Abstract
Background:
The burden of end-stage liver disease in older adults has increased; understanding trends in liver transplantation (LT) and outcomes for older recipients is imperative for evaluation, counseling, and appropriate referral of this vulnerable group of older adults.
Study design and setting:
We studied 8,627 older (age≥65) deceased donor liver-only transplant recipients using data from the Scientific Registry of Transplant Recipients (1/1/2003–12/31/2016). We evaluated temporal changes in recipient, donor, and transplant characteristics. We also evaluated post-LT length of stay (LOS), acute rejection, graft loss, and mortality using logistic regression and Cox proportional hazards.
Results:
LT in older adults increased almost 5-fold from 263 in 2003 (9.5% of total LT that year) to 1,144 in 2016 (20.7% of total LT). Recent recipients were more likely to be female, African American, and have a higher BMI and MELD score. Hepatitis C, non-alcoholic steatohepatitis, and hepatocellular carcinoma were the most common indications for LT in recent recipients. Comparing those in 2013–2016 to those in 2003–2006, odds of LOS>2 weeks decreased 34% (adjusted odds ratio [aOR]:0.66, 95%CI:0.57–0.76, P<.001), 1-year acute rejection decreased 30% (aOR:0.70, 95%CI:0.56–0.88, P=.002), all-cause graft loss decreased 54% (adjusted hazard ratio [aHR]:0.46, 95%CI:0.40–0.52, P<.001), and mortality decreased 57% (aHR:0.43, 95%CI:0.38–0.49, P<.001).
Conclusion:
Despite the substantial increase in number and severity of older adults undergoing LT, LOS, rejection, graft loss, and mortality have significantly decreased over time. These trends can help guide appropriate LT referral and counseling in older adults with end-stage liver disease.
Keywords: graft loss, mortality, older recipients, liver transplantation
INTRODUCTION
The burden of end-stage liver disease (ESLD) in older adults (aged ≥65) in the United States (US) is increasing,1–4 and older adults comprise 23.8% of the current liver transplant (LT) waitlist, up from 8% in 2002.2, 5 The increase in older adults with ESLD disease is driven by the aging population with hepatitis C virus cirrhosis along with the increase in nonalcoholic steatohepatitis and hepatocellular carcinoma, which typically affect older adults.3, 4, 6, 7 Historically, older adults were denied access to LT because of poor post-transplant survival,8–10 but there are more recent reports of LT in older adults, including small reports of LT even in octogenarians.11, 12
It is possible that advances in immunosuppression regimens and surgical techniques 13–16 may be leading to improved LT outcomes in older adults. However, older adults are uniquely susceptible after LT given increased comorbidity, higher prevalence of frailty, and physical impairment.17–19 Among older LT candidates and recipients, physical impairment, frailty, and older age are associated with an increased risk of mortality.2, 18, 20–22 Additionally, older adults have immunosenescence, leading to lower tolerance of post-LT immunosuppression. 23–25 Therefore, improvements in modern immunosuppression may not translate to improved post-transplant outcomes over time in older recipients. Further, poor outcomes in older LT recipients are typically due to cardiac complications, malignancy, and infection,8, 9, 26 so surgical and immunosuppression changes do not necessarily translate into improved outcomes for older recipients. A better understanding of the trends over time in outcomes for older LT recipients is warranted for appropriate LT referral, evaluation, and counseling prior to transplantation.
In light of the aging ESLD population, we sought to evaluate and understand the temporal trends in LT and post-LT outcomes for older recipients. To inform clinical practice, we used national registry data to: 1) characterize the changing landscape of LT in older adults, and 2) describe the trends over the last 15 years in LT length of stay, acute rejection, graft loss, and mortality for older recipients.
METHODS
Data Source
This study used data from the Scientific Registry of Transplant Recipients (SRTR) external release made available in March 2017. The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States submitted by members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere.27 All-cause graft loss and mortality were augmented through linkage with the Social Security Master Death File, data from Centers for Medicare and Medicaid Services (CMS), and waitlist data. The Health Resources and Services Administration (HRSA), the United States Department of Health and Human Services, provides oversight to the activities of OPTN and SRTR contractors.
Study Population
We identified 8627 older (age ≥65) deceased donor liver-only transplant recipients between January 1, 2003 and December 31, 2016 using data from SRTR. We grouped these recipients by year of LT into four strata for empirical reasons and to reflect changes in allocation policy and general evolution of immunosuppression regimens: 2003–2006, 2007–2009, 2010–6/18/2013 and 6/19/2013–2016. We divided the recent time periods at 6/18/2013 to evaluate trends before and after implementation of the Share 35 policy change, which increased regional liver allograft offers to patients with MELD score ≥35. The annual number and percent of liver transplants for older recipients was examined over time. Donor, recipient, and transplant characteristics were examined using t tests for continuous variables and χ2 tests for categorical variables.
Outcomes
LOS was defined as the duration of hospitalization during the initial transplant episode and analyzed as a binary variable ≤2 weeks or >2 weeks using adjusted multiple logistic regression; a cut-off previously used in abdominal solid organ transplantation.28, 29 Acute rejection within the first year of LT was analyzed as a binary variable using adjusted multiple logistic regression. All-cause graft loss and mortality were estimated at 1-, 3- and 5-years using the Kaplan-Meier method for each time stratum. Kaplan-Meier methods were also used to create unadjusted cumulative incidence curves of all-cause graft loss and mortality. Cox proportional hazards models for all-cause graft loss and mortality were used to adjust for changes in recipient, donor, and transplant characteristics. Proportional hazards assumptions were confirmed with visual inspection of complementary log-log plots and Schoenfeld residuals.
Statistical Analyses
To ensure proper risk adjustment, we adjusted each of the regression models for standard factors accounted for in the SRTR program specific reports. This included recipient factors—sex, age, race, body mass index (BMI), primary diagnosis, life support, hepatocellular carcinoma, non-hepatocellular carcinoma malignancy, hepatitis C virus, HIV status, diabetic, primary insurance, portal vein thrombosis, and split LT)—and donor factors—age, race, BMI, hepatitis C virus, donation after cardiac death, ABO compatibility, cold ischemia time. All analyses were two-tailed and α was set at 0.05. All analyses were performed using STATA 14.2/MP for Linux (College Station, Texas).
RESULTS
Study Population
Among 58,598 adult LT recipients, 8627 (14.7%) were older LT recipients between 2003–2016; 78% were aged 65–69, 20.1% were aged 70–74, 1.6% were aged 75–79, and 0.1% were aged ≥80. Also, 36.1% were female, and 6.4% were African-American (Table 1).
Table 1.
Characteristics of the older liver transplant (LT) recipients from 2003–2016.
| Characteristic | 2003–2006 | 2007–2009 | 2010– 06/18/2013 | 06/19/2013– 2016 | P value |
|---|---|---|---|---|---|
| N=1453 | N=1544 | N=2222 | N=3408 | ||
| Recipient characteristic | |||||
| Age (years), mean± SD | 68.0± 2.7 | 67.8± 2.6 | 67.8± 2.5 | 67.6± 2.4 | .001 |
| Female, % | 38.9 | 37.2 | 36.9 | 34.0 | .006 |
| BMI, mean± SD | 27.4± 4.9 | 27.9± 5.3 | 28.0± 5.1 | 28.4± 5.1 | <.001 |
| Race, % | |||||
| White | 77.7 | 74.1 | 73.8 | 73.8 | <.001 |
| African American | 3.9 | 5.1 | 6.7 | 7.8 | |
| Indication for LT, % | |||||
| Hepatitis C virus cirrhosis | 16.5 | 12.6 | 15.8 | 18.1 | <.001 |
| Alcoholic cirrhosis | 12.6 | 14.1 | 12.7 | 13.0 | |
| Non-alcoholic steatohepatitis | 5.8 | 13.0 | 14.2 | 19.5 | |
| Hepatocellular carcinoma | 18.4 | 27.9 | 29.1 | 28.9 | |
| Cholestatic liver disease | 10.0 | 8.3 | 7.8 | 6.7 | |
| Non-cholestatic cirrhosis | 26.4 | 18.2 | 13.8 | 8.6 | |
| MELD, % | |||||
| <10 | 3.0 | 1.4 | 0.6 | 0.4 | <.001 |
| 10–19 | 28.3 | 20.7 | 12.7 | 11.4 | |
| 20–29 | 53.8 | 62.7 | 58.3 | 52.9 | |
| 30–39 | 11.1 | 10.6 | 20.2 | 27.5 | |
| ≥40 | 1.9 | 3.6 | 6.4 | 6.7 | |
| Status 1/1A | 1.9 | 1.1 | 1.7 | 1.2 | |
| Life support (prior to LT) | 2.8 | 3.1 | 4.7 | 5.3 | <.001 |
| Ascites, % | 2.0 | 2.0 | 2.0 | 1.9 | .001 |
| Albumin (g/dL) | 1.1 | 1.1 | 1.1 | 1.2 | <.001 |
| Portal vein thrombosis, % | 5.0 | 8.9 | 14.3 | 14.6 | <.001 |
| Comorbidities, % | |||||
| HIV | 0.1 | 0.1 | 0.1 | 0.2 | .79 |
| Hepatitis C | 27.5 | 27.6 | 30.2 | 32.6 | <.001 |
| Diabetes mellitus | 31.0 | 34.5 | 33.5 | 37.4 | <.001 |
| Liver transplant characteristic | |||||
| Cold ischemia time (hours), % | |||||
| 0–8 | 72.3 | 80.6 | 88.4 | 88.5 | <.001 |
| 9–11 | 18.0 | 14.2 | 9.8 | 9.7 | |
| ≥12 | 9.7 | 5.2 | 1.8 | 1.8 | |
| ABO incompatible, % | 0.5 | 0.5 | 0.7 | 1.0 | .08 |
| Split graft, % | 1.2 | 1.9 | 1.7 | 1.5 | .37 |
| Donor characteristic | |||||
| Age, % | <.001 | ||||
| <18 | 6.2 | 6.2 | 5.1 | 5.1 | |
| 18–39 | 28.8 | 32.8 | 34.6 | 36.5 | |
| 40–49 | 18.7 | 17.5 | 16.9 | 17.1 | |
| 50–59 | 19.0 | 18.3 | 20.2 | 20.3 | |
| 60–69 | 15.4 | 13.8 | 15.0 | 14.3 | |
| ≥70 | 12.0 | 11.5 | 8.2 | 6.7 | |
| Female, % | 44.9 | 40.2 | 43.1 | 40.8 | .02 |
| Race, % | |||||
| White | 71.6 | 64.4 | 66.0 | 65.7 | <.001 |
| African American | 14.3 | 17.2 | 19.4 | 18.8 | |
| Donation after cardiac death, % | 4.8 | 6.4 | 5.4 | 7.2 | .003 |
| Hepatitis C, % | 1.2 | 1.8 | 2.3 | 5.3 | <.001 |
Increase in LT in Older Adults
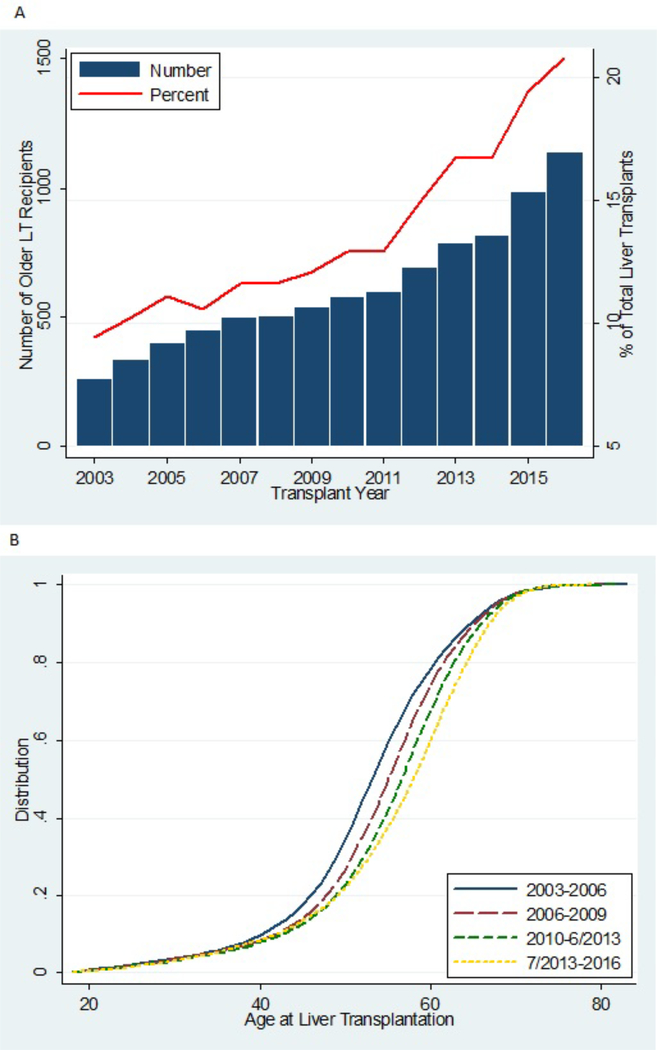
The annual number of LTs performed in older adults increased substantially throughout the study period (Figure 1A). In 2016, 1144 older adults received LTs (20.7% of all LT recipients), up from 263 older LT recipients in 2003 (9.5% of all LT recipients).
Figure 1.
Trends in 8,627 older liver transplant (LT) recipients according to year of transplant. (A) The number of older LT recipients is shown as a bar (left y-axis), and the percentage of total older LT recipients among 58,598 adult LT recipients is shown as a line (right y-axis). (B) For all older LT recipients from 2003–2016, the nested cumulative distribution of age at the time of LT is displayed according to year of transplant.
Changing Landscape of LT in Older Adults
LT recipients shifted toward older ages over time (Figure 1B). Older LT recipients became more likely to be male (66.0% in 2013–2016 vs 61.1% in 2003–2006, P =.006), African American (7.8 % vs 3.9%, P <.001), have MELD ≥30 (34.2% vs 13.0%, P <.001), have portal vein thrombosis (14.6% vs 5.0%, P <.001), and have non-alcoholic steatohepatitis (19.5% vs 5.8%) or hepatocellular carcinoma (28.9% vs 18.4%) as their indication for LT (Table 1). In addition, older LT recipients became more likely to receive a hepatitis C virus positive donor (5.3% vs 1.2%, p<0.001) or DCD donor (7.2% vs 4.8%, P=.003), and became less likely to receive a nationally shared donor (4.1% vs 10.6%, P <.001) (Table 1).
Length of Stay over Time
Median (interquartile range) LOS decreased from 10 (7–18) days in 2003–2006 to 9 (6–16) days in 2013–2016. LOS >2 weeks for older LT recipients decreased from 30.8% in 2003–2006 to 28.0% in 2013–2016. After adjusting for donor, recipient, and transplant factors, the odds for LOS >2 weeks in 2013–2016 was 34% lower than in 2003–2006 (adjusted odds ratio [aOR]:0.66, 95% CI:0.57–0.76, P <.001) (Table 3).
Table 3.
Length of stay, one-year acute rejection, all-cause graft loss, and mortality for older liver transplant (LT) recipients.
| Year of LT | Older recipients (N= 8,627) | |
|---|---|---|
| Length of stay >2 weeks | N | aOR (95% CI) P value |
| 2003–2006 | 1453 | Reference |
| 2007–2009 | 1544 | 0.83 (0.71, 0.98) P=.03 |
| 2010–06/18/2013 | 2222 | 0.73 (0.63, 0.85) P<.001 |
| 06/19/2013–2016 | 3408 | 0.66 (0.57, 0.76) P<.001 |
| One-year acute rejection | N | aOR (95% CI) P value |
| 2003–2006 | 1453 | Reference |
| 2007–2009 | 1544 | 1.00 (0.79, 1.27) P=.99 |
| 2010–06/18/2013 | 2222 | 0.74 (0.58, 0.93) P=.01 |
| 06/19/2013–2016 | 3408 | 0.70 (0.56, 0.88) P=.002 |
| All-cause graft loss | N | aHR (95% CI) P value |
| 2003–2006 | 1453 | Reference |
| 2007–2009 | 1544 | 0.83 (0.75, 0.92) P=.001 |
| 2010–06/18/2013 | 2222 | 0.68 (0.61, 0.75) P<.001 |
| 06/19/2013–2016 | 3408 | 0.46 (0.40, 0.52) P<.001 |
| Mortality | N | aHR (95% CI) P value |
| 2003–2006 | 1453 | Reference |
| 2007–2009 | 1544 | 0.83 (0.75, 0.93) P=0.001 |
| 2010–06/18/2013 | 2222 | 0.67 (0.60, 0.75) P<.001 |
| 06/19/2013–2016 | 3408 | 0.43 (0.38, 0.49) P<.001 |
Adjusted odds ratios (aORs) of one-year acute rejection loss and length of stay >2 weeks (relative to 2003–2006) in older were estimated using logistic regression. Adjusted hazard ratios (aHRs) of mortality and graft loss (relative to 2003–2006) in older recipients were estimated using Cox models. aHRs and aORs were adjusted for recipient factors (sex, age, race, body mass index (BMI), primary diagnosis, life support, hepatocellular carcinoma (HCC), non-HCC malignancy, hepatitis C virus (HCV), HIV status, diabetic, primary insurance, portal vein thrombosis, and split LT), and donor factors (age, race, BMI, HCV, donation after cardiac death (DCD), ABO compatibility, as well as donor and recipient geography).
The two latest time periods were split at 6/18/2103 after the allocation policy implementation of Share35. This policy increases regional liver allograft offers to patients with MELD score ≥35.
Acute Rejection over Time
One-year acute rejection decreased from 14.8% in 2003–2006 to 9.7% in 2013–2016. After adjusting for donor, recipient, and transplant factors, one-year acute rejection in 2013–2016 was 30% lower than in 2003–2006 (aOR:0.70, 95% CI:0.56–0.88, P =.002) (Table 3).
All-Cause Graft Loss over Time
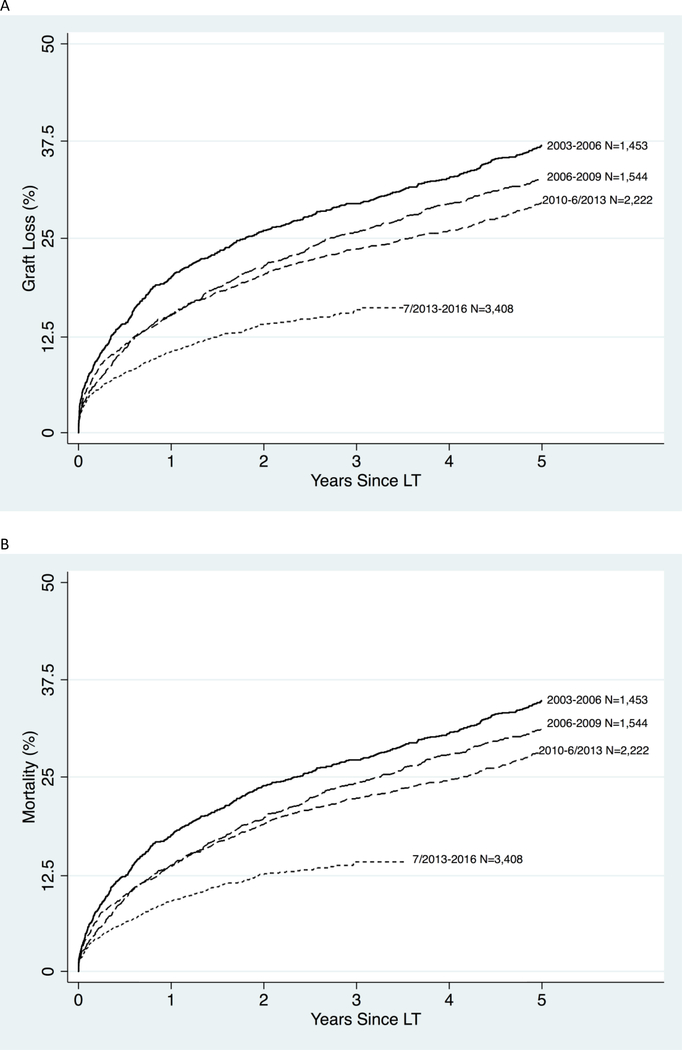
Graft survival in older LT recipients also improved over time (Figure 2A). One-year survival improved from 80% in 2003–2006 to 90% in 2013–2016; 3-year survival improved from 71% to 84%, and 5-year from 63% to 70% (Table 2). After adjusting for donor, recipient, and transplant factors, graft failure in 2013–2016 was 54% lower than it was in 2003–2006 (adjusted hazard ratio [aHR]:0.46, 95% CI:0.40–0.52, P <.001) (Table 3).
Figure 2.
Cumulative incidence of (A) all-cause graft loss and (B) mortality in older LT recipients by year. The year and number of LT recipients is seen to the right of the curve. The most recent time periods were split at 6/18/2013 after the allocation policy implementation of Share35. This policy increases regional liver allograft offers to patients with MELD score ≥35 to direct allografts to sicker candidates.
Table 2.
Patient and all-cause graft survival at 1-, 3-, and 5-year in older recipients according to year of liver transplantation (LT).
| Year of LT | Older recipients (N= 8,627) | ||||
|---|---|---|---|---|---|
| N | % | ||||
| 1-year | 3-year | 5-year | |||
| Graft survival | |||||
| 2003–2006 | 1453 | 80 | 71 | 63 | |
| 2007–2009 | 1544 | 85 | 74 | 67 | |
| 2010–06/18/2013 | 2222 | 85 | 76 | 70 | |
| 06/19/2013–2016 | 3408 | 90 | 84 | -- | |
| Patient survival | |||||
| 2003–2006 | 1453 | 82 | 73 | 65 | |
| 2007–2009 | 1544 | 87 | 76 | 69 | |
| 2010–06/18/2013 | 2222 | 86 | 78 | 72 | |
| 06/19/2013–2016 | 3408 | 91 | 86 | -- | |
The two latest time periods were split at 6/18/2103 after the allocation policy implementation of Share35. This policy increases regional liver allograft offers to patients with MELD score ≥35.
Mortality over Time
Patient survival in older LT recipients improved steadily over time (Figure 2B). One-year survival improved from 82% in 2003–2006 to 91% in; 3-year survival improved from 73% to 86%, and 5-year from 65% to 72% (Table 2). After adjusting for donor, recipient, and transplant factors, mortality in 2013–2016 was 57% lower than it was in 2003–2006 (aHR:0.43, 95% CI:0.38–0.49, P <.001) (Table 3).
DISCUSSION
In this national study of 8627 older LT recipients between 2003–2016, we have identified a changing landscape in transplantation for older adults, with a dramatic increase in number of LTs performed and a significant improvements in LOS, acute rejection, graft survival, and patient survival. There was almost a 5-fold increase in the number of older adults who underwent LT from 2003 (N=263) to 2016 (N=1144), and older adults accounted for 20.7% of total LT recipients in 2016. Older LT recipients were more likely to be male, African American, have higher a MELD score and portal vein thrombosis in 2013–2016 as compared to 2003–2006. Also, recent older recipients were more likely to undergo LT for hepatitis C virus, non-alcoholic steatohepatitis, or hepatocellular carcinoma, and more likely to receive a hepatitis C virus positive or donation after cardiac death graft compared to older LT recipients in 2003–2006. Despite an increase in the severity of liver disease and number of LTs performed in older recipients, from 2003 to 2016 there were significant improvements in acute rejection (aOR: 0.70, P=.002) and shorter LOS (aOR: 0.66, P<.001) along with graft loss and mortality (aHR: 0.46 and 0.43, both P<.001).
Our findings of a significant increase in the number of older adults undergoing LT are consistent with reports of increasing numbers of older adults undergoing kidney, heart, and lung transplantation. 30, 31 These studies described a substantial rise in the number and proportion of older adults undergoing transplantation, with up to 18.4% of kidney transplant recipients over the age of 65. 30 Our findings are also consistent with a report of increased LT in recipients over the age of 60 by Su et al; we extended their study by evaluating the trends over time in the characteristics and outcomes of older LT recipients and found that, despite the changing demographics, outcomes have dramatically improved.2 Also, the temporal improvement we observed in graft and patient survival for older LT recipients is consistent with improvement in graft and patient survival for older KT recipients,30 supporting our hypothesis that improvements in immunosuppression might play a role. Finally, we show a dramatic improvement in long-term outcomes for older LT recipients that is different from a recent paper that showed no improvement in long-term outcomes for LT recipients of all ages.32 However, this report did not stratify outcomes by age, but the majority of LT recipients are under age 65, so it seems to be driven by younger patients.
The strengths of this study include a large, unbiased, national cohort of LT recipients (i.e. every recipient in the United States) dating back to the implementation of the MELD allocation system. While we are limited by the general coarseness of comorbidity data in the national registry, it is unlikely that differences in comorbidities would explain the dramatically observed improvement in outcomes seen in recent years, especially given that older LT recipients are now sicker than those in the past (so any potential bias would be toward the null).
LT in older recipients increased dramatically in the last 15 years, with improvements in length of stay, acute rejection, graft survival, and patient survival in these recipients. Older patients with ESLD and their providers should be aware of these findings, and increased age per se should not prohibit access to LT in older adults.
ACKNOWLEDGEMENTS
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR, OPTN/UNOS, or the United States Government.
Funding
Funding for this study was provided by the National Institute of Diabetes and Digestive and Kidney Disease and the National Institute on Aging: grant numbers F32AG053025 (PI: Christine Haugen), F32DK109662 (PI: Courtenay Holscher), K01AG043501 (PI: Mara McAdams-DeMarco), R01AG055781 (PI: Mara McAdams-DeMarco), R01AG042504 (PI: Dorry Segev), and K24DK101828 (PI: Dorry Segev). Dr. Holscher was also funded by the American College of Surgeons Resident Research Scholarship.
Abbreviations:
- aHR
adjusted hazard ratio
- aOR
adjusted odds ratio
- BMI
body mass index
- ESLD
end-stage liver disease
- HRSA
Health Resources and Services Administration
- LOS
length of stay
- LT
liver transplantation
- MELD
model for end-stage liver disease
- OPTN
Organ Procurement and Transplantation Network
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Disclosures: Authors have no conflict of interest to report as described by Journal of the American Geriatrics Society.
Impact statement: We certify that this work is novel and can provide guidance for geriatricians treating patients with liver disease as well as geriatric general surgeons for appropriate referral of older patients with liver disease who were previously thought of as poor transplant candidates.
REFERENCES
- [1].Agopian VG, Petrowsky H, Kaldas FM, et al. The evolution of liver transplantation during 3 decades: analysis of 5347 consecutive liver transplants at a single center. Ann Surg. 2013;258: 409–421. [DOI] [PubMed] [Google Scholar]
- [2].Su F, Yu L, Berry K, et al. Aging of Liver Transplant Registrants and Recipients: Trends and Impact on Waitlist Outcomes, Post-Transplantation Outcomes, and Transplant-Related Survival Benefit. Gastroenterology. 2016;150: 441–453 e446; quiz e416. [DOI] [PubMed] [Google Scholar]
- [3].Biggins SW, Bambha KM, Terrault NA, et al. Projected future increase in aging hepatitis C virus-infected liver transplant candidates: a potential effect of hepatocellular carcinoma. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2012;18: 1471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hoshida Y, Ikeda K, Kobayashi M, et al. Chronic liver disease in the extremely elderly of 80 years or more: clinical characteristics, prognosis and patient survival analysis. Journal of hepatology. 1999;31: 860–866. [DOI] [PubMed] [Google Scholar]
- [5].OPTN National Data 2017.
- [6].Frith J, Day CP, Henderson E, Burt AD, Newton JL. Non-alcoholic fatty liver disease in older people. Gerontology. 2009;55: 607–613. [DOI] [PubMed] [Google Scholar]
- [7].Noureddin M, Yates KP, Vaughn IA, et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58: 1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Collins BH, Pirsch JD, Becker YT, et al. Long-term results of liver transplantation in older patients 60 years of age and older. Transplantation. 2000;70: 780–783. [DOI] [PubMed] [Google Scholar]
- [9].Herrero JI, Lucena JF, Quiroga J, et al. Liver transplant recipients older than 60 years have lower survival and higher incidence of malignancy. Am J Transplant. 2003;3: 1407–1412. [DOI] [PubMed] [Google Scholar]
- [10].Malinis MF, Chen S, Allore HG, Quagliarello VJ. Outcomes among older adult liver transplantation recipients in the model of end stage liver disease (MELD) era. Ann Transplant. 2014;19: 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Aduen JF, Sujay B, Dickson RC, et al. Outcomes after liver transplant in patients aged 70 years or older compared with those younger than 60 years. Mayo Clin Proc. 2009;84: 973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lipshutz GS, Busuttil RW. Liver transplantation in those of advancing age: the case for transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2007;13: 1355–1357. [DOI] [PubMed] [Google Scholar]
- [13].Wiesner R, Rabkin J, Klintmalm G, et al. A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2001;7: 442–450. [DOI] [PubMed] [Google Scholar]
- [14].Fisher RA, Ham JM, Marcos A, et al. A prospective randomized trial of mycophenolate mofetil with neoral or tacrolimus after orthotopic liver transplantation. Transplantation. 1998;66: 1616–1621. [DOI] [PubMed] [Google Scholar]
- [15].Watson CJ, Friend PJ, Jamieson NV, et al. Sirolimus: a potent new immunosuppressant for liver transplantation. Transplantation. 1999;67: 505–509. [DOI] [PubMed] [Google Scholar]
- [16].Llado L, Figueras J. Techniques of orthotopic liver transplantation. HPB (Oxford). 2004;6: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McAdams-DeMarco MA, Ying H, Olorundare I, et al. Individual Frailty Components and Mortality In Kidney Transplant Recipients. Transplantation. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang CW, Covinsky KE, Feng S, Hayssen H, Segev DL, Lai JC. Functional impairment in older liver transplantation candidates: From the functional assessment in liver transplantation study. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2015;21: 1465–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162: 2269–2276. [DOI] [PubMed] [Google Scholar]
- [20].Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14: 1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lai JC, Dodge JL, Sen S, Covinsky K, Feng S. Functional decline in patients with cirrhosis awaiting liver transplantation: Results from the functional assessment in liver transplantation (FrAILT) study. Hepatology. 2016;63: 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lai JC, Covinsky KE, McCulloch CE, Feng S. The Liver Frailty Index Improves Mortality Prediction of the Subjective Clinician Assessment in Patients With Cirrhosis. The American journal of gastroenterology. 2018;113: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Danovitch GM, Gill J, Bunnapradist S. Immunosuppression of the elderly kidney transplant recipient. Transplantation. 2007;84: 285–291. [DOI] [PubMed] [Google Scholar]
- [24].Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211: 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99: 805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sonny A, Kelly D, Hammel JP, Albeldawi M, Zein N, Cywinski JB. Predictors of poor outcome among older liver transplant recipients. Clin Transplant. 2015;29: 197–203. [DOI] [PubMed] [Google Scholar]
- [27].Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14: 1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Ann Surg. 2017;266: 1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Haugen CE, Mountford A, Warsame F, et al. Incidence, Risk Factors, and Sequelae of Post-kidney Transplant Delirium. J Am Soc Nephrol. 2018;29: 1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McAdams-DeMarco MA, James N, Salter ML, Walston J, Segev DL. Trends in kidney transplant outcomes in older adults. J Am Geriatr Soc. 2014;62: 2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Abecassis M, Bridges ND, Clancy CJ, et al. Solid-organ transplantation in older adults: current status and future research. Am J Transplant. 2012;12: 2608–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rana A, Ackah RL, Webb GJ, et al. No Gains in Long-Term Survival After Liver Transplantation Over the Past Three Decades. Ann Surg. 2018. [DOI] [PubMed] [Google Scholar]