Abstract
Background:
Polyomavirus-associated nephropathy is associated with high risk of kidney allograft loss. Whether the cause of native end-stage renal disease influences the risk of BK infection is unclear.
Methods:
A retrospective, single-center study of 2,741 adult kidney transplant recipients between 1994 to 2014 was performed. Recipients had end-stage renal disease due to polycystic kidney disease (PKD, n=549), diabetes mellitus (DM, n=947), hypertension (HTN, n=442), or glomerulonephritis (GN, n=803).
Results:
A total of 327 recipients (12%) developed post-transplant BK viremia over a median follow-up time of 5 years. The incidence rate of BK viremia was lowest in patients with PKD (1.46 per 100 person-years) compared to other causes of ESRD (DM = 2.06, HTN = 2.65, and GN = 2.01 per 100 person-years). A diagnosis of PKD was associated with a lower risk of post-transplant BK viremia (adjusted HR (95% CI) = 0.67 (0.48–0.95), P=0.02). BK nephropathy was significantly less common in patients with PKD (0.21 per 100 person-years) compared to those with HTN (0.80 per 100 person-years, P=<0.001). Among patients with PKD, the risk of BK viremia was lower in patients with nephrectomy, compared to those without nephrectomy (adjusted HR (95% CI) = 0.42 (0.19–0.92), P<0.05).
Conclusion:
ESRD due to PKD is associated with a lower risk of post-transplant BK infection. The renal tubular epithelial cells in PKD are unique; they are in a proliferative but non-differentiated state. Whether this characteristic of renal tubular epithelial cells alters the BK viral reservoir or replication in PKD patients warrants further study.
Keywords: Kidney transplant, BK virus, polycystic kidney disease
1. INTRODUCTION
BK nephropathy is a common cause of allograft dysfunction and loss following kidney transplant, occurring in 2–5% of renal transplant patients with progression to graft loss in an estimated 18–80%.1–3 Following mild or asymptomatic primary infection, typically early in life, BK virus remains latent in renal epithelial cells and uroepithelium.4,5 In the context of immunosuppression, particularly following kidney transplantation, BK viral reactivation and replication may occur and can progress to viruria, viremia, and nephropathy. Allograft dysfunction occurs due to leakage of urine from injured tubules into the surrounding interstitium and peritubular capillaries. 6 Though BK nephropathy occurs infrequently in patients following other solid organ transplant or bone marrow transplant, it is much more common after kidney transplantation.1,7
Coincident with the adoption of increasingly aggressive immunosuppressive regimens, the incidence and prevalence of BK nephropathy has increased in recent years compared to when the condition was first described in the mid-1990s.2 Higher-potency immunosuppression regimens are thought to create a permissive environment for BK viral replication and represent an important risk factor for viral reactivation. In addition to the overall degree of immunosuppression, certain agents have been associated with higher risk of BK nephropathy in studies, including rabbit antithymocyte globulin induction.8,9 and maintenance immunosuppression with tacrolimus and mycophenolate.1,2,7,9,10 Corticosteroid maintenance therapy has also been associated with increased risk of BK viremia or nephropathy in limited studies.2,8–10 Management of BK nephropathy predominantly involves reduction of immunosuppression as anti-BK effective therapies are limited.4
Risk factors for BK virus reactivation are incompletely understood, though a myriad of risk factors have been posited including characteristics pertaining to the graft, recipient, and type of immunosuppression regimen. Graft-related risk factors include ischemic allograft injury, prior acute rejection, degree of HLA mismatch, high BK-specific antibody titers, and BK serologic mismatch (seropositive donor and seronegative recipient), though serologic status is not routinely clinically assessed.2,11,12 Recipient-related risk factors for BK reactivation include older age, Caucasian ethnicity, and male sex.8,13 It is currently unknown whether the cause of ESRD affects BK viral reactivation or nephropathy. We hypothesized that patients with exposure to pre-transplant immunosuppression (patients with glomerulonephritis, GN) or relatively immunosuppressed patients (patients with diabetes mellitus, DM), would be more susceptible to the development of BK viremia and nephropathy in the post-transplant period. We sought to better characterize the risk of BK viremia and nephropathy in transplant recipients by comparing incidence of BK viremia and nephropathy by cause of ESRD. Better understanding of risk factors for BK reactivation and nephropathy could allow for identification of at-risk populations, improve predictive models and surveillance strategies, and potentially allow for optimization of risk factors and management of disease.
2. METHODS
2.1. Patient population
Patients eligible for inclusion in this study consisted of all consecutive adult (>18 years old) renal transplant recipients at the University of Wisconsin Hospital and Clinics from January 1, 1994 through December 31, 2014 with known etiology of end-stage renal disease. Patients were excluded if they had a previous renal transplant, multiorgan transplant, were less than 18 years old, or if the etiology of ESRD was unclear. Four patient groups were analyzed, based on the cause of ESRD. The four causes of ESRD included were hypertensive nephropathy (HTN), diabetic nephropathy (DM), polycystic kidney disease (PKD), and biopsy-proven glomerulonephritis (GN). This study was approved by the University of Wisconsin-Madison Institutional Review Board and the Human Subjects Committee. All clinical and research activities performed were in accordance with the 2000 Declaration of Helsinki and the Declaration of Istanbul 2008 ethical standards for human subjects.
2.2. Definitions of primary and secondary outcomes
Data were obtained from the Wisconsin Allograft Recipient Database (WisARD). The primary outcome was the first episode of BK viremia following renal transplantation, defined as detectable BK viral DNA in plasma by PCR analysis, regardless of viral quantitation. Time to BK viremia event was defined as time from renal transplantation to first positive test for BK viremia in each patient. The secondary outcome was first episode of BK nephropathy. Diagnosis of BK nephropathy was defined as positive SV-40 staining on renal biopsy as determined by a clinical renal pathologist. Patients were followed until graft loss (retransplant or return to dialysis), death, or last available follow-up.
2.3. Protocol for BK laboratory monitoring and immunosuppression medication adjustment
The protocol for laboratory monitoring of BK viremia and adjustment of immunosuppression at our center was performed as previously described.3,12 Briefly, plasma BK PCR was monitored every two weeks for the first three months post-transplant, then monthly until 6 months post-transplant, and then every three months until 18 months post-transplant. Allograft biopsy was performed if BK PCR was positive and/or creatinine was elevated 25% above baseline. If the BK PCR level was greater than 1,000 copies/mL, the anti-metabolite dose was reduced by 50%, followed by a reduction in calcineurin inhibitor dose. If BK PCR level continued to increase, the anti-metabolite was replaced with leflunomide ± intravenous immunoglobulin (IVIG). If a diagnosis of acute rejection was determined on allograft biopsy, BK PCR was monitored every two weeks for three months.
2.4. Statistical analysis
Continuous variables were compared between groups using t-tests and Kruskal-Wallis tests. Categorical variables were compared between groups with chi-square or Fisher exact tests. Time-to-event data estimates were obtained using Kaplan-Meier curves and log-rank test. Cox proportional hazard models were used to assess independent associations between demographics, baseline characteristics, BK viremia, and BK nephropathy. P value less than 0.05 were considered statistically significant. All analyses were performed using Stata Statistical Software: Release 13 (StataCorp; College Station, TX).
3. RESULTS
3.1. Baseline characteristics
Mean patient age in the group transplanted for ESRD secondary to glomerulonephritis was significantly younger (45.3 years ± 13.2) compared to those transplanted for other causes of ESRD (HTN, DM, PKD) (Table 1). While male patients comprised a majority of all of the groups, there were significantly fewer female patients in the HTN and DM groups. Caucasians made up a lower percentage of the HTN group (62%) and a higher percentage of the PKD group (93%) when compared with GN. GN and DM groups did not significantly differ in terms of racial composition. There were no significant differences in induction immunosuppression regimens between the four groups. Maintenance immunosuppression regimens at time of discharge were also similar among the groups, with the exception of a higher percentage of patients in the PKD group receiving mycophenolate for maintenance immunosuppression. HLA mismatch >2 was more common among the HTN and PKD groups. Delayed graft failure occurred at a significantly higher rate in the HTN and DM groups, but did not significantly differ between GN and PKD.
TABLE 1.
Baseline characteristics of kidney transplant recipients
| GN (n=803) |
HTN (n=442) |
DM (n=947) |
PKD (n=549) |
|
|---|---|---|---|---|
| Age (years), mean | 45.3 ± 13.2 | 53.2 ± 12.4* | 54.8 ± 10.1* | 52.7 ± 9.1* |
| Female, n (%) | 340 (42%) | 125 (28%)* | 322 (34%)* | 256 (47%) |
| White ethnicity, n (%) | 646 (80%) | 275 (62%)* | 788 (83%) | 510 (93%)* |
| Pre-transplant dialysis, n (%) | 592 (74%) | 378 (86%)* | 804 (85%)* | 324 (59%)* |
| Dialysis duration prior to transplant (months), mean |
19.6 ± 22.9 | 26.0 ± 25.1* | 20.7 ± 20.0* | 14.0 ± 24.8* |
| Panel reactive antibody >10%, n (%) | 71 (19%) | 37 (17%) | 82 (17%) | 50 (19%) |
| Deceased donor, n (%) | 422 (53%) | 327 (74%)* | 623 (66%)* | 301 (55%) |
| Donor age (years), mean | 41.8 ± 14.3 | 43.7 ± 15.1* | 43.9 ± 14.8* | 43.6 ± 14.3* |
| HLA mismatch >2, n (%) | 568 (71%) | 352 (80%)* | 675 (71%) | 427 (78%)* |
| Delayed graft function, n (%) | 111 (14%) | 115 (26%)* | 227 (24%)* | 74 (13%) |
| Induction, n (%) • Alemtuzumab • Basiliximab • Thymoglobulin • None |
114 (14%) 414 (52%) 131 (16%) 144 (18%) |
64 (15%) 226 (51%) 92 (21%) 60 (14%) |
168 (18%) 459 (48%) 200 (21%) 120 (13%) |
82 (15%) 323 (59%) 84 (15%) 60 (11%) |
| Maintenance immunosuppression, n (%) • Prednisone • CNI • Mycophenolate |
801 (100%) 730 (91%) 709 (88%) |
442 (100%) 397 (90%) 394 (89%) |
947 (100%) 844 (89%) 857 (90%) |
549 (100%) 495 (90%) 506 (92%)* |
GN, glomerulonephritis; HTN, hypertension; DM, diabetes mellitus; PKD, polycystic kidney disease; HLA, human leukocyte antigen; CNI, calcineurin inhibitor
P<0.05 compared to GN
3.2. Risk of BK viremia is lower in PKD compared with other causes of ESRD
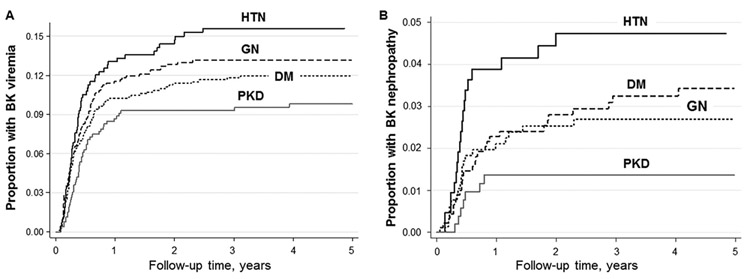
Out of a total of 2,741 kidney transplant patients, 327 (12%) developed BK viremia over a median follow up time of 5.1 years (Table 2). There were no significant differences in the incidence of acute rejection among the four groups (GN: 4.2 per 100 person-years, HTN: 5.6 per 100 person-years, DM: 4.1 per 100 person-years, and PKD: 3.9 per 100 person-years). The proportion of patients with less severe (Grade I) compared to more severe (Grade II or III) acute rejections prior to BK infection diagnosis was not significantly different between the patient groups (Grade I acute rejections: GN: n=68 (8%), HTN: n=46 (10%), DM: 56 (6%), and PKD: 37 (7%); Grade II or III acute rejections: GN: n=12 (2%), HTN: n=11 (2%), DM: n=24 (3%), and PKD: n=13 (2%); P=0.12). Also, the number of patients who developed ureteral stenosis requiring urinary stent placement prior to BK infection diagnosis was not different among the patient groups (GN: n=11 (1%), HTN: n=3 (1%), DM: n=5 (1%), and PKD: n=8 (1%); P=0.18). The proportion of patients who developed BK viremia was significantly lower in patients with PKD compared to the other causes of ESRD (P<0.005 for PKD versus HTN, P=0.05 for PKD versus GN, P=0.04 for PKD versus DM) (Figure 1A). The lowest incidence of BK viremia was noted among the PKD group relative to other causes of ESRD, though this did not achieve statistical significance. Risk of BK viremia did not significantly differ according to cause of ESRD in an unadjusted model; however, with adjustment for age there was a significantly lower risk of BK viremia noted among the PKD group compared to the reference group (age-adjusted relative hazard ratio (95% CI) = 0.68 (0.49–0.96), P=0.03). Additionally, a significantly lower risk of BK viremia was observed in PKD in a fully adjusted model (adjusted for patient age, sex, race, receipt of dialysis prior to transplant, duration of dialysis, donor status, donor age, delayed graft function, HLA mismatch, and induction immunosuppression; fully adjusted relative hazard ratio (95% CI) = 0.67 (0.48–0.95), P=0.02 compared to GN). The risk of BK viremia in the HTN and DM groups did not significantly differ from the reference group in the unadjusted model or in any of the multivariate analyses.
TABLE 2.
Incidence of BK viremia in kidney transplant recipients based on native kidney disease
| GN (n=803) |
HTN (n=442) |
DM (n=947) |
PKD (n=549) |
|
|---|---|---|---|---|
| Patients with BK viremia, n (%) | 103 (12.8%) |
64 (14.5%) |
107 (11.3%) |
53 (9.7%) |
| Incidence rate (per 100 person- years) |
2.01 | 2.65 | 2.06 | 1.46 |
| Time to viremia (median, months) | 4.2 | 4.1 | 3.6 | 4.8 |
| Unadjusted hazard ratio (95% CI) | 1.0 (Reference) |
1.17 (0.86 – 1.59) |
0.89 (0.68 – 1.17) |
0.73 (0.53 – 1.02) |
| Age-adjusted hazard ratio (95% CI) | 1.0 (Reference) |
1.08 (0.78 – 1.49) |
0.82 (0.61 – 1.08) |
0.68* (0.49 – 0.96) |
| Fully adjusted hazard ratioǂ (95% CI) |
1.0 (Reference) |
0.97 (0.70 – 1.34) |
0.83 (0.62 – 1.11) |
0.67* (0.48 – 0.95) |
GN, glomerulonephritis; HTN, hypertension; DM, diabetes mellitus; PKD, polycystic kidney disease.
Adjusted for age, sex, race, dialysis pre-transplant, duration of dialysis, donor status, donor age, DGF, HLA mismatch >2, and induction immunosuppression use
P <0.05 compared to GN
FIGURE 1. Kaplan-Meier curve of the proportion of kidney transplants with BK viremia or BK nephropathy over time, stratified by cause of ESRD.
A, The lowest proportion of patients with post-transplant BK infection was seen in PKD, when stratified by cause of ESRD (P<0.005 for PKD versus HTN, P=0.05 for PKD versus GN, P=0.04 for PKD versus DM). B, When stratified by cause of ESRD, BK nephropathy occurred in the lowest proportion of patients in the PKD group (P=0.003 for PKD versus HTN, P=0.19 for PKD versus GN, P=0.04 for PKD versus DM).
ESRD, end-stage renal disease; GN, glomerulonephritis; DM, diabetes mellitus; HTN, hypertension; PKD, polycystic kidney disease
3.3. Risk of BK nephropathy was lower in patients with PKD compared to HTN or DM
A total of 77 patients developed BK nephropathy. The lowest proportion of patients with BK nephropathy was observed in the PKD group, compared to other causes of ESRD (P=0.003 for PKD versus HTN, P=0.19 for PKD versus GN, P=0.04 for PKD versus DM), as shown in Figure 1B. The incidence rate of BK nephropathy was significantly lower in PKD compared to DM and HTN groups (P=0.02 and P=0.001, respectively, Table 3), though not significantly different when compared with the GN group. Although this trend persisted, it was not statistically significant in an age-adjusted model, minimally-adjusted model (with adjustments for age, sex, and race), or in subsequent multivariate analyses. BK nephropathy risk was not otherwise significantly different in the remaining groups compared with GN (in unadjusted or multivariate models).
TABLE 3.
Incidence of BK nephropathy in kidney transplant recipients based on native disease
| GN (n=803) |
HTN (n=442) |
DM (n=947) |
PKD (n=549) |
|
|---|---|---|---|---|
| Patients with BK nephropathy, n (%) |
20 (2.5%) | 20 (4.5%) | 29 (3.1%) | 8 (1.5%) |
| Incidence rate (per 100 person- years) |
0.53 | 0.80* | 0.37 | 0.21+,# |
| Time to BK nephropathy (median, months) |
5.1 | 5.0 | 7.7 | 5.6 |
| Unadjusted hazard ratio (95% CI) | 1.0 (Reference) |
1.91* | 1.27 | 0.58 |
| Age-adjusted hazard ratio (95% CI) |
1.0 (Reference) |
1.95* (1.03 - 3.70) |
1.30 (0.71 - 2.37) |
0.59 (0.26 - 1.36) |
| Fully adjusted hazard ratioǂ (95% CI) |
1.0 (Reference) |
1.68 (0.87 – 3.25) |
1.29 (0.70 – 2.38) |
0.58 (0.72 – 1.10) |
GN, glomerulonephritis; HTN, hypertension; DM, diabetes mellitus; PKD, polycystic kidney disease.
Adjusted for age, sex, race, dialysis pre-transplant, duration of dialysis, donor status, donor age, delayed graft function, HLA mismatch, and induction immunosuppression use
P<0.05 compared to GN
P<0.05 compared to DM
P<0.001 compared to HTN
3.4. PKD patients who underwent nephrectomy had a lower risk of BK viremia, compared to those without nephrectomy
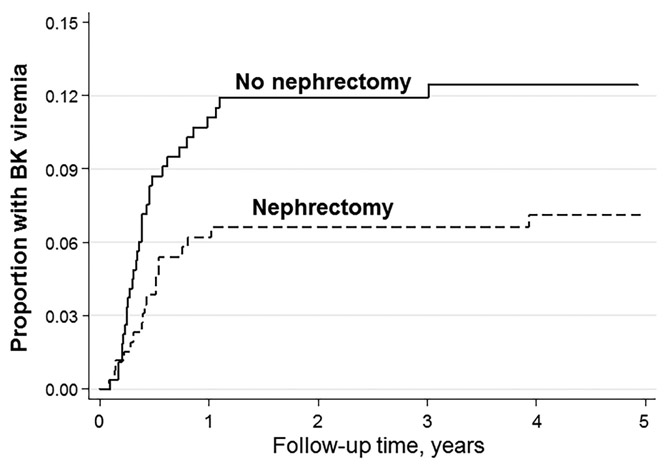
A total of 549 study patients received kidney transplants for ESRD due to PKD. Of these patients, 280 underwent surgical nephrectomy prior to diagnosis of BK viremia, and 269 did not undergo nephrectomy (Table 4). A total of 20 PKD patients who underwent nephrectomy developed BK viremia, compared to 33 PKD patients who did not undergo nephrectomy. The majority of BK viremia events occurred within one year post-transplant. As shown in the Kaplan-Meier curve in Figure 2, BK viremia occurred in a higher proportion of PKD patients who underwent nephrectomy, though this trend did not achieve statistical significance (P=0.053 for nephrectomy versus no nephrectomy group). The incidence rate of BK viremia was significantly lower in the nephrectomy subgroup compared to those who did not have a nephrectomy overall (0.01 versus 0.02 events per 100 person-years, respectively; P=0.02) and within the first year post-transplant (P=0.046) (Table 4). The unadjusted hazard ratio for BK viremia within the first year post-transplant was not significantly different between the two groups. Significance emerged in the fully adjusted model, as the nephrectomy subgroup had a significantly lower risk of BK viremia compared to the no nephrectomy reference group (adjusted hazard ratio (95% CI) = 0.42 (0.19–0.92), P=0.03).
TABLE 4.
Incidence of BK viremia in kidney transplant recipients with ESRD due to PKD, by nephrectomy status
| PKD (n=549) |
||
|---|---|---|
| No Nephrectomy (n=280) |
Nephrectomy (n=269) |
|
| Overall | ||
| Patients with BK viremia, n (%) | 33 (12%) | 20 (7%) |
| Incidence rate (per 100 person-years) | 0.02 | 0.01* |
| Time to BK viremia (median, months) | 4.6 | 5.6 |
| Within first year post-transplant | ||
| Patients with BK viremia, n (%) | 29 (10%) | 16 (6%) |
| Incidence rate (per 100 person-years) | 0.12 | 0.07* |
| Time to BK viremia (median, months) | 4.1 | 4.8 |
| Unadjusted hazard ratio (95% CI) | 1.0 (Reference) |
0.54 (0.30 – 1.00) |
| Fully adjusted hazard ratioǂ (95% CI) | 1.0 (Reference) |
0.42* (0.19 – 0.92) |
Adjusted for age, sex, race, dialysis pre-transplant, duration of dialysis, donor status, donor age, delayed graft function, and HLA mismatch
P<0.05
FIGURE 2. BK viremia in kidney transplant patients with PKD, stratified by nephrectomy status.
BK viremia occurred in a lower proportion of patients transplanted for PKD who underwent nephrectomy, compared to those who did not undergo nephrectomy (P=0.053 for nephrectomy versus no nephrectomy).
ESRD, end-stage renal disease, PKD, polycystic kidney disease
4. DISCUSSION
This study found a lower incidence rate of BK viremia in patients transplanted for ESRD due to polycystic kidney disease, compared to other etiologies of ESRD (HTN, DM, GN). A significantly lower incidence rate of BK nephropathy in PKD patients was also observed, compared to patients with ESRD due to HTN or DM. Patients with PKD who underwent nephrectomy were also noted to have a lower risk of BK viremia.
Numerous recipient-related factors have been studied as potential risk factors for BK viremia and nephropathy, with overall degree of immunosuppression emerging as the most salient. Specific immunosuppression regimens, including those with tacrolimus or mycophenolate, have also been shown to correlate with an increased risk of BK viremia.9–11 Other studied risk factors include male sex, non-African American race, older recipient age, treatment for rejection, prolonged cold ischemia, ureteral stents, BK serostatus of donor and recipient, degree of HLA mismatch, and the presence of HLA C7 alleles.14-20 As our study groups differed in terms of several baseline characteristics including age, sex, race, degree of HLA mismatch, and delayed graft failure, we adjusted for these factors in a multivariate model. In this model, a significantly lower incidence rate was observed in PKD patients, as well as among those patients with PKD who underwent nephrectomy prior to BK viremia development. Of note, there was no significant difference in either induction or maintenance immunosuppression regimens between the groups, aside from a higher rate of mycophenolate receipt for maintenance immunosuppression in the PKD group.
Our findings suggest a potential protective effect of PKD anatomy or physiology against post-transplant BK viremia and nephropathy, and are concordant with the findings of Mitterhofer et al.21 In this study of 47 renal transplant patients, a lower rate of BK viremia was observed among PKD patients compared to non-PKD patients at 12 hours, 3 months, and 6 months after transplantation. This difference is posited to be attributable to lower cellular permissivity of the renal tubular epithelial cells in PKD to BK viral replication; these cells in PKD are characteristically non-differentiated but remain capable of proliferating.21 In the majority of cases of PKD, mutations in the genes PKD1 or PKD2 lead to abnormalities in transmembrane proteins (polycystin 1 and polycystin 2), responsible for multiple cellular functions including maintenance of renal tubule cell differentiation. A study by Atencio and Villarreal lent support to the theory that BK virus infectivity is hindered by lack of renal tubule cell differentiation in PKD.22 Employing an adult mouse model of PKD, they demonstrated that tubule cell proliferation alone was not sufficient to permit polyomavirus infection, and proposed that terminal differentiation of the host cell is also required for BK infectivity.22 The 2011 study by Mitterhofer et al. also found higher discordance in blood and urine BK viral detection by PCR among PKD patients, who were more likely than non-PKD patients to lack BK viremia despite having BK virus-positive urine.21 These findings lend further support to the theory of decreased renal tubular cell permissivity to BK virus replication in PKD. Further characterization is needed to fully elucidate the underlying cellular and molecular mechanisms in PKD contributing to apparent decreased permissivity to BK virus infection. Multivariate subgroup analysis of PKD patients by nephrectomy status showed a lower incidence of post-transplant BK viremia among patients who underwent nephrectomy, compared to those who did not. This data suggests a potential protective role for nephrectomy in PKD patients with regards to development of BK viremia.
Uncertainty remains about whether reactivated BK virus predominantly originates from donor or recipient. Our finding of decreased BK viremia incidence in nephrectomized patients suggests the native kidney may also serve as an important reservoir for post-transplant BK viral infection. Donor origin of BK viral reactivation is supported by work by Hirsch et al., in which BK seronegativity prior to transplant was not shown to be a risk factor for BK nephropathy, and the majority of kidney transplant patients with decoy cell shedding in the urine were BK seropositive prior to transplantation.17 Other studies, in contrast, lend support to the donor kidney as the predominant reservoir for post-transplant BK viral reactivation. A prospective study found a higher rate of concordance of BK viral infection among matched pairs of renal transplant recipients who received kidneys from the same donor, compared to pairs of recipients with transplants from different donors, supporting a donor origin of early BK infection.15 Matching BK virus molecular fingerprints (shared gene sequences) were also noted among the BK-infected pairs with a shared donor, while these gene sequences often differed between pairs of BK-infected patients with differing donors. Additionally, of the patients who developed BK infection, a greater proportion had received kidneys from BK seropositive, rather than seronegative, donors.15
Limitations of this study include a lack of pre-transplant BK serologic data for kidney transplant donors and recipients, although, of note, this is not routinely obtained in clinical practice. Furthermore, quantitative comparisons were not made with regards to degree of BK viremia, raising the question of whether all detected instances of viremia indeed had comparable clinical significance and natural history. The study was also limited by sample size, which did not permit evaluation of the effect of nephrectomy on incidence of BK nephropathy in PKD patients, given the small number of cases of BK nephropathy. The study was additionally limited to a single center, and should be validated in a more diverse cohort.
In conclusion, we found a lower incidence of BK viremia and nephropathy among patients with PKD compared to other causes of renal failure, which is potentially attributable to decreased permissivity of non-differentiated renal tubular cells in PKD to BK viral infection. We also noted a lower incidence rate of BK viremia in PKD patients who underwent nephrectomy, compared to those who did not. As there are limited effective therapeutics for BK nephropathy, further study of the mechanisms that may confer a relative resistance to BK viral infection, alter the BK viral reservoir, or replication in the renal tubular epithelial cells in PKD are warranted.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Stephen Peery for his contributions to the data collection for this study and Dana Clark, MA, for her editorial assistance.
This project was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427, and KL2 training Award (KL2TR002374). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- CI
confidence interval
- DM
diabetes mellitus
- ESRD
end-stage renal disease
- GN
glomerulonephritis
- HLA
human leukocyte antigen
- HR
hazard ratio
- HTN
hypertension
- PKD
polycystic kidney disease
- PRA
panel reactive antibody
Footnotes
CONFLICTS OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose.
REFERENCES
- 1.Pham PT, Schaenman J, Pham PC. BK virus infection following kidney transplantation: an overview of risk factors, screening strategies, and therapeutic interventions. Curr Opin Organ Transplant. 2014;19(4): 401–412. [DOI] [PubMed] [Google Scholar]
- 2.Suwelack B, Malyar V, Koch M, Sester M, Sommerer C. The influence of immunosuppressive agents on BK virus risk following kidney transplantation, and implications for choice of regimen. Transplant Rev (Orlando). 2012;26(3): 201–211. [DOI] [PubMed] [Google Scholar]
- 3.Parajuli S, Astor BC, Kaufman D, et al. Which is more nephrotoxic for kidney transplants: BK nephropathy or rejection? Clin Transplant. 2018;32(4): e13216. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez S, Escobar-Serna DP, Suarez O, Benavides X, Escobar-Serna JF, Lozano E. BK Virus Nephropathy in Kidney Transplantation: An Approach Proposal and Update on Risk Factors, Diagnosis, and Treatment. Transplant Proc. 2015;47(6): 1777–1785. [DOI] [PubMed] [Google Scholar]
- 5.Pinto M, Dobson S. BK and JC virus: a review. J Infect. 2014;68 Suppl 1: S2–8. [DOI] [PubMed] [Google Scholar]
- 6.Nickeleit V, Singh HK, Mihatsch MJ. Polyomavirus nephropathy: morphology, pathophysiology, and clinical management. Curr Opin Nephrol Hypertens. 2003;12(6): 599–605. [DOI] [PubMed] [Google Scholar]
- 7.Shenagari M, Monfared A, Eghtedari H, et al. BK virus replication in renal transplant recipients: Analysis of potential risk factors may contribute in reactivation. J Clin Virol. 2017;96: 7–11. [DOI] [PubMed] [Google Scholar]
- 8.Dadhania D, Snopkowski C, Ding R, et al. Epidemiology of BK virus in renal allograft recipients: independent risk factors for BK virus replication. Transplantation. 2008;86(4): 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87(7): 1019–1026. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch HH, Vincenti F, Friman S, et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant. 2013;13(1): 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trofe-Clark J, Sawinski D. BK and Other Polyomaviruses in Kidney Transplantation. Semin Nephrol. 2016;36(5): 372–385. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed M, Parajuli S, Muth B, et al. In kidney transplant recipients with BK polyomavirus infection, early BK nephropathy, microvascular inflammation, and serum creatinine are risk factors for graft loss. Transpl Infect Dis. 2016;18(3): 361–371. [DOI] [PubMed] [Google Scholar]
- 13.Siguier M, Sellier P, Bergmann JF. BK-virus infections: a literature review. Med Mal Infect. 2012;42(5): 181–187. [DOI] [PubMed] [Google Scholar]
- 14.Bohl DL, Brennan DC, Ryschkewitsch C, Gaudreault-Keener M, Major EO, Storch GA. BK virus antibody titers and intensity of infections after renal transplantation. J Clin Virol. 2008;43(2): 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohl DL, Storch GA, Ryschkewitsch C, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant. 2005;5(9): 2213–2221. [DOI] [PubMed] [Google Scholar]
- 16.Hassig A, Roos M, Etter A, et al. Association of BK viremia with human leukocyte antigen mismatches and acute rejection, but not with type of calcineurin inhibitor. Transpl Infect Dis. 2014;16(1): 44–54. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347(7): 488–496. [DOI] [PubMed] [Google Scholar]
- 18.Kayler L, Zendejas I, Schain D, Magliocca J. Ureteral stent placement and BK viremia in kidney transplant recipients. Transpl Infect Dis. 2013;15(2): 202–207. [DOI] [PubMed] [Google Scholar]
- 19.Schold JD, Rehman S, Kayle LK, Magliocca J, Srinivas TR, Meier-Kriesche HU. Treatment for BK virus: incidence, risk factors and outcomes for kidney transplant recipients in the United States. Transpl Int. 2009;22(6): 626–634. [DOI] [PubMed] [Google Scholar]
- 20.Sood P, Senanayake S, Sujeet K, et al. Management and outcome of BK viremia in renal transplant recipients: a prospective single-center study. Transplantation. 2012;94(8): 814–821. [DOI] [PubMed] [Google Scholar]
- 21.Mitterhofer AP, Tinti F, Pietropaolo V, et al. Polyomavirus BK replication in adult polycystic kidney disease post-renal transplant patients and possible role of cellular permissivity. Transplant Proc. 2011;43(4): 1048–1051. [DOI] [PubMed] [Google Scholar]
- 22.Atencio IA, Villarreal LP. Polyomavirus replicates in differentiating but not in proliferating tubules of adult mouse polycystic kidneys. Virology. 1994;201(1): 26–35. [DOI] [PubMed] [Google Scholar]