Abstract
Trophoblast invasion and remodeling of the maternal spiral arteries are required for pregnancy success. Aberrant endothelium-trophoblast crosstalk may lead to preeclampsia, a pregnancy complication that has serious effects on both the mother and the baby. However, our understanding of the mechanisms involved in this pathology remains elementary because current in vitro models cannot describe trophoblast-endothelium interactions under dynamic culture. In this work, we developed a dynamic 3D placenta model by bioprinting trophoblasts and an endothelialized lumen in a perfusion bioreactor. We found the perfusion bioreactor system significantly augmented responses of endothelial cells by encouraging network formations and expressions of angiogenic markers CD31, MMP2, MMP9, and VEGFA. Bioprinting favored colocalization trophoblasts with endothelial cells, similar to in vivo observations. Additional analysis revealed that trophoblasts reduced the angiogenic responses by reducing network formation and motility rates while inducing apoptosis of endothelial cells. Moreover, presence of endothelial cells appeared to inhibit trophoblast invasion rates. These results clearly demonstrated the utility and potential of bioprinting and perfusion bioreactor system to model trophoblast-endothelium interactions in vitro. Our bioprinted placenta model represents a crucial step to develop advanced research approach that will expand our understanding and treatment options of preeclampsia and other pregnancy-related pathologies.
Keywords: placenta, endothelium, preeclampsia, tissue model, bioreactor
INTRODUCTION
Prior to the arrival of the embryo and fetal trophoblast, vascular remodeling occurs in the maternal endometrium in preparation for embryo implantation (Demir, Yaba, & Huppertz, 2010; Halasz & Szekeres-Bartho, 2013). Contractile activities of the uterus cause interstitial flow that are important for proper embryo implantation and pregnancy success, but the effect of fluid shear stress from interstitial flow on angiogenesis remain rarely investigated (Aguilar & Mitchell, 2010). Improper implantation can lead to preeclampsia, which is a leading cause of maternal and perinatal morbidity and mortality that affects 3 to 8% of all pregnancies (Eric A P Steegers, 2010). Preeclampsia is characterized by intense systemic vasoconstriction leading to maternal hypertension, a clinical hallmark for preeclampsia (American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy & American College of Obstetricians and Gynecologists, 2013). Globally, about 76,000 pregnant women and 500,000 infants are dying from preeclampsia and related hypertensive disorders (Kuklina, Ayala, & Callaghan, 2009). Early evidences of systemic endothelial dysfunction in preeclampsia came from measurement of plasma biomarkers of endothelial cell activation including von Willebrand factor (Bergmann, Rotmensch, Rosenzweig, How, & Chediak, 1991), plasma cellular fibronectin (Friedman, Degroot, Taylor, Golditch, & Roberts, 1994), thrombomodulin (Boffa et al., 1998), endothelin-1 (ET-1) (Schiff et al., 1992; Venkatesha et al., 2006), and soluble fms-like tyrosine kinase (sFlt-1) (Shibata et al., 2005). These findings demonstrate a clear correlation between endothelial dysfunction and preeclampsia, although the underlying mechanism remains unclear (Shibata et al., 2005).
After embryo implantation, fetal trophoblasts invade and continue to remodel the maternal spiral arteries to create a low-resistance, high-volume blood flow (Christopher W. Redman, 2005; Mohd Nordin Noraihan, 2005; Tinnakorn Chaiworapongsa, 2014; Young, Levine, & Karumanchi, 2010). Specifically, the fetal trophoblasts interdigitate between the endothelial cells and eventually replace the endothelial lining in the vessel walls of spiral arteries (Ashton et al., 2005). Histological data suggest that trophoblasts bind to, migrate, and transiently coexist on the walls of partially modified spiral arteries before replacing the endothelium (Enders & Blankenship, 1997; Pijnenborg, Dixon, Robertson, & Brosens, 1980). Impaired trophoblast invasion and remodeling of the spiral arteries are also associated with pregnancies complicated by preeclampsia (C. Y. Kuo et al., 2016). The detailed interactions between trophoblasts and the endothelial cells of the spiral arteries, which could be the fundamental cause of preeclampsia, have yet to be determined in normal or preeclamptic pregnancies.
Studies of spiral arteries have been confined primarily to immunohistochemical analysis of placental bed biopsies and these studies provides little mechanistic insights for trophoblast-mediated maternal vascular remodeling (Ashton et al., 2005). On the other hand, current in vitro studies have been hindered by the lack of suitable experimental models to directly examine cellular interactions between trophoblast and endothelial cells during invasion (Cartwright et al., 2002; Orendi et al., 2011). In this work, we developed a dynamic placenta model to address this challenge using bioprinting technology and a perfusion bioreactor system. We hypothesized that a dynamic placenta model can be developed to directly examine cellular interactions between trophoblast and endothelial cells. We tested the hypothesis by the following objectives: 1) To develop a dynamic placenta model with controlled fluid shear stress by combining the advantages of a peristaltic pump and 3D bioprinting; 2) To determine the effect of fluid shear stress on the angiogenic responses of endothelial cells in the dynamic placenta model; 3) To define the interactions between trophoblast and endothelial cells in the dynamic bioprinted placenta model.
We began by 3D printing customized bioreactor chambers to host the bioprinted placenta, and integrated with a peristaltic pump to establish the dynamic bioprinted placenta model base on our previous work (C.-Y. Kuo et al., 2018; C. Y. Kuo et al., 2016; Nguyen, Ko, Moriarty, Etheridge, & Fisher, 2016; Yeatts et al., 2014; Yeatts, Geibel, Fears, & Fisher, 2012; Yeatts, Gordon, & Fisher, 2011). The resulting shear stress profiles from the perfusion were physiologically relevant, as calculated via computational fluid dynamics. Next, we tested the angiogenic effect of the fluid shear stress using human umbilical vein endothelial cells (HUVEC) and found that the interstitial flow had a significant impact on endothelial cells located within 400 μm away from the lumen. Moreover, increasing shear stress led to higher angiogenic responses of endothelial cells. The enhanced angiogenic responses of endothelial cells were impaired when trophoblasts were bioprinted 4.2 mms away in an indirect coculture placenta model. When trophoblasts were bioprinted directly adjacent to the endothelial cells, the two cell populations interdigitated, mimicking ex vivo results (Ashton et al., 2005). Fluorescent staining and mRNA levels showed that the direct coculture of trophoblast and endothelial cells created a more apoptotic environment. These results suggest that endothelial cells and trophoblast interdigitated, and that apoptosis and angiogenesis are potential mechanisms for vascular remodeling during early placental development. Our novel dynamic placenta model enabled researchers to directly examine trophoblast-endothelium interactions in a dynamic environment for the first time, which will improve our fundamental understanding of preeclampsia and other human reproductive diseases.
MATERIALS AND METHODS
Cell Culture
Human Umbilical Vein Endothelial Cells (HUVEC) were purchased from Lonza, cultured in Endothelial Growth Medium Bullet Kits (EGM BulletKit; Lonza) according to manufacturer’s instructions, and used until passage 5. HTR8 cells, an extravillous trophoblast cell line, were purchased from American Type Culture Collection (ATCC) and cultured according to manufacturer’s instructions. For coculture experiments, bioprinted placenta models were cultured in complete endothelial growth medium.
Gelatin Methacrylate Synthesis
Gelatin methacrylate (GelMA) was synthesized according to previously published methods (C.-Y. Kuo et al., 2017). Briefly, type A porcine skin gelatin (Sigma-Aldrich; 300 bloom) was mixed at 10% (w/v) in phosphate buffered saline (PBS; Thermo Fisher Scientific) at 50°C for 20 minutes. Methacrylic anhydride (MA, Sigma-Aldrich) was then added at 0.6 g of MA / g of gelatin under rigorous stirring for one hour. The reactants were then diluted 2× with PBS to stop the reaction. The mixture was centrifuged, and the pellet was discarded. The supernatant was dialyzed against deionized water at 50°C using dialysis cassettes (10kDa molecular weight cut-off, Thermo Fisher Scientific) for at least 3 days. The deionized water was changed twice a day to remove salts and excess methacrylic acid. The dialyzed GelMA was then lyophilized for at least 3 days and store at −80°C until further use.
Bioprinted Placenta Model
We designed the bioprinted placenta model (BPM) based on previous published work (C.-Y. Kuo et al., 2018; C. Y. Kuo et al., 2016). The bioprinted placenta model is a gelatin-based, cylindrical hydrogel (diameter = 10 mm; height = 2 mm) with an patent central lumen (diameter = 1 mm; height = 2 mm) to model the spiral arteriole (1–2 mm in diameter (Jackson, Mayhew, & Boyd, 1992)). All bioprinting work was completed using a commercial 3D bioprinter (3D-Bioplotter; EnvisionTEC). To prepare the prepolymer solution, lyophilized GelMA was dissolved in complete endothelial growth media at 50°C for 20 minutes. Photoinitiator (2-hydroxy-1-(4-(hydroxyethoxy)phenyl)-2-methyl-1-propanone; Irgacure 2959; BASF) was then added into the GelMA solution at 0.1% (w/v) at 50°C for 15 minutes. The prepolymer solution was loaded into the low-temperature printer head and allowed to equilibrate for 20 minutes at 37°C. Fibronectin (50 μg/mL), HUVEC (10 million/mL), and/or extravillous trophoblast (HTR8, 2 million/mL) were then added. The final enriched prepolymer solutions were then loaded into printing cartridge and allowed to equilibrate to printing temperature (e.g. ~21°C) for another 30 minutes prior to printing. Printed constructs were UV-cured for 30 seconds (0.09 mW/cm2) using a UV box (VWR).
3D Printing of Customized Bioreactor Chambers
The bioreactor was designed using Solidworks 3D CAD software (Dassault Systèmes SolidWorks Corporation). The bioreactor is composed of two halves that form a single chamber when assembled together. Both halves of the bioreactor are separated by a FDA approved Viton Fluroelastomer O-ring (McMaster-Carr) to ensure a leak free seal when assembled. The CAD model was exported as a binary stereolithography (STL) file for 3D printing. A deviation tolerance of 0.50 μm and angular tolerance of 10 deg was chosen to preserve resolution during fabrication. The bioreactor was fabricated using the Connex500 3D printer (Stratasys) with Med610 polyjet material. Med610 was chosen for its biocompatibility to ensure the bioreactor did not adversely affect the bioprinted placenta model. After 3D printing, the bioreactors were cleaned with water and sodium hydroxide, and then disinfected with an isopropyl bath per the manufacture’s guidelines for Med610.
Perfusion Bioreactor System
The perfusion bioreactor system was assembled as described previously (Yeatts et al., 2014; Yeatts, Choquette, & Fisher, 2013; Yeatts et al., 2012; Yeatts et al., 2011) and illustrated in Figure 1d. Bioprinted placenta models were loaded into 3D printed bioreactor chamber (Figure 1f) and connected to the perfusion bioreactor system. The cell culture media was driven by an L/S Multichannel Pump System (Cole Parmer) with controlled flow rates and cultured for 3 days. At the end of the study, cells encapsulated in the bioprinted placenta model were isolated from GelMA scaffolds by dissolution in papain (4 mg/mL) for 30 min at 37°C. A cell pellet was formed by centrifugation and used for downstream analysis.
Figure 1. Development 3D Printed Perfusion Bioreactor System for the Dynamic Bioprinted Placenta Model.
(a) The 3D printed bioreactor chamber was composed a rack that the bioreactor chambers could slide into to keep them in place. (b) Flow was perfused through the center lumen of the bioreactor chamber (left) which induces shear stress along the wall. The wall shear stress was calculated by computational fluid dynamics (CFD, right). (c) CFD demonstrated a linear relationship between fluid shear stress and velocity in the physiologically relevant range. (d) The perfusion system was driven by a peristaltic pump (bottom) that drove the media from the media reservoirs through the 3D printed bioreactor chambers. The fluid then returned to the media reservoir to complete the circulation. The entire system was housed in a cell culture incubator with CO2, temperature, and humidity control. (e) Computational fluid dynamic predicts that oxygen concentration would reach equilibrium by 11 hours. (f) The picture on the top left demonstrated where the placenta model would be sitting relative to the 3D printed reactor chamber and was enlarged in the picture on the right. The bioprinted placenta model had a cylindrical shape (diameter = 10 mm; height = 2 mm) with a patent channel at the center (red arrow). Using a blue dye, we demonstrated that material diffused radially outwards from the central lumen after 12 hours of perfusion, which demonstrate the presence of interstitial flow.
Computational Fluid Dynamics (CFD)
The diffusion model developed in this study was generated using COMSOL with the following assumptions by assuming 1D radial diffusion in cylindrical coordinates. For diffusion of oxygen, we assumed a perfect source from the center of the disc and a no flux condition at the edge of the disc. The diffusion coefficient of oxygen in hydrogel (D=0.74 × 10−9m2s−1) was obtained from published literature (Vanstroebiezen, Everaerts, Janssen, & Tacken, 1993). In addition, the fluid shear stress from the bioreactor was calculated using SolidWorks 3D CAD software (Dassault Systèmes SolidWorks Corporation) simulation using water to model cell culture media. We assumed a no-slip boundary condition and that the pressure at the outlet of the bioreactor system was set at atmospheric pressure.
RNA Isolation and qRT-PCR
Cell pellets were dissolved in Trizol (Thermo Fisher Scientific). Total RNA was isolated using the RNeasy Plus Mini Kit (Qiagen) and then reverse transcribed to complementary DNA (cDNA) using a High Capacity cDNA Archive Kit (Thermo Fisher Scientific). Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was performed by combining the cDNA solution with a Universal Master Mix (Thermo Fisher Scientific), as well as oligonucleotide primers and Taqman probes for MMP2, MMP9, CASP3, CASP9 and the endogenous gene control glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Thermo Fisher Scientific). The reaction was performed using a 7900HT real-time PCR System (Applied Biosystems) at thermal conditions of 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 s at 95°C, and 1 min at 60°C. The relative gene expression level of each target gene was normalized to the mean of GAPDH in each group then the fold change was determined relative to appropriate controls. Fold change was calculated using the ΔΔCT relative comparative method as described previously (Nguyen et al., 2016).
Invasion Study
For the invasion experiments, phase-contrast images were taken on day 1 and day 3 using an inverted fluorescent microscope (Olympus). The distance between the periphery of the inner lumen of the bioprinted placenta model and the invasion fronts of endothelial cells were measured and averaged over time to obtain invasion/motility rates, as described previously.(C.-Y. Kuo et al., 2018; C. Y. Kuo et al., 2016; C. Y. Kuo, E. Wilson, et al., 2018)
Immunofluorescence staining of CD31 and Quantitative Image Analysis
Bioprinted placenta model were fixed in 3.7% formaldehyde solution for 15 minutes at room temperature and permeabilized with 0.3% Triton X-100 for 5 min. Hydrogels were blocked by 5% bovine serum albumin (BSA) solution for 30 min and incubated overnight with primary antibody (mouse anti-human CD31; 1:100 dilution; R&D systems). The stained HUVECs were imaged with a fluorescent microscope (Olympus). The endothelial cells were characterized by the number of linear aggregates, length of the aggregates, fraction of area covered by linear aggregates, and number of branching points using Image J.(Staton, Reed, & Brown, 2009) Linear aggregates were defined as aggregates of more than three cells with no more than two adjacent cells. At least 3 samples for each condition were processed for analysis.
Apoptosis Study
After 3 days of dynamic culture, bioprinted placenta model were harvested from the bioreactor system and stained with the Apoptosis/Necrosis Detection Kit (Abcam) according to manufacturer’s instruction. Briefly, harvested cell-laden bioprinted placenta models were washed twice with phosphate buffered saline (PBS) and incubated in a cocktail of fluorescently tagged antibodies of Apopxin Green Solution and CytoCalcein Violet 450 for 60 minutes. The stained bioprinted placenta models were observed under a fluorescent microscope (Olympus).
Statistical Analysis
Error bars were expressed as average ± standard deviation (SD) and * indicated statistically significant differences between groups (p<0.05). Data from all the studies were analyzed using student’s t-test and/or analysis of variance (ANOVA) using Minitab. All experiments were done in biological triplicates unless stated otherwise.
RESULTS
Utilizing Bioprinting and Perfusion Bioreactor System to Develop the Dynamic Placenta Model
Previous study demonstrated that interstitial flow can be generated near the surface of the hydrogels that was exposed to bulk fluid flow (Chen, Buckley, Cohen, Bonassar, & Awad, 2012). In the present work, we 3D printed a customized bioreactor chamber to fit the bioprinted placenta model based on a perfusion bioreactor system (Figure 1a,d) (C. Y. Kuo et al., 2016; Nguyen et al., 2016). The resulting fluid shear stress profile for the dynamic bioprinted placenta model was calculated through computational fluid dynamics (Figure 1b). By adjusting the flow velocity, we tuned the resulting shear stress to model that of the placental spiral arteriole (1 – 3 dyne/cm2 (Burton, Woods, Jauniaux, & Kingdom, 2009), Figure 1c). Diffusion of materials in the dynamic placenta model was qualitatively assessed by perfusing with blue dye. As shown, the central lumen was patent and visible (in Figure 1f, top two images; red arrow; d = 1 mm) at t = 0. After two hours of perfusion, limited radial diffusion was observed from the central lumen while the bulk of the 3D printed gel remain translucent. After 12 hours of perfusion, there was significant radial diffusion of the blue dye (Figure 1f). Computational fluid dynamics was again utilized to quantitatively assessed the mass transport phenomena within the placenta model (Figure 1e). We found a gradient in oxygen concentration only during the first 11 hours of dynamic culture, after which normoxic conditions were established throughout the bioprinted placenta model.
Position Influenced Proliferation and Angiogenic Responses of Endothelial Cells
To determine effect of position on angiogenesis of endothelial cells, we bioprinted placenta model with HUVEC homogenously distributed. Then we divided the placenta model into three zones according to their distances from the central lumen for quantitative image analysis (I = 0–200 μm, II = 200–400 μm, and III = 400–600 μm; Figure 2a). These regions were selected based on a previously published study that provided insights into shear stress and mass transport in a bioreactor-cultured hydrogels (Chen et al., 2012). In Figure 2b, fluorescent intensity of DAPI, which correlates with number of cells, decreased as distance from the central lumen increased (I = 0.92±0.08; II = 0.77±0.11; III = 0.66±0.15; p<0.05) which was consistent with previous work (Dimmeler, Assmus, Hermann, Haendeler, & Zeiher, 1998). Similarly, angiogenic responses such as number of linear aggregates (I = 92.67±37.69, II = 50.68±22.92, III = 8.61±5.51 /mm2; p<0.05) and fraction of area covered by HUVEC network (I = 7.9±2.81, II = 4.32±0.94, III = 0.74±0.92 %; p<0.05) significantly decreased as distance from the central lumen increased (Figure 2c,d). Altogether, we found that the angiogenic effects of flow on HUVEC residing more than 400 μm away from the lumen were significantly reduced compare to other groups. Therefore, all analyses for HUVEC for the rest of the work were focused on regions within 400 μm away from the lumen.
Figure 2. Position of HUVEC Influenced Proliferation and Angiogenesis in Dynamic Culture.
(a) The cross section of bioprinted placenta model was divided into 3 zones from the central lumen (I=0–200 μm, II=200–400 μm, and III=400–800 μm) for quantitative image analysis. (b) Average DAPI Fluorescent Intensity (FI), which correlated with the number of cells, was measured. The FI decreased as the distance from the central channel increased. (c) The number of linear aggregates/area of HUVEC decreased as the distance away from the central channel increased. (d) The fraction of area covered by HUVEC network decreased as distance from central channel increased. All quantitative assays were done in triplicates and * indicated significant difference between groups (p<0.05).
Dosage-Dependent Effects of Interstitial Fluid Shear Stress on Angiogenic Response and Outgrowth of Endothelial Cells
Even though fluid shear stress is known to promote angiogenesis, the impact of interstitial flow in endothelial cells remains poorly understood. This is relevant because interstitial flow can arise from uterine contractile activities that are important for proper placental development (Aguilar & Mitchell, 2010). To investigate this aspect, we bioprinted a layer of endothelial cells (~450 μm wide) around the lumen of the placenta model. After 3 days of perfusion, the angiogenic responses of endothelial cells were assessed and compared against static control. HUVEC under 0, 0.1, and 1 dyne/cm2 were stained with CD31, a angiogenic marker (DeLisser et al., 1997), and DAPI as a counter stain (Figure 3a–c, respectively). We found the expression of CD31 and DAPI seemed to increase as shear stress increased. Quantitative image analysis revealed that normalized fluorescent intensity (FI) of CD31 of endothelial cells was positively correlated with shear stress (static = 1±0.001, 0.1 dyne/cm2 = 2.43±0.01 and 1 dyne/cm2 = 2.84±0.02; p<0.05; Figure 3d). Additional image analysis demonstrated a significant (p<0.05) dosage-dependent angiogenic responses of endothelial cells towards fluid shear stresses indicated by key angiogenic marker such as length of linear aggregates, number of linear aggregates, fraction of area covered by HUVEC network and number of branching points/area (Staton et al., 2009) (Figure 3e–g, respectively).
Figure 3. Fluid Shear Stress Augmented Angiogenesis Responses of HUVEC.
(a) Fluorescent image of HUVECs in static culture showed no expression of CD31 (blue = DAPI stain, green = CD31). (b) Fluorescent image of HUVECs under 0.1 dyne/cm2 of interfacial fluid stress showed relatively moderate expression of CD31 (blue = DAPI, green = CD31). (c) Fluorescent image of HUVECs under 1 dyne/cm2 of interfacial fluid shear stress showed relatively high expression of CD31 (blue = DAPI, green = CD31). (d-h) The Interstitial flow from the bioreactor induced angiogenic responses of HUVECs in a dosage dependent-manner by increasing the expression of CD31, the number, length, and area of the aggregates. All quantitative assays were performed up to 400 μm away from the lumen. All measurements were done in triplicates and * indicates significant difference between groups (p<0.05).
Another key angiogenic phenotype was the outgrowth of endothelial cells (Staton et al., 2009). Bright field images showed dynamic flow at 1 dyne/cm2 induced more prominent invasion of endothelial cells compare to static control (Figure 4a,b) with significant differences in the outgrowth rates (static = 16.77±2.30 μm/day vs. dynamic = 31.88±7.48 μm/day; p<0.05; Figure 4c). mRNA level of angiogenic markers such as MMP2 (static = 1±0.06 vs. dynamic = 2.74±0.12; p<0.05), MMP9 (static = 1±0.05 vs. dynamic =17.18±4.03; p<0.05), and VEGFA (static = 1±0.06 vs. dynamic = 1.75±0.08; p<0.05) were all upregulated when exposed to interstitial fluid shear stress (Figure 4d–f). Altogether these results demonstrated that we have developed a dynamic placenta model that can induce proliferation, angiogenic response, and outgrowth of endothelial cells.
Figure 4. Fluid Shear Stress Promoted Outgrowth of HUVEC.
(a) After 3 days of static culture, HUVECs remained circular with limited outgrowth. (b) Outgrowth of HUVECs occurred in the direction of interstitial flow (yellow arrow) with network formation after 3 days of dynamic culture. (c) Interstitial flow enhanced HUVECs’ outgrowth rates significantly (p<0.05). Additionally, interstitial flow significantly increased gene expressions of angiogenic markers such as (d) MMP2, (e) MMP9 and (f) VEGFA. All quantitative assays were done in triplicates and * indicates significant difference between groups (p<0.05).
Endothelial Cells Outgrowth and Angiogenic Response were Impaired by Trophoblasts in Indirect Coculture
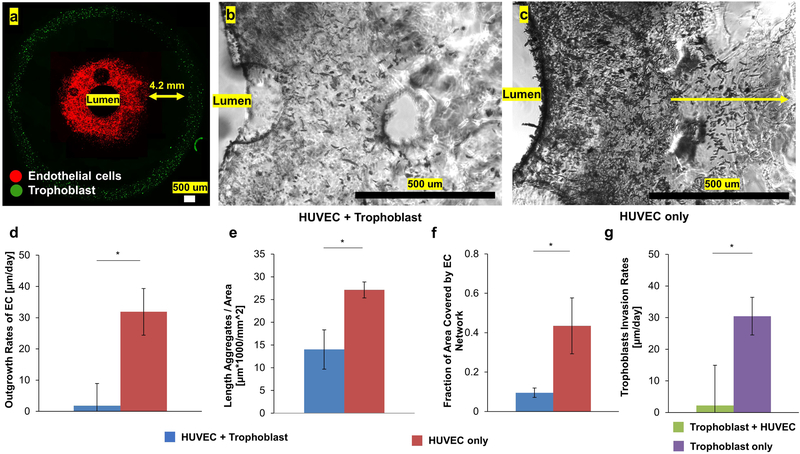
Next, we used the placenta model to describe endothelium-trophoblast interactions, a critical process in placental development and preeclampsia (Ahmed, Dunk, Ahmad, & Khaliq, 2000; Christopher W. Redman, 2005; Eric A P Steegers, 2010; Fraser & Lunn, 2000). We started by using an indirect coculture approach. Utilizing the advantages of bioprinting, we patterned a ring of trophoblasts (green in Figure 5a) that was 4.2 mm away from the endothelial cells to establish an indirect coculture system. HUVEC remained along the periphery of the inner lumen (red in Figure 5a) which was exposed to a defined fluid shear stress of 1 dyne/cm2 to model the spiral arteriole (Jackson et al., 1992). Compare to HUVEC homotypic cultures, trophoblasts significantly reduced the outgrowth and network formation of HUVEC (Figure 5b,c). Quantitative image analysis showed the outgrowth rate of HUVEC was significantly reduced in the presence of trophoblasts (HUVEC + trophoblast = 1.82±7.11 μm/day vs. HUVEC only = 30.47±5.99 μm/day; Figure 5d; p<0.05). Further analysis in Figure 5e,f demonstrated that the distanced trophoblasts reduced the length of HUVEC aggregates (HUVEC + trophoblast = 14.03±4.32 μmx1000/mm2 vs. HUVEC only = 27.11±1.77 ×1000μm/mm2; p<0.05) and fraction of area covered by HUVEC network (HUVEC + trophoblast = 0.095±0.024 vs. HUVEC only = 0.43±0.14; p<0.05). On the other hand, the presence of endothelial cells also reduced the invasion rates of trophoblast (trophoblast + HUVEC = 2.21±17.3 vs. trophoblast only = 31.9±7.3 μm/day, Figure 5g). Taken together, these results showed the presence of trophoblasts impaired the flow-induced angiogenic response of endothelial cells in an indirect coculture system.
Figure 5. Trophoblast Reduced Outgrowth and Angiogenic Responses of HUVEC in Indirect Coculture.
(a) Representative images showing HUVECs (red) were bioprinted along the periphery of the inner lumen while trophoblasts(green) were bioprinted along the outer periphery. (b) In the presence of trophoblasts, HUVECs showed limited outgrowth and network formation. (c) When HUVECs were cultured without trophoblast, they showed significantly higher rates of proliferation and network formation. Quantitative image analysis showed that the presence of trophoblasts significantly (d) impaired the outgrowth rates of HUVECs; (e) reduced the length of linear aggregates of HUVECs; (f) decreased the fraction of area covered by networks of HUVECs. (g) The presence of HUVECs reduced the motility rate of trophoblast. All quantitative image analysis on HUVECs was performed regions less than 400 μm away from the lumen. All quantitative assays were done in triplicates and * indicates significant difference between groups (p<0.05).
Trophoblasts and Endothelial Cells Interdigitated and Stimulated a Proapoptotic Environment in Direct Coculture
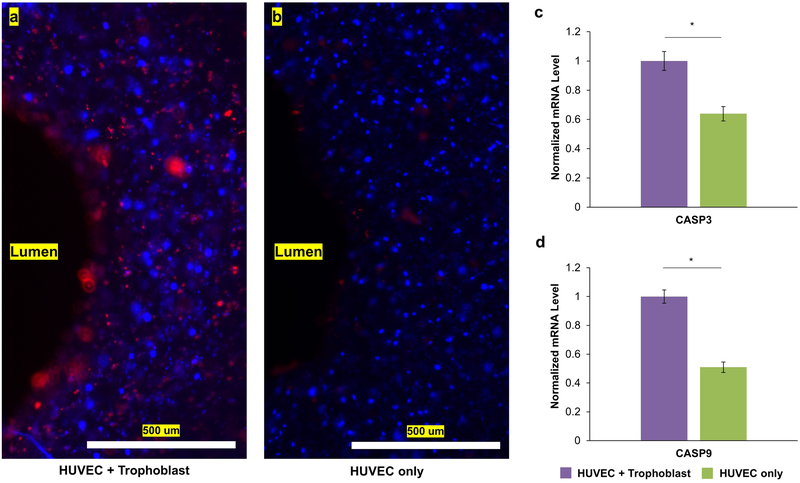
Finally, we bioprinted trophoblast and HUVEC adjacent to investigate their direct interactions. We successfully bioprinted two distinct cell populations (trophoblast in green and HUVEC in red, Figure 6b). After 3 days of dynamic culture, trophoblasts and HUVEC appeared to interact, formed linear aggregates, and interdigitated with each other (Figure 6c). Placenta models that did not have trophoblasts were bioprinted as controls and showed network formation after 3 days of dynamic culture (Figure 6d–f). Additional fluorescent staining showed upregulation of phosphatidylserine (red), an early marker of apoptosis, when HUVECs were bioprinted with trophoblasts in comparison to HUVEC homotypic cultures (Figure 7a, b). Moreover, overall mRNA levels of CASP3 and CASP9, genes that played central role in the execution phase of apoptosis, were upregulated when trophoblasts were bioprinted directly next to HUVEC (Figure 7c,d). These results suggest direct coculture of trophoblasts and endothelial cells induced a significantly more apoptotic environment in the dynamic bioprinted placenta model.
Figure 6. Trophoblast and HUVEC Interdigitated under Direct Coculture in the Dynamic Bioprinted Placenta Model.
(a) Schematic image of placenta model with HUVECs and trophoblast bioprinted directly adjacent with each other. (b) Initially, before dynamic culture, HUVECs (red) and trophoblasts (green) were circular in two distinct groups. (c) After 3 days of dynamic culture, HUVECs (red) and trophoblasts (green) invaded and tangled into with each other. In addition, HUVECs and trophoblast both formed linear aggregates. (d) Schematic image of placenta model with HUVECs only as a control group. (e) Initially, only HUVECs were bioprinted along the lumen of the placenta model with circular morphology. (f) After 3 days of dynamic culture, endothelial cells formed networks of linear aggregates.
Figure 7. Direct Coculture of Trophoblast and HUVEC Promoted an Apoptotic Environment.
(a) Expression of phosphatidylserine, a marker of early apoptosis (red), was higher in HUVEC + trophoblast cocultures compared to (b) homotypic HUVEC cultures after 3 days of dynamic culture. The addition of trophoblast increased the overall mRNA levels of (c) CASP3 and (d) CASP9 compare to HUVEC only control. All quantitative assays were done in biological triplicates and * indicates significant difference between groups (p<0.05).
DISCUSSIONS
Loss of endothelial cells from the lumen of the maternal spiral arteries is necessary and critical to pregnancy success (Ashton et al., 2005; Christopher W. Redman, 2005). Current knowledge in trophoblast invasion and remodeling of spiral arteries is mostly derived from biopsies from placental bed (Ashton et al., 2005). These studies concluded that the interdigitation and invasion of maternal spiral arteries by extravillous trophoblasts were vital in pregnancy success, but they provided limited insights into the cellular dynamics and molecular mechanisms involved (Ashton et al., 2005; Enders & Blankenship, 1997; Pijnenborg et al., 1980). In vitro assays are effective tools for mechanistic studies but they are mostly performed in 2D culture systems (e.g. petri dish, multiwell plates) that are unable to capture the complex interactions between trophoblast and endothelial cells occurring in the native 3D microenvironment (Cartwright et al., 2002; Edmondson, Broglie, Adcock, & Yang, 2014; Orendi et al., 2011). Moreover, current in vitro placenta models are mostly static and do not consider the effects of fluid shear stress, an essential regulator of endothelium homeostasis and proper maternal vascular remodeling (Dimmeler et al., 1998; James, Whitley, & Cartwright, 2011a; Kim, Chung, Ahn, Lee, & Jeon, 2016).
To address these shortcomings, in this work we developed a dynamic 3D bioprinted placenta model that directly examines cellular interactions and provides mechanistic insights in trophoblasts-endothelial interactions under fluid flow. We used stereolithography-based 3D printing to fabricate a customized bioreactor chamber to fit the bioprinted placenta model and apply controlled fluid shear stress in its lumen (Figure 1, red arrow). Since we used 3D printing as our fabrication technique, bioreactor chambers with different architecture can be easily developed as needed. To ensure the perfusion bioreactor system induced the desired biological effect on endothelial cells, we tested the angiogenic responses of HUVEC as a function of position and shear stress (Figure 2 and Figure 3). We found that the interstitial flow from the perfusion bioreactor system can influence angiogenesis of endothelial cells that reside within 400 μm from the lumen of the placenta model (Figure 2). Such information is useful for fabricating tissue engineered grafts for regenerative medicine because they will ultimately be implanted into the human body, where interstitial fluid is ubiquitous. In addition, we found that increasing fluid shear stress promoted network formation, expressions of CD31, MMP2 and MMP9, and the outgrowth of HUVEC, which are key angiogenic markers (Staton et al., 2009). Even though the upregulation of MMPs is not unique to angiogenesis, they are still relevant in angiogenesis and therefore can serve as supporting evidences. Considering all the results above, we concluded we have established a dynamic bioprinted placenta model that could apply physiologically relevant fluid shear stress to upregulate angiogenic responses of endothelial cells.
Next, we used the dynamic bioprinted placenta model to examine interactions between endothelial cells and trophoblasts, a biological process that is critical in preeclampsia (Young et al., 2010). By utilizing the capability of extrusion-based bioprinting, we demonstrated spatial control of trophoblasts and HUVEC by coculturing the two populations indirectly (Figure 5a) and directly adjacent (Figure 6b). The different spatial arrangements of biomaterials can be leveraged for different purpose. For instance, cell motility rates and detailed image analysis on different cell populations can be easily assessed in indirect cocultures (Figure 5). In normal pregnancy, fetal trophoblasts have to penetrate the maternal decidua to reach the spiral arteriole and the thickness of decidua is approximately 4.2 mm in early pregnancy (Wong & Cheung, 2010), which is what we attempted to recreate in the indirect coculture model. On the other hand, direct biophysical interactions between cell populations can be investigated when trophoblast and HUVECs are cocultured directly (Figure 6), which occurs when trophoblasts have successfully reached the spiral arteriole via interstitial invasion. However, since the positions of the cell populations are difficult to track in direct coculture, extensive image analysis on a specific cell population has proven to be impractical, which is necessary to determine motility rates and angiogenesis in vitro.
In this study, we found that the paracrine signaling from trophoblasts in indirect cocultures reduced angiogenic response and outgrowth of endothelial cells, which is consistent with published results (Loegl et al., 2016; Sokolov et al., 2017). This is most likely a mechanism to control and limit feto-placental angiogenesis, vascular expansion and remodeling during pregnancy. Moreover, presence of endothelial cells millimeters away also reduced the invasion rates of extravillous trophoblasts (Figure 5g). Several studies have reported endothelial cells can influence motility of trophoblasts with conflicting results (Campbell, Rowe, Jackson, & Gallery, 2004; Soghomonians, Barakat, Thirkill, & Douglas, 2005), and our results suggest that paracrine signaling from the endothelium reduces the invasion of extravillous trophoblasts. When trophoblasts were bioprinted directly adjacent to endothelial cells, the two cell populations interdigitated in our dynamic bioprinted placenta model, similar to what was observed in vivo (Ashton et al., 2005). Further analysis found that bioprinting trophoblasts next to endothelial cells led to upregulation of apoptotic markers at both protein and gene levels. Since the apoptotic stains were observed within 400 μm away from the lumen (Figure 7a) while trophoblast invasion rates were less than 10 μm/day, we concluded the stains were most likely from the endothelial cells. During early pregnancy, the trophoblasts replace the endothelial lining in the maternal spiral arterioles to create a high-flow, low-resistance circulation (Ashton et al., 2005; James, Whitley, & Cartwright, 2011b). However, the detailed cellular interactions that causes the vascular remodeling is not well understood and our results suggest apoptosis may be a mechanism that govern the loss of endothelial lining in spiral arteriole during early pregnancy.
Altogether these results suggest that trophoblast-mediated remodeling of spiral arteries were regulated by angiogenic and apoptotic pathways. While the involvement of these pathways in the remodeling of spiral arteries is already known (Ashton et al., 2005; Campbell et al., 2004; Loegl et al., 2016; Soghomonians et al., 2005; Sokolov et al., 2017), these phenomena have not been accurately mimicked in 3D hydrogels. Our placenta model offers the possibility to interrogate the cellular dynamics and molecular mechanism governing angiogenesis of endothelial cells and trophoblast-mediated vascular remodeling within a controlled bioengineered environment. Leveraging the advantages of bioprinting, placenta models describing further microenvironmental cues (e.g. stromal cells, extracellular matrix (ECM), and growth factors) with different spatial arrangements can be fabricated to investigate other biological processes such as the role of uterine natural killer cells in placental development and maternal vascular remodeling. Moreover, leveraging the spatial control of biomaterials enabled by our dynamic placenta model, we can investigate the effect of relative spatial orientation of trophoblasts and endothelial cells on their motility, proliferation, and gene/protein expression.
Our current model, however, can still be improved to reach its full potential. Although HTR8 used in this study have proven to be effective for recapitulating key aspects of extravillous trophoblasts (Qiu, Yang, Tsang, & Gruslin, 2004; Shan et al., 2015), questions remain regarding the validity of using these immortalized cell lines to represent the in vivo environment (Orendi et al., 2011). Similarly, the use of HUVECs to model endothelial lining of spiral arteriole in early pregnancy is not ideal. To address these limitations, future iterations of this system should include culture of primary extravillous trophoblast cells and spiral arteriole endothelial cells from early pregnancy for improved physiological relevance. In addition, the endothelial cells were bioprinted with multiple layers rather than a monolayer of endothelium lining. Current bioprinting technology does not permit single cell printing, and we expect to improve the dynamic placenta model as biofabrication technology advances. Lastly, the extracellular matrix material used in current study was not tissue-specific, which can be improved in the future based on a recently published work (C.-Y. Kuo et al., 2018). Despite these limitations, however, the results presented in this work undoubtedly demonstrated that our dynamic placenta model is an effective tool to examine cellular dynamics and molecular mechanism involved in placental development and preeclampsia.
CONCLUSIONS
In this study, we developed an advanced bioprinted placenta model that can directly examine cell-cell interactions and simultaneously apply controlled fluid shear stress in its endothelialized lumen, which were not possible in our previous work (C. Y. Kuo et al., 2016; C. Y. Kuo, T. Guo, et al., 2018). We successfully modeled key events in trophoblast-mediated vascular remodeling, which validated and confirmed our dynamic placenta model as a physiologically more relevant platform to study preeclampsia than conventional 2D placenta models. The results described here clearly demonstrate the potential of 3D bioprinting and perfusion bioreactor system to study of trophoblast invasion and placental development. Altogether, our dynamic bioprinted placenta model is a crucial step that will lead to advanced research approaches to expand our understanding and to develop treatment options for preeclampsia and other pregnancy-related complications.
ACKNOWLEDGEMENTS
This research was funded by the Sheikh Zayed Institute for Pediatric Surgical Innovation of Children’s National Health System, the National Institute of Biomedical Imaging and Bioengineering / National Institutes of Health (NIBIB/NIH) Center for Engineering Complex Tissues (P41 EB023833), and the American Heart Association (17PRE33670295).
Grant Number(s): National Institute of Biomedical Imaging and Bioengineering / National Institutes of Health (NIBIB/NIH) Center for Engineering Complex Tissues (P41 EB023833)
REFERENCES
- Aguilar HN, & Mitchell BF (2010). Physiological pathways and molecular mechanisms regulating uterine contractility. Human Reproduction Update, 16(6), 725–744. doi:10.1093/humupd/dmq016 [DOI] [PubMed] [Google Scholar]
- Ahmed A, Dunk C, Ahmad S, & Khaliq A (2000). Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen - A review. Placenta, 21, S16–S24. doi:10.1053/plac.1999.0524 [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy, & American College of Obstetricians and Gynecologists (2013). Hypertension in pregnancy. Washington, DC: American College of Obstetricians and Gynecologists. [DOI] [PubMed] [Google Scholar]
- Ashton SV, Whitley GSJ, Dash PR, Wareing M, Crocker IP, Baker PN, & Cartwright JE (2005). Uterine spiral artery remodeling involves endothelial apoptosis induced by extravillous trophoblasts through Fas/FasL interactions. Arteriosclerosis Thrombosis and Vascular Biology, 25(1), 102–108. doi:10.1161/01.atv.0000148547.70187.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann F, Rotmensch S, Rosenzweig B, How H, & Chediak J (1991). THE ROLE OF VONWILLEBRAND-FACTOR IN PREECLAMPSIA. Thrombosis and Haemostasis, 66(5), 525–528. [PubMed] [Google Scholar]
- Boffa MC, Valsecchi L, Fausto A, Gozin D, D’Angelo SV, Safa O, … D’Angelo A (1998). Predictive value of plasma thrombomodulin in preeclampsia and gestational hypertension. Thrombosis and Haemostasis, 79(6), 1092–1095. [PubMed] [Google Scholar]
- Burton GJ, Woods AW, Jauniaux E, & Kingdom JCP (2009). Rheological and Physiological Consequences of Conversion of the Maternal Spiral Arteries for Uteroplacental Blood Flow during Human Pregnancy. Placenta, 30(6), 473–482. doi:10.1016/j.placenta.2009.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Rowe J, Jackson CJ, & Gallery EDM (2004). Interaction of cocultured decidual endothelial cells and cytotrophoblasts in preeclampsia. Biology of Reproduction, 71(1), 244–252. doi:10.1095/biolreprod.103.026716 [DOI] [PubMed] [Google Scholar]
- Cartwright JE, Kenny LC, Dash PR, Crocker IP, Aplin JD, Baker PN, & Whitley GS (2002). Trophoblast invasion of spiral arteries: a novel in vitro model. Placenta, 23(2–3), 232–235. doi:10.1053/plac.2001.0760 [DOI] [PubMed] [Google Scholar]
- Chen T, Buckley M, Cohen I, Bonassar L, & Awad HA (2012). Insights into interstitial flow, shear stress, and mass transport effects on ECM heterogeneity in bioreactor-cultivated engineered cartilage hydrogels. Biomechanics and Modeling in Mechanobiology, 11(5), 689–702. doi:10.1007/s10237-011-0343-x [DOI] [PubMed] [Google Scholar]
- Redman Christopher W., S. IL (2005). Latest Advances in Understanding Preeclampsia. SCIENCE, 308, 1592–1594. [DOI] [PubMed] [Google Scholar]
- DeLisser HM, ChristofidouSolomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, … Albelda SM (1997). Involvement of endothelial PECAM-1/CD31 in angiogenesis. American Journal of Pathology, 151(3), 671–677. [PMC free article] [PubMed] [Google Scholar]
- Demir R, Yaba A, & Huppertz B (2010). Vasculogenesis and angiogenesis in the endometrium during menstrual cycle and implantation. Acta Histochemica, 112(3), 203–214. doi:10.1016/j.acthis.2009.04.004 [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Assmus B, Hermann C, Haendeler J, & Zeiher AM (1998). Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells - Involvement in suppression of apoptosis. Circulation Research, 83(3), 334–341. [DOI] [PubMed] [Google Scholar]
- Edmondson R, Broglie JJ, Adcock AF, & Yang LJ (2014). Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay and Drug Development Technologies, 12(4), 207–218. doi:10.1089/adt.2014.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders AC, & Blankenship TN (1997). Modification of endometrial arteries during invasion by cytotrophoblast cells in the pregnant macaque. Acta Anatomica, 159(4), 169–193. [DOI] [PubMed] [Google Scholar]
- Eric AP Steegers P. v. D., Johannes J Duvekot, Robert Pijnenborg. (2010). Pre-eclampsia. The Lancet, 376, 631–644. doi:10.1016/s01406736(10)60279-6 [DOI] [PubMed] [Google Scholar]
- Fraser HM, & Lunn SF (2000). Angiogenesis and its control in the female reproductive system. British Medical Bulletin, 56(3), 787–797. doi:10.1258/0007142001903364 [DOI] [PubMed] [Google Scholar]
- Friedman SA, Degroot CJM, Taylor RN, Golditch BD, & Roberts JM (1994). PLASMA CELLULAR FIBRONECTIN AS A MEASURE OF ENDOTHELIAL INVOLVEMENT IN PREECLAMPSIA AND INTRAUTERINE GROWTH-RETARDATION. American Journal of Obstetrics and Gynecology, 170(3), 838–841. [DOI] [PubMed] [Google Scholar]
- Halasz M, & Szekeres-Bartho J (2013). The role of progesterone in implantation and trophoblast invasion. Journal of reproductive immunology, 97(1), 43–50. doi:10.1016/j.jri.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Jackson MR, Mayhew TM, & Boyd PA (1992). QUANTITATIVE DESCRIPTION OF THE ELABORATION AND MATURATION OF VILLI FROM 10 WEEKS OF GESTATION TO TERM. Placenta, 13(4), 357–370. doi:10.1016/0143-4004(92)90060-7 [DOI] [PubMed] [Google Scholar]
- James JL, Whitley GS, & Cartwright JE (2011a). Shear stress and spiral artery remodelling: the effects of low shear stress on trophoblast-induced endothelial cell apoptosis. Cardiovascular research, 90(1), 130–139. doi:10.1093/cvr/cvq396 [DOI] [PubMed] [Google Scholar]
- James JL, Whitley GS, & Cartwright JE (2011b). Shear stress and spiral artery remodelling: the effects of low shear stress on trophoblast-induced endothelial cell apoptosis. Cardiovascular Research, 90(1), 130–139. doi:10.1093/cvr/cvq396 [DOI] [PubMed] [Google Scholar]
- Kim S, Chung M, Ahn J, Lee S, & Jeon NL (2016). Interstitial flow regulates the angiogenic response and phenotype of endothelial cells in a 3D culture model. Lab on a Chip, 16(21), 4189–4199. doi:10.1039/c6lc00910g [DOI] [PubMed] [Google Scholar]
- Kuklina EV, Ayala C, & Callaghan WM (2009). Hypertensive Disorders and Severe Obstetric Morbidity in the United States. Obstetrics and Gynecology, 113(6), 1299–1306. [DOI] [PubMed] [Google Scholar]
- Kuo C-Y, Guo T, Cabrera-Luque J, Arumugasaamy N, Bracaglia L, Garcia-Vivas A, … Kim P (2018). Placental basement membrane proteins are required for effective cytotrophoblast invasion in a 3D bioprinted placenta model J Biomed Mater Res Part A, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C-Y, Wilson E, Fuson A, Gandhi N, Reza M, Jenkins A, … Reilly B (2017). Repair of Tympanic Membrane Perforations with Customized, Bioprinted Ear Grafts Using Chinchilla Models. Tissue Engineering Part A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CY, Eranki A, Placone JK, Rhodes KR, Aranda-Espinoza H, Fernandes R, … Kim PCW (2016). Development of a 3D Printed, Bioengineered Placenta Model to Evaluate the Role of Trophoblast Migration in Preeclampsia. Acs Biomaterials Science & Engineering, 2(10), 1817–1826. doi:10.1021/acsbiomaterials.6b00031 [DOI] [PubMed] [Google Scholar]
- Kuo CY, Guo T, Cabrera-Luque J, Arumugasaamy N, Bracaglia L, Garcia-Vivas A, … Kim P (2018). Placental basement membrane proteins are required for effective cytotrophoblast invasion in a three-dimensional bioprinted placenta model. Journal of Biomedical Materials Research Part A, 106(6), 1476–1487. doi:10.1002/jbm.a.36350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CY, Wilson E, Fuson A, Gandhi N, Monfaredi R, Jenkins A, … Reilly B (2018). Repair of Tympanic Membrane Perforations with Customized Bioprinted Ear Grafts Using Chinchilla Models. Tissue Engineering Part A, 24(5–6), 527–535. doi:10.1089/ten.tea.2017.0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loegl J, Nussbaumer E, Hiden U, Majali-Martinez A, Ghaffari-Tabrizi-Wizy N, Cvitic S, … Huppertz B (2016). Pigment epithelium-derived factor (PEDF): a novel trophoblast-derived factor limiting feto-placental angiogenesis in late pregnancy. Angiogenesis, 19(3), 373–388. doi:10.1007/s10456-016-9513-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noraihan Mohd Nordin, S. P a. A. B. EJ (2005). Report of 50 cases of eclampsia. 31(4), 302–309. [DOI] [PubMed] [Google Scholar]
- Nguyen BNB, Ko H, Moriarty RA, Etheridge JM, & Fisher JP (2016). Dynamic Bioreactor Culture of High Volume Engineered Bone Tissue. Tissue Engineering Part A, 22(3–4), 263–271. doi:10.1089/ten.tea.2015.0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orendi K, Kivity V, Sammar M, Grimpel Y, Gonen R, Meiri H, … Huppertz B (2011). Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta, 32 Suppl, S49–54. doi:10.1016/j.placenta.2010.11.023 [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Dixon G, Robertson WB, & Brosens I (1980). TROPHOBLASTIC INVASION OF HUMAN DECIDUA FROM 8 TO 18 WEEKS OF PREGNANCY. Placenta, 1(1), 3–&. doi:10.1016/s0143-4004(80)80012-9 [DOI] [PubMed] [Google Scholar]
- Qiu Q, Yang M, Tsang BK, & Gruslin A (2004). EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction, 128(3), 355–363. doi:10.1530/rep.1.00234 [DOI] [PubMed] [Google Scholar]
- Schiff E, Benbaruch G, Peleg E, Rosenthal T, Alcalay M, Devir M, & Mashiach S (1992). IMMUNOREACTIVE CIRCULATING ENDOTHELIN-1 IN NORMAL AND HYPERTENSIVE PREGNANCIES. American Journal of Obstetrics and Gynecology, 166(2), 624–628. [DOI] [PubMed] [Google Scholar]
- Shan N, Zhang X, Xiao X, Zhang H, Tong C, Luo X, … Qi H (2015). Laminin alpha 4 (LAMA4) expression promotes trophoblast cell invasion, migration, and angiogenesis, and is lowered in preeclamptic placentas. Placenta, 36(8), 809–820. doi:10.1016/j.placenta.2015.04.008 [DOI] [PubMed] [Google Scholar]
- Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, … Hubel CA (2005). Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. The Journal of clinical endocrinology and metabolism, 90(8), 4895–4903. doi:10.1210/jc.2004-1955 [DOI] [PubMed] [Google Scholar]
- Soghomonians A, Barakat AI, Thirkill TL, & Douglas GC (2005). Trophoblast migration under flow is regulated by endothelial cells. Biology of Reproduction, 73(1), 14–19. doi:10.1095/biolreprod.104.036509 [DOI] [PubMed] [Google Scholar]
- Sokolov DI, Lvova TY, Okorokova LS, Belyakova KL, Sheveleva AR, Stepanova OI, … Sel’kov SA (2017). Effect of Cytokines on the Formation Tube-Like Structures by Endothelial Cells in the Presence of Trophoblast Cells. Bulletin of Experimental Biology and Medicine, 163(1), 148–158. doi:10.1007/s10517-017-3756-4 [DOI] [PubMed] [Google Scholar]
- Staton CA, Reed MWR, & Brown NJ (2009). A critical analysis of current in vitro and in vivo angiogenesis assays. International Journal of Experimental Pathology, 90(3), 195–221. doi:10.1111/j.1365-2613.2008.00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinnakorn Chaiworapongsa PC, Lami Yeo and Roberto Romero. (2014). Pre-eclampsia part 1: current understanding of its paothophsiology. Nature Nephrology, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstroebiezen SAM, Everaerts FM, Janssen LJJ, & Tacken RA (1993). DIFFUSION-COEFFICIENTS OF OXYGEN, HYDROGEN-PEROXIDE AND GLUCOSE IN A HYDROGEL. Analytica Chimica Acta, 273(1–2), 553–560. doi:10.1016/0003-2670(93)80202-v [Google Scholar]
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, … Karumanchi SA (2006). Soluble endoglin contributes to the pathogenesis of preeclampsia. Nature medicine, 12(6), 642–649. doi:10.1038/nm1429 [DOI] [PubMed] [Google Scholar]
- Wong HS, & Cheung YK (2010). Sonographic study of the decidua basalis in early pregnancy loss. Ultrasound in Obstetrics & Gynecology, 36(3), 362–367. doi:10.1002/uog.7736 [DOI] [PubMed] [Google Scholar]
- Yeatts AB, Both SK, Yang WX, Alghamdi HS, Yang F, Fisher JP, & Jansen JA (2014). In Vivo Bone Regeneration Using Tubular Perfusion System Bioreactor Cultured Nanofibrous Scaffolds. Tissue Engineering Part A, 20(1–2), 139–146. doi:10.1089/ten.tea.2013.0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatts AB, Choquette DT, & Fisher JP (2013). Bioreactors to influence stem cell fate: Augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochimica Et Biophysica Acta-General Subjects, 1830(2), 2470–2480. doi:10.1016/j.bbagen.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatts AB, Geibel EM, Fears FF, & Fisher JP (2012). Human mesenchymal stem cell position within scaffolds influences cell fate during dynamic culture. Biotechnology and Bioengineering, 109(9), 2381–2391. doi:10.1002/bit.24497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatts AB, Gordon CN, & Fisher JP (2011). Formation of an Aggregated Alginate Construct in a Tubular Perfusion System. Tissue Engineering Part C-Methods, 17(12), 1171–1178. doi:10.1089/ten.tec.2011.0263 [DOI] [PubMed] [Google Scholar]
- Young BC, Levine RJ, & Karumanchi SA (2010). Pathogenesis of preeclampsia. Annu Rev Pathol, 5, 173–192. doi:10.1146/annurev-pathol-121808-102149 [DOI] [PubMed] [Google Scholar]