Abstract
Objectives:
Endoscopic resection is preferred for duodenal carcinoids <20 mm, however the efficacy of simple polypectomy has not been compared to advanced endoscopic resection techniques.
Methods:
We performed a retrospective review of 33 patients who underwent endoscopic duodenal carcinoid resection (10 simple, 23 endoscopic mucosal resection) at the Hospital of the University of Pennsylvania between 1/1/2006 and 6/15/2017. The primary outcomes were resection margin positivity and local tumor recurrence.
Results:
There were no significant differences in demographics or tumor functionality. Lesions managed with simple polypectomy had smaller median gross specimen size (6.0 mm vs. 8.0 mm, P = 0.043). There was no significant difference in pathology resection margins between simple polypectomy and endoscopic mucosal resection (86% vs. 68% positive, P = 0.64). Local recurrence on surveillance endoscopy was also similar (14.3% vs. 17.7%, respectively, P = 1.000), with median time to recurrence 2.3 months (interquartile range, 1.2–5.4 months). The median follow-up time in patients without local recurrence was 21.4 months (interquartile range, 7.1–39.6 months).
Conclusions:
Simple polypectomy may be adequate treatment for small duodenal carcinoids, although further studies are needed for validation and to define the upper limits of tumor size that can be managed with this technique.
Keywords: duodenal carcinoid, simple polypectomy, endoscopic mucosal resection, pathology margins
Introduction
Carcinoids describe an array of neuroendocrine neoplasms that most commonly develop in the pulmonary or gastrointestinal tracts. Duodenal carcinoid tumors arise from enterochromaffin neuroendocrine cells and are extremely rare. They account for <3% of intestinal carcinoids,1,2 with an annual age-adjusted incidence between 0.07 and 0.19 per 100,000 population.3,4 Epidemiological studies show that male sex and black race are positive risk factors for duodenal carcinoids. There are also a number of syndromic associations, including multiple neuroendocrine neoplasia type 1 (MEN-1) and neurofibromatosis type 1 (NF-1). Importantly, duodenal carcinoid tumors may be “functional,” reflecting the symptomatic expression of excess endogenous hormones such as gastrin or somatostatin.
Owing to their rarity, the natural history of duodenal carcinoids remains poorly defined. However, these notoriously slow-growing tumors are known to bear malignant potential. Previously identified risk factors for lymph node spread and metastasis are tumor invasion into the muscularis propria, diameter ≥20 mm, and a high rate of mitotic figures on pathology.5,6 There are conflicting data as to the risk of metastasis in lesions ≤10 mm,7,8 but if endoscopic ultrasound rules out muscularis propria involvement then endoscopic resection is recommended.9 On the other end of the spectrum, surgery is usually favored for lesions ≥20 mm, while the best approach to managing tumors between 10 mm and 20 mm represents an ongoing area of uncertainty.7 For such lesions confined to the submucosa, a number of case series have documented successful advanced endoscopic resection.10–13
Several groups have reported the safety and efficacy of both endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) for managing duodenal carcinoid tumors ≤20 mm and confined to the submucosa, with the largest single center experience comprising 38 patients.14–16 However, no study to date has compared the outcomes of simple polypectomy to advanced endoscopic resection techniques. We postulated that smaller lesions might be adequately treated with simple polypectomy, and possibly with fewer complications as compared to EMR for all lesions. At our center, both simple polypectomy and EMR have been used to remove duodenal carcinoids. As such, we sought to characterize the nature of lesions addressed with each technique, and to evaluate their efficacy of resection as well as the rate of complications.
MATERIALS AND METHODS
Patient Selection
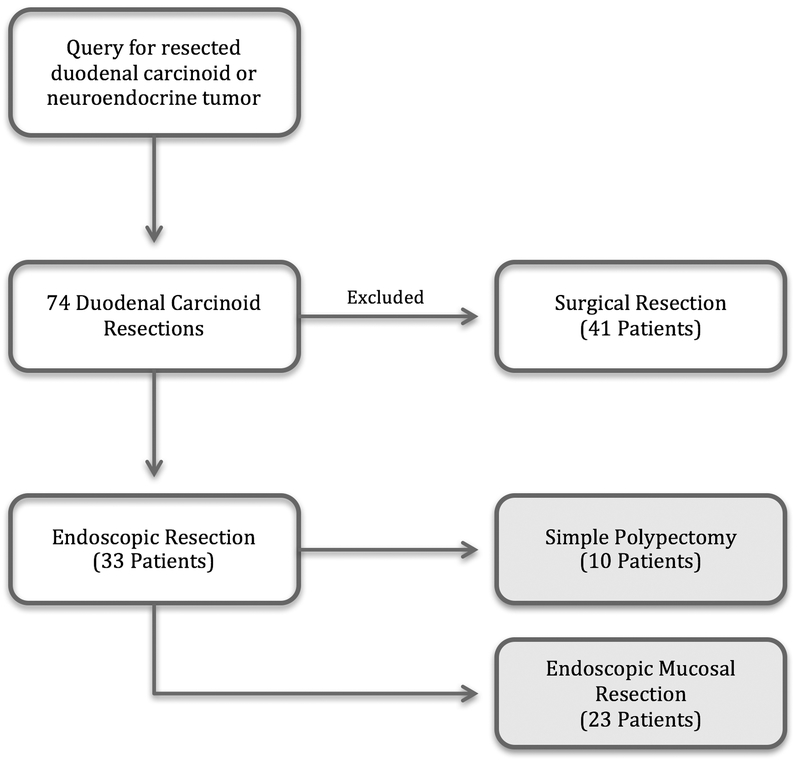
A retrospective review of patients who underwent endoscopic resection of duodenal carcinoid tumors was conducted at the Hospital of the University of Pennsylvania in Philadelphia, Pennsylvania. In order to define the cohort of patients, a Cerner (North Kansas City, Mo) pathology database query was performed to identify resection specimens between 1/1/2006 and 6/15/2017 that described duodenal neuroendocrine/carcinoid tumors. Specimens from tumors that were labeled as biopsy specimens (as opposed to resection specimens) were not included. This returned a total of 74 patients. Those who received surgical management (41) were excluded, leaving 33 patients for analysis who underwent endoscopic resection (Fig. 1). Of note, we included patients who underwent dedicated resection procedures, including those who had biopsy-proven duodenal carcinoid on a prior endoscopy. We also included those who had incidentally resected duodenal carcinoid lesions.
FIGURE 1.
Patient flow diagram.
Chart Review and Variable Definitions
An extensive chart review was performed of each record to ascertain the circumstances of the patient’s carcinoid resection. Sociodemographic data including age, sex, race, and ethnicity were collected for all patients. Clinical data included the functional status of the tumor, hormones produced, associated genetic disorders (i.e. MEN-1 or NF-1), and presence/absence of metastatic disease. Endoscopy reports were reviewed to collect depth of tumor invasion on endoscopic ultrasound, type of resection performed (simple polypectomy versus EMR), en bloc resection (i.e. in one piece) or piecemeal resection, incidental versus planned resection, and both intra- and post-procedural complications (aspiration, perforation, significant bleeding, escalation of care). Simple polypectomy was defined as cold forceps biopsy (using standard forceps), cold snare polypectomy, or hot snare polypectomy, while EMR comprised conventional injection-assisted, cap-assisted, and ligation-assisted techniques. All EMR resections were performed by advanced endoscopists, while simple polypectomy cases were performed by either general or advanced endoscopists.
Gross resection specimen size (i.e. the size of the resected specimen) and pathologic tumor size (i.e. the measured size of the tumor itself) were obtained from pathology records, in addition to World Health Organization (WHO) tumor pathology grade, degree of tumor differentiation, mitotic count, Ki-67 expression index, and immunohistochemical stain results. Lateral and vertical pathology resection margins were examined, with margins classified as positive, negative, or unknown if indeterminate. Categories of tumor size and specimen size were also created for categorical analysis (<5 mm, 5 to <10 mm, ≥10 mm). Finally, local tumor recurrence events were defined on subsequent endoscopy and were biopsy-confirmed. At our institution, the general recommended follow-up protocol after duodenal carcinoid resection is repeat upper endoscopy within approximately one year. However, these recommendations could vary at the clinician’s discretion. As such, durations of actual clinical and upper endoscopic follow-up were recorded. Expedited Institutional Review Board (IRB) approval was obtained for this study protocol, with added permission to contact patients by telephone in order to complete follow-up documentation, where necessary.
Primary and Secondary Analyses
The primary outcomes of the study were to compare pathology resection margins, local tumor recurrence, and complications between simple polypectomy and EMR. Secondary analyses included comparisons of tumor size, gross specimen size, rate of piecemeal excision, complications, and survival. Time to endoscopic tumor recurrence, duration of negative endoscopic follow-up, and overall length of clinical follow-up were also compared between groups.
Statistical Methods
Given the small sample size, normality was not assumed in the continuous data variables. Descriptive statistics were thus computed as medians and interquartile ranges (IQRs), where applicable. The Wilcoxon rank-sum test and Fisher’s exact test were used to compare continuous and categorical variables, respectively, with an alpha level of 0.05 regarded to be statistically significant. Where relevant, missing elements of data were assumed to be missing completely at random, and were excluded from analysis. Data management and computations were performed using STATA/IC version 14.2 (College Station, Texas).
RESULTS
Patient Characteristics and Resection Approach
The characteristics of patients undergoing simple polypectomy versus endoscopic mucosal resection are shown in Table 1. There were no significant differences between groups in age, race, ethnicity, tumor functionality, MEN-1 status, or known metastatic disease. The overall median age was 57.7 years (IQR, 52.5–67.8 years). The cohort was predominantly male (63.6%) and predominantly white (57.6% vs. 36.4% black and 6% other). Two patients had confirmed diagnoses of MEN-1 and two patients were diagnosed with hormonally-active functional carcinoid tumors, both of which were gastrinomas. There were no patients in the cohort with NF-1, and none had known metastatic disease at the time of endoscopic resection, although three patients did not have prior imaging to definitively rule this out.
TABLE 1.
Patient Characteristics
| Variable | Simple Resection (n = 10) |
EMR (n = 23) |
P |
|---|---|---|---|
| Age, median (IQR), y | 66.5 (56.9–73.3) | 56.7 (52.1–65.4) | 0.066 |
| Sex, n (%) | 0.110 | ||
| Male | 4 (40) | 17 (74) | |
| Female | 6 (60) | 6 (26) | |
| Race, n (%) | 0.190 | ||
| White | 4 (40) | 15 (65) | |
| Black | 6 (60) | 6 (26) | |
| Other | 0 (0) | 2 (9) | |
| Ethnicity, n (%) | 1.000 | ||
| Non-Hispanic | 10 (100) | 22 (96) | |
| Hispanic | 0 (0) | 1 (4) | |
| Functional tumor, n (%) | 2 (20) | 0 (0) | 0.100 |
| MEN-1 syndrome, n (%) | 1 (10) | 1 (4) | 0.520 |
| Metastases at time of resection, n (%) | 1.000 | ||
| No | 10 (100) | 21 (91) | |
| Unknown | 0 (0) | 2 (9) |
EMR indicates endoscopic mucosal resection; MEN, multiple endocrine neoplasia
Endoscopic Resection and Lesion Size
Most of the endoscopically identified duodenal carcinoids were located in the bulb (90.9%), with the remainder in the second portion of the duodenum (9.1%). The distribution of endoscopic resection methods is given in Table 2. Cold forceps biopsy was the most commonly employed method of simple polypectomy (60%), and most advanced resections were injection-assisted EMRs (87%). As compared to simple polypectomy, EMR resections were more often preceded by EUS (87% versus 30%, P = 0.002) and achieved a significantly higher rate of en bloc resection (87% versus 50%, p = 0.036). Median gross specimen size (6.0 mm vs. 8.0 mm, P = 0.043) and median pathologic tumor size (3.0 mm vs. 6.0 mm, P = 0.010) were significantly smaller for lesions managed with simple polypectomy. These results are summarized in Table 3.
TABLE 2.
Resection Methods
| Variable | Simple Resection (n = 10) |
EMR (n = 23) |
|---|---|---|
| Method of Resection, n (%) | ||
| Biopsy polypectomy | 6 (60) | 0 (0) |
| Cold snare polypectomy | 1 (10) | 0 (0) |
| Hot snare polypectomy | 3 (30) | 0 (0) |
| Injection-assisted EMR | 0 (0) | 20 (87) |
| Cap-assisted EMR | 0 (0) | 2 (9) |
| Ligation-assisted EMR | 0 (0) | 1 (4) |
EMR indicates endoscopic mucosal resection
TABLE 3.
Endoscopic Resection and Outcomes Data
| Variable | Simple Resection (n = 10) |
EMR (n = 23) |
P |
|---|---|---|---|
| Incidental Lesion, n (%) | 5 (50) | 0 (0) | 0.001* |
| EUS Performed, n (%) | 3 (30) | 20 (87) | 0.002* |
| Depth of involvement on EUS, n (%) | 1.000 | ||
| Lamina propria | 0 (0) | 1 (7) | |
| Muscularis mucosae | 1 (50) | 6 (43) | |
| Submucosa | 1 (50) | 6 (43) | |
| Muscularis propria | 0 (0) | 1 (7) | |
| Not reported | 1 | 6 | |
| Resection, n (%) | 0.036* | ||
| En bloc | 5 (50) | 20 (87) | |
| Piecemeal | 5 (50) | 3 (13) | |
| Tumor size, median (IQR), mm | 3.0 (2.0–4.0) | 6.0 (4.0–8.0) | 0.010* |
| Tumor Size, mm, n (%) | 0.051 | ||
| <5 | 6 (86) | 6 (32) | |
| 5 to <10 | 1 (14) | 10 (53) | |
| ≥10 | 0 (0) | 3 (16) | |
| Gross size, median (IQR), mm | 6.0 (4.0–8.0) | 8.0 (6.0–12.0) | 0.043* |
| Gross size, mm, n (%) | 0.084 | ||
| <5 | 4 (40) | 2 (9) | |
| 5 to <10 | 5 (50) | 13 (57) | |
| ≥10 | 1 (10) | 8 (35) | |
| Resection Margins, n (%) | 0.640 | ||
| Negative | 1 (14) | 7 (32) | |
| Positive | 6 (86) | 15 (68) | |
| Not reported | 3 | 1 | |
| Local Recurrence, n (%) | 1 (14.3) | 3 (18) | 1.000 |
| Unknown | 3 | 6 | |
| Survival, n (%) | 7 (78) | 17 (94) | 0.250 |
| Unknown | 1 | 5 | |
| Total follow-up duration, median (IQR), mo | 12.1 (2.8–37.4) | 28.7 (8.2–54.0) | 0.090 |
| Time to endoscopic recurrence, median (IQR), mo | 1.0 (1.0–1.0) | 3.2 (1.4–7.6) | 0.180 |
| Negative endoscopic follow-up duration, median (IQR), mo | 15.7 (4.0–23.8) | 22.2 (8.2–39.2) | 0.400 |
Statistically significant at the alpha = 0.05 level.
†EMR, indicates endoscopic mucosal resection; EUS, endoscopic ultrasound; IQR, interquartile range.
Resection Margins, Tumor Recurrence, and Complications
Simple polypectomy was more frequently used in cases where a duodenal carcinoid was incidentally resected (50% versus 0% EMR, P = 0.001). There was no significant difference in the pathology resection margins between simple polypectomy and EMR (86% positive versus 68% positive, respectively, P = 0.64; Table 3). When this analysis was limited to polyps <10 mm in size, there remained no difference in positive resection margins (simple polypectomy 55.6% versus EMR 73.3%, P = 0.45). The number of patients diagnosed with local recurrence on surveillance upper endoscopy was also similar (14.3% versus 17.7%, respectively, P = 1.000). Additionally, there was no clear relationship between tumor recurrence and depth of invasion on EUS (P = 0.41), pathology margin (P = 0.530), gross specimen size category (P = 0.155), or tumor size category (P = 1.00).
Overall there were very few complications associated with endoscopic duodenal carcinoid resection (Table 4). Among the patients who received simple polypectomy, there were no instances of aspiration, perforation, bleeding, or escalation of care. However, two patients underwent EMR that was complicated by significant post-procedural bleeding, one of whom required intensive care unit admission. None of these complications resulted in death, and the rate of complications was not statistically significantly different between groups (see Table 4).
TABLE 4.
Complications
| Variable | Simple Resection (n = 10) |
EMR (n = 23) |
P |
|---|---|---|---|
| Aspiration, n (%) | 0 (0) | 0 (0) | |
| Perforation, n (%) | 0 (0) | 0 (0) | |
| Significant bleeding during endoscopy, n (%) | 0 (0) | 0 (0) | |
| Significant bleeding after procedure, n (%) | 0 (0) | 2 (8.7) | 0.479 |
| Need for escalation of care, n (%) | 0 (0) | 1 (4.35) | 0.697 |
| No complications, n (%) | 10 (100) | 21 (91.3) | 0.479 |
Additional Pathology Characteristics
The remaining pathology characteristics of the duodenal carcinoid tumors are summarized in Table 5. All tumors were well differentiated on pathology. In total only three were WHO grade II, with the remainder WHO grade I. Low mitotic rates and Ki-67 proliferation indices were also noted, consistent with the low WHO pathology grade assigned to the specimens. There were no statistically significant differences in these pathology findings between simple polypectomy and EMR resections (Table 5).
TABLE 5.
Additional Pathology Characteristics
| Variable | Simple Resection (n = 10) |
EMR (n = 23) |
P |
|---|---|---|---|
| Tumor differentiation, n (%) | |||
| Well-differentiated | 8 (100) | 23 (100) | |
| Not reported | 2 | 0 | |
| WHO tumor grade, n (%) | 1.00 | ||
| I (low) | 8 (80) | 17 (74) | |
| II (intermediate) | 1 (10) | 2 (9) | |
| Not reported | 1 | 4 | |
| Mitotic count (per HPF), n (%) | 0.34 | ||
| <2 | 7 (70) | 20 (87) | |
| Not reported | 3 (30) | 3 (13) | |
| Ki-67 proliferative index, n (%) | 1.00 | ||
| ≤2% | 6 (60) | 15 (65) | |
| 3–20% | 1 (10) | 2 (9) | |
| Not reported | 3 | 6 |
WHO indicates World Health Organization; HPF, high power field.
Survival and Duration of Follow-up
The overall median duration of clinical follow-up was 24.0 months (IQR, 6.5–48.6 months). Over this time three patients died, with a median time to death of 6.9 months (IQR, 0.2–18.7 months). In all of these cases, patients died of causes unrelated to their diagnosis of duodenal carcinoid. Local tumor recurrence was diagnosed in three patients managed with EMR (13%) as compared to one patient with simple polypectomy (10%). The median time to diagnosed recurrence was 2.3 months (IQR, 1.2–5.4 months). In all of these patients repeat endoscopic resection was pursued rather than surgical management. Finally, among patients without evidence of recurrence on surveillance endoscopy, the median duration of negative endoscopic follow-up was 21.4 months (IQR, 7.1–39.6 months). There were no significant differences between simple polypectomy and EMR among any of these variables (Table 3).
DISCUSSION
Endoscopic resection of duodenal carcinoids is generally considered appropriate for smaller lesions that are at least <20 mm (ideally <10 mm) and confined to the submucosa and without lymph node or distant spread. What remains unclear is the ideal technique and approach to endoscopic resection, and the criteria by which different techniques should be favored. Prior case reports and series have primarily focused on advanced endoscopic resection such as EMR. This makes intuitive sense, as duodenal carcinoids frequently invade into the submucosa. However, it is intriguing to note that local recurrence rates after EMR are minimal despite a high proportion of positive pathology margins at initial resection. For example, in one recent study comparing different EMR techniques, the proportion of positive pathology margins ranged from 25–56%, but there were no local tumor recurrences at 17 months of mean follow up time.16 This finding led our group to question whether resection to the submucosa was indeed necessary for all lesions, especially given the known slow-growing and indolent nature of most duodenal carcinoids.
Our data suggest that simple polypectomy may be equally effective in reducing the risk of local recurrence as compared to EMR for selected lesions. This is plausible, as two prior studies have shown efficacy of non-EMR techniques in small duodenal carcinoid resection.17,18 Although en bloc resection rates were notably lower for simple polypectomy as compared to EMR in our study, both groups had similar proportions of positive pathology margins and local recurrence. As in prior case series,14–16 the rates of local recurrence were very low overall despite moderate proportions of positive margins. It is worth noting that when local recurrences were identified in our cohort, repeat endoscopic management was pursued. For example, the sole patient with local recurrence in the simple polypectomy group underwent further endoscopic resection with subsequent negative surveillance endoscopy. Importantly, simple polypectomy was applied for lesions that were consistently smaller than those resected with EMR (median 6 mm versus 8 mm diameter). As such the recurrence rates of larger lesions treated with simple polypectomy remains unknown.
Another crucial consideration in evaluating the potential benefit of simple resection techniques for smaller carcinoid lesions is the likelihood of complications. Although there was no statistically significant difference in the rate of complications in our study, likely because of sample size, the only complications noted were in the EMR group. In one case a patient suffered a life-threatening bleed mandating an intensive care unit stay and aggressive endoscopic therapy. Furthermore, it has been shown previously that EMR in general has a higher complication rate than simple polypectomy techniques.19 As such simple polypectomy is expected to be safer from a procedural standpoint as compared to advanced endoscopic resection for duodenal carcinoids.
Finally, a confirmatory finding of our study pertains to endoscopic surveillance intervals. All local recurrences were diagnosed within eight months of the index resection, with a median time to recurrence of 2.4 months. This is to be contrasted with the median duration of negative endoscopic follow-up, which was 21.4 months. These findings serve to reinforce the general practice for repeat endoscopic surveillance within approximately one year of the index resection. If there is no recurrence at this time point, then recurrence appears less likely moving forward. However, longer-term data are still lacking to define the time horizon of local tumor recurrence in this slow-growing neoplasm.
We acknowledge several limitations to this work. As with all studies to date addressing endoscopic resection of duodenal carcinoids, this study is primarily limited by a small sample size. Although this clearly restrains the nature of conclusions that can be drawn, our results are in line with aforementioned case series with regard to rates of positive margins and local recurrence. A second limitation is the degree of loss-to-follow up that we encountered in our cohort. This primarily occurred in patients who underwent endoscopic resection nearly 10 years prior, and for whom contact information was no longer current. In several of these instances, the patients were referred to our hospital system only for resection of their duodenal carcinoid, and were not seen subsequently in our practice. Third, there is a possibility of selection bias among the groups. Endoscopic ultrasound was performed in far more cases where EMR was ultimately performed. This may reflect referral of select cases to more skilled endoscopists, possibly for lesions previously biopsied and confirmed to be duodenal carcinoids, or for lesions that were felt to be too challenging to resect with simple polypectomy. Furthermore, all EMR resections were performed by advanced endoscopists, in contrast to simple polypectomy. The effect of these biases, however, would be to favor more complete resection in the EMR group, making the conclusions in this study even more striking. Finally, missing data are of some concern in this cohort. With respect to the primary analysis evaluating resection margins and complications, there were no missing data. However, several patients lacked endoscopic surveillance data, and it is possible that a small number of local recurrences could have been missed. We would expect these differences to be non-differential between groups. Additionally, 30% of the patients in the simple polypectomy group did not have pathology margins reported. These were primarily cold forceps biopsy specimens. It is likely that the true margins would be positive in these cases, as all were piecemeal resections. As such, the missing data in this case would bias towards a null hypothesis; that is, simple polypectomy likely has a higher rate of margin positivity as compared to EMR, which would be expected.
In summary, our study provides intriguing findings that simple polypectomy may be adequate treatment for very small duodenal carcinoids (~6 mm or smaller), although this conclusion should be interpreted with caution given the limitations presented. Further studies are clearly needed to reevaluate this premise, but over time fewer complications are to be expected with simple polypectomy as compared to EMR. This is critical to optimize the procedural risk/benefit ratio for a condition that harbors a generally low risk profile at its outset.
Acknowledgments
Financial Support Acknowledgement:
Nadim Mahmud is supported by a National Institutes of Health T32 grant (2-T32-DK007740-21A1).
Footnotes
Disclosure:
The authors declare no conflict of interest.
REFERENCES
- 1.Riddell R, Petras R, Williams G, et al. Lymphoproliferative disorders of the intestines Tumors of the Intestines. Washington, DC: Armed Forces Institute of Pathology; 2003;395–430. [Google Scholar]
- 2.Modlin IM, Lye KD, Kidd M. A 5‐decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 4.Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease E-Book: Pathophysiology, Diagnosis, Management, Expert Consult Premium Edition-Enhanced Online Features. 9th edition. Vol 1 Philadelpha, PA: Saunders Elsevier Health Sciences; 2010. [Google Scholar]
- 5.Burke A, Sobin L, Federspiel B, et al. Carcinoid tumors of the duodenum. A clinicopathologic study of 99 cases. Arch Pathol Lab Med. 1990;114:700–704. [PubMed] [Google Scholar]
- 6.Klöppel G, Rindi G, Anlauf M, et al. Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Archiv. 2007;451:9–27. [DOI] [PubMed] [Google Scholar]
- 7.Zyromski NJ, Kendrick ML, Nagorney DM, et al. Duodenal carcinoid tumors: how aggressive should we be? J Gastrointest Surg. 2001;5:588–593. [DOI] [PubMed] [Google Scholar]
- 8.Endocrinocarcinomas Soga J. (carcinoids and their variants) of the duodenum. An evaluation of 927 cases. J Exp Clin Cancer Res. 2003;22:349–363. [PubMed] [Google Scholar]
- 9.Dalenbäck J, Havel G. Local endoscopic removal of duodenal carcinoid tumors. Endoscopy. 2004;36:651–655. [DOI] [PubMed] [Google Scholar]
- 10.Tai WP, Yue H. Endoscopic mucosa resection of a duodenum carcinoid tumor of 1.2 cm diameter: a case report. Med Oncol. 2009;26:319–321. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama S, Takifuji K, Tani M, et al. Endoscopic resection of duodenal bulb neuroendocrine tumor larger than 10 mm in diameter. BMC Gastroenterol. 2011;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alberti-Flor JJ, Hernandez ME, Ferrer JP. Endoscopic polypectomy of a large duodenal carcinoid. Gastrointest Endosc. 1993;39:853–854. [DOI] [PubMed] [Google Scholar]
- 13.Pungpapong S, Woodward TA, Wallace MB, et al. EUS-assisted EMR of a large duodenal carcinoid tumor. Gastrointest Endosc. 2006;63:703–704. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto S, Miyatani H, Yoshida Y, et al. Duodenal carcinoid tumors: 5 cases treated by endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1152–1156. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Park CH, Ki HS, et al. Endoscopic treatment of duodenal neuroendocrine tumors. Clin Endosc. 2013;46:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim GH, Kim JI, Jeon SW, et al. Endoscopic resection for duodenal carcinoid tumors: a multicenter, retrospective study. J Gastroenterol Hepatol. 2014;29:318–324. [DOI] [PubMed] [Google Scholar]
- 17.Scherer JR, Holinga J, Sanders M, et al. Small duodenal carcinoids: a case series comparing endoscopic resection and autoamputation with band ligation. J Clin Gastroenterol. 2015;49:289–292. [DOI] [PubMed] [Google Scholar]
- 18.Yoshikane H, Goto H, Niwa Y, et al. Endoscopic resection of small duodenal carcinoid tumors with strip biopsy technique. Gastrointest Endosc. 1998;47:466–470. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad NA, Kochman ML, Long WB, et al. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390–396. [DOI] [PubMed] [Google Scholar]