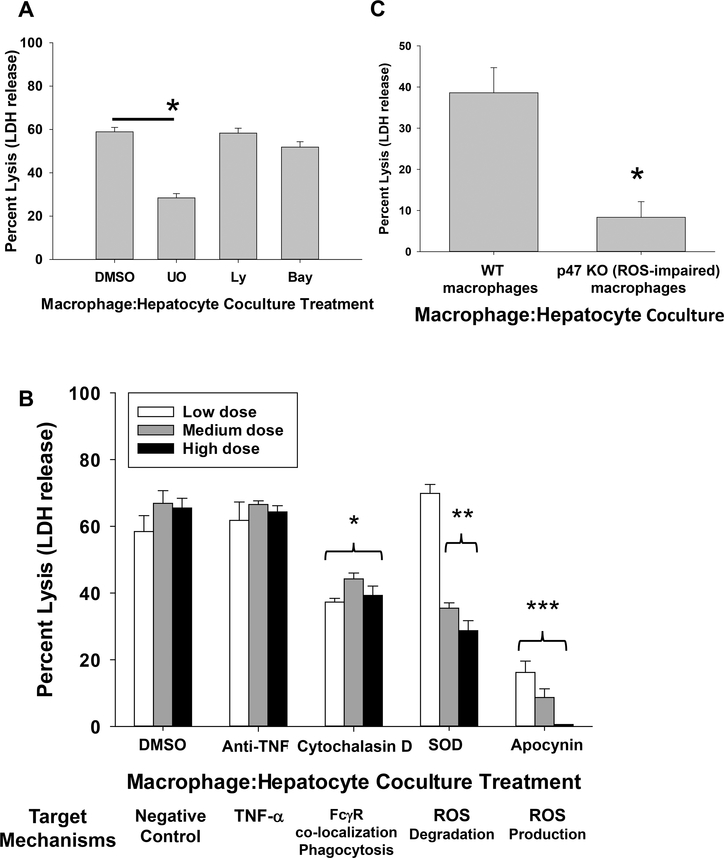
We tested the capacity of intracellular signaling inhibitors to block in vitro alloantibody-dependent, macrophage-mediated hepatocytotoxicity. The inhibitors tested included UO126 (inhibits MAPK/Erk kinases MEK1 and MEK2), LY294002 (inhibits PI3K), BAY11–7058 (inhibits IKK) in comparison to DMSO (vehicle control). Only incubation with UO126 (5 μM) significantly inhibited macrophage-mediated hepatocytotoxicity (28.4±2.0%, p<0.0001) as compared to DMSO control (58.9±2.1%; Figure 4A). Treatment with LY294002 (20 μM; 58.3±2.3%) or BAY11–7085 (5 μM; 51.9±2.5%) did not inhibit macrophage-mediated cytotoxicity. Furthermore, higher doses of LY294002 (40, 80 μM) and BAY11–7085 (10, 20 μM) failed to inhibit cytotoxicity (data not shown). These three inhibitors target multiple cytotoxic effector pathways including phagocytosis and cytokines (46–48). However, in contrast to the other agents, UO126 has been shown to target ROS (49, 50).
Figure 4. In vitro macrophage-mediated hepatocytotoxicity is reactive oxygen species-dependent.
All macrophage:hepatocyte co-cultures consisted of FVB/N hepatocytes, activated macrophages and alloserum. RAW 264.7 macrophages and BMM were activated by pretreatment with IFN-γ (2.5 ng/mL; 18 hours). A) During co-culture of hepatocytes and RAW macrophages, cultures were treated with DMSO control, UO126, LY294002, or BAY11–7058. While treatment with LY294002 (58.3±2.3%; n=12, 20 μM; p=ns) and BAY11–7085 (51.9±2.5%; n=12, 5 μM, p=ns) did not inhibit macrophage-mediated hepatocytotoxicity compared to positive control (58.9±2.1%; DMSO control, n=20), treatment with UO126 (28.4±2.0%; n=14, 5 μM; p<0.0001, as denoted by “*”) significantly inhibited macrophage-mediated cytotoxicity of alloantibody-incubated hepatocytes. Data is combined from triplicate experiments. B) In a separate cohort, co-cultures were incubated with DMSO (vehicle control), anti-TNF-α mAb, cytochalasin D, superoxide dismutase (SOD), or apocynin. Both superoxide dismutase (200 U/mL= 69.8±2.7%; 500 U/mL= 35.4±1.6%; 1,000 U/mL=28.7±3.0%; n=4 for all, p<0.0005 for 500 and 1,000 U/mL, as denoted by “**”) and apocynin (0.25 mM=16.9±4.0%; 0.5 mM=9.6±3.0%; 1.0 mM=0±0%; n=5 for all, p<0.0001 for all, as denoted by “***”) significantly inhibited macrophage-mediated hepatocytotoxicity (apocynin abrogated cytotoxicity at 1.0 mM) as compared to DMSO-treated co-cultures (0.1%=62.6±3.3%, 0.5%= 66.9±3.8%, 1.0%= 65.5±2.9%; n=6 for all). RAW cells incubated with anti-TNF-α mAb at all doses tested (5 μg/mL=61.8±5.5%; 10 μg/mL=66.6±1.1%; 20 μg/mL=64.3±1.9%; n=4 for all) had no effect on macrophage-mediated hepatocytotoxicity. Cytochalasin D (inhibitor of actin polymerization and FcγR co-localization) significantly impaired macrophage-mediated hepatocytotoxcity (1 μg/mL=37.3±1.1%; 5 μg/mL=44.2±1.8%; 10 μg/mL=39.3±2.8%; n=4 for all, p<0.009 for all doses, as denoted by “*”). Data is combined from duplicate experiments. C) Bone marrow macrophages (BMM) were isolated from WT and p47 KO mice and co-cultured with allogeneic hepatocytes and alloserum. Macrophage-mediated hepatocytotoxicity was significantly impaired in co-cultures containing p47-deficient BMM (8.3±3.8%; n=6) which cannot produce ROS compared to WT BMM (38.6±6.1%; n=6, p=0.0009, as denoted by “*”). Data is combined from duplicate experiments.
Results with UO126 prompted studies to more specifically investigate the role of ROS in alloantibody-dependent, macrophage-mediated hepatocytotoxicity. In order to do this, superoxide dismutase (an enzyme that degrades ROS), and apocynin (inhibits NADPH oxidase and ROS production) were used to interfere with ROS-mediated effects in the macrophage-mediated hepatocytotoxicity assay. Macrophages were treated with superoxide dismutase (200, 500, 1,000 U/mL) or apocynin (0.25, 0.5, 1.0 mM) in co-culture with alloantibody-incubated hepatocytes. Treatment with superoxide dismutase resulted in a dose-dependent inhibition of macrophage-mediated hepatocytotoxicity (cytotoxicity at doses 200 U/mL=69.8±2.7%, 500 U/mL=35.4±1.6%, 1,000 U/mL=28.7±3.0%; p<0.0005 for 500 and 1,000 U/mL doses) compared to DMSO treated control macrophages (cytotoxicity at DMSO doses 0.1%=58.4±4.8%, 0.5%=66.9±3.8%, 1.0%=65.5±2.9%). Apocynin, at all concentrations tested, significantly inhibited macrophage-mediated hepatocytoxicity (cytotoxicity at doses 0.25 mM=16.2±3.4%, 0.5 mM=8.7±2.6%, 1.0 mM=0±0%; p<0.0001 for all) and was abrogated at the 1.0 mM concentration (Figure 4B). Treatment with anti-TNF-α mAb, tested across a wide range of doses, did not interfere with macrophage-mediated hepatocytoxicity (cytotoxicity at doses 5 μg/mL=61.8±5.5%; 10 μg/mL=66.6±1.1%; 20 μg/mL=64.3±1.9%). Cytochalasin D (an inhibitor of actin polymerization), which inhibits FcγR co-localization and phagocytosis, significantly reduced macrophage-mediated hepatocytotoxicity (cytotoxicity at doses 1 μg/mL=37.3±1.1%; 5 μg/mL=44.2±1.8%; 10 μg/mL=39.3±2.8%; p<0.0009 for all doses). Altogether these results identify the critical role of FcγR-signaling and ROS in alloantibody-dependent, macrophage-mediated hepatocytotoxicity.
To further investigate the role of macrophage-derived ROS, we tested WT and p47-deficient bone marrow-derived macrophages (BMM) in the in vitro macrophage-mediated hepatocytotoxicity assay. Since p47 is a subunit of NADPH oxidase, it is required for ROS production (51). Co-culture of hepatocytes with BMM that are p47-deficient led to significantly reduced hepatocytotoxicity (8.3±3.8%) compared to co-culture with WT BMM (38.6±6.1%; p<0.0009; Figure 4C). Collectively, these studies implicate ROS as critically important cytotoxic effector molecules released by macrophages downstream of alloantibody-dependent FcγR-mediated signaling.
Alloantibody-dependent macrophage-mediated hepatocyte rejection is FcγR and ROS-dependent.
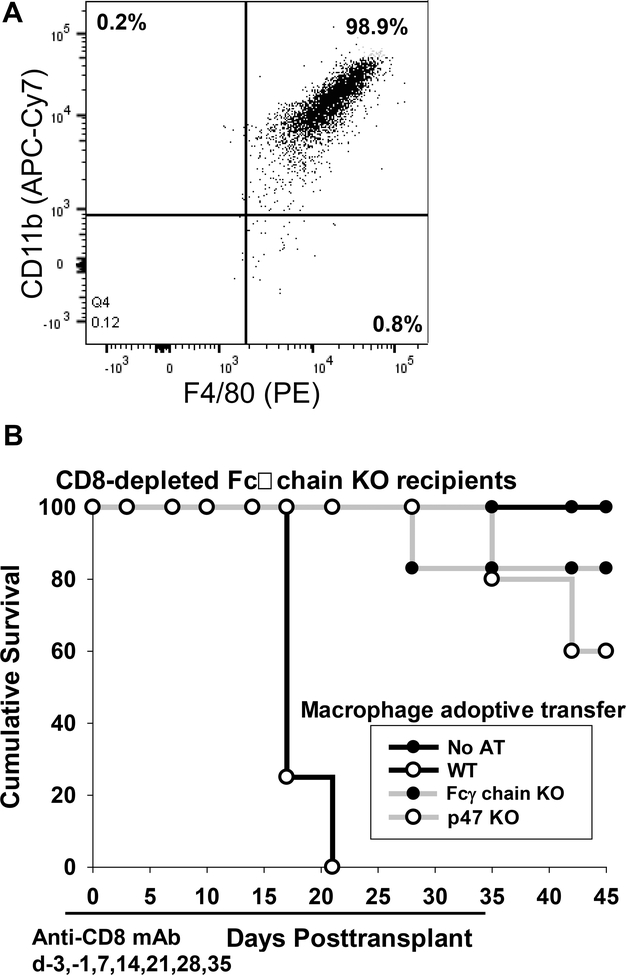
To investigate the role of ROS on in vivo alloantibody-dependent, macrophage mediated hepatocyte rejection, CD8-depleted Fcγ chain KO mice (H-2b) were transplanted with allogeneic FVB/N hepatocytes (H-2q) on day 0. CD8-depletion occurred by intraperitoneal administration of anti-CD8 mAb on days −3, −1, and weekly post transplant until day 35. Fcγ chain KO recipients were adoptively transferred with WT, p47-deficient, or control Fcγ chain KO BMM (5×106 cells) immediately following transplant. BMM preparations were >98% pure as shown by F4/80 and CD11b dual expression by flow cytometry (Figure 5A). CD8-depleted WT and Fcγ chain KO recipients both produce high amounts of alloantibody (peak on day 14 post transplant) with the same predominant IgG1 and low titer IgG3 alloantibody isotype profile (Supplemental Figure 1). Adoptive transfer of WT BMM led to rapid rejection of allogeneic hepatocytes (MST=day 17) with all recipients rejecting by day 21 (Figure 5B). In contrast, adoptive transfer of p47-deficient BMM (which do not produce ROS) into CD8-depleted Fcγ chain KO recipients did not precipitate acute hepatocyte rejection in the majority of recipients (MST>45 days). In fact, eighty-percent of recipients which received adoptive transfer of p47-deficient BMM had ongoing graft survival at post transplant day 35 study endpoint (when anti-CD8 mAb therapy was discontinued) which was significantly prolonged compared to the recipients which received WT BMM (p<0.0001) (Figure 5B). Likewise, the adoptive transfer of control Fcγ chain KO BMM did not precipitate rejection in the majority of recipients (MST>45 days). Eighty-three percent of recipients which received Fcγ chain KO BMM had ongoing graft survival at post transplant day 35 study endpoint. Following the cessation of anti-CD8 mAb treatment on day 35 post transplant in recipients adoptively transferred with p47-deficient or Fcγ chain KO BMM, hepatocyte rejection occurred in all recipients by day 56 post transplant which correlated with the repopulation of peripheral CD8+ T cells. In order to investigate the possibility that impaired in vivo macrophage-mediated hepatocyte rejection by p47-deficient or Fcγ chain KO BMM compared to WT BMM was due to impaired trafficking to the site of allogeneic hepatocyte engraftment in the host liver, we performed studies to determine the localization of fluorescently-labeled BMM after intravenous injection. Bone marrow macrophages were stained with CFSE before adoptive transfer by intravenous injection into hepatocyte transplant recipients. Recipients of CFSE+ BMM from all three groups were detected within the host liver by multiphoton microscopy. Thus, we found that WT, Fcγ chain KO, and p47 KO BMM appear to have comparable trafficking since macrophages from all three groups were detected in the host liver (Supplemental Figure 2). Altogether, these results support a humoral alloimmune mechanism of hepatocyte rejection which is mediated by macrophage FcγR signaling and macrophage-derived, ROS-mediated hepatocytotoxicity.
Figure 5. Macrophage-mediated alloantibody-dependent hepatocyte rejection is mediated by FcγR and ROS.
Fcγ chain KO (H-2b) mice were transplanted with allogeneic FVB/N hepatocytes (H-2q) on day 0. All recipients were CD8-depleted (days −3, −1, and weekly post transplant until day +35) to suppress cell-mediated rejection. On the same day of transplant, recipients were adoptively transferred (AT) with bone marrow macrophages from WT, Fcγ chain KO, or p47-deficient mice. A) Representative data shows that adoptively transferred bone marrow macrophages cells were >98% macrophages as determined by dual expression of F4/80 and CD11b by flow cytometry. B) Adoptive transfer of WT bone marrow macrophages precipitated rapid rejection (MST= 17; n=4) while adoptive transfer of Fcγ chain KO (n=6) and p47-deficient (n=5) bone marrow macrophages did not (MST > 45 days, p<0.0001 for both).
Discussion
We have previously reported that allogeneic hepatocytes initiate a robust humoral alloimmune response and that alloantibody (in the absence of cell-mediated rejection) is sufficient to mediate hepatocellular allograft rejection in a dose-dependent fashion (8). In the setting of high alloantibody titers without the use of immunosuppressants, hepatocellular allograft rejection occurs between 10 and 17 days post transplant (9). Allogeneic hepatocytes express predominantly MHC class I (52), though in some studies hepatocytes may be induced to express MHC class II (53). In our murine hepatocyte transplant model, we have detected predominantly MHC class I reactive alloantibodies (unpublished observations). However, results from clinical studies have reported the detection of both MHC class I and MHC class II reactive alloantibodies in association with the loss of hepatocellular allograft function after hepatocyte transplantation (4). Thus, both experimental and clinical studies implicate the importance of humoral alloimmune injury to the short-lived survival of transplanted hepatocytes.
We previously reported a critical role for macrophages in mediating alloantibody-dependent hepatocytotoxicity in vitro and alloantibody-dependent hepatocyte rejection in vivo (8, 9). In the current studies, we found that alloantibody-dependent, macrophage-mediated cytotoxicity to allogeneic hepatocytes in co-cultures is critically dependent on cell contact and the presence of Fcγ receptors. Blockade of both FcγRI and FcγRIII abrogated in vitro alloantibody-dependent, macrophage-mediated hepatotoxicity. Alloserum used in the co-cultures were collected from high alloantibody producing CD8-deficient hepatocyte recipients (both WT and Fcγ chain KO recipients) and is largely IgG1 with low level IgG3 and undetectable levels of IgG2a/IgG2b (26). Therefore, in the in vitro studies we targeted FcγRI and FcγRIII since these are the only FcγRs which recognize IgG3 and IgG1 isotypes, respectively (30).
In these studies macrophage-hepatocytotoxicity assays were performed with RAW cells (Balb/c, H-2d) or BMM (C57BL/6, H-2b). The occurrence of in vitro macrophage-mediated hepatocytotoxicity only occurs in macrophage-hepatocyte co-cultures containing allogeneic hepatocytes (FVB/N, Balb/c, or C57BL/6) and allosera (but not third-party sera; Supplemental Figure 3A,B). However, macrophage-mediated hepatocytotoxicity does not require concordance between macrophage strain and the host strain source of allosera. For example, C57BL/6 BMM mediate hepatocytotoxicity of FVB/N hepatocytes when co-cultured with allosera from FVB/N-primed Balb/c mice (Supplemental Figure 3C). This observation likely reflects the fact that both IgG Fc domain and FcγR are highly conserved [reviewed in (54, 55)].
In vivo studies also highlighted the importance of FcγRs in alloantibody-dependent hepatocyte rejection since AMR did not occur in CD8-depleted Fcγ chain KO recipients (unlike in CD8-depleted WT recipients) despite high alloantibody titers. The difference observed in hepatocellular allograft survival in CD8-depleted WT versus Fcγ chain KO recipients cannot be attributed to differences in alloantibody isotypes produced since isotypes in both WT and Fcγ chain KO recipient mice are comparable (Supplemental Figure 1). Instead we found that adoptive transfer of WT FcγR-bearing macrophages (but not BMM from Fcγ KO mice) into CD8-depleted Fcγ chain KO hepatocyte recipients is sufficient to precipitate acute rejection in all recipients by three weeks post transplant. However, once anti-CD8 mAb immunotherapy was stopped, acute rejection occurred in all recipients within three weeks indicating that these hosts were not tolerant and engrafted hepatocytes in these hosts remained vulnerable to CD8-mediated rejection. FcγRs are expressed on cell types other than macrophages; however we did not investigate their role on NK cells or neutrophils since we previously determined that neither of these cell subsets significantly contribute to in vivo alloantibody-dependent hepatocyte rejection (9). Nevertheless, our studies do not definitively exclude a potential role of other FcγR-bearing cell types in alloantibody-dependent hepatocyte rejection.
While macrophages can be categorized into pro-inflammatory classic M1 or anti-inflammatory “alternative” M2 phenotypes, they are known to respond to different environmental cues with a high degree of plasticity (56) and have multiple effector pathways including phagocytosis, cytokine production, NO and/or ROS production (reviewed in (57)). FcγR signaling in monocytes and macrophages drives the activation of numerous signaling cascades, including the MAPK (mitogen-activated protein kinase) pathway (including Erk and Jnk). This signaling mediates phagocytosis, NADPH oxidase assembly, and ROS production (29). Inhibitors of MEK1/2 (including UO126 used in these studies) prevent ROS production (49, 50) but also target multiple other cytotoxic effector pathways (46). ROS, including O2●-, H2O2, ●OH, etc., activate Jnk and caspases important in mediating apoptotic cell death in vitro (58) and necrosis in vitro (59). Parenchymal cells, including hepatocytes and renal tubules, are susceptible to ROS-mediated apoptosis in vivo (60). RAW cells and BMM [as well as peritoneal exudate cells (9)] used in our in vitro hepatotoxicity assay are all known to release ROS (61, 62). Substantial inhibition of macrophage-mediated hepatocytotoxicity in vitro with U0126, directed subsequent studies to further evaluate the role of macrophage-derived ROS using ROS-deficient BMM from p47 KO mice. No hepatocytotoxicity was observed in macrophage-hepatocyte co-cultures when macrophages were ROS-deficient. Similarly, adoptive transfer of ROS-deficient macrophages into CD8-depleted Fcγ chain KO hepatocyte recipients was not sufficient to precipitate acute rejection. Thus, both in vitro and in vivo results support a paradigm of alloantibody-dependent hepatocyte rejection which occurs by an antibody dependent, cell-mediated cytotoxic mechanism triggered by FcγR signaling on host macrophages which induces ROS-dependent cytotoxic damage of allogeneic liver parenchymal cells.
Reactive oxygen species have been implicated in a variety of processes mediating early immune injury of transplanted cells and organs including ischemia reperfusion injury and initiation of a pro-inflammatory cascade (60, 63) which promotes alloreactive immune responses [reviewed in (64)]. Transplantation of cells such as pancreatic islets are particularly susceptible to ROS-mediated damage not only following transplantation but also prior to transplant during the cell isolation process [reviewed in (65)]. Consequently, much attention has been focused on approaches to reduce early ROS-mediated allograft injury including the use of redox scavengers such superoxide dismutase mimetics, anti-oxidants, ion chelators, organ ischemic pre-conditioning as well as strategies to boost endogenous anti-oxidant defenses (66–68). Whereas the role of ROS in initiating early allograft damage is well recognized, the current report is the first to associate ROS as an effector mechanism of AMR and to identify macrophage-derived ROS as a critical effector in alloantibody mediated hepatocyte rejection.
It is likely that the dominant mediators of alloantibody-dependent, macrophage-mediated hepatocyte rejection are infiltrating host macrophages rather than residential liver macrophages (i.e., Kupffer cells). First, it is known that the ROS response is minimal in Kupffer cells (69, 70) and their primary effector function is phagocytosis (71). In contrast, infiltrating macrophages are known to have a strong ROS response (72). Second, this premise is also supported by our prior work where we found that macrophage-deficient recipients (MCSF KO, op/op) (73) are resistant to alloantibody-mediated hepatocyte rejection despite high alloantibody titers (9). MCSF KO mice are known to have impaired macrophage infiltration to sites of inflammation (74, 75). However, Kupffer cells (liver resident macrophages) are present in MCSF KO mice [albeit reduced in number (73, 76)] and retain normal phagocytic capability, killing activity, and exhibit enhanced NO release (75). Therefore, evidence to date suggests that infiltrating, rather than residential macrophages are the main cellular effectors of alloantibody mediated hepatocyte rejection.
Collectively, these studies highlight the critical role of host macrophage FcγR signaling and ROS mediated cytotoxicity in alloantibody-mediated hepatocyte rejection. Our results may have relevance not only for transplanted hepatocytes (4), but also for AMR of other cell types such as pancreatic islets (77, 78), bone marrow (79) and potentially for solid organ transplants when the integrity of the endothelial vasculature is compromised (9). For example, it is interesting to note that macrophages are detected in the interstitium and peritubular capillaries concurrent with other pathologic features diagnostic of AMR after kidney transplantation (80, 81). Similar studies have reported that detection of intravascular macrophages correlates with AMR after cardiac (82, 83) and liver (84) transplantation. Furthermore, in the presence of pathogenic autoantibodies as in lupus nephritis, macrophages are deleterious to renal parenchymal cells (85) and macrophage infiltration into the renal tubules and glomeruli is associated with a poor outcome in lupus nephritis (86). Thus, results in the current studies may be relevant to parenchymal cell immune damage in other conditions involving pathogenic antibodies. These results raise the possibility of developing novel immunotherapeutic strategies targeting macrophages, selected FcγRs and/or downstream cytotoxic effector molecules such as ROS to inhibit ongoing AMR. ROS scavengers have been shown to prolong graft survival in models of skin transplant (87) and it will be interesting to test these and other strategies to mitigate alloantibody mediated hepatocyte rejection. Additional studies in other cell and solid organ transplant models will be required to determine the generalizability of our findings to other allografts. A report by Sunay et al. raises caution regarding potential untoward effects of targeting FcγRIII. In their studies using a murine model of cardiac transplantation, they unexpectedly found accelerated rejection of cardiac allografts which were transplanted into FcγRIII KO recipients (88). These “paradoxical” results were attributed to overall pro-inflammatory immune responses, enhanced alloantibody production, and complement activation arising from disruption of FcγRIII-dependent macrophage-mediated clearance of apoptotic bodies. The differences observed between these two studies could be related to other non-FcγR related defects associated with the FcγRIII KO host, differences in AMR mechanisms for cell and solid organ transplant, differences in alloantibody isotypes (in our studies alloantibody was predominantly non-complement activating IgG1 whereas alloantibody produced in the cardiac transplant model was predominantly complement activating IgG2a and IgG2b isotypes) and/or differences associated with targeting a single FcγR (FcγRIII) versus all FcγRs.
Supplementary Material
Acknowledgments
Support: This work was supported in part by grants from the ASTS-NKF (National Kidney Foundation) Folkert Belzer, MD, Research Award (to T.A.P.), and National Institutes of Health grants DK072262 and AI083456 (to G.L.B.), and F32 DK082148 (NIDDK; to J.M.Z.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Nonstandard Abbreviations:
- AMR
antibody-mediated rejection
- BMM
bone marrow macrophage
- FcγR
Fc gamma receptor
- hA1AT
human alpha-1 antitrypsin
- LDH
lactate dehydrogenase
- SOT
solid organ transplant
- ROS
reactive oxygen species
References
- 1.Colvin RB, and Smith RN 2005. Antibody-mediated organ-allograft rejection. Nat. Rev. Immunol 5: 807–817. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz M, Regele H, Schillinger M, Exner M, Rasoul-Rockenschaub S, Wahrmann M, Kletzmayr J, Silberhumer G, Horl WH, and Bohmig GA 2004. Risk factors for capillary C4d deposition in kidney allografts: evaluation of a large study cohort. Transplantation. 78: 447–452. [DOI] [PubMed] [Google Scholar]
- 3.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, and Shapiro AM 2005. Five-year follow-up after clinical islet transplantation. Diabetes. 54: 2060–2069. [DOI] [PubMed] [Google Scholar]
- 4.Jorns C, Nowak G, Nemeth A, Zemack H, Mork LM, Johansson H, Gramignoli R, Watanabe M, Karadagi A, Alheim M, Hauzenberger D, van Dijk R, Bosma PJ, Ebbesen F, Szakos A, Fischler B, Strom S, Ellis E, and Ericzon BG 2016. De Novo Donor-Specific HLA Antibody Formation in Two Patients With Crigler-Najjar Syndrome Type I Following Human Hepatocyte Transplantation With Partial Hepatectomy Preconditioning. Am. J. Transplant 16: 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colvin RB 2007. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J. Am. Soc. Nephrol 18: 1046–1056. [DOI] [PubMed] [Google Scholar]
- 6.Cai J, and Terasaki PI 2005. Humoral theory of transplantation: mechanism, prevention, and treatment. Hum. Immunol 66: 334–342. [DOI] [PubMed] [Google Scholar]
- 7.Haas M 2014. An updated Banff schema for diagnosis of antibody-mediated rejection in renal allografts. Curr. Opin. Organ Transplant 19: 315–322. [DOI] [PubMed] [Google Scholar]
- 8.Horne PH, Lunsford KE, Eiring AM, Wang Y, Gao D, and Bumgardner GL 2005. CD4+ T-cell-dependent immune damage of liver parenchymal cells is mediated by alloantibody. Transplantation. 80: 514–521. [DOI] [PubMed] [Google Scholar]
- 9.Horne PH, Zimmerer JM, Fisher MG, Lunsford KE, Nadasdy G, Nadasdy T, van Rooijen N, and Bumgardner GL 2008. Critical role of effector macrophages in mediating CD4-dependent alloimmune injury of transplanted liver parenchymal cells. J. Immunol 181: 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond AS, and Gill RG 2000. An essential contribution by IFN-gamma to CD8+ T cell-mediated rejection of pancreatic islet allografts. J. Immunol 165: 247–255. [DOI] [PubMed] [Google Scholar]
- 11.Lunsford KE, Jayanshankar K, Eiring AM, Horne PH, Koester MA, Gao D, and Bumgardner GL 2008. Alloreactive (CD4-Independent) CD8+ T cells jeopardize long-term survival of intrahepatic islet allografts. Am. J. Transplant 8: 1113–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmerer JM, Horne PH, Fiessinger LA, Fisher MG, Pham TA, Saklayen SL, and Bumgardner GL 2012. Cytotoxic Effector Function of CD4-Independent, CD8+ T Cells Is Mediated by TNF-alpha/TNFR. Transplantation. 94: 1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerer JM, Horne PH, Fisher MG, Pham TA, Lunsford KE, Ringwald BA, Avila CL, and Bumgardner GL 2016. Unique CD8+ T Cell-Mediated Immune Responses Primed in the Liver. Transplantation. 100: 1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Sousa MV, Goncalez AC, Zollner RL, and Mazzali M 2018. Effect of Preformed or De Novo Anti-HLA Antibodies on Function and Graft Survival in Kidney Transplant Recipients. Ann. Transplant. 23: 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quintella A, Lasmar MF, Fabreti-Oliveira RA, and Nascimento E 2018. Delayed Graft Function, Predictive Factors, and 7-Year Outcome of Deceased Donor Kidney Transplant Recipients With Different Immunologic Profiles. Transplant Proc. 50: 737–742. [DOI] [PubMed] [Google Scholar]
- 16.Barten MJ, Schulz U, Beiras-Fernandez A, Berchtold-Herz M, Boeken U, Garbade J, Hirt S, Richter M, Ruhpawar A, Sandhaus T, Schmitto JD, Schonrath F, Schramm R, Schweiger M, Wilhelm M, and Zuckermann A 2018. The clinical impact of donor-specific antibodies in heart transplantation. Transplant. Rev doi:10.1016/j.trre.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Nozaki T, Amano H, Bickerstaff A, Orosz CG, Novick AC, Tanabe K, and Fairchild RL 2007. Antibody-mediated rejection of cardiac allografts in CCR5-deficient recipients. J. Immunol 179: 5238–5245. [DOI] [PubMed] [Google Scholar]
- 18.Kovandova B, Slavcev A, Sekerkova Z, Honsova E, and Trunecka P 2018. Antibody-Mediated rejection after liver transplantation - relevance of C1q and C3d-Binding antibodies. HLA. doi:10.111/tan.13354. [DOI] [PubMed] [Google Scholar]
- 19.Roux A, Bendib Le Lan I, Holifanjaniaina S, Thomas KA, Picard C, Grenet D, De Miranda S, Douvry B, Beaumont-Azuar L, Sage E, Devaquet J, Cuquemelle E, Le Guen M, Suberbielle C, Gautreau C, Stern M, Rossetti M, Hamid AM, and Parquin F 2017. Characteristics of Donor-Specific Antibodies Associated With Antibody-Mediated Rejection in Lung Transplantation. Front. Med. 4: 155. doi: 10.3389/fmed.2017.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Gouveia RH, Vitorino E, Ramos S, Rebocho MJ, Queiros EMJ, Martins AP, and Moura ML 2009. C4d-the witness of humoral rejection. Transplant. Proc 41: 866–867. [DOI] [PubMed] [Google Scholar]
- 21.Bickerstaff A, Nozaki T, Wang JJ, Pelletier R, Hadley G, Nadasdy G, Nadasdy T, and Fairchild RL 2008. Acute humoral rejection of renal allografts in CCR5(−/−) recipients. Am. J. Transplant 8: 557–566. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerer JM, Horne PH, Fiessinger LA, Fisher MG, Jayashankar K, Garcia SF, Abdel-Rasoul M, van Rooijen N, and Bumgardner GL 2013. Inhibition of recall responses through complementary therapies targeting CD8+ T-cell- and alloantibody-dependent allocytotoxicity in sensitized transplant recipients. Cell Transplant. 22: 1157–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kfoury AG, Miller DV, Snow GL, Afshar K, Stehlik J, Drakos SG, Budge D, Fang JC, Revelo MP, Alharethi RA, Gilbert EM, Caine WT, McKellar S, Molina KM, and Hammond MEH 2016. Mixed cellular and antibody-mediated rejection in heart transplantation: In-depth pathologic and clinical observations. J. Heart Lung Transplant. 35: 335–341. [DOI] [PubMed] [Google Scholar]
- 24.Thrush PT, Pahl E, Naftel DC, Pruitt E, Everitt MD, Missler H, Zangwill S, Burch M, Hoffman TM, Butts R, and Mahle WT 2016. A multi-institutional evaluation of antibody-mediated rejection utilizing the Pediatric Heart Transplant Study database: Incidence, therapies and outcomes. J. Heart Lung Transplant 35: 1497–1504. [DOI] [PubMed] [Google Scholar]
- 25.Reeve J, Bohmig GA, Eskandary F, Einecke G, Lefaucheur C, Loupy A, Halloran PF, and M. M.-K. s. group. 2017. Assessing rejection-related disease in kidney transplant biopsies based on archetypal analysis of molecular phenotypes. JCI Insight. e94197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerer JM, Pham TA, Sanders VM, and Bumgardner GL 2010. CD8+ T cells negatively regulate IL-4-dependent, IgG1-dominant posttransplant alloantibody production. J. Immunol. 185: 7285–7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, Singer K, and Lumeng CN 2013. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 62: 2762–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dijstelbloem HM, van de Winkel JG, and Kallenberg CG 2001. Inflammation in autoimmunity: receptors for IgG revisited. Trends Immunol. 22: 510–516. [DOI] [PubMed] [Google Scholar]
- 29.Nimmerjahn F, and Ravetch JV 2008. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol 8: 34–47. [DOI] [PubMed] [Google Scholar]
- 30.Bruhns P 2012. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 119: 5640–5649. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerer JM, Swamy P, Sanghavi PB, Wright CL, Abdel-Rasoul M, Elzein SM, Brutkiewicz RR, and Bumgardner GL 2014. Critical role of NKT cells in posttransplant alloantibody production. Am. J. Transplant 14: 2491–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gavin AL, Tan PS, and Hogarth PM 1998. Gain-of-function mutations in FcgammaRI of NOD mice: implications for the evolution of the Ig superfamily. EMBO J. 17: 3850–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancardi DA, Iannascoli B, Hoos S, England P, Daeron M, and Bruhns P 2008. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J. Clin. Invest 118: 3738–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daeron M 1997. Fc receptor biology. Annu. Rev. Immunol 15: 203–234. [DOI] [PubMed] [Google Scholar]
- 35.Mendoza-Coronel E, and Ortega E 2017. Macrophage Polarization Modulates FcgammaR- and CD13-Mediated Phagocytosis and Reactive Oxygen Species Production, Independently of Receptor Membrane Expression. Front. Immmunol. 8: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeap WH, Wong KL, Shimasaki N, Teo ECY, Quek JKS, Yong HX, Diong CP, Bertoletti A, Linn YC, and Wong SC 2016. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep 6: 34310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bumgardner GL, Heininger M, Li J, Xia D, Parker-Thornburg J, Ferguson RM, and Orosz CG 1998. A Functional Model of Hepatocyte Transplantation for in Vivo Immunologic Studies. Transplantation. 65: 53–61. [DOI] [PubMed] [Google Scholar]
- 38.Bumgardner GL, Gao D, Li J, Baskin JH, Heininger M, and Orosz CG 2000. Rejection responses to allogeneic hepatocytes by reconstituted SCID mice, CD4, KO, and CD8 KO mice. Transplantation. 70: 1771–1780. [DOI] [PubMed] [Google Scholar]
- 39.Rajaram MV, Butchar JP, Parsa KV, Cremer TJ, Amer A, Schlesinger LS, and Tridandapani S 2009. Akt and SHIP modulate Francisella escape from the phagosome and induction of the Fas-mediated death pathway. PLoS One. 4: e7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guicciardi ME, Malhi H, Mott JL, and Gores GJ 2013. Apoptosis and necrosis in the liver. Compr. Physiol. 3: 977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergstrom JJ, and Heyman B 2015. IgG Suppresses Antibody Responses in Mice Lacking C1q, C3, Complement Receptors 1 and 2, or IgG Fc-Receptors. PLoS One. 10: e0143841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Montfoort N, t Hoen PA, Mangsbo SM, Camps MG, Boross P, Melief CJ, Ossendorp F, and Verbeek JS 2012. Fcgamma receptor IIb strongly regulates Fcgamma receptor-facilitated T cell activation by dendritic cells. J. Immunol 189: 92–101. [DOI] [PubMed] [Google Scholar]
- 43.Horne PH, Koester MA, Jayashankar K, Lunsford KE, Dziema HL, and Bumgardner GL 2007. Disparate primary and secondary allospecific CD8+ T cell cytolytic effector function in the presence or absence of host CD4+ T cells. J. Immunol 179: 80–88. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Gao D, Lunsford KE, Frankel WL, and Bumgardner GL 2003. Targeting LFA-1 synergizes with CD40/CD40L blockade for suppression of both CD4-dependent and CD8-dependent rejection. Am. J. Transplant 3: 1251–1258. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerer JM, Horne PH, Fisher MG, Pham TA, Lunsford KE, Ringwald BA, Avila CL, and Bumgardner GL 2016. Unique CD8+ T Cell-Mediated Immune Responses Primed in the Liver. Transplantation. 100: 1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scherle PA, Jones EA, Favata MF, Daulerio AJ, Covington MB, Nurnberg SA, Magolda RL, and Trzaskos JM 1998. Inhibition of MAP kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J. Immunol 161: 5681–5686. [PubMed] [Google Scholar]
- 47.Mendes Sdos S, Candi A, Vansteenbrugge M, Pignon MR, Bult H, Boudjeltia KZ, Munaut C, and Raes M 2009. Microarray analyses of the effects of NF-kappaB or PI3K pathway inhibitors on the LPS-induced gene expression profile in RAW264.7 cells: synergistic effects of rapamycin on LPS-induced MMP9-overexpression. Cell. Signal 21: 1109–1122. [DOI] [PubMed] [Google Scholar]
- 48.Avni D, Glucksam Y, and Zor T 2012. The phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 modulates cytokine expression in macrophages via p50 nuclear factor kappaB inhibition, in a PI3K-independent mechanism. Biochem. Pharmacol 83: 106–114. [DOI] [PubMed] [Google Scholar]
- 49.Chatterjee S, Feinstein SI, Dodia C, Sorokina E, Lien YC, Nguyen S, Debolt K, Speicher D, and Fisher AB 2011. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J. Biol. Chem. 286: 11696–11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, and Dekel N 2011. Reactive oxygen species are indispensable in ovulation. Proc. NAtl. Acad. Sci. U.S.A. 108: 1462–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, and Shah AM 2002. Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ. Res. 90: 143–150. [DOI] [PubMed] [Google Scholar]
- 52.Bumgardner GL, Chen S, Hoffman R, Cahill DC, So SK, Platt J, Bach FH, and Ascher NL 1989. Afferent and efferent pathways in T cell responses to MHC class I+, II-hepatocytes. Transplantation. 47: 163–170. [DOI] [PubMed] [Google Scholar]
- 53.Franco A, Barnaba V, Natali P, Balsano C, Musca A, and Balsano F 1988. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology. 8: 449–454. [DOI] [PubMed] [Google Scholar]
- 54.Ravetch FN a. J. V. 2007. Fc- Receptors as Regulators of Immunity. Adv. Immunol. 96: 179–204. [DOI] [PubMed] [Google Scholar]
- 55.Brian Moldt AJH 2014. FcyRs Across Species In Antibody Fc Linking Adaptive and Innate Immunity, 1st ed. Ackerman M and Nimmerjahn F, eds. Academic Press, San Diego, CA: p. 145–157. [Google Scholar]
- 56.Mosser DM, and Edwards JP 2008. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol 8: 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dale DC, Boxer L, and Liles WC 2008. The phagocytes: neutrophils and monocytes. Blood. 112: 935–945. [DOI] [PubMed] [Google Scholar]
- 58.Jaeschke H 2011. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol Hepatol. 26: Suppl 1: 173–179. [DOI] [PubMed] [Google Scholar]
- 59.Hong JY, Lebofsky M, Farhood A, and Jaeschke H 2009. Oxidant stress-induced liver injury in vivo: role of apoptosis, oncotic necrosis, and c-Jun NH2-terminal kinase activation. Am. J. Physiol. Gastrointest. Liver Physiol. 296: G572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W, Wang M, Xie HY, Zhou L, Meng XQ, Shi J, and Zheng S 2007. Role of reactive oxygen species in mediating hepatic ischemia-reperfusion injury and its therapeutic applications in liver transplantation. Transplant. Proc.39: 1332–1337. [DOI] [PubMed] [Google Scholar]
- 61.Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, and Lee SY 2005. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 106: 852–859. [DOI] [PubMed] [Google Scholar]
- 62.Horio F, Fukuda M, Katoh H, Petruzzelli M, Yano N, Rittershaus C, Bonner-Weir S, and Hattori M 1994. Reactive oxygen intermediates in autoimmune islet cell destruction of the NOD mouse induced by peritoneal exudate cells (rich in macrophages) but not T cells. Diabetologia. 37: 22–31. [DOI] [PubMed] [Google Scholar]
- 63.Linas SL, Whittenburg D, Parsons PE, and Repine JE 1995. Ischemia increases neutrophil retention and worsens acute renal failure: role of oxygen metabolites and ICAM 1. Kidney Int. 48: 1584–1591. [DOI] [PubMed] [Google Scholar]
- 64.Ingulli E 2010. Mechanism of cellular rejection in transplantation. Pediatr. Nephrol. 25: 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barra JM, and Tse HM 2018. Redox-Dependent Inflammation in Islet Transplantation Rejection. Front. Endocrinol 9: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi S, and Xue F 2016. Current Antioxidant Treatments in Organ Transplantation. Oxid. Med. Cell Longev. 2016: 8678510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ambrosi N, Guerrieri D, Caro F, Sanchez F, Haeublein G, Casadei D, Incardona C, and Chuluyan E 2018. Alpha Lipoic Acid: A Therapeutic Strategy that Tend to Limit the Action of Free Radicals in Transplantation. Int. J. Mol. Sci 19: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bilska A, and Wlodek L 2005. Lipoic acid - the drug of the future? Pharmacol. Rep. 57: 570–577. [PubMed] [Google Scholar]
- 69.ten Hagen TL, van Vianen W, and Bakker-Woudenberg IA 1996. Isolation and characterization of murine Kupffer cells and splenic macrophages. J. Immunol. Methods 193: 81–91. [DOI] [PubMed] [Google Scholar]
- 70.Lepay DA, Nathan CF, Steinman RM, Murray HW, and Cohn ZA 1985. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J. Exp. Med 161: 1079–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knolle P, Lohr H, Treichel U, Dienes HP, Lohse A, Schlaack J, and Gerken G 1995. Parenchymal and nonparenchymal liver cells and their interaction in the local immune response. Z. Gastroenterol 33: 613–620. [PubMed] [Google Scholar]
- 72.Koller CA, and LoBuglio AF 1981. Monocyte-mediated antibody-dependent cell-mediated cytotoxicity: the role of the metabolic burst. Blood. 58: 293–299. [PubMed] [Google Scholar]
- 73.Naito M, Hayashi S, Yoshida H, Nishikawa S, Shultz LD, and Takahashi K 1991. Abnormal differentiation of tissue macrophage populations in ‘osteopetrosis’ (op) mice defective in the production of macrophage colony-stimulating factor. Am. J. Pathol 139: 657–667. [PMC free article] [PubMed] [Google Scholar]
- 74.Wiktor-Jedrzejczak WW, Ahmed A, Szczylik C, and Skelly RR 1982. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J. Exp. Med 156: 1516–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schonlau F, Schlesiger C, Ehrchen J, Grabbe S, Sorg C, and Sunderkotter C 2003. Monocyte and macrophage functions in M-CSF-deficient op/op mice during experimental leishmaniasis. J. Leukoc. Biol 73: 564–573. [DOI] [PubMed] [Google Scholar]
- 76.Witmer-Pack MD, Hughes DA, Schuler G, Lawson L, McWilliam A, Inaba K, Steinman RM, and Gordon S 1993. Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J. Cell Sci 104 (Pt 4): 1021–1029. [DOI] [PubMed] [Google Scholar]
- 77.Mohanakumar T, Narayanan K, Desai N, Ramachandran S, Shenoy S, Jendrisak M, Susskind BM, Olack B, Benshoff N, Phelan DL, Brennan DC, Fernandez LA, Odorico JS, and Polonsky KS 2006. A significant role for histocompatibility in human islet transplantation. Transplantation. 82: 180–187. [DOI] [PubMed] [Google Scholar]
- 78.Rickels MR, Kamoun M, Kearns J, Markmann JF, and Naji A 2007. Evidence for allograft rejection in an islet transplant recipient and effect on beta-cell secretory capacity. J. Clin. Endocrinol. Metab 92: 2410–2414. [DOI] [PubMed] [Google Scholar]
- 79.Xu H, Chilton PM, Tanner MK, Huang Y, Schanie CL, Dy-Liacco M, Yan J, and Ildstad ST 2006. Humoral immunity is the dominant barrier for allogeneic bone marrow engraftment in sensitized recipients. Blood. 108: 3611–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mannon RB 2012. Macrophages: contributors to allograft dysfunction, repair, or innocent bystanders? Curr. Opin. Organ Transplant 17: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fahim T, Bohmig GA, Exner M, Huttary N, Kerschner H, Kandutsch S, Kerjaschki D, Brambock A, Nagy-Bojarszky K, and Regele H 2007. The cellular lesion of humoral rejection: predominant recruitment of monocytes to peritubular and glomerular capillaries. Am. J. Transplant 7: 385–393. [DOI] [PubMed] [Google Scholar]
- 82.Ratliff NB, and McMahon JT 1995. Activation of intravascular macrophages within myocardial small vessels is a feature of acute vascular rejection in human heart transplants. J. Heart Lung Transplant. 14: 338–345. [PubMed] [Google Scholar]
- 83.Fishbein GA, and Fishbein MC 2012. Morphologic and immunohistochemical findings in antibody-mediated rejection of the cardiac allograft. Human Immunol. 73: 1213–1217. [DOI] [PubMed] [Google Scholar]
- 84.Dankof A, Schmeding M, Morawietz L, Gunther R, Krukemeyer MG, Rudolph B, Koch M, Krenn V, and Neumann U 2005. Portal capillary C4d deposits and increased infiltration by macrophages indicate humorally mediated mechanisms in acute cellular liver allograft rejection. Virchows Arch. 447: 87–93. [DOI] [PubMed] [Google Scholar]
- 85.Chalmers SA, Chitu V, Herlitz LC, Sahu R, Stanley ER, and Putterman C 2014. Macrophage depletion ameliorates nephritis induced by pathogenic antibodies. J. Autoimmun. 57: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hill GS, Delahousse M, Nochy D, Remy P, Mignon F, Mery JP, and Bariety J 2001. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int. 59: 304–316. [DOI] [PubMed] [Google Scholar]
- 87.Tocco G, Illigens BM, Malfroy B, and Benichou G 2006. Prolongation of alloskin graft survival by catalytic scavengers of reactive oxygen species. Cell. Immunol 241: 59–65. [DOI] [PubMed] [Google Scholar]
- 88.Erdinc Sunay MM, Fox-Talbot K, Velidedeoglu E, Baldwin WM 3rd, and Wasowska BA 2013. Absence of FcgammaRIII results in increased proinflammatory response in FcgammaRIII-KO cardiac recipients. Transplantation. 96: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.