Abstract
Objective:
To better understand decision role preferences in women diagnosed with breast cancer at a young age for return of results of genome sequencing in research and clinical settings.
Methods:
Participants were surveyed about communication and decision-making preferences related to genome sequencing results and factors that may affect these preferences. The primary outcome was decision role preference (Control Preference Scale) for selecting what results to receive within medical care or within a research study.
Results:
For results returned as part of medical care, most patients preferred a collaborative (N=481, 45%) or active (N=488, 45%) role with only 107 (10%) choosing a passive role. When making the decision as part of a research study, most patients preferred an active role (N=617, 57%), 350 (33%) choosing a collaborative role, and110 (10%) choosing a passive role.
Conclusion:
Most women in this study preferred to share in decision making. Participants had somewhat different role preferences for clinical and research contexts, with greater preference for active roles in the research context.
Practice Implications:
We advocate for practice guidelines that incorporate discussion of decision role as an integral part of patient centered care and shared decision-making and recognize that more work is needed to inform guidelines.
Keywords: breast cancer, BRCA, genetic knowledge, advanced genetic testing, decision making, decision role preferences
1. INTRODUCTION
Genome sequencing is on the rise in both the clinical and research settings [1, 2], since rapidly improving technology has decreased the costs and time needed. This has been an important step towards personalized medicine. This test provides vast amounts of information, but there are scientific limitations to interpreting gene variants for clinical use. As such, it is uncertain how to best use the data and how to communicate results to patients, even in the research setting [3–5]. There are many types of information that can result from the use of sequencing technologies including information about: risk of preventable disease, risk of non-preventable disease, risk of future disease for relatives, and efficacy of medications to name a few [1, 5–7]. Clinical sequencing holds the promise to provide much needed, clinically relevant information. However, what is currently clinically relevant is dwarfed by information with potential personal utility for patients, but marginal or no clinical utility. The resultant ethical and legal implications of these tests can be difficult for patients and providers to consider since the results may affect not just the individual, but the entire family [8, 9]. Because this is a difficult situation to navigate, it is important to understand the role(s) patients want to play in decisions about results to be returned.
In making this type of decision, patients may prefer different levels of participation, with some preferring to make the decision themselves, some preferring to have the provider make the decision, and some wanting to share the responsibility for the decision with the provider [10–15]. Shared decision-making is defined as a collaborative process in which the patient and provider each contribute to the final decision. It is advocated as the most patient-centered decision making process and decision role preference is a key part of the process [16–19]. When it comes to decision preferences for returning test results generated by sequencing technologies, there is very little known about how patients want to engage in the process and what factors might be associated with these role preferences. Studies in other clinical decision-making contexts have shown that the role a patient wants to play in the decision is associated with multiple demographic factors with younger patients and more educated patients preferring to take on more active roles where they take primary responsibility for the decision. Role preference, however, is likely dynamic, and depends on contextual variables such as type of disease, age, clinic setting, gender, and dominant cultural values [10, 13, 20–32]. With genome sequencing becoming more common, there are many opportunities for understanding how patients want to make decisions about testing and how they want the information communicated back to them (i.e. return of results). Specific disease contexts, such as breast cancer, where single gene testing has long been available, but more comprehensive genetic testing such as genome sequencing is new, provide rich resources for understanding these processes. Because sequencing results may generate personalized data related to multiple health conditions, patient decisional preferences for receiving these results may differ from their preferences for receiving results from disease specific genetic tests.
Breast cancer is the most commonly diagnosed malignancy in women of all ages [33]. Women diagnosed at a young age (40 or younger) are recommended to undergo genetic testing [34]. These women are more likely than women over 40 to carry genetic mutations that put them at increased risk for developing breast cancer. While the best known and highest risk of these mutations are in the BRCA genes, the list of gene mutations for which to test is ever evolving and increasing. Genetic testing for high risk mutations at the time of a breast cancer diagnosis can have clear impacts on treatment choice whereas the clinical usefulness of other types of information generated by genome sequencing is much less clear [1, 2, 6, 7].
Decisions about what results patients would like to receive and how to communicate the results can be even murkier in the research setting where the usefulness of the results to an individual is less immediate [35, 36]. Breast cancer patients may have greater comfort with genetic testing and its implications, but it is not clear that this familiarity with genetic testing leads to a desire for more active participation in decisions to receive results from less familiar testing technologies such as genome sequencing. While prior opinion surveys of the general public have indicated that most individuals want as much information as possible, this may not apply to actual cancer patients [36]. To better understand how a population for whom sequencing is increasingly relevant might approach this issue, we surveyed women who had been diagnosed with breast cancer at or younger than age 40 about their preferred role in return of results decisions about genome sequencing and evaluated what factors might be associated with their preferred role. Since the context of the decision may matter, we looked at role preference in decisions for return of results from sequencing in a research or a clinical setting.
2. METHODS
2.1. Study Participants
Description of the study participants has been provided in a prior publication [37]. Study participants were recruited from an existing nationwide cohort (Young Women’s Breast Cancer Program; YWBCP; https://siteman.wustl.edu/ywbcp) comprised of women diagnosed with breast cancer at the age of 40 or younger who had agreed to be contacted about other studies. Before any contact, we searched the Social Security Death Index and removed those individuals who were deceased. We mailed letters to 1,778 YWBCP members inviting them to participate in a survey online, by telephone, or by mail, followed by an email invitation that contained a link to the online survey. We sent two follow-up emails with links to the survey to those who did not opt-out of further contact, followed by a mailed paper version of the survey. Sixteen participants without an email address were mailed a paper survey at baseline and follow up. Each participant completed a single survey. All participants reviewed a consent information sheet or were read the information by phone and gave consent to participate. Participants received a $10 gift card in recognition of their time spent. The study was approved by the Human Research Protections Office at Washington University in St. Louis.
2.2. Survey Procedures
Participants were surveyed on their communication and decision-making preferences related to genome sequencing results and factors that might affect these preferences (e.g., genetics-related knowledge, self-efficacy, worry, and beliefs; cancer-related worry; health information seeking and orientation; health consciousness; numeracy; clinical and sociodemographic characteristics). These factors were selected based on prior research linking them to clinical treatment decisions and decision role preference, and expert opinion.
2.3. Measures
The primary outcome of this analysis was decision role preference for selecting what results to receive from genome sequencing as part of medical care or as part of a research study. Decision role preference was measured using the Control Preferences Scale which asks the participant to choose from one of five roles: two passive roles (provider takes primary responsibility for the decision with varying degrees of involvement from the patient), one collaborative role (the decision is shared between the two parties), and two active roles (patient takes primary responsibility for the decision with varying degrees of involvement from the provider).
We tested sociodemographic variables, clinical and disease variables, genetic testing variables, genetic self-efficacy, genetic-related beliefs, subjective numeracy, and personal health perception as possible factors affecting decision-making preferences. Sociodemographic variables included current age, biological children, brothers and sisters, family history, race and ethnicity, education level, marital status, and income. Clinical variables included age at diagnosis, having more than one cancer and other types of cancer. Variables related to genetic testing were also analyzed including having met with a genetic counselor, having had cancer genetic testing, type of testing done, and BRCA mutation status. The survey did not ask about the actionability of genetic changes in the context of decision role preferences. Genetic self-efficacy was measured using three Likert-type items: assessing the role of genes in health, assessing genetic risk of disease, and comfort explaining genetic issues to others [38]. Genetic-related beliefs were measured with an adaptation of the Illness Perception Questionnaire with 5 items measuring beliefs around possible genetic and environmental causes of breast cancer with higher scores indicating stronger beliefs in genetic causality (i.e., belief that genetic makeup determines whether a woman will get breast cancer) [39–41]. Subjective numeracy was measured with the eight-item Subjective Numeracy Scale [42]. Personal health perception was measured using a single item asking participants to rate their overall health as poor, fair, good, very good, or excellent.
2.4. Analysis
Descriptive statistics of characteristics for the study participants are shown in Table 1. Bivariate analyses between each of the factors and the categorical outcomes was carried out using multinomial logistic regression. Using factors that were associated in bivariate analyses at p<0.1, multivariable models were built for each of the outcomes using stepwise multinomial logistic regression, with a passive role as the reference for each outcome (preferred role for decisions related to return of results from whole genome sequencing as part of medical care, and preferred role for return of results as part of research). Statistical significance was assessed at p<0.05. All analyses were conducted using SAS/STAT 9.4 (SAS Institute Inc., Cary, North Carolina).
Table 1.
Study participant characteristics.
| N | % | |
|---|---|---|
| Genetic related beliefs | ||
| Genetic make-up affects getting breast cancer (n=1079) | ||
| High genetic causal belief* | 299 | 27.21 |
| Low genetic causal belief | 780 | 72.29 |
| Family history affects getting breast cancer (n=1079) | ||
| High family history causal belief | 394 | 36.52 |
| Low family history causal belief | 685 | 63.48 |
| Received genetic testing - clinical record (n=1080) | ||
| No | 245 | 22.69 |
| Yes | 835 | 77.31 |
| Family history of breast cancer (n=1078) | ||
| Unknown or Low | 568 | 52.69 |
| Moderate | 207 | 19.20 |
| Strong | 303 | 28.11 |
| Mutation on BRCA1 or BRCA2 (n=1080) | ||
| Positive | 120 | 11.11 |
| Negative/Variant | 712 | 65.93 |
| Unknown | 248 | 22.96 |
| Mutation / family history categories (n=1080) | ||
| No mutation and strong family history | 190 | 17.59 |
| No mutation and low or moderate family history | 525 | 48.61 |
| Mutation positive | 120 | 11.11 |
| No genetic testing | 245 | 22.69 |
| Biological children (n=1080) | ||
| Yes | 740 | 68.52 |
| No | 340 | 31.48 |
| Siblings related by blood (n=1080) | ||
| Yes | 999 | 92.50 |
| No | 81 | 7.50 |
| Race (n=1080) | ||
| Non-Hispanic White | 994 | 92.04 |
| Other | 86 | 7.96 |
| Education (n=1079) | ||
| Some college or less | 222 | 20.57 |
| College degree | 376 | 34.85 |
| Beyond college | 481 | 44.58 |
| Marital status (n=1077) | ||
| Married/Living as married | 839 | 77.90 |
| Widowed/Divorced/Separated | 132 | 12.26 |
| Never been married | 106 | 9.84 |
| Annual household income (n=947) | ||
| < $100,000 | 468 | 49.42 |
| $100,000 and over | 479 | 50.58 |
| General perception of health (n= 1080) | ||
| Poor/Fair/Good | 458 | 42.41 |
| Very good/Excellent | 622 | 57.59 |
| Medical professional has told that have a genetic condition (n=1044) | ||
| No | 843 | 80.75 |
| Yes | 201 | 19.25 |
| More than one primary cancer (n=1079) | ||
| No other cancer | 907 | 84.06 |
| Yes, breast cancer recurrence or second breast | 113 | 10.47 |
| Cancer |
| Continuous Covariates | Mean (Range) | SD |
|---|---|---|
| N | % | |
| Age - current (n=1080) | 45.93 (26–82) | 9.15 |
| Age - at diagnosis (n=959) | 34.88(21–41) | 4.17 |
| Time since diagnosis (n=959) | 10.43(0–57) | 7.87 |
| Genetic self-efficacy (n= 1080) | 2.89(1.00–5.00) | 1.08 |
| Subjective numeracy scale (n=1080) | 4.92(1.25–6.00) | 0.84 |
| Ability subscale (n=1080) | 4.95(1.00–6.00) | 1.09 |
| Preference subscale (n=1079) | 4.90(1.25–6.00) | 0.91 |
Stronger beliefs in genetic causes over environmental causes in the development of breast cancer
3. RESULTS
Of those contacted, 1,080 (61%) women completed the survey. Comparing the respondents to the YWCBP cohort, we found no significant difference in age at diagnosis. The sample had a lower proportion of African American women (1.5%) than the cohort (3.8%; p < .01). There were no significant differences in the other racial and ethnic groups. As shown in Table 1, the majority of participants were non-Hispanic white (92%), married (78%), and had biological children (69%). Half had an annual household income of greater than $100,000 per year and 79% had a college degree or higher. Over half considered themselves to be in very good or excellent health (58%).
The mean age at the time of their breast cancer diagnosis was 35 years old (standard deviation (SD)=4 years), with a mean of 10 years (SD=8 years) since their diagnosis. Most of the women (84%) had not had a recurrence of their cancer or a new breast cancer. The majority (77%) had received cancer genetic testing, 11% had a known deleterious mutation in the BRCA genes, and18% had no mutation, but a strong family history of breast cancer. Most (72%) had lower genetic causal beliefs, responding “somewhat,” “a little,” or “not at all” to a causal role for genes in breast cancer. Slightly fewer women had lower causal beliefs related to family history (63%). Most scored in the middle for genetic self-efficacy (mean=2.89, SD=1.08) and had fairly high subjective numeracy (mean=4.92, SD=0.84).
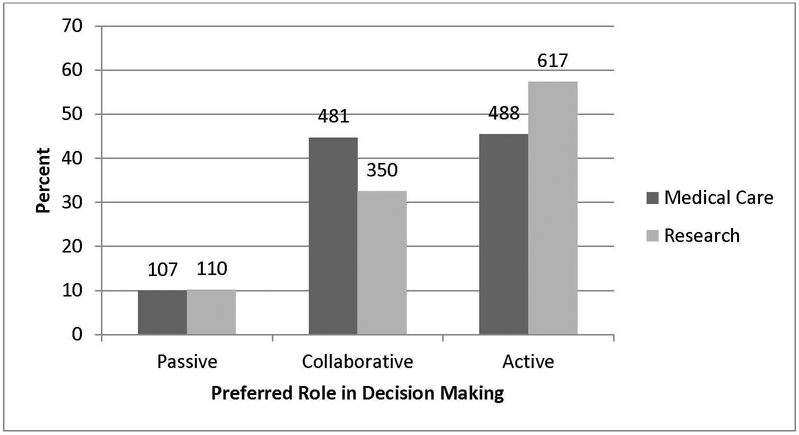
When asked to identify their preferred role in decisions to have genome sequencing results returned to them as part of medical care, most patients preferred either a collaborative (N=481, 45%) or active (N=488, 45%) role with only 107 (10%) choosing a passive role. When making the decision for return of genome sequencing results as part of a research study, most patients preferred an active role (N=617, 57%), with 110 (10%) choosing a passive role, and 350 (33%) choosing one of the collaborative roles(Figure 1).
Figure 1.
Preferred role of women diagnosed with breast cancer at a young age in decisions related to return of results from whole genome sequencing as part of medical care versus research.
3.1. Role Preference for Return of Results as Part of Medical Care
In bivariate analysis of factors related to decision making preferences for return of genome sequencing results as part of medical care (Table 2), women who perceived their health to be very good or excellent were more likely to prefer an active (OR: 1.91, 95%CI: 1.25–2.91, p<0.01) or collaborative role (OR: 1.52, 95% CI: 0.99–2.31, p=0.05) when compared to a passive role. Those who had higher genetic self-efficacy (OR: 1.38, 95% CI: 1.13–1.67, p<0.01), or those with increased time since diagnosis (OR: 1.04, 95% CI: 1.01–1.07, p=0.02) were significantly more likely to prefer an active role compared to a passive role. Women with higher genetic causal beliefs (OR: 0.63 95% CI: 0.40–0.99, p=0.04), and those who had biological children (OR: 0.57, 95% CI: 0.35–0.91, p=0.02) were significantly less likely to prefer an active role compared with a passive role. Those with more than one primary cancer (OR: 0.67, 95% CI: 0.47–0.97, p=0.03) were less likely to prefer a collaborative role compared with a passive role.
Table 2.
Factors associated with preferred role for decisions related to return of results from whole genome sequencing as part of medical care.
| Preferred Role (passive as reference) | Active Role | Collaborative Role | ||
|---|---|---|---|---|
| Bivariate Analysis | ||||
| OR (95% Cl) | p-value | OR (95% Cl) | p-value | |
| High genetic causal belief | 0.627 (0.398 – 0.988) |
0.044 | 0.926 (0.592–1.449) |
0.737 |
| Have more than one primary cancer | 0.840 (0.592–1.193) |
0.330 | 0.672 (0.467 – 0.966) |
0.032 |
| Biological children | 0.567 (0.354–0.911) |
0.019 | 0.902 (0.558–1.458) |
0.673 |
| General perception of health: Very good/Excellent | 1.906 (1.250–2.905) |
0.003 | 1.515 (0.995–2.307) |
0.053 |
| Genetic self-efficacy | 1.375 (1.132–1.669) |
0.001 | 1.055 (0.870–1.278) |
0.588 |
| Time since diagnosis | 1.037 (1.005–1.071) |
0.023 | 1.024 (0.992–1.057) |
0.149 |
| Multivariable analysis1 | ||||
|---|---|---|---|---|
| OR (95% Cl) | p-value | OR (95% Cl) | p-value | |
| Biological children | 0.573 (0.349 – 0.940) |
0.028 | 0.893 (0.540–1.475) |
0.658 |
| General perception of health: Very good/Excellent |
1.730 (1.107–2.705) |
0.016 | 1.563 (1.001 −2.441) |
0.049 |
| Time since diagnosis | 1.039 (1.006–1.072) |
0.020 | 1.021 (0.989–1.054) |
0.209 |
Model controlling for genetic causal belief and subjective numeracy
In multivariable analysis, factors associated with preferring an active role compared with a passive role were perceiving one’s health to be very good or excellent (OR: 1.73, 95% CI: 1.11–2.71, p=0.02) and longer time since diagnosis (OR: 1.04, 95% CI: 1.01–1.07, p=0.02). Those who had biological children (OR: 0.57, 95% CI: 0.35–0.94, p=0.03) were less likely to prefer an active role than a passive one. Perceiving one’s health as very good or excellent was also significantly related to preferring a collaborative role compared with a passive role (OR: 1.56, 95% CI: 1.00–2.44, p<0.05).
3.2. Role Preference for Return of Results as Part of a Research Study
For decision making preferences related to return of results for genome sequencing as part of a research study (see Table 3), bivariate analysis showed that women who perceived their health to be very good or excellent (OR: 2.14, 95% CI: 1.42–3.23, p<0.01), had higher genetic self-efficacy (OR: 1.29, 95% CI: 1.07–1.55, p<0.01), had an older current age (OR: 1.06, 95% CI: 1.03–1.08, p<0.01), were older at diagnosis (OR: 1.06, 95% CI: 1.01–1.11, p=0.01), had more time since diagnosis (OR: 1.07, 95% CI: 1.03–1.11, p<0.01), or had higher subjective numeracy (OR: 1.32, 95% CI: 1.04–1.66, p<0.01) were significantly more likely to prefer an active role compared with a passive role. Those who perceived their health to be very good or excellent (OR: 1.88, 95% CI: 1.22–2.90, p<0.01), had an older current age (OR: 1.06, 95% CI: 1.03–1.09, p<0.01), were older at diagnosis (OR: 1.09, 95% CI: 1.04–1.15, p=0.01), or had more time since diagnosis (OR: 1.07, 95% CI: 1.03–1.12, p<0.01) were also significantly more likely to prefer a collaborative role compared with a passive role.
Table 3.
Factors associated with preferred role for decisions related to return of results from whole genome sequencing as part of research.
| Preferred Role (passive as reference) | Active Role | p-value | Collaborative Role | p-value |
|---|---|---|---|---|
| Bivariate Analysis | ||||
| OR (95% Cl) | p-value | OR (95% Cl) | p-value | |
| General perception of health: Very good/Excellent |
2.141 (1.418–3.233) |
<0.001 | 1.877 (1.216–2.897) |
0.005 |
| Genetic self-efficacy | 1.285 (1.066–1.549) |
0.009 | 1.160 (0.953–1.412) |
0.138 |
| Age | 1.055 (1.027–1.084) |
<0.001 | 1.063 (1.034–1.093) |
<0.001 |
| Age at diagnosis | 1.062 (1.013–1.113) |
0.013 | 1.092 (1.037–1.150) |
<0.001 |
| Time since diagnosis | 1.072 (1.032–1.113) |
<0.001 | 1.074 (1.034–1.117) |
<0.001 |
| Subjective numeracy scale | 1.317 (1.043–1.661) |
0.021 | 1.061 (0.834–1.351) |
0.630 |
| Multivariable Analysis1 | ||||
|---|---|---|---|---|
| OR (95% Cl) | p-value | OR (95% Cl) | p-value | |
| General perception of health: Very good/Excellent |
1.858 (1.199–2.878) |
0.006 | 1.673 (1.051 −2.665) |
0.030 |
| Age at diagnosis | 1.063 (1.011 −1.117) |
0.016 | 1.089 (1.032–1.149) |
0.002 |
| Time since diagnosis | 1.071 (1.031 −1.113) |
<0.001 | 1.073 (1.032–1.117) |
<0.001 |
| Subjective numeracy preference subscale | 1.309 (1.042–1.646) |
0.021 | 1.095 (0.863–1.390) |
0.454 |
Model controlling for genetic causal belief and biological children
In multivariable analysis, women who perceived their health to be very good or excellent (OR: 1.86, 95% CI: 1.20–2.88, p<0.01), were older at diagnosis (OR: 1.06, 95% CI: 1.01–1.12, p=0.02), had more time since diagnosis (OR: 1.06, 95% CI: 1.01–1.12, p<0.01), or had higher subjective numeracy (OR: 1.31, 95% CI: 1.04–1.65, p=0.02) were more likely to prefer an active role than a passive role. Those who perceived their health to be very good or excellent (OR: 1.67, 95% CI: 1.05–2.67, p=0.03), were older at diagnosis (OR: 1.09, 95% CI: 1.03–1.15, p<0.01), or had more time since diagnosis (OR: 1.07, 95% CI: 1.03–1.12, p<0.01) were also more likely to prefer a collaborative than a passive role.
4. DISCUSSION AND CONCLUSION
4.1. Discussion
This survey of a large group of women diagnosed with breast cancer at a young age provides valuable insights into the decision-making preferences of these patients for return of genome sequencing results in the clinical and research contexts. Consistent with past studies on role preferences in decision-making, most women prefer to share in the process with their providers to some degree. The results from this study show that a number of different variables were associated with these preferences [28, 43, 44].
We found that participants had somewhat different decision-making preferences for clinical and research contexts, with a greater preference for active roles in the research context. This may be due to differences in the immediate applicability of the results or differences in levels of trust and longer relationships for doctors versus researchers. Results in a research context are generally not used to make decisions about current health issues unless clinically verified, but may be viewed as potentially relevant in the future. This potential future application requires patients to project what may be important to them in the future which may impact the amount of control they want to have over their results in making future decisions. Results from research may also have more implications for the individual’s relatives rather than the individual and this may also lead to differences in the preferred level of control in these decisions. Participants may want more control over return of results when they are less immediate and less useful for the individual.
In addition, different factors were associated with preferred role depending on whether the sequencing would be done as part of medical care or as part of research. The participating women’s perceptions of themselves as healthy, with a good understanding of how to use and communicate about genetic information (higher genetic self-efficacy), and a good understanding of how to use numbers (greater subjective numeracy) illustrates this group of women as well-educated. This is coupled with a longer time to have processed their disease experience as shown by being diagnosed at younger ages and having more time since their diagnosis. All of these factors were associated with their decision making preference, though in different ways depending on the medical care or research context. Based on prior studies, these factors are what one might predict to be associated with a desire to be more involved in decisions to participate in research studies and the return of results that may come from their participation. Younger age and higher educational level have been associated with preference for more active roles in past studies [45–47], but this study is the first to look at perceptions of health and genetic-related variables. In most past studies of breast cancer patient role preferences, about half of patients preferred the collaborative role with the other half split between the more active and passive roles [46, 47]. In our study, preferences were skewed towards the more active roles, but this was much more striking in the research study context.
On the other hand, it is somewhat surprising that women with biological children were less likely to prefer active or collaborative roles compared with passive roles when making the decision to have results returned from genomic sequencing as part of medical care, since the results may have implications for their children’s disease risks. We hypothesize that this may be due to a parental desire to know the information, but discomfort in deciding what information should be important to their children and possibly fear of what the results may predict about their future leading them to feel less inclined to actively make the decision. We do not know of any past studies that have explored how familial variables influence decision role preferences and this is an area for future work.
This study has several limitations. It is known that role preferences can vary by race and ethnicity and education level [10, 25, 26, 45, 46, 48] and our study population is limited in this regard. Our population is reflective of the breast cancer patient population in general [49] and consistent with populations in prior studies, but there is evidence that responses to genomic risk information do vary by race and ethnicity [50] and role preferences and associations may be different if studied in a more diverse population. There may also be important differences in role preference for return of actionable versus non-actionable results.Though the larger survey did distinguish between actionable and non-actionable results, the questions specifically regarding decision role preferences did not so this cannot be assessed in this study. Also, this survey asks the participants to imagine hypothetical scenarios and it is not known if they would want the same role if they were faced with this decision in actual practice or research. Future work should address this by looking at role preferences in people who are approached for genome sequencing in clinical and research settings. It would also be interesting to reassess preferences among participants from this study who go on to receive genome sequencing to see if their preferences were stable. It is not clear if these results would translate to people making decisions about genome sequencing who are healthy or have a history of other conditions.
4.2. Conclusion
What these data very clearly show is that decision making preferences are not easily categorized and reinforce the idea that preferred role is not easily predicted based on demographic factors alone [13, 15, 25]. It is critical for providers to recognize that because someone is young (or old) or well-educated (or not) or has children (or doesn’t), they can’t assume what role the patient wants to play in the decision-making process in a particular context. Models of patient-centered care should incorporate questions about decision role preferences across contexts, recognizing that this is a dynamic preference that needs to be re-addressed regularly. This is not a preference that can be easily known without direct questioning. Elicitation of decision role preferences is not routinely done which contributes to discordance between patient and provider perceptions of role. Aligning perceptions and helping patients achieve their preferred role could improve care delivery in as yet undefined ways. This is also an area for future exploration.
4.3. Practice Implications
There are many types of genetic variants that can be discovered through genome sequencing. Patients prefer different roles in deciding what information they would like to receive and this does vary by the context of the testing. We advocate for practice guidelines that incorporate discussion of decision role as an integral part of engaging in patient-centered care and promoting shared decision-making. This study demonstrates that most patients do want to play a collaborative or active role in these decisions, but there is variability in role preferences for return of results. This provides important data that can be used by providers, researchers, and policy makers when developing policies and procedures for returning results from genetic tests.
HIGHLIGHTS.
Availability and use of whole genome sequencing is expanding
Patients prefer different roles when deciding what results to receive
There is no consistent variable that predicts what role a person prefers
ACKNOWLEDGEMENTS
Jingsong Zhao, MPH for assistance in editing and writing the manuscript.
FUNDING SOURCES
This work was supported by the National Cancer Institute, National Institutes of Health (R01CA168608) and the Intramural Research Program of the National Human Genome Research Institute. It was also supported by the Building Interdisciplinary Research Careers in Women’s Health Program of the National Institutes of Health under award number K12HD085852 and the Agency for Healthcare Research and Quality award number R03HS024784
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Fiore RN and Goodman KW, Precision medicine ethics: selected issues and developments in next-generation sequencing, clinical oncology, and ethics. Curr Opin Oncol, 2016. 28(1): p. 83–7. [DOI] [PubMed] [Google Scholar]
- 2.Foley SB, et al. , Use of Whole Genome Sequencing for Diagnosis and Discovery in the Cancer Genetics Clinic. EBioMedicine, 2015. 2(1): p. 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelbaum PS, et al. , Models of consent to return of incidental findings in genomic research. Hastings Cent Rep, 2014. 44(4): p. 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarvik GP, et al. , Return of genomic results to research participants: the floor, the ceiling, and the choices in between. Am J Hum Genet, 2014. 94(6): p. 818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marian AJ, Challenges in medical applications of whole exome/genome sequencing discoveries. Trends Cardiovasc Med, 2012. 22(8): p. 219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewey FE, et al. , Clinical interpretation and implications of whole-genome sequencing. Jama, 2014. 311(10): p. 1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feero WG, Genomics in medicine: maturation, but not maturity. Jama, 2013. 309(14): p. 1522–4. [DOI] [PubMed] [Google Scholar]
- 8.Ayuso C, et al. , Informed consent for whole-genome sequencing studies in the clinical setting. Proposed recommendations on essential content and process. Eur J Hum Genet, 2013. 21(10): p. 1054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knoppers BM, Zawati MH, and Senecal K, Return of genetic testing results in the era of whole-genome sequencing. Nat Rev Genet, 2015. 16(9): p. 553–9. [DOI] [PubMed] [Google Scholar]
- 10.Almyroudi A, et al. , Decision-making preferences and information needs among Greek breast cancer patients. Psycho-Oncology, 2011. 20(8): p. 871–879. [DOI] [PubMed] [Google Scholar]
- 11.Aoki A, et al. , Matching of patients’ actual and desired roles in treatment decision making and trust in physicians. Arthritis and Rheumatism, 2012. 64: p. S867. [Google Scholar]
- 12.Ashraf AA, et al. , Patient involvement in the decision making process improves satisfaction and quality of life in postmastectomy breast reconstruction. Journal of Surgical Research, 2013. 179(2). [DOI] [PubMed] [Google Scholar]
- 13.Bilodeau BA and Degner LF, Information needs, sources of information, and decisional roles in women with breast cancer. Oncol Nurs Forum, 1996. 23(4): p. 691–6. [PubMed] [Google Scholar]
- 14.Degner LF, Sloan JA, and Venkatesh P, The Control Preferences Scale. Can J Nurs Res, 1997. 29(3): p. 21–43. [PubMed] [Google Scholar]
- 15.Entwistle VA and Watt IS, Patient involvement in treatment decision-making: the case for a broader conceptual framework. Patient Educ Couns, 2006. 63(3): p. 268–78. [DOI] [PubMed] [Google Scholar]
- 16.Bakshi N, et al. , Shared decision making or physician advocate for a particular treatment option: A spectrum of approaches to decision making about disease modifying therapies in sickle cell disease. Blood, 2016. 128(22). [Google Scholar]
- 17.Blair L and Legare F, Is Shared Decision Making a Utopian Dream or an Achievable Goal? Patient, 2015. 8(6): p. 471–6. [DOI] [PubMed] [Google Scholar]
- 18.Bouniols N, Leclere B, and Moret L, Evaluating the quality of shared decision making during the patient-carer encounter: a systematic review of tools. BMC Res Notes, 2016. 9: p. 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elwyn G, et al. , shared decision making: a model for clinical practice. J Gen Intern Med, 2012. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambigapathy R, Chia YC, and Ng CJ, Patient involvement in decision-making: A cross-sectional study in a Malaysian primary care clinic. BMJ Open, 2016. 6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blinman P, et al. , The preferred and actual levels of involvement in decision-making among patients considering adjuvant chemotherapy (ACT) for non-small-cell lung cancer (NSCLC). Journal of Thoracic Oncology, 2013. 8: p. S157. [Google Scholar]
- 22.Albrecht K, et al. , Shared decision making in dermato-oncology-preference for involvement of melanoma patients. Psycho-Oncology, 2013. 22: p. 351. [DOI] [PubMed] [Google Scholar]
- 23.Davison BJ and Breckon EN, Impact of health information-seeking behavior and personal factors on preferred role in treatment decision making in men with newly diagnosed prostate cancer. Cancer Nurs, 2012. 35(6): p. 411–8. [DOI] [PubMed] [Google Scholar]
- 24.Decker C, et al. , Role of peripheral arterial disease patient in shared decision-making: Congruence of preference with actual involvement. European Journal of Cardiovascular Nursing, 2016. 15: p. S7. [Google Scholar]
- 25.Hawley ST, et al. , Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst, 2009. 101(19): p. 1337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawley ST, et al. , Latina patient perspectives about informed treatment decision making for breast cancer. Patient Educ Couns, 2008. 73(2): p. 363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heesen C, et al. , Decisional role preferences, risk knowledge and information interests in patients with multiple sclerosis. Multiple Sclerosis, 2004. 10(6): p. 643–650. [DOI] [PubMed] [Google Scholar]
- 28.Janz NK, et al. , Patient-physician concordance: Preferences, perceptions, and factors influencing the breast cancer surgical decision. Journal of Clinical Oncology, 2004. 22(15): p. 3091–3098. [DOI] [PubMed] [Google Scholar]
- 29.Kumar R, et al. , Decision-making role preferences among patients with HIV: Associations with patient and provider characteristics and communication behaviors. Journal of General Internal Medicine, 2010. 25(6): p. 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moth E, et al. , Patients’ preferred and perceived roles in making decisions about adjuvant chemotherapy for non-small-cell lung cancer. Lung Cancer, 2016. 95: p. 8–14. [DOI] [PubMed] [Google Scholar]
- 31.Sekimoto M, et al. , Patients’ preferences for involvement in treatment decision making in Japan. BMC Family Practice, 2004. 5: p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart DE, et al. , Gender Differences in Health Information Needs and Decisional Preferences in Patients Recovering from an Acute Ischemic Coronary Event. Psychosomatic Medicine, 2004. 66(1): p. 42–48. [DOI] [PubMed] [Google Scholar]
- 33.Ferlay J, et al. , Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer, 2010. 127(12): p. 2893–917. [DOI] [PubMed] [Google Scholar]
- 34.Menen RS and Hunt KK, Considerations for the Treatment of Young Patients with Breast Cancer. Breast J, 2016. 22(6): p. 667–672. [DOI] [PubMed] [Google Scholar]
- 35.Haga SB and Zhao JQ, Stakeholder views on returning research results. Adv Genet, 2013. 84: p. 41–81. [DOI] [PubMed] [Google Scholar]
- 36.Kaphingst KA, et al. , How, who, and when: preferences for delivery of genome sequencing results among women diagnosed with breast cancer at a young age. Mol Genet Genomic Med, 2016. 4(6): p. 684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaphingst KA, et al. , Preferences for learning different types of genome sequencing results among young breast cancer patients: Role of psychological and clinical factors. Transl Behav Med, 2018. 8(1): p. 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parrott R, et al. , Behavioral health outcomes associated with religious faith and media exposure about human genetics. Health Commun, 2004. 16(1): p. 29–45. [DOI] [PubMed] [Google Scholar]
- 39.Ashida S, et al. , Age differences in genetic knowledge, health literacy and causal beliefs for health conditions. Public Health Genomics, 2011. 14(4–5): p. 307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McBride CM, et al. , Putting science over supposition in the arena of personalized genomics. Nat Genet, 2008. 40(8): p. 939–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinman J, et al. , The illness perception questionnaire: A new method for assessing the cognitive representation of illness. Psychology & Health, 1996. 11(3): p. 431–445. [Google Scholar]
- 42.Fagerlin A, et al. , Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making, 2007. 27(5): p. 672–80. [DOI] [PubMed] [Google Scholar]
- 43.Hillyer GC, et al. , A survey of breast cancer physicians regarding patient involvement in breast cancer treatment decisions. Breast, 2013. 22(4): p. 548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen F, et al. , Treatment decision-making in the medical encounter: Comparing the attitudes of French surgeons and their patients in breast cancer care. Patient Education and Counseling, 2014. 94(2): p. 230–237. [DOI] [PubMed] [Google Scholar]
- 45.Johnson JD, et al. , Breast cancer patients’ personality style, age, and treatment decision making. Journal of Surgical Oncology, 1996. 63(3): p. 183–186. [DOI] [PubMed] [Google Scholar]
- 46.Janz NK, et al. , Patient-physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol, 2004. 22(15): p. 3091–8. [DOI] [PubMed] [Google Scholar]
- 47.Degner LF, et al. , Information needs and decisional preferences in women with breast cancer. Jama, 1997. 277(18): p. 1485–92. [PubMed] [Google Scholar]
- 48.Tariman JD, et al. , Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol, 2010. 21(6): p. 1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prevention, C.f.D.C.a. United States Cancer Statistics: Data Visualizations. 2018. [cited 2018 July 2]; Available from: https://gis.cdc.gov/Cancer/USCS/DataViz.html.
- 50.Kaphingst KA, et al. , Effects of racial and ethnic group and health literacy on responses to genomic risk information in a medically underserved population. Health Psychol, 2015. 34(2): p. 101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]