Abstract
Recent research has demonstrated that cardiovascular deconditioning (i.e., cardiac atrophy and hypovolemia) contributes significantly to the Postural Orthostatic Tachycardia Syndrome (POTS) and its functional disability. Therefore, physical reconditioning with exercise training and volume expansion via increased salt and fluid intake should be initiated early in the course of treatment for patients with POTS if possible. The use of horizontal exercise (e.g., rowing, swimming, recumbent bike, etc.) at the beginning is a critical strategy, allowing patients to exercise while avoiding the upright posture that elicits their POTS symptoms. As patients become increasingly fit, the duration and intensity of exercise should be progressively increased, and upright exercise can be gradually added as tolerated. Supervised training is preferable to maximize functional capacity. Other non-pharmacological interventions, which include: 1) chronic volume expansion via sleeping in the head-up position; 2) reduction in venous pooling during orthostasis by lower body compression garments extending at least to the xiphoid or with an abdominal binder; and 3) physical countermeasure maneuvers, such as squeezing a rubber ball, leg crossing, muscle pumping, squatting, negative-pressure breathing, etc., may also be effective in preventing orthostatic intolerance and managing acute clinical symptoms in POTS patients. However, randomized clinical trials are needed to evaluate the efficacies of these non-pharmacological treatments of POTS.
Keywords: deconditioning, exercise intolerance, physical activity, volume expansion, venous pooling, physical countermeasures
1. INTRODUCTION
The pathophysiological mechanisms underlying the Postural Orthostatic Tachycardia Syndrome (POTS) are heterogeneous (Benarroch 2012); however, cardiovascular deconditioning (i.e., cardiac atrophy and hypovolemia) appears to be a common feature or a final common pathway in patients with this disorder regardless of the inciting mechanisms (Fu et al. 2010; Fu et al. 2011; Galbreath et al. 2011; Garland et al. 2015; Joyner and Masuki 2008; Parsaik et al. 2012; Shibata et al. 2012) and plays a substantial role in the physical disability associated with POTS. Indeed, the heart size and mass are much smaller in patients with POTS or POTS like syndromes compared to age and sex-matched healthy sedentary individuals (Fu et al. 2010; Miwa and Fujita 2011; Raj and Levine 2013). Reductions in plasma and blood volume have also been reported in many POTS patients (Fu et al. 2010; Raj et al. 2005; Ruzieh et al. 2017; Stewart et al. 2006a). In addition, most of these patients have significant limitations to even low-intensity physical activity (Masuki et al. 2007; Parsaik et al. 2012; Shibata et al. 2012). The small heart coupled with reduced blood volume results in a large fall in stroke volume during orthostasis as a function of the Frank-Starling mechanism (Levine et al. 1997), leading to an excessive increase in heart rate via the baroreflex (i.e., reflex tachycardia) in patients with POTS.
Since POTS is fundamentally a condition dependent on gravity, clues regarding the etiology of POTS can be found in research studying spaceflight and its ground based analog, bedrest deconditioning. Many studies have demonstrated that real and/or simulated microgravity exposure, such as spaceflight and head-down bed rest elicit a “POTS-like” syndrome even in previously healthy, physically fit individuals, and many patients with POTS report a significant degree of bedrest during the course of their illness. In contrast, exercise training combined with volume repletion expand plasma and blood volume (Saltin et al. 1968), increase cardiac size and mass (Dorfman et al. 2007a), and therefore, prevent the physiology of “cardiovascular deconditioning” and orthostatic intolerance after microgravity exposure (Dorfman et al. 2007b; Hastings et al. 2012; Levine et al. 1997; Perhonen et al. 2001a; Perhonen et al. 2001b; Shibata et al. 2010b). In this regard, exercise training along with volume expansion via increased salt and fluid intake should be started early in the treatment of POTS if possible (Raj and Levine 2013).
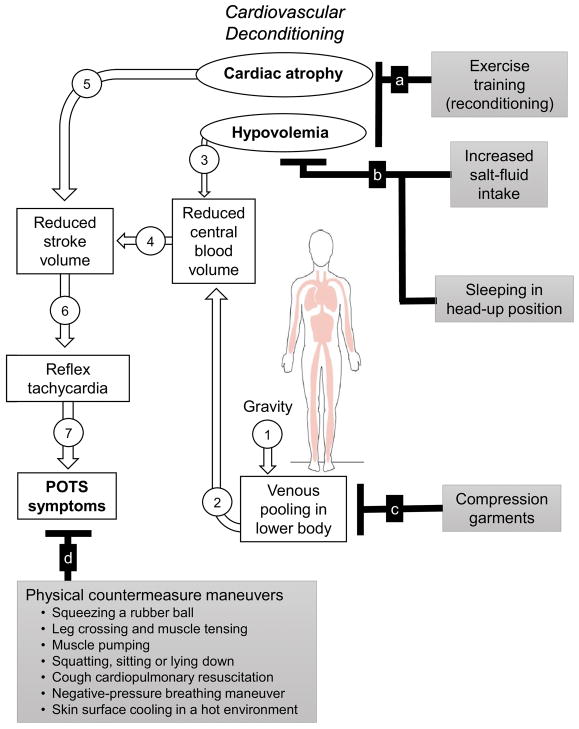
In this article, we review a “structured” exercise training program specifically designed for patients with POTS. Other non-pharmacological interventions, such as volume expansion, use of the lower body compression garment to reduce venous pooling, and physical countermeasures in the prevention of orthostatic intolerance and management of clinical symptoms in POTS patients are also reviewed. Exercise and non-pharmacological interventions should be considered for most patients if tolerated (Sheldon et al. 2015), because they are simple and cost-effective and have no or minimal side effects. Figure summarizes the rationale for exercise training and non-pharmacological interventions in the treatment of POTS.
Figure.
The rationale for exercise and non-pharmacological treatments of the Postural Orthostatic Tachycardia Syndrome (POTS).
2. EXERCISE TRAINING
Physical reconditioning with regular exercise is the cornerstone of treatment for POTS (Sheldon et al. 2015) especially in the chronic state when physical disability has been compounded by cardiovascular deconditioning. A structured exercise program featuring endurance (aerobic) reconditioning with some resistance (strength) training for the lower body described below in detail is recommended for patients with POTS, and supervised exercise training is preferable to maximize functional capacity in these patients (Fu and Levine 2013; Fu and Levine 2015). This carefully structured exercise training program was initially developed for spaceflight and/or head-down bed rest countermeasures (Hastings et al. 2012; Holt et al. 2016; Iwasaki et al. 2003; Lee et al. 2014; Ploutz-Snyder et al. 2014; Shibata et al. 2010a), which has been demonstrated to be effective for the treatment of POTS in our previous studies in a research setting (Fu et al. 2010; Fu et al. 2011; Galbreath et al. 2011; Shibata et al. 2012), as well as in a community environment (George et al. 2016). We found that short-term (e.g., 3 months) exercise training increased peak oxygen uptake (an indication of physical fitness) by 8%, cardiac size and mass by 12% and 8%, and blood volume by 6% in patients with POTS (Fu et al. 2010; Fu et al. 2011; Galbreath et al. 2011; Shibata et al. 2012). More importantly, in contrast to standard pharmacological therapies such as β-blockers, exercise training improved patient overall well-being and quality of life (Fu et al. 2010; Fu et al. 2011; George et al. 2016; Shibata et al. 2012). After 3 months of training, 53% of the patients in our research studies (Fu et al. 2010; Fu et al. 2011; Galbreath et al. 2011; Shibata et al. 2012) and 71% in the POTS registry (George et al. 2016) no longer met objective criteria for POTS and were considered to be “in remission”. The completion rate of training was 76% in the research setting (Fu et al. 2010) and 41% in the community environment (George et al. 2016); thus, approximately 45% of the overall POTS patients were able to complete the program. Factors for inability to continue with exercise training in our previous studies included other medical problems (e.g., mitochondrial disorders, Lyme disease, epilepsy, trauma unrelated to training, etc.), personal reasons (e.g., inability to afford a gym membership, lack of access to work out equipment, etc.), and the training was considered “too difficult” in some patients. Previous studies showed that the adherence to other medical interventions such as antihypertensive drug treatment, or to cardiac rehabilitation ranges from 35% to 65% (Flack et al. 1996; Sharp and Freeman 2009; Sloan et al. 2009). The retention rate for the proposed POTS exercise intervention is thus comparable to the findings of these studies, suggesting that compliance with this approach is within the range of the drug or nondrug interventions in common diseases. Exercise training is a Heart Rhythm Society Expert Consensus Statement (HRSECS) Class II-a recommendation in POTS (Sheldon et al. 2015).
2.1 Endurance training
A structured endurance exercise program for the initial 3 months of training is displayed in Table 1. Based on the predicted maximal heart rate (e.g., (take 220 – age) ± 5 bpm) and resting heart rate, three training zones are determined; base pace, maximal steady state, and recovery (Fu et al. 2010; Fu et al. 2011; George et al. 2016). The majority of the training sessions, particularly during the early phases are prescribed as “base pace” training with target heart rate equivalent to approximately 75% of maximal predicted heart rate and a Rating of Perceived Exertion (RPE) of 13–15 corresponding to the words “somewhat hard” to “hard”. Initially, patients train 3 to 4 times per week for 25 to 30 minutes per session by using rowing, swimming or a recumbent bike. The use of recumbent or semi-recumbent training is critical in the beginning, allowing patients to exercise while avoiding upright posture and eliciting POTS symptoms. As the patient becomes increasingly fit, the duration of the base pace training is prolonged and subsequently sessions of increased intensity (i.e., maximal steady state) are added first once per two weeks and then once per week, and are always followed by recovery sessions. The RPE for “maximal steady state” training is 16–18 corresponding to the word “very hard”, whereas the RPE for “recovery” training is 6–12 corresponding to the word “fairly easy”. By the end of the second month or the beginning of the third month, upright exercise, such as upright bicycle, walking on a treadmill, or jogging is added. By the end of the third month, patients exercise 5 to 6 times per week and 45 to 60 minutes per session. Endurance training is preceded by a 5-minute warm-up and followed by a 5-minute cool down. Modifications of the program are expected with some patients. For example, if the patient cannot complete 30 minutes of maximal steady state without a break, he/she will be allowed to break it down to two training sessions, namely, 15 minutes each, and the patient can take 10 minutes of rest in between. Patients are encouraged to monitor their heart rate during every session of training by using a chest strap based heart rate monitor.
Table 1.
A structured endurance training program
| Workout zone | RPE | Target HR | Month 1 | Month 2 | Month 3 |
|---|---|---|---|---|---|
| Base pace | 13–15 | ~75% of maximal HR | 10@25–30 min | 6@30 min 3@35–40 min |
5@35 min 4@45–60 min |
| Maximal steady state | 16–18 | (220–age) ± 5 bpm | 1@20 min 1@25 min |
1@25 min 1@30 min 1@35 min |
1@30 min 1@35 min 1@40 min |
| Recovery | 6–12 | < lowest base pace HR | 2@30 min | 3@35 min | 3@40 min |
| Cardio modes | Rowing Swimming Recumbent bike |
Month 1 modes plus upright bike | Month 1 and 2 modes plus elliptical and treadmill walking |
RPE, rating of perceived exertion (subjective rating of the entire cardio workout on a scale of 6–20: 6 is very, very easy; 11 is fairly easy; 13 is somewhat hard; 15 is hard; 17 is very hard; 19 is very, very hard). HR, heart rate.
It is important to emphasize that these are only guidelines that in some patients, such as those who are taking β-blockers or other medications or with underlying autonomic disorders, may affect the heart rate response to exercise and may not accurately reflect exercise intensity. If the patients choose to continue β-blockers while following the training, it may be more difficult to follow the heart rate zones; rather, they will need to gauge each workout based on the RPE. In our POTS registry, most healthcare providers chose to wean patients off medications they had been prescribed before starting the training or during the training (George et al. 2016).
Patients with both POTS and the Ehlers-Danlos Syndrome (hypermobility type) usually have joint hypermobility, joint instability complications and widespread musculoskeletal pain, and these clinical symptoms can limit their exercise capacity. Patients should start with non-weight bearing exercise, such as swimming or rowing at mild intensity levels (e.g., base pace training), and they are recommended to wear elbow and knee braces for joint protection during exercise. Exercise intensity should be slowly and carefully increased, and each increment depends on successful completions of the last. Expert guidance and/or supervision from spine specialists during exercise training are preferable. Physical therapy should be incorporated along with supervised exercise training in this particular patient population to avoid worsening joint damage, joint instability and pain.
There are several tips for utilizing this endurance program. First, starting with the horizontal mode of training is key. Second, rowing with a flywheel based device is preferred because it mimics open water rowing best and allows for the rhythmic contraction of large muscle groups. Open water rowers have the largest hearts out of all competitive athletes, and rowing is great to strengthen the heart muscle and induce cardiac hypertrophy (Baggish et al. 2011; Spirito et al. 1994; Urhausen et al. 1996; Wasfy et al. 2015). Third, keep the workouts spread out throughout the week, and this is more beneficial than bunching them up and then taking several days off from exercising. Fourth, try not to take more than 2 days off from exercising. Fifth, if patients cannot complete all the sessions for that week, they need to repeat that entire week again before moving forward. If for some reason the patient misses a period of workouts due to illness, injury, or other reasons, it is best to back up in the schedule and repeat cardio workouts. If the patient stops training for more than two weeks, he/she may want to consider beginning all over again. The patient may also need to return to training with the first more horizontal modes of training (i.e., recumbent bike, swimming, rowing only) before moving forward in the program again. Finally, many patients experience increased fatigue or exacerbation of other POTS symptoms when they start the exercise program. In our experience, they should be encouraged to persevere, and be reassured that the exercise is not harming them, even if they find it uncomfortable. As they work through the program, these untoward symptoms abate, and most patients will start to feel better.
For long-term maintenance training, patients could just maintain the level of training laid out in Month 3 indefinitely, as this amount of exercise meets the AHA/ACC/ACSM consensus guidelines for physical activity to maximize cardiorespiratory fitness and cardiovascular health. Patients can use whatever modes they enjoy most, continuing to more upright activities, and still using the rower 1 to 2 times per week if they like it. Jogging and stair stepping should be saved for after they are able to complete workouts on the elliptical or fast walking at an incline completely symptom free, and they never have to do either of these activities if they never want to.
2.2 Resistance training
In addition to endurance training, resistance training focusing on lower body and core using weight lifting is also recommended. This is intentional, since lower body muscles act as pumps when they contract to increase venous return to the heart during orthostasis. Weight lifting starts from once a week, 15 to 20 minutes per session, and gradually increases to twice a week, 30 to 40 minutes per session. All resistance training sessions should be done on seated equipment, and the use of free weights needs to be avoided until the patient has become stronger and fitter. For those who are unfamiliar with weight training, a personal trainer is recommended to help utilize proper form and technique on each machine.
We recommend to perform 2 sets of 10 repetitions of the following: seated leg press, leg curl, leg extension, calf raise, chest press, and seated row. Patients should do as many repetitions as they can on the second set. When the patient can do more than 10 on the second set, he/she needs to increase the weight lifted for the next training session. Patients are also recommended to perform exercises for core, such as abdominal crunches, back extensions, side planks or anything Pilates based that they can do on the floor (2 sets, 10 to 20 repetitions as they are able to). This is the minimum strength training exercises that are recommended as patients are getting started. After the first month, if they would like to add new weight training exercises, we recommend them to consult their personal trainers/therapists, but do so slowly. If patients do not have access to any seated strength training equipment, they may consider home strength training exercises to strengthen legs and core. These will usually utilize a floor mat, resistance bands, or a physio-ball, and again anything Pilates based that can be done on the floor is great.
There are several tips for resistance training. First, resistance training can make muscle sore in the beginning, especially two days after the workout. The sore sensation will be improved as training continues. Second, it is fine to perform weight training at the end of the cardio workouts instead of on separate days if the patient prefers. Third, take at least a day off between resistance training workouts to allow muscles to recover. A detailed video discussion regarding this program between a POTS patient and our exercise physiologist can be found at: http://www.ieemphd.org/patient-care/syncope-and-autonomic-dysfunction
3. VOLUME EXPANSION
Many POTS patients have reduced plasma and blood volume (Fu et al. 2010; Raj et al. 2005; Ruzieh et al. 2017; Stewart et al. 2006a), which contribute significantly to a small stroke volume and reflex tachycardia during orthostasis. Chronic volume expansion via increased salt and fluid intake, and/or sleeping in the head-up position amplify the plasma volume expanding effect of exercise training and are recommended to patients with POTS. Yet, the efficacies of these interventions independently remain to be evaluated in large randomized clinical trials.
3.1 Increased salt and fluid intake
Salt loading in patients with posturally-related syncope has been shown to increase plasma volume and orthostatic tolerance (Mtinangi and Hainsworth 1998; Wieling et al. 2002). Thus, POTS patients who have normal cardiac and renal-adrenal function are recommended to gradually increase their daily salt intake by using dietary salt up to 10 grams per day, if tolerated (Fu et al. 2010; Fu et al. 2011; George et al. 2016; Sheldon et al. 2015). A slow, progressive increase in daily sodium intake in/on the food and eating salty snacks are recommended. However, salt tablets should be avoided because they are very concentrated and can induce an osmotic load into the stomach which may cause nausea, vomiting and dehydration, leading to reduced rather than expanded plasma and blood volume. Patients are also encouraged to increase water intake up to 3 liters per day (Fu et al. 2010; Fu et al. 2011; George et al. 2016). Increasing salt and water intake throughout the day and consuming them together are recommended, as water alone is not effective in long-term volume expansion. Glucose-salt rehydration solution may be more effective in the expansion of plasma and blood volume, but this speculation needs to be verified in randomized clinical trials. Increased salt and fluid intake is a HRSECS Class II-b recommendation in POTS (Sheldon et al. 2015), and should be started prior to or at the time when exercise training is initiated.
3.2 Sleeping in the head-up position
Patients are also encouraged to elevate the head of the bed off the ground 4 to 6 inches in order to increase circulating plasma and blood volume. Large phone books, blocks of wood, or bed risers placed under the feet at the top of the bed work best for placing the entire body at a slight angle during sleeping at night. This approach is different from sleeping on a few extra pillows under the head. The rationale behind this approach is that mild orthostatic stress induces fluid shift to the lower body and decreases central blood volume and the effective circulating blood volume (Wieling et al. 2002), which activate the renin-angiotensin-aldosterone system, leading to salt-water retention and volume expansion. An earlier case report showed that head-up tilt at night caused chronic volume expansion by activation of the renin-angiotensin-aldosterone system in patients with autonomic failure (van Lieshout et al. 1991; van Lieshout et al. 2000). It was also found that head-up sleeping for a period of 3–4 months improved orthostatic tolerance in patients with syncope (Cooper and Hainsworth 2008). However, the magnitude of volume expansion with this approach is unknown and needs to be investigated in future research.
4. REDUCTION IN VENOUS POOLING
Gravity shifts 700 to 900 mL of blood from the central to lower body during orthostasis (Rowell 1993), while excessive venous pooling is considered one major cause for orthostatic intolerance in humans (Gaw et al. 2012; Streeten 1999; Streeten and Scullard 1996). Indeed, previous studies showed that patients with POTS have increased venous pooling in the leg during upright posture, which may be attributed to inappropriate vascular tone and abnormal vasomotor control (Stewart 2002; Stewart and Weldon 2001a; Stewart and Weldon 2001b; Stewart and Weldon 2003). Excessive splanchnic pooling at rest and during upright posture was also reported in patients with POTS (Stewart et al. 2006b; Tani et al. 2000).
Gradient compression garments have been used as a countermeasure to post-spaceflight orthostatic intolerance in astronauts (Platts et al. 2009; Stenger et al. 2010). It was demonstrated that the compression garment decreased venous pooling, increased systolic blood pressure, attenuated the reductions in stroke volume and cardiac output (Stenger et al. 2010), and prevented tachycardia with standing after spaceflight (Stenger et al. 2014; Stenger et al. 2013). The compression garment was also found to be effective in the prevention of orthostatic intolerance in orthostatically intolerant athletes (Privett et al. 2010), and to render the tilt-test negative in patients with a clinical diagnosis of recurrent syncope (Dos Santos et al. 2013).
Compression of all lower body compartments (calf, thigh and low abdomen) seems to be the most efficacious, followed by abdominal compression, whereas leg compression alone appears to be less effective, presumably reflecting the large capacity of the abdomen relative to the legs (Denq et al. 1997; Garvin et al. 2014). Thus, abdomen-high rather than knee or thigh-high compression garments should be recommended for patients with POTS. The commercially-available gradient compression garment is one-piece, which is difficult to put on and take off even with assistance, leading to low compliance of its use. A custom-fit abdomen-high three-piece gradient compression garment developed by the National Aeronautics and Space Administration (NASA) in collaboration with a commercial partner addresses these issues directly.
The NASA compression garment consists of three separate pieces (e.g., two leg stockings and a pair of biker-style shorts), which is far easier to don than the commercially-available one-piece garment. Further, the NASA compression garment includes the addition of strategically-placed zippers near the ankle and in the shorts to relieve the compression during the donning process. These modifications may improve patient compliance with the garment use and efficacy of treatment. Pilot studies from our laboratory showed that the NASA compression garment reduced heart rate by approximately 10 bpm and eliminated clinical symptoms during a 5-minute stand test in patients with POTS. These preliminary observations need to be confirmed in more patients.
5. PHYSICAL COUNTERMEASURES
Physical countermeasure maneuvers to increase orthostatic tolerance have been reviewed in an excellent article by Wieling et al (2015). Physical countermeasures may be effective in the management of acute clinical symptoms and prevention of orthostatic intolerance or syncope/near-syncope in patients with POTS. These maneuvers are simple and easy to learn, require no or limited equipment, and should be recommended to all patients. Table 2 depicts the commonly used countermeasure maneuvers along with their action mechanisms.
Table 2.
Physical countermeasure maneuvers
| Maneuvers | Brief description | Action mechanisms |
|---|---|---|
| Squeezing a rubber ball | Static or rhythmic muscle contraction to increase mean arterial pressure and prevent orthostatic intolerance or syncope | Sympathetic activation, vagal withdraw, or both via the exercise pressor reflex |
| Leg crossing and muscle tensing | Crossing one foot in front of the other and squeezing the thighs and gluteal muscles together | Restoration of venous return and prevention of further blood pooling in the lower body |
| Muscle pumping | Swaying, shifting, tiptoeing, or walking | Activation of the muscle pump in the legs to increase venous return |
| Squatting, sitting, lying down | Squatting is a combination of sitting, bending and muscle tensing; sitting and lying down to reduce/eliminate gravitational stress | Facilitating venous return from the legs to the heart and increasing central blood volume |
| Cough cardiopulmonary resuscitation | Forceful coughing | Increasing intrathoracic pressure to force blood out of the chest into the aorta and its braches |
| Negative-pressure breathing maneuver | Breathe through an inspiratory impedance threshold device | Using endogenous respiratory pump to increase venous return and central blood volume |
| Skin surface cooling | Spray cold water, use fan and cooling towel to cool the skin in a hot environment | Decreasing blood supply to the skin and reducing clinical symptoms |
5.1 Squeezing a rubber ball
Static or rhythmic skeletal muscle contraction increases mean arterial pressure through sympathetic activation, vagal withdrawal, or both (the so-called exercise pressor reflex) (Mitchell 1990; Mitchell and Victor 1996). Squeezing a rubber ball by hand using static hand-gripping combined with contraction of leg and abdominal muscle (Krediet et al. 2005), would be equally or perhaps more effective for increasing mean arterial pressure. This maneuver could be used to counter or delay the onset of neurally mediated syncope, which is common among young women including patients with POTS.
5.2 Leg crossing and muscle tensing
Crossing one foot in front of the other and squeezing the thighs and gluteal muscles together, the so-called Dutch leg-crossing maneuver, is very potent at restoring venous return, preventing further blood pooling in the lower body, and increasing cardiac output and mean arterial pressure in the upright positon (Krediet et al. 2006; Wieling et al. 2004). This maneuver increases intramuscular pressure and decreases transmural pressure of the vein, as such venous distension is reduced and blood is shifted centrally, and thus, cardiac output increases. Results regarding systemic vascular resistance responses are controversial, but remains largely unchanged (Wieling et al. 2015). The physiological effects of leg crossing and muscle tensing are mainly mechanical, but neural control is also involved as evidenced by an increase in heart rate during the maneuver (Krediet et al. 2005; van Dijk et al. 2005). The instantaneous increase in heart rate at the onset of leg crossing and muscle tensing is a reflex effect produced by a combination of the muscle mechanoreflexes and central command with inhibition of vagal tone to the heart (Wieling et al. 2015). Cardiac contractility may be increased under this condition, which may also contribute to the increases in cardiac output and mean arterial pressure during leg crossing and muscle tensing.
5.3 Muscle pumping
Patients are encouraged to learn to sway and shift so that the pumping action of the muscles, especially leg muscles can be used to counter gravitational displacement of blood by squeezing venous blood from the leg upward (Claydon and Hainsworth 2005; Wieling et al. 2015). Tiptoeing or walking activates the muscle pump of the legs in the presence of competent venous valves, which can increase venous return to the heart, and thereafter, increase cardiac output and mean arterial pressure. Indeed, the leg muscle pump is considered a “second heart” and is capable of translocating blood against a substantial pressure gradient (e.g., >90 mmHg) (Wieling et al. 2015).
5.4 Squatting, sitting or lying down
Squatting is a combination of sitting, bending and muscle tensing, which facilitates venous return from the legs and increases central blood volume and cardiac output (Krediet et al. 2005; Lewis et al. 1980; Sharpey-Schafer 1956). The greater the amount of blood pooled in the legs, the more robust the effect of squatting (Wieling et al. 2015). Sitting decreases the gravitational stress and increases venous return to the heart, resulting in increases in cardiac filling pressure, stroke volume, cardiac output, and mean arterial pressure. Lying down eliminates the gravitational stress and shifts blood centrally, leading to increases in cardiac output and mean arterial pressure.
5.5 Cough cardiopulmonary resuscitation
Forceful coughing can maintain circulation for a brief period of time during cardiac arrest by generating sufficient blood pressure that perfuse the brain and vital organs (Girsky and Criley 2006). Each forceful cough abruptly raises intrathoracic pressure, forcing blood out of the chest, into the aorta and its branches. Between coughs, deep inspiration draws blood into the right heart. Thus, a circulation is established (Jafary 2008). Initiation of cough cardiopulmonary resuscitation at the first warning of hemodynamic collapse can maintain consciousness until conventional cardiopulmonary resuscitation can be delivered. Cough cardiopulmonary resuscitation can acutely increase mean arterial pressure to prevent loss of consciousness if patients get dizzy in the upright posture (Criley et al. 1976; Niemann et al. 1980).
5.6 Negative-pressure breathing maneuver
Inspiratory resistance through the use of an impedance threshold device (ITD; set to open at 0.7 kPa or 7-cm H2O pressure) has been found to be effective in the treatment of orthostatic intolerance, orthostatic hypotension, and hemorrhagic shock (Convertino et al. 2005a; Convertino et al. 2005b; Cooke and Convertino 2005; Rickards et al. 2008). The ITD acutely increases central blood volume by forcing the thoracic muscles to develop increased negative pressure, thus drawing venous blood from extrathoracic cavities into the heart and lungs, leading to increases in cardiac output, stroke volume, and mean arterial pressure (Convertino et al. 2005a). A recent randomized, single-blind, cross-over trial demonstrated that increasing negative intrathorasis pressure with ITD breathing increased stroke volume and attenuated the heart rate response during upright posture in patients with POTS (Gamboa et al. 2015). However, the long-term effects of ITD on POTS need to be determined.
5.7 Skin surface cooling
Whole-body skin surface cooling increases orthostatic tolerance in the heated-stressed humans and individuals following prolonged bed rest deconditioning by reducing skin vascular conductance, decreasing blood supply to the skin, and attenuating the drop in central blood volume (Cui et al. 2005; Durand et al. 2004; Keller et al. 2011; Schlader et al. 2016; Wilson et al. 2009; Wilson et al. 2002). In addition, resetting baroreflex curves to higher pressure (Cui et al. 2007) and the Frank-Starling mechanism (Wilson et al. 2009) may also contribute to the increased orthostatic tolerance associated with skin surface cooling. Though whole-body skin surface cooling cannot be easily implemented in a free-living setting, local cooling (e.g., face, neck, chest, etc.) may be helpful in the management of acute clinical symptoms in POTS patients in a hot environment. Patients can fill a spray bottle with water and keep it in the refrigerator for a few hours, and spay the cold water to the face when they are in the hot environment. Placing a cooling towel around the neck and/or wearing a cooling vest may also be effective in reducing clinical symptoms. Finally, using small portable, battery-powered fans, attached to a water bottle that spays a cooling mist to help circulate air may also be effective. The effectiveness of these approaches needs to be evaluated in randomized clinical trials.
6. A MULTIDISCIPLINARY APPROACH
A recent study demonstrated that an interdisciplinary or multidisciplinary program was effective in improving overall functional ability and psychological distress in adolescents with POTS (Bruce et al. 2016). The treatment program consisted of an intensive 3-week outpatient hospital-based multidisciplinary rehabilitation intervention, which included biofeedback, physical therapy, occupational therapy, recreational therapy, relaxation training, stress management, wellness instruction (e.g., sleep hygiene, healthy diet), as well as pain and physical symptom management training. As the heart rate response to upright posture was not assessed in the study by Bruce et al (2016), it remains to be determined whether this multidisciplinary program is effective in the treatment of POTS.
7. SUMMARY
Exercise and non-pharmacological interventions should be considered early in the treatment of POTS. The use of horizontal exercise at the beginning is a critical strategy, and supervised training is preferable to maximize functional capacity in patients with POTS. Other non-pharmacological interventions, such as volume expansion, reduction in venous pooling, and physical countermeasures may also be effective in preventing orthostatic intolerance and managing acute clinical symptoms. Though the therapeutic effects of exercise in POTS have been evaluated in clinical trials, the efficacies of other non-pharmacological treatments of POTS remain to be investigated.
Acknowledgments
Support for this work was provided in part by the National Institutes of Health (K23 HL075238 grant).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baggish AL, Hale A, Weiner RB, Lewis GD, Systrom D, Wang F, Wang TJ, Chan SY. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol. 2011;589(Pt 16):3983–3994. doi: 10.1113/jphysiol.2011.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87(12):1214–1225. doi: 10.1016/j.mayocp.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce BK, Harrison TE, Bee SM, Luedtke CA, Porter CJ, Fischer PR, Hayes SE, Allman DA, Ale CM, Weiss KE. Improvement in Functioning and Psychological Distress in Adolescents With Postural Orthostatic Tachycardia Syndrome Following Interdisciplinary Treatment. Clin Pediatr (Phila) 2016;55(14):1300–1304. doi: 10.1177/0009922816638663. [DOI] [PubMed] [Google Scholar]
- Claydon VE, Hainsworth R. Increased postural sway in control subjects with poor orthostatic tolerance. J Am Coll Cardiol. 2005;46(7):1309–1313. doi: 10.1016/j.jacc.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Cooke WH, Lurie KG. Inspiratory resistance as a potential treatment for orthostatic intolerance and hemorrhagic shock. Aviat Space Environ Med. 2005a;76(4):319–325. [PubMed] [Google Scholar]
- Convertino VA, Ratliff DA, Crissey J, Doerr DF, Idris AH, Lurie KG. Effects of inspiratory impedance on hemodynamic responses to a squat-stand test in human volunteers: implications for treatment of orthostatic hypotension. Eur J Appl Physiol. 2005b;94(4):392–399. doi: 10.1007/s00421-005-1344-1. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Convertino VA. Cardiovascular consequences of weightlessness promote advances in clinical and trauma care. Curr Pharm Biotechnol. 2005;6(4):285–297. doi: 10.2174/1389201054553671. [DOI] [PubMed] [Google Scholar]
- Cooper VL, Hainsworth R. Head-up sleeping improves orthostatic tolerance in patients with syncope. Clin Auton Res. 2008;18(6):318–324. doi: 10.1007/s10286-008-0494-8. [DOI] [PubMed] [Google Scholar]
- Criley JM, Blaufuss AH, Kissel GL. Cough-induced cardiac compression. Self-administered from of cardiopulmonary resuscitation. JAMA. 1976;236(11):1246–1250. [PubMed] [Google Scholar]
- Cui J, Durand S, Crandall CG. Baroreflex control of muscle sympathetic nerve activity during skin surface cooling. J Appl Physiol (1985) 2007;103(4):1284–1289. doi: 10.1152/japplphysiol.00115.2007. [DOI] [PubMed] [Google Scholar]
- Cui J, Durand S, Levine BD, Crandall CG. Effect of skin surface cooling on central venous pressure during orthostatic challenge. Am J Physiol Heart Circ Physiol. 2005;289(6):H2429–2433. doi: 10.1152/ajpheart.00383.2005. [DOI] [PubMed] [Google Scholar]
- Denq JC, Opfer-Gehrking TL, Giuliani M, Felten J, Convertino VA, Low PA. Efficacy of compression of different capacitance beds in the amelioration of orthostatic hypotension. Clin Auton Res. 1997;7(6):321–326. doi: 10.1007/BF02267725. [DOI] [PubMed] [Google Scholar]
- Dorfman TA, Levine BD, Tillery T, Peshock RM, Hastings JL, Schneider SM, Macias BR, Biolo G, Hargens AR. Cardiac atrophy in women following bed rest. J Appl Physiol. 2007a;103(1):8–16. doi: 10.1152/japplphysiol.01162.2006. [DOI] [PubMed] [Google Scholar]
- Dorfman TA, Levine BD, Tillery T, Peshock RM, Hastings JL, Schneider SM, Macias BR, Biolo G, Hargens AR. Cardiac atrophy in women following bed rest. J Appl Physiol (1985) 2007b;103(1):8–16. doi: 10.1152/japplphysiol.01162.2006. [DOI] [PubMed] [Google Scholar]
- Dos Santos RQ, Smidt L, Suzigan BH, De Souza LV, Barbisan JN. Efficacy of lower limb compression in the management of vasovagal syncope--randomized, crossover study. Pacing Clin Electrophysiol. 2013;36(4):451–455. doi: 10.1111/pace.12069. [DOI] [PubMed] [Google Scholar]
- Durand S, Cui J, Williams KD, Crandall CG. Skin surface cooling improves orthostatic tolerance in normothermic individuals. Am J Physiol Regul Integr Comp Physiol. 2004;286(1):R199–205. doi: 10.1152/ajpregu.00394.2003. [DOI] [PubMed] [Google Scholar]
- Flack JM, Novikov SV, Ferrario CM. Benefits of adherence to anti-hypertensive drug therapy. Eur Heart J. 1996;17(Suppl A):16–20. doi: 10.1093/eurheartj/17.suppl_a.16. [DOI] [PubMed] [Google Scholar]
- Fu Q, Levine BD. Exercise and the autonomic nervous system. Handb Clin Neurol. 2013;117:147–160. doi: 10.1016/B978-0-444-53491-0.00013-4. [DOI] [PubMed] [Google Scholar]
- Fu Q, Levine BD. Exercise in the postural orthostatic tachycardia syndrome. Auton Neurosci. 2015;188:86–89. doi: 10.1016/j.autneu.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Vangundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, Bhella PS, Levine BD. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2010;55(25):2858–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension. 2011;58(2):167–175. doi: 10.1161/HYPERTENSIONAHA.111.172262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbreath MM, Shibata S, VanGundy TB, Okazaki K, Fu Q, Levine BD. Effects of exercise training on arterial-cardiac baroreflex function in POTS. Clin Auton Res. 2011;21(2):73–80. doi: 10.1007/s10286-010-0091-5. [DOI] [PubMed] [Google Scholar]
- Gamboa A, Paranjape SY, Black BK, Arnold AC, Figueroa R, Okamoto LE, Nwazue VC, Diedrich A, Plummer WD, Dupont WD, et al. Inspiratory resistance improves postural tachycardia: a randomized study. Circ Arrhythm Electrophysiol. 2015;8(3):651–658. doi: 10.1161/CIRCEP.114.002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EM, Celedonio JE, Raj SR. Postural Tachycardia Syndrome: Beyond Orthostatic Intolerance. Curr Neurol Neurosci Rep. 2015;15(9):60. doi: 10.1007/s11910-015-0583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin NM, Levine BD, Raven PB, Pawelczyk JA. Pneumatic antishock garment inflation activates the human sympathetic nervous system by abdominal compression. Exp Physiol. 2014;99(1):101–110. doi: 10.1113/expphysiol.2013.072447. [DOI] [PubMed] [Google Scholar]
- Gaw CE, Shields RW, Jr, Mayuga KA, Gornik HL, Fouad-Tarazi F. POTS due to excessive venous pooling in an enlarged inferior vena cava. Clin Auton Res. 2012;22(4):197–198. doi: 10.1007/s10286-012-0157-7. [DOI] [PubMed] [Google Scholar]
- George SA, Bivens TB, Howden EJ, Saleem Y, Galbreath MM, Hendrickson D, Fu Q, Levine BD. The international POTS registry: Evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm. 2016;13(4):943–950. doi: 10.1016/j.hrthm.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Girsky MJ, Criley JM. Images in cardiovascular medicine. Cough cardiopulmonary resuscitation revisited. Circulation. 2006;114(15):e530–531. doi: 10.1161/CIRCULATIONAHA.106.620773. [DOI] [PubMed] [Google Scholar]
- Hastings JL, Krainski F, Snell PG, Pacini EL, Jain M, Bhella PS, Shibata S, Fu Q, Palmer MD, Levine BD. Effect of rowing ergometry and oral volume loading on cardiovascular structure and function during bed rest. J Appl Physiol (1985) 2012;112(10):1735–1743. doi: 10.1152/japplphysiol.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JA, Macias BR, Schneider SM, Watenpaugh DE, Lee SM, Chang DG, Hargens AR. WISE 2005: Aerobic and resistive countermeasures prevent paraspinal muscle deconditioning during 60-day bed rest in women. J Appl Physiol (1985) 2016;120(10):1215–1222. doi: 10.1152/japplphysiol.00532.2015. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Zhang R, Zuckerman JH, Levine BD. Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J Appl Physiol. 2003;95(4):1575–1583. doi: 10.1152/japplphysiol.00482.2003. [DOI] [PubMed] [Google Scholar]
- Jafary FH. Cough-assisted maintenance of perfusion during asystole. Can J Cardiol. 2008;24(10):e76. doi: 10.1016/s0828-282x(08)70693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ, Masuki S. POTS versus deconditioning: the same or different? Clin Auton Res. 2008;18(6):300–307. doi: 10.1007/s10286-008-0487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Low DA, Davis SL, Hastings J, Crandall CG. Skin surface cooling improves orthostatic tolerance following prolonged head-down bed rest. J Appl Physiol (1985) 2011;110(6):1592–1597. doi: 10.1152/japplphysiol.00233.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krediet CT, de Bruin IG, Ganzeboom KS, Linzer M, van Lieshout JJ, Wieling W. Leg crossing, muscle tensing, squatting, and the crash position are effective against vasovagal reactions solely through increases in cardiac output. J Appl Physiol (1985) 2005;99(5):1697–1703. doi: 10.1152/japplphysiol.01250.2004. [DOI] [PubMed] [Google Scholar]
- Krediet CT, van Lieshout JJ, Bogert LW, Immink RV, Kim YS, Wieling W. Leg crossing improves orthostatic tolerance in healthy subjects: a placebo-controlled crossover study. Am J Physiol Heart Circ Physiol. 2006;291(4):H1768–1772. doi: 10.1152/ajpheart.00287.2006. [DOI] [PubMed] [Google Scholar]
- Lee SM, Schneider SM, Feiveson AH, Macias BR, Smith SM, Watenpaugh DE, Hargens AR. WISE-2005: Countermeasures to prevent muscle deconditioning during bed rest in women. J Appl Physiol (1985) 2014;116(6):654–667. doi: 10.1152/japplphysiol.00590.2013. [DOI] [PubMed] [Google Scholar]
- Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96(2):517–525. doi: 10.1161/01.cir.96.2.517. [DOI] [PubMed] [Google Scholar]
- Lewis BS, Lewis N, Gotsman MS. Effect of standing and squatting on echocardiographic left ventricular function. Eur J Cardiol. 1980;11(6):405–412. [PubMed] [Google Scholar]
- Masuki S, Eisenach JH, Schrage WG, Johnson CP, Dietz NM, Wilkins BW, Sandroni P, Low PA, Joyner MJ. Reduced stroke volume during exercise in postural tachycardia syndrome. J Appl Physiol. 2007;103(4):1128–1135. doi: 10.1152/japplphysiol.00175.2007. [DOI] [PubMed] [Google Scholar]
- Mitchell JH. J.B. Wolffe memorial lecture. Neural control of the circulation during exercise. Med Sci Sports Exerc. 1990;22(2):141–154. [PubMed] [Google Scholar]
- Mitchell JH, Victor RG. Neural control of the cardiovascular system: insights from muscle sympathetic nerve recordings in humans. Med Sci Sports Exerc. 1996;28(10 Suppl):S60–69. doi: 10.1097/00005768-199610000-00036. [DOI] [PubMed] [Google Scholar]
- Miwa K, Fujita M. Small heart with low cardiac output for orthostatic intolerance in patients with chronic fatigue syndrome. Clin Cardiol. 2011;34(12):782–786. doi: 10.1002/clc.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtinangi BL, Hainsworth R. Early effects of oral salt on plasma volume, orthostatic tolerance, and baroreceptor sensitivity in patients with syncope. Clin Auton Res. 1998;8(4):231–235. doi: 10.1007/BF02267786. [DOI] [PubMed] [Google Scholar]
- Niemann JT, Rosborough J, Hausknecht M, Brown D, Criley JM. Cough-CPR: documentation of systemic perfusion in man and in an experimental model: a “window” to the mechanism of blood flow in external CPR. Crit Care Med. 1980;8(3):141–146. doi: 10.1097/00003246-198003000-00011. [DOI] [PubMed] [Google Scholar]
- Parsaik A, Allison TG, Singer W, Sletten DM, Joyner MJ, Benarroch EE, Low PA, Sandroni P. Deconditioning in patients with orthostatic intolerance. Neurology. 2012;79(14):1435–1439. doi: 10.1212/WNL.0b013e31826d5f95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, Peshock RM, Weatherall PT, Levine BD. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol (1985) 2001a;91(2):645–653. doi: 10.1152/jappl.2001.91.2.645. [DOI] [PubMed] [Google Scholar]
- Perhonen MA, Zuckerman JH, Levine BD. Deterioration of left ventricular chamber performance after bed rest: “cardiovascular deconditioning” or hypovolemia? Circulation. 2001b;103(14):1851–1857. doi: 10.1161/01.cir.103.14.1851. [DOI] [PubMed] [Google Scholar]
- Platts SH, Tuxhorn JA, Ribeiro LC, Stenger MB, Lee SM, Meck JV. Compression garments as countermeasures to orthostatic intolerance. Aviat Space Environ Med. 2009;80(5):437–442. doi: 10.3357/asem.2473.2009. [DOI] [PubMed] [Google Scholar]
- Ploutz-Snyder LL, Downs M, Ryder J, Hackney K, Scott J, Buxton R, Goetchius E, Crowell B. Integrated resistance and aerobic exercise protects fitness during bed rest. Med Sci Sports Exerc. 2014;46(2):358–368. doi: 10.1249/MSS.0b013e3182a62f85. [DOI] [PubMed] [Google Scholar]
- Privett SE, George KP, Whyte GP, Cable NT. The effectiveness of compression garments and lower limb exercise on post-exercise blood pressure regulation in orthostatically intolerant athletes. Clin J Sport Med. 2010;20(5):362–367. doi: 10.1097/JSM.0b013e3181f20292. [DOI] [PubMed] [Google Scholar]
- Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111(13):1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- Raj SR, Levine BD. Postural tachycardia syndrome (POTS) diagnosis and treatment: Basics and new developments. 2013 http://crmcardiosourceorg/Learn-from-the-Experts/2013/02/POTS-Diagnosis-and-Treatment.
- Rickards CA, Cohen KD, Bergeron LL, Burton L, Khatri PJ, Lee CT, Ryan KL, Cooke WH, Doerr DF, Lurie KG, et al. Inspiratory resistance, cerebral blood flow velocity, and symptoms of acute hypotension. Aviat Space Environ Med. 2008;79(6):557–564. doi: 10.3357/asem.2149.2008. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Passive effects of gravity. Human Cardiovascular Control 1993 [Google Scholar]
- Ruzieh M, Baugh A, Dasa O, Parker RL, Perrault JT, Renno A, Karabin BL, Grubb B. Effects of intermittent intravenous saline infusions in patients with medication-refractory postural tachycardia syndrome. J Interv Card Electrophysiol. 2017;48(3):255–260. doi: 10.1007/s10840-017-0225-y. [DOI] [PubMed] [Google Scholar]
- Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38(5 Suppl):VII1–78. [PubMed] [Google Scholar]
- Schlader ZJ, Wilson TE, Crandall CG. Mechanisms of orthostatic intolerance during heat stress. Auton Neurosci. 2016;196:37–46. doi: 10.1016/j.autneu.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J, Freeman C. Patterns and predictors of uptake and adherence to cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2009;29(4):241–247. doi: 10.1097/HCR.0b013e3181adcf0f. [DOI] [PubMed] [Google Scholar]
- Sharpey-Schafer EP. Effects of squatting on the normal and failing circulation. Br Med J. 1956;1(4975):1072–1074. doi: 10.1136/bmj.1.4975.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon RS, Grubb BP, 2nd, Olshansky B, Shen WK, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12(6):e41–63. doi: 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Fu Q, Bivens TB, Hastings JL, Wang W, Levine BD. Short-term exercise training improves the cardiovascular response to exercise in the postural orthostatic tachycardia syndrome. J Physiol. 2012;590(Pt 15):3495–3505. doi: 10.1113/jphysiol.2012.233858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Perhonen M, Levine BD. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. J Appl Physiol. 2010a;108(5):1177–1186. doi: 10.1152/japplphysiol.01408.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Perhonen M, Levine BD. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. J Appl Physiol (1985) 2010b;108(5):1177–1186. doi: 10.1152/japplphysiol.01408.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, DeMeersman RE, Bagiella E, Brondolo EN, McKinley PS, Slavov I, Fang Y, Myers MM. The effect of aerobic training and cardiac autonomic regulation in young adults. Am J Public Health. 2009;99(5):921–928. doi: 10.2105/AJPH.2007.133165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirito P, Pelliccia A, Proschan MA, Granata M, Spataro A, Bellone P, Caselli G, Biffi A, Vecchio C, Maron BJ. Morphology of the “athlete’s heart” assessed by echocardiography in 947 elite athletes representing 27 sports. Am J Cardiol. 1994;74(8):802–806. doi: 10.1016/0002-9149(94)90439-1. [DOI] [PubMed] [Google Scholar]
- Stenger MB, Brown AK, Lee SM, Locke JP, Platts SH. Gradient compression garments as a countermeasure to post-spaceflight orthostatic intolerance. Aviat Space Environ Med. 2010;81(9):883–887. doi: 10.3357/asem.2781.2010. [DOI] [PubMed] [Google Scholar]
- Stenger MB, Lee SM, Ribeiro LC, Phillips TR, Ploutz-Snyder RJ, Willig MC, Westby CM, Platts SH. Gradient compression garments protect against orthostatic intolerance during recovery from bed rest. Eur J Appl Physiol. 2014;114(3):597–608. doi: 10.1007/s00421-013-2787-4. [DOI] [PubMed] [Google Scholar]
- Stenger MB, Lee SM, Westby CM, Ribeiro LC, Phillips TR, Martin DS, Platts SH. Abdomen-high elastic gradient compression garments during post-spaceflight stand tests. Aviat Space Environ Med. 2013;84(5):459–466. doi: 10.3357/asem.3528.2013. [DOI] [PubMed] [Google Scholar]
- Stewart JM. Pooling in chronic orthostatic intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation. 2002;105(19):2274–2281. doi: 10.1161/01.cir.0000016348.55378.c4. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Glover JL, Medow MS. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin Sci (Lond) 2006a;110(2):255–263. doi: 10.1042/CS20050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Medow MS, Glover JL, Montgomery LD. Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2006b;290(2):H665–673. doi: 10.1152/ajpheart.00784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Weldon A. Reflex vascular defects in the orthostatic tachycardia syndrome of adolescents. J Appl Physiol (1985) 2001a;90(6):2025–2032. doi: 10.1152/jappl.2001.90.6.2025. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Weldon A. The relation between lower limb pooling and blood flow during orthostasis in the postural orthostatic tachycardia syndrome of adolescents. J Pediatr. 2001b;138(4):512–519. doi: 10.1067/mpd.2001.112170. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Weldon A. Contrasting neurovascular findings in chronic orthostatic intolerance and neurocardiogenic syncope. Clin Sci (Lond) 2003;104(4):329–340. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Streeten DH. Orthostatic intolerance. A historical introduction to the pathophysiological mechanisms. Am J Med Sci. 1999;317(2):78–87. doi: 10.1097/00000441-199902000-00002. [DOI] [PubMed] [Google Scholar]
- Streeten DH, Scullard TF. Excessive gravitational blood pooling caused by impaired venous tone is the predominant non-cardiac mechanism of orthostatic intolerance. Clin Sci (Lond) 1996;90(4):277–285. doi: 10.1042/cs0900277. [DOI] [PubMed] [Google Scholar]
- Tani H, Singer W, McPhee BR, Opfer-Gehrking TL, Haruma K, Kajiyama G, Low PA. Splanchnic-mesenteric capacitance bed in the postural tachycardia syndrome (POTS) Auton Neurosci. 2000;86(1–2):107–113. doi: 10.1016/S1566-0702(00)00205-8. [DOI] [PubMed] [Google Scholar]
- Urhausen A, Monz T, Kindermann W. Sports-specific adaptation of left ventricular muscle mass in athlete’s heart. I. An echocardiographic study with combined isometric and dynamic exercise trained athletes (male and female rowers) Int J Sports Med. 1996;17(Suppl 3):S145–151. doi: 10.1055/s-2007-972916. [DOI] [PubMed] [Google Scholar]
- van Dijk N, de Bruin IG, Gisolf J, de Bruin-Bon HA, Linzer M, van Lieshout JJ, Wieling W. Hemodynamic effects of leg crossing and skeletal muscle tensing during free standing in patients with vasovagal syncope. J Appl Physiol (1985) 2005;98(2):584–590. doi: 10.1152/japplphysiol.00738.2004. [DOI] [PubMed] [Google Scholar]
- van Lieshout JJ, ten Harkel AD, van Leeuwen AM, Wieling W. Contrasting effects of acute and chronic volume expansion on orthostatic blood pressure control in a patient with autonomic circulatory failure. Neth J Med. 1991;39(1–2):72–83. [PubMed] [Google Scholar]
- van Lieshout JJ, ten Harkel AD, Wieling W. Fludrocortisone and sleeping in the head-up position limit the postural decrease in cardiac output in autonomic failure. Clin Auton Res. 2000;10(1):35–42. doi: 10.1007/BF02291388. [DOI] [PubMed] [Google Scholar]
- Wasfy MM, DeLuca J, Wang F, Berkstresser B, Ackerman KE, Eisman A, Lewis GD, Hutter AM, Weiner RB, Baggish AL. ECG findings in competitive rowers: normative data and the prevalence of abnormalities using contemporary screening recommendations. Br J Sports Med. 2015;49(3):200–206. doi: 10.1136/bjsports-2014-093919. [DOI] [PubMed] [Google Scholar]
- Wieling W, Colman N, Krediet CT, Freeman R. Nonpharmacological treatment of reflex syncope. Clin Auton Res. 2004;14(Suppl 1):62–70. doi: 10.1007/s10286-004-1009-x. [DOI] [PubMed] [Google Scholar]
- Wieling W, van Dijk N, Thijs RD, de Lange FJ, Krediet CT, Halliwill JR. Physical countermeasures to increase orthostatic tolerance. J Intern Med. 2015;277(1):69–82. doi: 10.1111/joim.12249. [DOI] [PubMed] [Google Scholar]
- Wieling W, Van Lieshout JJ, Hainsworth R. Extracellular fluid volume expansion in patients with posturally related syncope. Clin Auton Res. 2002;12(4):242–249. doi: 10.1007/s10286-002-0024-z. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Brothers RM, Tollund C, Dawson EA, Nissen P, Yoshiga CC, Jons C, Secher NH, Crandall CG. Effect of thermal stress on Frank-Starling relations in humans. J Physiol. 2009;587(Pt 13):3383–3392. doi: 10.1113/jphysiol.2009.170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol (1985) 2002;93(1):85–91. doi: 10.1152/japplphysiol.01043.2001. [DOI] [PubMed] [Google Scholar]