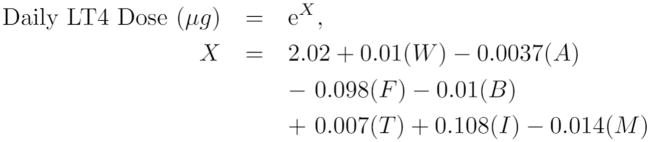Abstract
Background
Patients often struggle to attain euthyroidism after thyroidectomy, and multiple dosing schemes have been proposed to supplant the standard weight-based approach for initial levothyroxine (LT4) dosing. The objectives of this study were to review the literature for existing LT4 dosing schemes and compare estimation accuracies with novel schemes developed with machine learning.
Methods
This study retrospectively analyzed 598 patients who attained euthyroidism after total or completion thyroidectomy for benign disease. A scoping review identified existing LT4 dosing schemes. 13 machine learning algorithms estimated euthyroid dose. Using 10-fold cross-validation, we compared schemes by the proportion of patients having a predicted dose within 12.5 μg/day of their euthyroid dose.
Results
Of 264 reviewed articles, 7 articles proposed retrospectively implementable dosing schemes. A novel Poisson regression model proved most accurate, correctly predicting 64.8% of doses. Incorporating 7 variables, Poisson regression was significantly more accurate than the best scheme in the literature (BMI/weight based) that correctly predicted 60.9% of doses (p=0.031). Standard weight-based dosing (1.6 μg/kg/day) correctly predicted 51.3% of doses, and the least effective scheme (age/sex/weight based) correctly predicted 27.4% of doses.
Conclusion
Using readily available variables, a novel Poisson regression dosing scheme outperforms other machine learning algorithms and all existing schemes in estimating LT4 dose.
BACKGROUND
Levothyroxine (LT4) is the most widely prescribed drug in the United States.(1) LT4 is prescribed after thyroidectomy with the intent to restore normal thyroid hormone function, and for patients with benign thyroid disease the typical starting dose of LT4 is 1.6 to 1.7 μg/kg.(2) Often this weight-based initial dosing is inadequate, with about 70% of patients requiring dose adjustments at first post-surgery follow-up.(3–5) Overdosing of LT4 increases patient risk of accelerated bone loss, fractures, heat intolerance, diarrhea, and arrhythmias.(6–9) Underdosing of LT4 results in symptoms of hypothyroidism, including fatigue and weight gain.(10) Hence, incorrect thyroid hormone dosing after thyroidectomy greatly impacts patient quality of life. Many authors have proposed alternative LT4 dosing schemes to improve initial LT4 dosing after thyroidectomy to reduce the time patients spend in either a hypo- or hyperthyroid state postoperatively.
The literature contains a range of approaches for LT4 dosing. Some authors propose modified weight-based schemes;(3, 11) others incorporate body weight and one or two additional patient factors, including body mass index (BMI), age, sex, or lean body mass (LBM).(4, 5, 12–14) We have previously developed and tested a BMI-based regression algorithm that was superior to simple, weight-based dosing.(5) However, prospective evaluation indicated that it still incorrectly dosed 61.1% of patients.(14) Overall, the existing dosing schemes lack accuracy and no consensus exists for the optimal dosing scheme.
One potential method to improve prediction of the correct LT4 dose is with machine learning (ML). ML approaches focus on the creation of a predictive model using labeled datasets; predicting a dependent variable from explanatory factors. In clinical medicine, ML approaches are increasingly used to improve the accuracy of decision-making. For example, ML can successfully detect hyperparathyroidism(15) and identify risk of breast cancer from mammographic findings(16). To date, ML has not been applied to LT4 dosing. Moreover, existing LT4 dosing schemes have not been compared to more sophisticated ML methods utilizing all relevant patient factors.
The purpose of this study is two-fold: 1) to evaluate the accuracy of ML approaches in predicting LT4 dose, and 2) compare the accuracy of LT4 dosing schemes proposed in the literature. We hypothesized that a predictive model developed with supervised ML could improve initial LT4 dosing after thyroidectomy. We propose a predictive model that correctly doses significantly more patients than all other existing approaches.
METHODS
This is a two part study: 1) a scoping review to identify LT4 dosing schemes proposed in existing literature and 2) the development of predictive algorithms using a retrospective dataset, and comparing their performance to previously proposed schemes identified in the scoping review. The Institutional Review Board of the University of Wisconsin School of Medicine and Public Health approved data collection for this HIPAA compliant study.
Scoping Review
The scoping review identified existing LT4 dosing schemes after thyroidectomy for benign disease. Databases PubMed, Cochrane, Scopus, and Web of Science were queried using variations of the following search terms: levothyroxine, thyroidectomy, dosing, and algorithm. Additional articles were identified through citations of articles known to be relevant. To be included in our study, the article must have proposed a method to dose LT4 in adults (>=18 years of age) after non-cancerous total or completion thyroidectomy, or dose LT4 for adult patients needing full thyroid hormone replacement. Additionally, the article had to be written in English with no timeframe restrictions. Articles were excluded if they proposed schemes which could not be implemented retrospectively. Articles returned from the search were independently reviewed and agreed upon by three authors for relevance.
Study Sample and Definitions
We performed a retrospective review of our institution’s existing thyroid surgery database containing records for 598 patients who underwent total or completion thyroidectomy with pathology showing benign thyroid disease. Included were patients who achieved euthyroidism, defined as a serum thyroid-stimulating hormone (TSH) level of 0.45 – 4.50 mIU/mL, between 2008 and May of 2017. Excluded were those patients who were under 18 years old, taking liothyronine in addition to levothyroxine, had thyroid cancers greater than 1 cm in size (as these patients need adjusted doses for TSH suppression), or who did not achieve euthyroidism by the time of data collection.
Recommended starting dose of LT4 was generally 1.6 μg/kg/day, although starting from October 2012, patients were dosed according to our BMI-based algorithm.(5) TSH values were checked 6–8 weeks after surgery and levothyroxine doses were adjusted if necessary to meet TSH goal. Subsequent dose titration occurred at 6–8 week intervals.
44 variables were collected for each patient, including initial and euthyroid dose; clinical demographics such as age, gender, race, weight, BMI; pertinent medical history such as autoimmune thyroid disease, co-morbidities, medications; surgical pathology, and laboratory data such as pre-operative TSH and post-operative parathyroid hormone levels.
ML Model Development and Evaluation
We evaluated the ability of various supervised ML predictive models to predict daily LT4 requirements after thyroidectomy. A wide variety of predictive models were examined, including but not limited to, support vector machines, Bayesian recurrent neural networks, decision trees, random forests, ordinary least squares regression, Poisson regression, gamma regression, ridge regression, and the Lasso.
A subset of explanatory variables were selected as predictors based on the following process. First, a random forest (built on the entire dataset) generated feature importance values for all predictor variables. Next, the least important feature was omitted from the building of another random forest built on the entire dataset and the out-of-bag mean squared error (MSE) was noted. This step was repeated successively for the two least important features, then the three least important features, and so on. The variables from the random forest with the lowest out-of-bag MSE were chosen as the model building features.
Before any comparison or evaluation, all predictions were rounded to the nearest 12.5 μg to represent a practical initial dose, since 12.5 μg is the smallest increment between dosing strengths. We evaluated each algorithm’s predictive accuracy by calculating the percentage of patients whose predicted LT4 dose was within 12.5 μg of their actual euthyroid dose. In this case, we say the algorithm correctly predicted the LT4 dose. We determined the most accurate model to be the one with the highest proportion of correct predictions.
Statistical Analysis
All predictive algorithms were evaluated using repeated 10-fold cross validation. We formally tested the difference in performance between the best ML algorithm and the best scheme proposed in the literature using the method outlined by Bouckaert17 for cross validated results. A p value of 0.05 or less defined statistical significance. All models and descriptive statistics were generated with R (version 3.4.2; Vienna, Austria), a free and open source software environment for statistical computing.(18) The caret package for R was used extensively for building machine learning models.(19)
RESULTS
Scoping Review
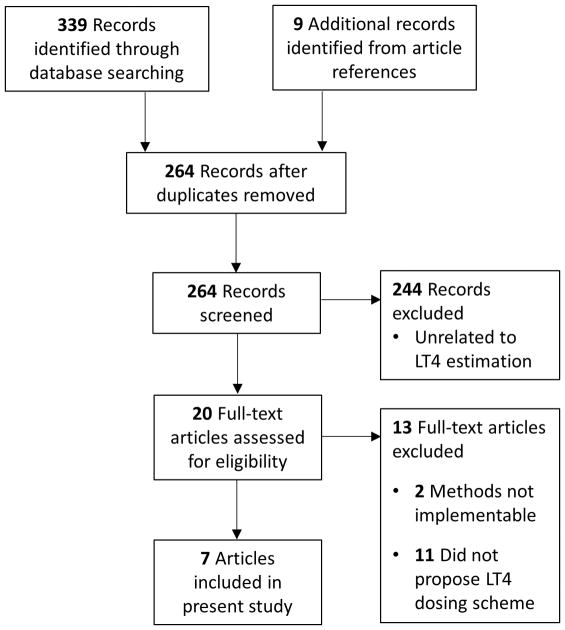
Figure 1 details the results of the literature search. From an initial pool of 264 articles meeting our search criteria, a total of 9 articles proposed full replacement LT4 dosing schemes. Ultimately 7 articles proposed schemes that could be implemented retrospectively and compared. The methods proposed by Sukumar/Agarwal et al.(20) and Banovac et al.(21) were excluded because they used a dual absorption X-ray densitometry machine to measure LBM and hence could not be implemented retrospectively. Table 1 details the 7 proposed schemes identified in the literature and included in this comparison.
Figure 1.
Scoping review search results
LT4 = Levothyroxine
Table 1.
Existing Schemes Proposed in the Literature for the Estimation of LT4 Requirements after Thyroidectomy
| Author/location | Formula or Scheme | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cunningham et al. 1984/New Haven, Connecticut |
|
|||||
|
| ||||||
| Olubowale et al. 2005/Chesterfield, United Kingdom |
|
|||||
|
| ||||||
| Mistry et al. 2011/Hull, United Kingdom |
|
|||||
|
| ||||||
| Ojomo et al. 2013/Madison, Wisconsin |
|
|||||
|
| ||||||
| Jin et al. 2013/Cleveland, Ohio |
|
|||||
|
| ||||||
| Di Donna et al. 2014/Rome, Italy | LT4 dose (μg/kg/day) = | BMI | ≤ 23 | 23–28 | > 28 | |
|
| ||||||
| Age | ||||||
| ≤ 40 | 1.8 | 1.7 | 1.6 | |||
| > 40–55 | 1.7 | 1.6 | 1.5 | |||
| > 55 | 1.6 | 1.5 | 1.4 | |||
|
| ||||||
| Elfenbein et al. 2015/Madison, Wisconsin | LT4 dose (μg/kg/day) = | Male | Female | |||
|
| ||||||
| BMI | ||||||
| < 21 | 2.1 | 1.8 | ||||
| 22–26 | 1.9 | 1.7 | ||||
| 27–32 | 1.7 | 1.6 | ||||
| 33–40 | 1.5 | 1.4 | ||||
| >40 | 1.3 | 1.2 | ||||
LT4 = levothyroxine;
LBM = lean body mass;
BMI = body mass index. All weights are in kg.
Cohort Characteristics
A total of 598 patients were included in the retrospective cohort – 504 (84.3%) were female, the median age was 51 years (range 18–84 years), and the median weight was 81 kg (range 38–206 kg) with a median BMI value of 29.3. Most (57.5%) underwent thyroidectomy for thyroid nodular disease or multinodular goiter. The second most common indications for thyroidectomy was hyperthyroidism (42.5%). In this cohort, 10.5% of thyroidectomy specimens contained a micropapillary thyroid carcinoma although this was not the initial indication for surgery. The median time to euthyroidism was 17 weeks (range 2–123 weeks). Nearly a fifth of the cohort (18.7%) were smokers, 103 (17.2%) patients were taking LT4 pre-operatively, and 79 (13.2%) were taking multivitamin-mineral supplements. Baseline characteristics of the cohort are summarized in Table 2.
Table 2.
Baseline Characteristics of the Cohort
| Cohort Characteristics | |
|---|---|
|
| |
| Sex, % (n) | |
| Male | 15.7% (94) |
| Female | 84.3% (504) |
|
| |
| Weight, kg (median ± SD*) | 80.7 ± 22.6 |
|
| |
| Height, in (median ± SD) | 65.0 ± 3.3 |
|
| |
| Age, years (median ± SD) | 51.1 ± 14.1 |
|
| |
| BMI†, kg/m2 (median ± SD) | 29.3 ± 8.1 |
|
| |
| Smoker, % (n) | |
| Yes | 18.7% (112) |
| No | 81.3% (486) |
|
| |
| Pre-operative TSH‡ (median ± SD) | 0.8 ± 2.4 |
|
| |
| Indication for Surgery, % (n) | |
| -Thyroid Nodular Disease/Multinodular Goiter | 57.5% (344) |
| -Hyperthyroidism | 42.5% (254) |
| -Graves’ Hyperthyroidism | 66.5% (169) |
|
| |
| Hashimoto’s Disease | 18.4% (110) |
|
| |
| Iron Supplementation, % (n) | |
| Yes | 2.5% (15) |
| No | 97.5% (583) |
|
| |
| Multivitamin-mineral use, % (n) | |
| Yes | 13.2% (79) |
| No | 86.8% (519) |
|
| |
| Weeks to Euthyroidism (median ± SD) | 16.5 ± 17.9 |
|
| |
| Initial LT4§ Dose, μg/day (median ± SD) | 125 ± 29.2 |
|
| |
| Euthyroid LT4 Dose, μg/day (median ± SD) | 125 ± 38.9 |
|
| |
| Pre-operative LT4 use, % (n) | |
| Yes | 17.2% (103) |
| No | 82.8% (495) |
SD = standard deviation;
BMI = body mass index;
TSH = thyroid-stimulating hormone;
LT4 = levothyroxine
Machine Learning Results
Seven patient variables were identified by the random forest variable selection process to develop ML algorithms: weight, age, BMI, sex, preoperative TSH, iron supplementation use, and multivitamin/mineral use. After testing multiple predictive algorithms, we found Poisson regression to be most successful at predicting LT4 dose, correctly predicting 64.8% of LT4 doses. Poisson regression is a simple extension of the ubiquitous ordinary least squares linear regression, wherein the dependent variable is assumed to have a Poisson distribution. Other linear algorithms provided similar but less accurate results, such as ordinary least squares (OLS) and gamma regression, each correctly predicting 64.4% of doses. Non-linear methods, such as random forests and Gaussian kernel support vector machines, performed relatively poorly with 51.3% and 55.6% correctly predicted doses, respectively. Table 3 describes the performance for 13 different machine learning algorithms.
Table 3.
Accuracy of Various Machine Learning Algorithms in Predicting LT4 Requirements
| Method | % Euthyroid doses |
|---|---|
| Random Forest | 51.3% |
| SVM* (Gaussian Kernel) | 55.6% |
| Ordinal Regression | 57.0% |
| K Nearest Neighbors | 59.5% |
| Boosted Decision Trees | 60.5% |
| SVM (Polynomial Kernel) | 63.2% |
| Lasso | 63.4% |
| Ridge Regression | 64.0% |
| Bayesian Recurrent Neural Networks | 64.0% |
| Boosted GLM | 64.2% |
| Gamma Regression | 64.4% |
| OLS Regression† | 64.4% |
| Poisson Regression | 64.8% |
SVM = support vector machine;
OLS = ordinary least squares
Comparison and Evaluation of Dosing Schemes
Poisson regression LT4 dosing correctly dosed 64.8% of patients, proving significantly more accurate than the best existing dosing scheme in the literature (proposed by Ojomo et al.) that correctly predicted 60.9% of doses (p=0.031). Standard weight-based dosing (1.6 μg/kg/day) correctly dosed 51.3% of patients, and the least effective dosing scheme proposed in the literature (proposed by Cunningham et al.) correctly dosed 27.4% of patients.
Poisson dosing provided the lowest rate of extreme over- or under-replacement (errors greater than 25 μg) at 19.1%, and the lowest rate of any over-replacement at 19.5%. Of all schemes performing better than standard weight-based dosing, Poisson dosing resulted in the lowest rate of under-replacement at 15.7%. The comparison between Poisson dosing and existing methods is shown in Table 4.
Table 4.
Efficacy of Existing LT4 Prediction Schemes and a Novel Poisson Dosing Algorithm
| Scheme | % Euthyroid doses | % Dose errors > 25 μg | % Hypothyroid | % Hyperthyroid |
|---|---|---|---|---|
|
|
||||
| 1.6 μg/kg/day | 51.3% | 26.9% | 16.4% | 32.3% |
| Cunningham et al. | 27.4% | 46.5% | 7.0% | 65.6% |
| Olubowale et al. | 43.5% | 25.9% | 15.7% | 40.8% |
| Mistry et al. | 40.1% | 40.0% | 9.2% | 50.7% |
| Ojomo et al. | 60.9% | 23.1% | 16.1% | 23.1% |
| Jin et al. | 53.2% | 25.1% | 24.4% | 22.4% |
| Di Donna et al. | 60.0% | 21.6% | 18.4% | 21.6% |
| Elfenbein et al. | 60.2% | 22.1% | 17.4% | 22.4% |
| Poisson Dosing | 64.8% | 19.1% | 15.7% | 19.5% |
Since we previously described the difficulties in achieving euthyroidism for patients at the extremes of BMI, we further evaluated dosing schemes by BMI subsets (tertiles). Table 5 describes the accuracy of each scheme within each BMI tertile. Poisson dosing outperforms all existing methods within each BMI group. Notably, standard weight-based dosing dosed 64.1% of the lower BMI patients correctly, but only 38.0% of the upper BMI patients; the 26.1% difference across BMI extremes is the highest of any method.
Table 5.
Accuracy of Existing LT4 Prediction Schemes and a Novel Poisson Dosing Algorithm by BMI Tertile Subsets
| Scheme | BMI* Tertile | ||
|---|---|---|---|
| ≤ 26 | 27–32 | > 32 | |
|
|
|||
| 1.6 μg/kg/day | 64.1% | 52.8% | 38.0% |
| Cunningham et al. | 35.9% | 19.3% | 25.9% |
| Olubowale et al. | 47.1% | 45.5% | 38.4% |
| Mistry et al. | 41.7% | 39.2% | 39.4% |
| Ojomo et al. | 72.3% | 55.1% | 54.6% |
| Jin et al. | 57.3% | 55.7% | 47.2% |
| Di Donna et al. | 70.4% | 60.2% | 50.0% |
| Elfenbein et al. | 68.4% | 53.4% | 57.9% |
| Poisson Dosing | 73.3% | 63.6% | 59.7% |
BMI = body mass index
Additionally, we observed how Poisson dosing performed in other patient subsets: Poisson dosing had an accuracy of 70.1% for patients with hyperthyroidism, 62.2% for patients with thyroid nodule disease, and 60.0% for patients with Hashimoto’s disease. Figure 2 presents the Poisson regression formula for predicting daily LT4 requirements.
Figure 2.
Poisson regression formula. LT4 is levothyroxine; W is patient weight, in kilograms; A is patient age, in years; F patient sex (1 for female, 0 for males); B is patient body mass index (BMI); T is pre-operative thyroid-stimulating hormone (TSH) value; I is iron supplementation (1 for supplementation, 0 otherwise); M is multi-vitamin/mineral supplementation (1 for supplementation, 0 otherwise).
DISCUSSION
Seven LT4 dosing schemes after thyroidectomy were identified in the literature, each using some combination of patient body weight, age, sex, or BMI. The Poisson regression LT4 dosing algorithm we developed correctly predicted significantly more doses compared to all other schemes proposed in the literature. Additionally, the algorithm was more accurate across patient BMI levels and made less large dosing errors than existing schemes. This comparison is the second and most extensive review of LT4 dosing schemes.
Previously, Di Donna et al. compared several LT4 dosing schemes using a retrospective cohort of 92 patients.(13) Our comparison extends upon Di Donna’s study by examining a comprehensive collection of LT4 schemes on a substantially larger retrospective cohort of 598 patients. Our comparison agrees with Di Donna’s in finding the scheme proposed by Ojomo et al. to be the most accurate of existing schemes, and in finding the scheme proposed by Mistry et al. to be less accurate than standard weight-based dosing. As detailed here, the Poisson regression method outperforms the scheme proposed by Ojomo et al.
The seven identified schemes predicted LT4 requirements using 3 or fewer explanatory factors. Our ML approach resulted in a predictive LT4 algorithm using the 4 factors found in proposed schemes (body weight, age, sex, and BMI), and using 3 additional factors: pre-operative TSH, iron supplementation, vitamin-mineral supplementation. The influence of the supplemental factors is consistent with recent research demonstrating iron and various vitamins interfere with LT4 therapy.(22, 23) Although each ML algorithm used these same 7 predictors, their accuracy varied substantially – reflecting the importance of their varying assumptions. Poisson regression assumes a model most aligned with LT4 estimation, and is correspondingly the most accurate ML algorithm.
Two of the most predictive schemes of those in the literature were proposed by Ojomo et al. and Elfenbein et al. These two schemes represent prior iterations of progressive inquiry at our institution and were developed with an earlier version of the dataset used in this study. The Ojomo scheme was developed first and estimated LT4 needs using linear regression, with BMI and weight as predictors.(5) After prospectively evaluating this scheme, differences in LT4 needs were observed between men and women, and a new scheme was developed by Elfenbein et al. – also using linear regression – to adjust for these sex differences.(14) Yet, as noted here, a significant portion of incorrect dosing persisted, and we therefore hypothesized that more advanced ML techniques could improve the accuracy of dosing prediction. Additionally, we collected all potential variables that could impact dosing accuracy such as medication and supplement use, labs, and postoperative parathyroid function. Accordingly, this current study expanded upon these earlier schemes and used ML to develop an even more accurate scheme that adjusts for additional variables influencing LT4 requirements.
To promote provider use of LT4 dosing with Poisson regression, we have developed an easy to use web application allowing users to input patient characteristics in order to estimate LT4 needs. A snapshot of this application is shown in Figure 3. Going further, our dosing algorithm could be implemented within electronic health systems as either an easy to use clinical decision-support tool, or automatic calculation using variables within the electronic health record. These implementations would make it convenient for providers to prescribe LT4 according to our proposed algorithm thereby reducing time to euthyroidism after thyroidectomy.
Figure 3.
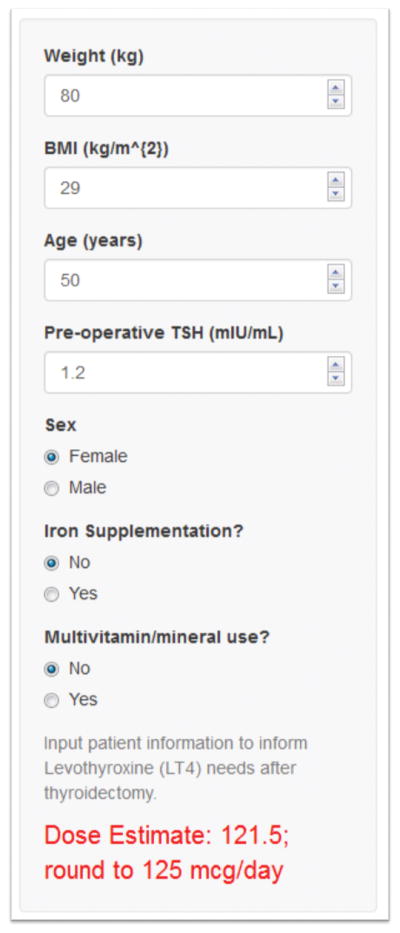
Web application to estimate daily levothyroxine (LT4) requirements after thyroidectomy using Poisson regression. The URL for this application is https://zaborek-uwsurgery.shinyapps.io/ShinyDosing/. BMI = body mass index; TSH = thyroid-stimulating hormone.
Our study has important limitations. First, the proposed algorithm has not been tested prospectively. Although our algorithm was developed using cross-validation and a substantially larger dataset than other authors, we have yet to observe how our proposed algorithm performs in clinical practice. Second, the dataset comes from a single institution, subjecting our results to the uncontrollable biases inherent in our patient population, thereby limiting the generalizability of our algorithm. However, our results are similar to those of the comparison conducted by Di Donna et al., a study using a cohort from another country;(13) this suggests that LT4 dosing requirements are robust to variant populations and adds credibility to the generalizability of our results. Third, explanatory variables of our dataset are limited to those readily collected during clinic. Research has shown associations between LT4 needs and LBM measured by Dual Energy X-ray Absorptiometry, as well as genetic factors and TSH serum levels in patients undergoing LT4 therapy.(20, 21, 24) Absence of these predictors in our model development leaves space for future algorithm improvement. However, these predictors require more invasive or expensive testing and our goal was to develop a method with readily obtainable data. Our starting dataset included over 40 predictive variables to represent a robust set of potential factors that could impact thyroid hormone dosing. Since 36% of patients still required dose adjustment, other non-measured factors clearly impact LT4 dosing. Such factors might include patient compliance and individual metabolism of LT4.
To address these limitations, we intend to pursue a prospective application of our algorithm in a multi-site clinical trial to determine its predictive ability in practice.
Conclusions
Using readily available variables, our novel Poisson regression dosing scheme outperforms other machine learning algorithms and all existing dosing schemes in calculating LT4 dose. Defining a correct prediction as a dose within 12.5 μg of the euthyroid dose, Poisson regression yielded significantly better dose estimates, including better estimation across patient BMI levels. Use of Poisson dosing by providers to calculate LT4 needs, either within electronic medical systems or with online calculators, could potentially reduce morbidity associated with LT4 replacement after thyroidectomy.
Acknowledgments
The authors would like to thank Mary Hitchcock for her assistance with the scoping review. This study was supported by NIH UL1TR000427 and NIH KL2TR000428.
Abbreviations
- LT4
Levothyroxine
- BMI
Body Mass Index
- LBM
Lean Body Mass
- ML
Machine Learning
- OLS
Ordinary Least Squares
- TSH
Thyroid-stimulating hormone
Footnotes
This manuscript has been accepted for an oral presentation at the 39th Annual Meeting of the American Association of Endocrine Surgeons in Durham, North Carolina. The meeting will take place from Sunday, May 6 through Tuesday, May 8, 2018.
References
- 1.Aitken M, Kleinrock M. Medicines Use and Spending in the U.S. IQVIA Institute for Human Data Science; 2017. [Google Scholar]
- 2.Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316(13):764–70. doi: 10.1056/NEJM198703263161302. [DOI] [PubMed] [Google Scholar]
- 3.Olubowale O, Chadwick DR. Optimization of thyroxine replacement therapy after total or near-total thyroidectomy for benign thyroid disease. Br J Surg. 2006;93(1):57–60. doi: 10.1002/bjs.5157. [DOI] [PubMed] [Google Scholar]
- 4.Mistry D, Atkin S, Atkinson H, Gunasekaran S, Sylvester D, Rigby AS, et al. Predicting thyroxine requirements following total thyroidectomy. Clin Endocrinol (Oxf) 2011;74(3):384–7. doi: 10.1111/j.1365-2265.2010.03940.x. [DOI] [PubMed] [Google Scholar]
- 5.Ojomo KA, Schneider DF, Reiher AE, Lai N, Schaefer S, Chen H, et al. Using body mass index to predict optimal thyroid dosing after thyroidectomy. J Am Coll Surg. 2013;216(3):454–60. doi: 10.1016/j.jamcollsurg.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biondi B, Fazio S, Carella C, Amato G, Cittadini A, Lupoli G, et al. Cardiac effects of long term thyrotropin-suppressive therapy with levothyroxine. J Clin Endocrinol Metab. 1993;77(2):334–8. doi: 10.1210/jcem.77.2.8345037. [DOI] [PubMed] [Google Scholar]
- 7.Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331(19):1249–52. doi: 10.1056/NEJM199411103311901. [DOI] [PubMed] [Google Scholar]
- 8.Bauer DC, Ettinger B, Nevitt MC, Stone KL Group SoOFR. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med. 2001;134(7):561–8. doi: 10.7326/0003-4819-134-7-200104030-00009. [DOI] [PubMed] [Google Scholar]
- 9.Uzzan B, Campos J, Cucherat M, Nony P, Boissel JP, Perret GY. Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. J Clin Endocrinol Metab. 1996;81(12):4278–89. doi: 10.1210/jcem.81.12.8954028. [DOI] [PubMed] [Google Scholar]
- 10.Vigário PoS, Vaisman F, Coeli CM, Ward L, Graf H, Carvalho G, et al. Inadequate levothyroxine replacement for primary hypothyroidism is associated with poor health-related quality of life-a Brazilian multicentre study. Endocrine. 2013;44(2):434–40. doi: 10.1007/s12020-013-9886-1. [DOI] [PubMed] [Google Scholar]
- 11.Jin J, Allemang MT, McHenry CR. Levothyroxine replacement dosage determination after thyroidectomy. American Journal of Surgery. 2013;205(3):360–4. doi: 10.1016/j.amjsurg.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham JJ, Barzel US. Lean body mass is a predictor of the daily requirement for thyroid hormone in older men and women. J Am Geriatr Soc. 1984;32(3):204–7. doi: 10.1111/j.1532-5415.1984.tb02003.x. [DOI] [PubMed] [Google Scholar]
- 13.Di Donna V, Santoro MG, de Waure C, Ricciato MP, Paragliola RM, Pontecorvi A, et al. A new strategy to estimate levothyroxine requirement after total thyroidectomy for benign thyroid disease. Thyroid. 2014;24(12):1759–64. doi: 10.1089/thy.2014.0111. [DOI] [PubMed] [Google Scholar]
- 14.Elfenbein DM, Ojomo KA, Schaefer S, Shumway C, Chen H, Sippel RS, et al. Prospective Intervention of a Novel Levothyroxine Dosing Protocol based on Body Mass Index after Thyroidectomy. Journal of the American College of Surgeons. 2014;219(3):S125-S. doi: 10.1016/j.jamcollsurg.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somnay YR, Craven M, McCoy KL, Carty SE, Wang TS, Greenberg CC, et al. Improving diagnostic recognition of primary hyperparathyroidism with machine learning. Surgery. 2017;161(4):1113–21. doi: 10.1016/j.surg.2016.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnside ES, Rubin DL, Fine JP, Shachter RD, Sisney GA, Leung WK. Bayesian network to predict breast cancer risk of mammographic microcalcifications and reduce number of benign biopsy results: initial experience. Radiology. 2006;240(3):666–73. doi: 10.1148/radiol.2403051096. [DOI] [PubMed] [Google Scholar]
- 17.Bouckaert RR. Choosing between two learning algorithms based on calibrated tests. Proceedings of the Twentieth International Conference on International Conference on Machine Learning; Washington, DC, USA. AAAI Press; 2003. pp. 51–8. 3041845. [Google Scholar]
- 18.Team RC. R Foundation for Statistical Computing. R Foundation for Statistical Computing; 2017. R: A Language and Environment for Statistical Computing. 3.4.2 ed. [Google Scholar]
- 19.Kuhn M. R package version 6.0-77 ed. R Foundation for Statistical Computing; 2017. caret: Classification and Regression Training. [Google Scholar]
- 20.Sukumar R, Agarwal A, Gupta S, Mishra A, Agarwal G, Verma AK, et al. Prediction of lt4 replacement dose to achieve euthyroidism in subjects undergoing total thyroidectomy for benign thyroid disorders. World Journal of Surgery. 2010;34(3):527–31. doi: 10.1007/s00268-009-0345-3. [DOI] [PubMed] [Google Scholar]
- 21.Banovac K, Carrington SAB, Levis S, Fill MD, Bilsker MS. Determination of Replacement and Suppressive Doses of Thyroxine. Journal of International Medical Research. 1990;18(3):210–8. doi: 10.1177/030006059001800305. [DOI] [PubMed] [Google Scholar]
- 22.Irving SA, Vadiveloo T, Leese GP. Drugs that interact with levothyroxine: an observational study from the Thyroid Epidemiology, Audit and Research Study (TEARS) Clin Endocrinol (Oxf) 2015;82(1):136–41. doi: 10.1111/cen.12559. [DOI] [PubMed] [Google Scholar]
- 23.Jubiz W, Ramirez M. Effect of vitamin C on the absorption of levothyroxine in patients with hypothyroidism and gastritis. J Clin Endocrinol Metab. 2014;99(6):E1031–4. doi: 10.1210/jc.2013-4360. [DOI] [PubMed] [Google Scholar]
- 24.Brigante G, Spaggiari G, Santi D, Cioni K, Gnarini V, Diazzi C, et al. The TRHR Gene Is Associated with Hypothalamo-Pituitary Sensitivity to Levothyroxine. Eur Thyroid J. 2014;3(2):101–8. doi: 10.1159/000358590. [DOI] [PMC free article] [PubMed] [Google Scholar]