Abstract
The role of sleep in brain physiology is poorly understood. Recently rodent studies have shown that the glymphatic system clears waste products from brain more efficiently during sleep compared to wakefulness due to the expansion of the interstitial fluid space facilitating entry of cerebrospinal fluid (CSF) into the brain. Here, we studied water diffusivity in the brain during sleep and awake conditions, hypothesizing that an increase in water diffusivity during sleep would occur concomitantly with an expansion of CSF volume - an effect that we predicted based on preclinical findings would be most prominent in cerebellum. We used MRI to measure slow and fast components of the apparent diffusion coefficient (ADC) of water in the brain in 50 healthy participants, in 30 of whom we compared awake versus sleep conditions and in 20 of whom we compared rested-wakefulness versus wakefulness following one night of sleep-deprivation. Sleep compared to wakefulness was associated with increases in slow-ADC in cerebellum and left temporal pole and with decreases in fast-ADC in thalamus, insula, parahippocampus and striatal regions, and the density of sleep arousals was inversely associated with ADC changes. The CSF volume was also increased during sleep and was associated with sleep-induced changes in ADCs in cerebellum. There were no differences in ADCs with wakefulness following sleep deprivation compared to rested-wakefulness. Although we hypothesized increases in ADC with sleep, our findings uncovered both increases in slow ADC (mostly in cerebellum) as well as decreases in fast ADC, which could reflect the distinct biological significance of fast- and slow-ADC values in relation to sleep. While preliminary, our findings suggest a more complex sleep-related glymphatic function in the human brain compared to rodents. On the other hand, our findings of sleep-induced changes in CSF volume provide preliminary evidence that is consistent with a glymphatic transport process in the human brain.
Introduction
The human brain utilizes 25% of the body’s total energy and generates an estimated 7 grams of potentially toxic protein waste daily (Nedergaard and Goldman, 2016); yet the mechanisms by which the brain clears waste products such as Aβ are not properly understood. Recently a peri-vascular cerebrospinal fluid (CSF) transport system in the brain referred to as the glymphatic system, which facilitates waste removal and that is most active during sleep, was described in rodents (Iliff et al., 2012). These studies showed that clearance of soluble Aβ increased 2-fold during slow-wave sleep when compared to wakefulness, which was associated with an increase in the interstitial fluid (ISF) space (Xie et al., 2013). Expansion of the ISF volume during sleep is proposed to facilitate access of more CSF transport through brain parenchyma thereby enhancing brain waste elimination. However, it is currently unknown if a glymphatic system as described in rodents (Iliff et al., 2012) exists in the human brain. Also, recent work has suggested alternative mechanisms involved in clearance of waste products in the brain (Abbott et al., 2018), or opposite results to those associated with glymphatic clearance during sleep; including greater parenchymal CSF circulation during wakefulness than during anesthesia (Gakuba et al., 2018).
To explore evidence of glymphatic clearance in the human being during sleep, here we tested the hypothesis that water diffusivity (as assessed by diffusion MRI) would be increased during sleep compared to the awake state. Our hypothesis is based on the observation that in rodents, cortical ISF volume increases (~40%) during sleep and is a major determinant of glymphatic transport efficiency (Kress et al., 2014; Xie et al., 2013); and further, that quantitative diffusion MRI is sensitive to changes in ISF volume (Benveniste et al., 1992; Davis et al., 1994; Sotak, 2004; van Gelderen et al., 1994; Verheul et al., 1994). We hypothesized that during sleep, ISF volume will expand thereby enhancing entry of CSF into the brain when compared to the awake state. The ISF volume enlargement would be associated with an increase in CSF volume and detectable as an increase in water diffusivity in the brain.
We predicted that the cerebellum would show the largest changes in ADC with sleep since in rodents it shows enhanced glymphatic CSF transport compared to other brain regions (Iliff et al., 2013). We also hypothesized that an individual’s sleep quality would have an effect on sleep-changes in ADC, such that participants with multiple awakenings during the night would show decreased ADC during sleep and vice versa. If correct, this hypothesis could help explain recent findings of an association between frequent arousals from sleep-disordered breathing and beta amyloid deposition in the brain (Alexander et al., 2001; Cedernaes et al., 2017; Sharma et al., 2018).
To test these hypotheses, we conducted two separate MRI studies in 50 healthy controls in whom we measured both “fast” and “slow” apparent di ffusion coefficients (ADC) (Le Bihan et al., 1988; Silva et al., 1997). MRI studies of diffusivity have reported different compartmental diffusion characteristics of tissue when implementing a range of sensitizing diffusion gradients, b-values, which have been distinguished as ‘fast’ and ‘slow’ ADCs. The fast ADC is reported to be more sensitive to changes in cerebral blood flow (CBF) (MacFall et al., 1991; Silva et al., 1997) whereas the slow ADC is considered to be representative of non-CBF components, such as neural or slower tissue-level diffusion changes (Abe et al., 2017a).
In the first study (n=30) we compared diffusivity and CSF volumes while participants were awake (AWAKE condition after a good night rest) versus when they were asleep (SLEEP condition after 24 hours of sleep deprivation). Participants were sleep-deprived the night prior to the SLEEP condition in order to facilitate their sleep in the MRI scanner. In a second study (n=20), to evaluate the effect of acute sleep deprivation (SD) on diffusivity, we compared diffusivity while participants were awake during rested wakefulness (A-RW) versus when they were awake but after a night of SD (A-SD). We also collected electroencephalography (EEG) based individual sleep behavior during two nights of sleep while in a clinical setting (NIH’s Clinical Center).
Materials and Methods
The study was approved by the Ethics Committee of the National Institutes of Health (Combined Neurosciences White Panel) and was in accordance with the Declaration of Helsinki. All subjects gave informed written consent before participating in the study.
Participants
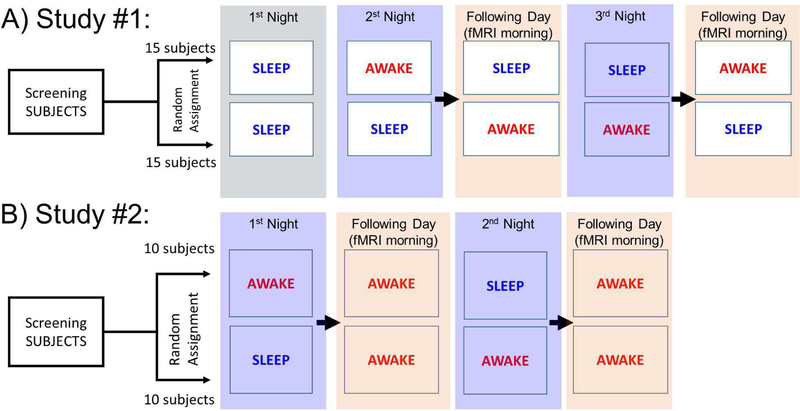
A total of fifty healthy participants were recruited for two separate MRI studies (Figure 1), 30 completed Study #1 (15 females; age 41.5±11.3y and 15 males; age 40.5±13.2y), and 20 completed Study #2 (10 females, age 40.0±14.5y and 10 males; age 42.7±12.6y). Participants were initially screened to exclude ferromagnetic implants, psychoactive medications and major medical problems, current psychiatric diagnosis as assessed by an abbreviated Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) including drug abuse or dependence, medical conditions that may alter cerebral function (i.e., cardiovascular, endocrinological, oncological, neurological, or autoimmune diseases), current use of prescribed or over-the-counter medications, and/or head trauma with loss of consciousness of more than 30 min. STAT urine tests were performed to identify drug use (cocaine, methamphetamine, stimulant medications, opioids, cannabinoids, benzodiazepines and barbiturates) and to rule out pregnancy in females.
Figure 1.
Experimental design: Participants in STUDY #1 slept in the inpatient unit the first night (for ACCLIMATIZATION). In the RESTED night, they also slept in the inpatient unit and were scanned the following morning while awake (AWAKE). The night of sleep deprivation (SD), was followed the following morning by a scanning session where they were encouraged to fall asleep in the magnet (SLEEP). Participants in STUDY #2 slept in the inpatient unit in the RESTED night and were scanned the following morning while awake (A-RW). The night of SD was followed the next morning by a scanning session where they were asked to stay awake in the magnet (A-SD).
Procedure
Study Design
STUDY #1 (Figure 1A):
Participants were admitted to the Clinical Center for a one-night inpatient stay three different times (total of 3 nights). The first night (referred to as ACCLIMATIZATION), was to acclimatize participants to sleeping in the inpatient unit and to characterize their sleep (i.e., duration of sleep stages, number of awakenings during sleep, total hours of sleep per night). Participants were then randomized into two groups so that for one of the groups the “AWAKE” scan was done first following a night of rested sleep (second night) and the “SLEEP” scan was done last following a night of SD (third night), which was done to facilitate their falling asleep inside the magnet. For the other half of the participants, the order was reversed and the “SLEEP” scan was done first after SD (second night) and the “AWAKE” scan was done last after a night of rested sleep (third night). For the SD night, participants were encouraged to read, watch television or a movie to help them stay awake and a nurse stayed by their side to ensure they did not fall asleep. The MRI scans for the AWAKE or SLEEP conditions were carried between 8AM to 12PM (average scan start time = 09:43AM). For the AWAKE scan, we asked participants to stay awake during the scan and for the SLEEP scan, we asked participants to try to fall asleep in the scanner.
Sleep Measures
During the nights that the participants spent at the Clinical Center they wore the Sleep Profiler (SP; Advanced Brain Monitoring, Inc., Carlsbad, CA), an FDA approved ambulatory EEG monitor used to detect sleep stages (Popovic et al., 2014). The SP uses three forehead electrodes to record EEG signals. For the first three participants studied, we validated the correspondence between visually obtained sleep scoring with the raw data from the sleep profiler output by looking at the band-pass signal in the low frequency delta (<4Hz), spindle frequency (12–16Hz), and high frequency gamma (>18Hz) bands and their amplitude changes. Recordings were initiated prior to 10PM and continued until 7AM. Total sleep duration, sleep disturbances (number of awakenings more 30 seconds), and duration of sleep in Non-rapid (NREM; Stage-1, Stage-2, and Stage-3) and rapid eye movement (REM) were measured. Most participants slept well during both nights (Supplementary Material 1). Measures of sleep quality were calculated for the two nights of sleep in the Clinical Center by averaging the number of awakenings per hour lasting more than 30s (Awakenings >30s was also correlated with awakenings >90s, Supplementary Figure 1). We assessed the Pearson’s r between these sleep quality measures and the change of ADC (∆ADC; SLEEP-AWAKE).
Simultaneous EEG-MRI recordings to assess sleep within the MRI were discontinued after one of the participants suffered a mild scalp burn from the electrodes (presumably due to excessive RF power deposition after 180 degree RF pulse, flipping within the transverse plane of the diffusion-weighted imaging) and notification to the IRB during the development phase of the protocol. Instead, we corroborate whether participants were asleep or awake by monitoring their response to a continuous task and by measuring eye closures, eye blinks and eye movements while in the MRI scanner with an infrared camera (procedures described below).
Additionally, after each night of sleep in the Clinical Center (i.e., ACCLIMATIZATION, and the night preceding the AWAKE scan), and at the end of the SLEEP scan, participants were asked to rate: i) “How deep did you sleep?” and ii) “How rested do you feel?” on a scale from 1–10, where 1 indicated “not at all” and 10 “extremely”.
STUDY #2 (Figure 1B):
Participants were scanned twice - once while awake following a night of rested sleep (awake with rested wakefulness or A-RW) and once while awake but following a night of SD (A-SD). For this purpose, participants spent two nights in the Clinical Center; for the night A-RW participants were asked to sleep normally, and for the A-SD night they were asked to stay awake until completion of MRI. The setting and sleep recordings were similar to STUDY #1. The following morning, they underwent MRI scans, taking part between 8AM to 11AM (average scan start time = 08:22AM), and were asked to stay awake during MRI procedures for both scanning sessions. This allowed us to control for the effects of SD as separate from the effects of sleep itself.
MRI acquisition
Scanning was performed on a Siemens 3T Prisma scanner (Siemens Medical Solutions USA, Inc., Malvern, PA) equipped with a 32-channel head coil. Scanner room temperature was controlled at 21˚C. For Study #1, MRI started with a resting-state functional magnetic resonance (rfMRI) time-series with a multiplexed echo-planar imaging sequence (Moeller et al., 2010) using multiband factor = 8, anterior-posterior phase encoding, TR/TE = 720/37ms, FA = 52deg, matrix = 104, 72 slices with 2 mm isotropic voxels and 820 time points leading to 590.4 seconds of data collection while participants were relaxed with their eyes open. A fixation cross was presented on a dark background under dimmed room lighting using a liquid-crystal display screen (BOLDscreen 32, Cambridge Research Systems; UK). This scan was followed by six diffusion-weighted imaging (DWI) time series with diffusion weighting stepping (b-values= 0, 50, 300, and 1000 s/mm2) along each of the 3 orthogonal axes, TR/TE=5000/52ms, 3mm isotropic voxels field-of-view (FOV) =240 × 240 mm, and 50 axial slices and integrated parallel acquisition technique (iPAT) with generalized auto-calibrating partially parallel acquisition (GRAPPA=2) (Griswold et al., 2002). Each DWI time-series consisted of 192 volumes and lasted 16 minutes. A T2w scan (1.1×1.1×1.7mm voxel size, TR/TE=8000/72ms, phase encoding direction: AP, 94 slices; fat saturation) was collected for DWI post-processing. The three-dimensional, magnetization-prepared rapid gradient-echo (Mugler and Brookeman, 1990) (3D MP-RAGE; TR/TE = 2400/2.24 ms, FA = 8 deg, TI=1060ms), and Sampling Perfection with Application optimized Contrasts by using different flip angle Evolutions (SPACE, Siemens; TR/TE = 3200/564 ms) pulse sequences were used to acquire high-resolution anatomical brain images with 0.8 mm isotropic voxels field-of-view (FOV) = 240 × 256 mm, matrix = 300 × 320, and 208 sagittal slices. For STUDY #2, we collected 10-minutes eyes-open resting state time series followed by one sixteen-minute DWI time series with the same parameters as in Study #1.
For both studies, and before the imaging sessions, polyvinyl pyrrolidine (PVP) phantom images with the same diffusion imaging parameters as the participant scanning were collected each day to control for potential artifacts due to magnetic field differences between scan days (Pierpaoli et al., 2009; Pullens et al., 2017). The PVP phantom comprises a stable aqueous polymer solution with a concentration of at least 30% (by weight). In our experiment, room temperature was 21˚C and there were no significant differences between sessions in the PVP phantom analysis for any of the ADC measures (see Supplementary Material 2).
Arousal and physiological measures during MRI scans
STUDY #1:
During the DWI scans, a white fixation cross was presented in the middle of the screen that occasionally changed color (to a dull green) for 10 seconds (jittered 45–70s, 16 values during each scan, a total of 96 triggers during whole diffusion scan). For the AWAKE scan, participants were asked to press a button with their right index finger whenever the fixation cross changed color and had 10 seconds to respond before it changed back to white. For the SLEEP scan, participants were encouraged to fall asleep. They were instructed to press the response button if they could not fall asleep and noticed the color change. Button press responses were used to monitor arousal during MRI. Additionally, a video of the right eye was recorded throughout the scan with an infrared mono-color single MRI-compatible camera (model: 12M-i, MRC Systems GmbH, Heidelberg, Germany) mounted on the head coil at a sampling rate of 30 frames/s and a resolution of 640×480 pixels. This video was used to compute eye-lid closure and eye blink measures and when they closed their eyes to monitor eye motions under the eye-lid related to non-rapid- and rapid-eye-movement periods. For the SLEEP condition we rated the eye motions under the eye-lid every minute as slow, fast, still, and eye-open states for every subject. Respiration, electrocardiography (ECG), and photoplethysmography (PPG) signals were recorded with BioPac MRI compatible sensors using an M150 amplifier (BIOPAC Systems, Inc., CA, USA) at a rate of 5kHz (https://www.biopac.com/research/).
STUDY #2:
Participants were asked to stay awake for both the A-RW and the A-SD scans. We used the same procedures as for STUDY #1 to monitor whether they were awake or asleep (detection of changes in cross and video recording of eye closures, blinks and movements).
Voxel-Based Morphometry and Structural Change Analyses
The Computational Analysis Toolbox (CAT12) (Gaser and Dahnke, 2016) was used to assess potential sleep-related changes in brain structure. We quantified whole brain total intracranial volume (TIV), grey (GM) and white matter (WM) volumes, and cerebrospinal fluid (CSF) volume. Voxel-based morphometry (VBM) analysis of T1 images -collected in the SLEEP and AWAKE sessions- was carried in CAT12 included; (i) spatial registration to a reference MNI template brain, (ii) tissue classification (segmentation) into grey and white matter and CSF, (iii) bias correction of intensity non-uniformities, and (iv) segmentations modulated by scaling with the amount of volume changes due to spatial registration. In addition, high-resolution T1 and T2 images were processed with Freesurfer software (version 6, http://surfer.nmr.mgh.harvard.edu/) to generate full segmentation algorithm including subject-specific white matter, cortical and subcortical gray matter brain masks (Glasser et al., 2013), sulci and gyri (Desikan et al., 2006) and of subcortical structures (Fischl et al., 2002) .
Image processing
Tolerably Obsessive Registration and Tensor Optimization Indolent Software Ensemble (TORTOISE) software, version 3 (Pierpaoli et al., 2010) was used to process DWI images. The T2w image with the highest quality (i.e., based on visual inspection) among the two scan sessions was selected and AC-PC aligned in Medical Image Processing, Analysis, and Visualization (MIPAV) software (https://mipav.cit.nih.gov/), and used as a structural image for alignment and distortion correction in TORTOISE. For each DWI scan, we corrected for rigid body motion, eddy current distortions, and B0 distortions, using the T2-structural image for alignment in TORTOISE. Finally, high-resolution T1 images processed in freesurfer software and the DWI images were aligned (T1 to DWI) in Statistical Parametric Mapping (SPM12; Welcome Trust Centre for Neuroimaging) with a 12-parameter affine transformation for each scan session.
Calculation of ADC values
Conventional ADC was calculated according to the Stejskal–Tanner (Stejskal and Tanner, 1965) equation:
where b is the b-value, S0 is signal intensity obtained in the region of interest for b=0, and S is the signal intensity obtained with larger b-values. For two b-values, this corresponds to a slope of a line fit between the two b-values. We estimated, two ADCs measures for each voxel by calculating averaged diffusion values of the x, y and z for isotropic diffusion (i.e., “trace”) using the linear fitting for the natural logarithmic conversion of the diffusion values obtained for b for i) fast-ADC, obtained with the b of 0, 50 and 300 s/mm2, and ii) slow-ADC, obtained with the b of 300 and 1000 s/mm2. Note that the higher b-values in our study are lower than most of the b-values reported in the literature that measured ‘very slow’ ADC values, but nonetheless can be used to approximate non-Gaussian component of (e.g., >1700 s/mm2) (Mulkern et al., 1999; Sehy et al., 2002). In this paper, ADC values will be reported in units of 10−3mm2/s. Freesurfer aparc+aseg.mgz segmentations were used to extract mean ADC values within each segment for each participant and session. For voxel-wise analyses, spatial normalization to the stereotactic space of the Montreal Neurological Institute (MNI) and spatial smoothing (4mm) were carried in SPM12.
Statistical analysis
All the statistical analyses were conducted in R version 3.4.3 (RCoreTeam, 2013) except the voxel-wise ADC analysis, which was conducted in SPM12. For that analysis, a two-step cluster-wise threshold correction approach using voxel-threshold of p=0.005, and a cluster size of minimum 100 voxels were used for the AWAKE-SLEEP and SLEEP-AWAKE t-contrasts. Family-wise error corrected clusters were then reported (pFWE<0.05; see Table 2). We also used individual ADC values from individual brain segments extracted from freesurfer software, and correlated these values with the individual sleep measure ‘awakenings’. For partial correlation we used “ppcor” package in R (Kim, 2015).
Table 2.
Fast and slow ADC results from the SPM analysis for the differences between AWAKE versus SLEEP, and SLEEP versus AWAKE contrasts. L=left, R=right
| Fast-ADC | X | Y | Z | PFWE-corr | PFDR-corr | KE | |
|---|---|---|---|---|---|---|---|
| L. parahippocampus | AWAKE-SLEEP | −12 | −32 | −10 | 0.0001 | 0.0001 | 442 |
| R. globus palidus | AWAKE-SLEEP | 18 | 0 | −6 | 0.001 | 0.0001 | 209 |
| R. insula | AWAKE-SLEEP | 42 | 0 | 14 | 0.0001 | 0.00001 | 256 |
| L. thalamus | AWAKE-SLEEP | −20 | −28 | 2 | 0.0001 | 0.0001 | 417 |
|
L. cerebellum (Lobules VII / VIII) |
AWAKE-SLEEP | −36 | −50 | −46 | 0.0001 | 0.0001 | 375 |
| L. temporal pole | SLEEP-AWAKE | −22 | 10 | −32 | 0.0001 | 0.0001 | 391 |
| Slow-ADC | X | Y | Z | PFWE-corr | PFDR-corr | KE | |
| L. parahippocampus | AWAKE-SLEEP | −22 | −50 | 4 | 0.0001 | 0.0001 | 254 |
|
L. cerebellum (Lobule VI/Cyrus I) |
SLEEP-AWAKE | −22 | −64 | −32 | 0.0001 | 0.0001 | 615 |
| L. temporal pole | SLEEP-AWAKE | −22 | 10 | −32 | 0.0001 | 0.0001 | 438 |
|
R. cerebellum (Lobules VI/VII) |
SLEEP-AWAKE | 44 | −52 | −40 | 0.001 | 0.0001 | 233 |
For VBM based whole brain analysis, we used the total intracranial volume as a covariate and CSF, grey and white matter volumes as dependent variables, and AWAKE and SLEEP as independent variables in Analysis of Covariance (ANCOVA). Pearson correlation across subjects was used to assess the pair-wise association between the changes in brain CSF volume, number of awakenings during sleep, and the ADC measures, and p-values < 0.05 were reported as significant. ADC and VBM analyses in STUDY#2 were conducted with the same statistical methodologies as for STUDY#1.
Results
Behavioral Results
Sleep Measures:
The scores on the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989), the sleep assessment questionnaire, and the clinic sleep measure (i.e., Sleep Profiler) for STUDY #1 and #2 are reported in Table 1. Inspection of the Sleep Profiler data and the reports for the nights preceding the SLEEP scan indicated that participants stayed awake as instructed (i.e., the total amount of time that any subject might have intermittently fallen asleep during a night was less than a few minutes, generally classified as Stage-1 sleep). Self-reported measures of sleep during the ACCLIMATIZATION night and the night preceding the AWAKE scan were in good agreement with the Sleep Profiler data.
Table 1.
Recording of sleep measures for Study 1 (n=28) and Study 2 (n=19): Pittsburgh Sleep Quality Index (PSQI) item for total sleep duration per night in daily life; Self-reports for the questions: i) “How deep did you sleep?” and, ii) “How rested do you feel?” after the sleeping periods in the Clinical Center (ACCLIMATIZATION, AWAKE and A-RW mornings) from a scale 1–10 and Sleep Profiler (SP) total sleep duration. A-SD has no sleep measures since participants stayed awake during the night and the scanning session. SLEEP* refers to the scores obtained after participants slept in the scanner (SLEEP scan). Self-report of “How rested” was lower after the SLEEP than the AWAKE scan session (p<.01). Subjective “sleep depth” did not differ between the nights of the ACCLIMATIZATION and prior to the AWAKE scan and that of the SLEEP scan condition, although the variability during the SLEEP scan was higher compared to the AWAKE conditions. Values correspond to means and standard deviations.
| PSQI total sleep time per night (hours) |
How deep did you sleep? 1–10 |
How rested do you feel? 1–10 |
SP total Sleep duration (hours) |
||
|---|---|---|---|---|---|
| STUDY #1 | 7.32 (0.98) | ACCLIMATIZATION | 7.16 (1.76) | 7.91 (1.52) | 6.97 (2.05) |
| AWAKE | 7.3 (1.25) | 7.91 (1.62) | 7.42 (1.59) | ||
| SLEEP* | 7.26 (2.36) | 5.93 (2.31)* | NA | ||
| STUDY #2 | 7.43 (0.92) | A-RW | 7.21 (1.21) | 7.89 (1.49) | 7.12 (1.87) |
| A-SD | NA | NA | NA |
Response times in the scanner:
For STUDY #1, the responses to color changes of the cross during the DWI scans revealed that one subject in the AWAKE scan missed a very high number of response-time points (MISSES 40 out of 96) and one subject in the SLEEP scan was not asleep and pressed the response button many times (RESPONSES 50 out of 96). We also confirmed participants’ behavior via eye videos recorded during the experiment. Thus, these subjects were excluded from further analyses (final N=28). For STUDY #2, the responses to color changes of the cross revealed that one subject in the A-RW scan missed a very high number of response-time points (14 out of 16) (final N=19). Eye video confirmed this behavior, thus this subject was excluded from further analyses. (See Supplementary Material 3, and Supplementary Figures 2 and 3 for the representation of the reaction times and accuracy across scan for each subject as well as the ratings of eye movements recorded when subjects closed their eyes during the SLEEP scan, see Supplementary Material 4).
STUDY #1
Voxel-Based Morphometry (VBM):
Using data from 24 participants (4 additional subjects were excluded due to technical and motion related issues during the T-1 structural scan at least in one of the sessions), we found a significantly larger CSF volume in SLEEP; average value = 316.21(11.21) cm3 compared to AWAKE; average value = 312.11(10.66) cm3, t(23) = 2.62, p=0.015. There were no differences in GM and WM volumes between SLEEP and AWAKE conditions (Figure 2).
Figure 2.

VBM based CSF, GM, and WM volume changes between SLEEP – AWAKE. CSF volume was larger in SLEEP compared to AWAKE (paired t-test (23) = 2.62, p=0.015). GM and WM volumes did not differ between the two states.
Whole brain ADC differences
We computed overall brain ADC measures calculated in native space extracted through freesurfer segmentation algorithms and found no differences between AWAKE and SLEEP in fADC;[ AWAKE; mean = 1.0359 (0.0433), SLEEP; mean = 1.0318 (0.0401)], or in sADC; [AWAKE; mean=0.7910 (0.0172), SLEEP; sADC=0.7902 (0.0151)].
Regional ADC SPM analysis
Though there were no significant differences between SLEEP vs AWAKE for the whole brain ADC measures there were significant regional differences with increases in some brain regions with SLEEP (as hypothesized) and decreases in others as found by SPM statistical contrasts. Further, the SLEEP vs AWAKE ADC results differed for the fast- and slow-ADC components (see below).
Fast-ADC (fADC) was lower in striatal regions, in insular regions, parahippocampus, thalamus and left ventral sections of the cerebellum, lobules VII/VIII (Table 2), and higher in the left temporal pole (Figure 3A) for SLEEP than for the AWAKE scans.
Figure 3.
A) Fast ADC results; Increases in fADC for AWAKE > SLEEP were observed in left ventral cerebellar sections, insular regions, subcortical regions (thalamus and striatum) and parahippocampus increases in SLEEP > AWAKE in left anterior temporal region. B) Slow ADC results; Increases in sADC for SLEEP > AWAKE were observed in left anterior temporal and dorsal cerebellar regions and increases for AWAKE > SLEEP were observed in the parahippocampus.
Slow-ADC (sADC) was higher in dorsal cerebellar (left lobules VI/Cyrus I; right lobules VI/VII) and left temporal pole, and was lower in left parahippocampus (Figure 3B, Table 2) for SLEEP than for the AWAKE scans. The only regions that showed similar findings for sADC and fADC were the left parahippocampus (decreased with SLEEP) and the left temporal pole (increased with SLEEP).
Cerebellar ADC changes and their correlations with sleep measures and brain CSF changes
We computed changes in cerebellar ADC (∆ADC; SLEEP-AWAKE), and their correlations with sleep quality (awakenings), Stage-1 and Stage-3 sleep durations and total brain CSF changes.
ADC changes were first calculated for the whole cerebellar gray matter freesurfer segments extracted from each individual’s brain native space and ADC values were extracted before the images were transformed to MNI space. As a second step, we extracted delta-ADCs in the cerebellar clusters found in the previous group-level SPM analysis to contrast between SLEEP and AWAKE conditions.
Cerebellar grey matter ADC changes
The comparisons for fADC and sADC between SLEEP and AWAKE in the freesurfer grey matter segmentation showed a trend for an increase in sADC with SLEEP (p=0.065 level) and no differences for fADC (see Supplementary Figures 4 and 5 for ADC changes in cerebellum and subcortical regions).
Cerebellar grey matter ∆ADC correlations
We next examined the relationship between cerebellar gray matter freesurfer segment ∆ADCs with sleep measures. We found a significant negative correlation between ∆ADC and awakenings both for slow- and fast-ADC. Specifically, participants with more awakenings showed smaller changes in ADC in cerebellum grey matter (fADC: r=−0.569, p<0.01; sADC: r= −0.41, p< 0.05; Figure 4, A and B), and this effect remained significant after controlling for age with partial correlation analysis (fADC: p<0.001 and sADC: p<0.01). We also observed a negative correlation between Stage-1 sleep duration and fADC in cerebellar grey matter (r=−0.396, p<0.05; Figure 4C), but the correlation with sADC was not significant (Figure 4D). Cerebellar grey matter ∆ADCs did not correlate with total Stage-3 sleep or with delta CSF. (Also note that the correlation between awakenings and SLEEP-AWAKE change in total brain CSF was also not significant, p>0.14.)
Figure 4.

Measures of sleep quality were calculated for the two nights of sleep in the inpatient unit of the Clinical Center by averaging Stage-1 sleep durations and the number of awakenings per hour lasting more than 30s. We assessed the correlation between these sleep quality measures and the change of ADC (∆ADC; SLEEP-AWAKE) in the freesurfer cerebellum. Participants with more arousals showed less ADC change with correlation values corresponding for fADC to r=−0.569 (p=.0016) (A), and for sADC to r= −0.41 (p= 0.0345) (B). A similar negative correlation was observed between Stage-1 sleep duration and fADC in cerebellum (r=−0.396, p=0.0368) (C), but the correlation with sADC was not significant (D). ADC units are in 10−3mm2/s.
Cerebellar SPM clusters’ ∆ADC correlations
We then computed the correlations between ∆ADCs in the cerebellar clusters that were significant in the SPM statistical analysis with the sleep measures and CSF changes. fADC cluster (in the left cerebellum) ∆ADC was significantly correlated with awakenings (r=0.491, p=0.008) (Supplementary Figure 6), but the correlation with sADC clusters (mean or each of the left and right cerebellum clusters) was not significant (p>0.2) Neither Stage-1 nor Stage-3 sleep durations correlated with cerebellar cluster ∆ADCs.
The correlation between sADC in the cerebellar clusters and CSF changes were significant (left; r=0.413, p<0.05; right, 0.404, p=0.0503; average of right and left cerebellar clusters, r=0.431, p=0.0354 (Figure 5), such that the greater the increases in brain CSF the greater the increases in sADC in these regions during SLEEP compared to AWAKE. The correlation between the cerebellar fADC cluster and CSF was not significant.
Figure 5.

The average sADC cluster (averaged over right and left sADC SPM cerebellum clusters) showing increases in sADC for SLEEP vs AWAKE was selected to examine the relationship with changes in total brain CSF. This analysis showed a significant association between the two measures; r=0.431, p=0.0354, such that the greater the increases in brain CSF the greater the increases in sADC in cerebellum during SLEEP as compared to AWAKE. ADC units are in 10−3mm2/s, CSF volume in ml.
∆ADC correlations in other SPM clusters
The correlations for the other clusters that showed significant differences in SPM between SLEEP vs AWAKE with the sleep measures and CSF is shown in Supplementary Material 5 and Supplementary Figure 7. After Bonferroni correction for multiple comparisons (i.e., fADC clusters, 0.05/6=0.0084, and for sADC clusters 0.05/5=0.01, applied for each sleep measure separately), awakenings were positively correlated with the right globus palidus cluster ∆fADC (r=0.489, p=0.008). In addition, both ∆fADC and ∆sADC in the left parahippocampus cluster were positively correlated with Stage-3 sleep (r=0.591, p=0.001, and r=0.673, p<0.001, respectively).
STUDY #2
There were no significant differences between A-RW and A-SD for any of the ADC components (data not shown) indicating that one-night of SD by itself did not affect ADC. We also compared CSF, WM, and GM volumes, and none of these measures differed between A-RW and A-SD sessions (p>0.2). In addition, to assess if the significant clusters found in STUDY #1 showed an effect of SD, we performed a 2-way ANOVA using State (A-SD/A-RW) and Cluster for sADC and for fADC, and showed no significant effects of State or of an interaction between State and Cluster neither for sADC nor for fADC (Fs<1). Nevertheless, separate t-test analyses revealed that the only region for which there was a trend of a decrease with A-SD compared to A-RW was for fADC cluster in the left cerebellum (p=0.032, uncorrected), however it did not survive correction for multiple comparisons (see Supplementary Material 6).
Discussion
This study explored whether sleep could induce ADC and CSF volume changes in the brain compared to wakefulness, which would provide preliminary support for the existence of a glymphatic system in the human brain. In addition, our study investigated how sleep disturbances (awakenings) would influence ADC changes. We found that sleep led to an increase in whole brain CSF volume, to an increase in sADC in the dorsal cerebellum as well as to an increase in both fast and slow ADC in left temporal pole, and that the sleep-associated increases in cerebellar sADC were associated with the increases in CSF volumes. In contrast and opposite to our hypothesis, we also observed significant decreases in fADC in right globus pallidus and insula, and in left thalamus, parahippocampus and ventral cerebellum in SLEEP compared to AWAKE state. Awakenings and total Stage-1 sleep were inversely correlated with changes in whole cerebellar grey matter sADC and fADC. In contrast, awakenings correlated positively with changes in fADC in left ventral cerebellum and right globus palidus SPM clusters. Finally, Stage-3 sleep duration was positively correlated with left parahippocampal fADC changes. Overall our findings indicated the existence of multiple, region-specific cortical and sub-cortical sleep-related diffusion changes.
Based on findings in the rodent brain of a 40% increase in ISF space that facilitates clearance of waste by the glymphatic system during sleep, we predicted that the ADC would increase during sleep compared to the awake state (Xie et al., 2013). Our hypothesis was also based on studies reporting that increases and decreases in the brain ISF volume fraction as measured by real-time iontophoretic methods (Nicholson and Sykova, 1998; Sykova, 2004) reflect similar changes in the ADC as measured with MRI. For example, during brain development the ISF volume fraction in cortex decrease from 0.43, at the post-natal age of 10 days, to 0.23 at 22 days (Vorisek and Sykova, 1997); and these changes are paralleled by decreases in the ADC (Sizonenko et al., 2007). Similarly, during acute ischemia the ISF volume fraction decreases abruptly by 50% or more at the time of the anoxic depolarization and is coincident with an ADC decrease (Davis et al., 1994; de Crespigny et al., 1999; Decanniere et al., 1995; Harris et al., 2000; Sevick et al., 1992). Note that the ADC values reported in these studies fall under 1×10−3mm2/s, and could be approximated to the slow-ADC we report in this manuscript.
We reasoned therefore, that a ~40% expansion of the ISF volume (Xie et al., 2013) accompanied by an increase in CSF into the brain would promote free water diffusivity and thereby an increase in the ADC (Sotak, 2004) and that increases in CSF would correlate with changes in the ADC. Of these hypotheses, we were able to corroborate the increase in sADC in lobules VI, VII and Cyrus I of the cerebellum (but also observed in temporal pole) and the increase in CSF volume during sleep, which we found correlated with one another. However, we also observed decrease in ADC with sleep, predominantly for fADC in parahippocampus, subcortical regions, insula and ventral cerebellar lobules VII and VIII. Recently, Gakuba et. al. (2018) reported that transport of contrast agents was decreased during anesthesia (presumably emulating sleep) relative to wake periods, which would be consistent with our findings of decreases in fADC in subcortical regions and insula and of sADC and fADC in left parahippocampus with SLEEP.
Although the differential contribution of changes in interstitial versus intracellular water volumes for the changes in sADC vs that for fADC remains unclear, the fADC is reported to be most sensitive to changes in CBF or microcirculation (MacFall et al., 1991; Silva et al., 1997), which would be in agreement with our results showing that the fADC tended to be higher during AWAKE than SLEEP conditions. Alternatively, the fact that fADC tended to be higher during AWAKE than SLEEP conditions could also be reflective of increases in cell volume associated with increased cell activity (Abe et al., 2017b).
Overall the sADC appeared to be the most sensitive for capturing proposed ADC increases with sleep. While the dorsal cerebellum and temporal pole regions showed increases in ADC mostly for sADC, subcortical regions, ventral cerebellum, insula, and parahippocampus were characterized by decreases in fADC during sleep. On the basis of previous findings showing enhanced cerebellar glymphatic CSF transport -compared to the other brain regions observed in rodent studies (Iliff et al., 2013)- and containing dense aquaporin 4 water channels (Hoddevik et al., 2017), cerebellum was an a priori region where we expected to observe the largest ADC; only the sADC increased in SLEEP (dorsal cerebellum) but the fADC decreased (ventral cerebellum). Thus sleep induced sADC increases might reflect overall more efficient CSF transport and glymphatic clearance in this region (Ratner et al., 2017). Indeed, this particular CSF transport pattern could explain why the cerebellum is one of the last brain regions to show beta-amyloid accumulation with the progression of Alzheimer’s disease (Calderon-Garcidueñas and Duyckaerts, 2018).
The difference between our results with ADC in the human brain during sleep and those obtained in the rodent brain during slow-wave sleep or anesthesia with ketamine/xylazine could reflect differences between species and techniques targeting ISF. Thus, the large increase (40%) in ISF documented in the cortex of the rat was obtained using the real-time iontophoresis method with tetramethylammonium ions (TMA) and ion-selective microelectrodes whereby the interstitial volume fraction and tortuosity can be calculated (Nicholson and Sykova, 1998; Xie et al., 2013). The ADC measured by diffusion MRI in this study, might not be sufficiently sensitive to pick up potentially small and transient changes in the ISF volume.
Alternatively, differences between the human and rodent brain sleep data are also likely to reflect the differences between the progressive and disrupted patterns of sleep that we observed in our participants, which might have been better controlled in mice as reported by Xie et. al. (2013). Indeed, the significant negative correlation that we observed between the number of awakenings and the changes in fADC and sADC in cerebellum suggest that the dynamics of changes in ISF are likely to be sensitive to sleep quality. We measured ADC only for the first 90 minutes upon sleep initiation and it might be that brain regions might require longer time periods to modify ISF, depending on their content of AQP4 or their proximity to larger CSF reservoirs, but further studies are required to disambiguate these potential confounds.
We showed that the number of arousals per hour for a given subject had a significant contribution to the ADC changes in whole cerebellum. It is possible that subjects in our study characterized by uninterrupted sleep, who had the largest sleep-related ADC changes, might have overall more benefit from sleep and also exhibit increased waste clearance from the brain compared to those who have frequent sleep interruptions. Recently, it was shown that arousal during the night related to sleep-disordered breathing was associated with beta amyloid accumulation in brain (Alexander et al., 2001; Cedernaes et al., 2017; Osorio et al., 2014; Sharma et al., 2018) consistent with impaired clearance. These finding highlight the importance of monitoring individual differences in sleep quality when assessing glymphatic clearance mechanisms in the human brain.
We did not observe any diffusion or structural changes between sleep-deprivation (A-SD) and rested-wakefulness (A-RW) in STUDY #2 in the whole brain SPM analysis. This is distinct from findings recently reported by Elvsåshagen et al. who showed effects of SD on mean diffusivity and cortical thickness (Elvsashagen et al., 2015; Elvsashagen et al., 2017). This discrepancy might reflect differences in experimental methodologies and participant composition. Specifically, Elvsåshagen et al. studied only male subjects who were much younger (mean age=21 y old) than our cohort (age= 41 y old), whereas we also included females. Their comparisons for sleep and SD were done on two different groups of subjects (sample from Elvsashagen et al., 2015 is a subset of the Elvsashagen et al., 2017 study, reporting only the sleep restricted group) whereas we compared both conditions in the same subjects. Elvsashagen et al. did not control for sleep behaviors prior to the fMRI scan, which might have resulted in interindividual variance from distinct sleep/arousal schedules prior to the study. Both studies used permutation based statistics, and the 2015 study reported significant clusters with 1 or 2 voxels (see Table 1 in Elvsashagen et al., 2015) whereas we restricted significance to clusters with >100 voxels, and the main focus in their study was diffusion in the white matter tracks, whereas we focused on grey matter. Furthermore, in the 2017 study, there was no interaction between group (sleep x deprivation) and time, suggesting as discussed by the authors that other mechanisms rather than sleep deprivation accounted for cortical thinning. Nevertheless, exploring each cluster found in STUDY #1 to differ between SLEEP vs AWAKE we showed that there was a non-significant trend for a decrease in fADC in the left cerebellum cluster. While this finding is consistent with a potential, but weak effect of sleep deprivation on cerebellar ADC, SLEEP was associated mostly with increases in cerebellar sADC in our study, suggesting that the differences we observed between SLEEP and AWAKE are not due to the effects of sleep deprivation, but rather due to sleep itself.
In addition, our findings differ from those by Bernardi et al. who reported mean diffusivity changes under intense overnight cognitive training (~12h) compared to pre-training rested non sleep deprived sessions (Bernardi et al., 2016), indicating potential influence of SD on diffusion measures. In contrast in our study we did not subject participants to a cognitively demanding task but instead encouraged them to do activities they enjoyed and distracted them. Thus it is probable that intense and long lasting cognitive performance might have increased cellular size and affected diffusion in the participants studied by Bernardi et al. Moreover, intense overnight cognitive training might have engaged neurotransmitters involved in arousal, learning, attention, and motivation (i.e., dopamine, norepinephrine, and adenosine etc.) that in turn might have influenced diffusivity. Intriguingly, Bernardi et al.’s findings of decreases in CSF (assessed by decreases in ventricle volumes) during SD that increased after sleep are consistent with the CSF volumetric changes we report during sleep.
We observed a negative association between the sleep-induced increases in the whole grey matter cerebellum fADC and sADC with awakenings, which we interpret to reflect the interference of glymphatic function with multiple awakenings. A-priori we had predicted that the increases in ADC with sleep would be strongest in cerebellum, which we corroborated. A more efficient glymphatic function in cerebellum has been postulated to contribute to its resilience to beta amyloid accumulation and might explain why it is one of the last brain regions to show beta amyloid plaques in advanced Alzheimer’s disease (Calderon-Garciduenas and Duyckaerts, 2017). However, though an association was also observed for fADC measure extracted from the cerebellar SPM cluster, the association for sADC in cerebellar SPM clusters and awakenings was not significant. These differences between fADC and sADC in cerebellum could reflect the existence of distinct sleep-related physiological dynamics. On the other hand, the difference in the cerebellar sADC findings between the SPM extracted clusters and the larger gray matter cerebellar space might reflect the fact that the calculations in the larger cerebellar space average the inter-subject variability in the precise location within the cerebellum where changes occur, which might be captured only when focusing on a more restricted region. However, these differences could also reflect regional specificity, which could also explain why we observed a significant difference in sADC between SLEEP and AWAKE for the SPM clusters, while the difference in the cerebellar gray matter showed only a trend for significance (p=0.065). Similarly, the positive correlation between the sleep-induced increases in sADC in the cerebellar SPM clusters and the whole brain CSF changes -an effect which was missing for the cerebellar grey matter- also supports the possibility of the existence of distinct cerebellar partitioning relating to sleep-related diffusion processes.
We also observed significant increases in sADC (and in fADC) with SLEEP in the left temporal pole, which differs from the cerebellum in that it is one of the first brain regions to show beta amyloid accumulation in Alzheimer’s disease (Arnold et al., 1991), suggesting that other factors contribute to such differences. For example, the cerebellum also differs with respect to the synthesis of beta amyloid degradation enzyme (i.e., IDE), which might protect against beta amyloid accumulation during aging (Caccamo et al., 2005). Moreover, the cerebellum also showed increases in fADC in ventral nuclei that might reflect differential activity of cerebellar regions in sleep (Canto et al., 2017) including slow wave sleep (Dang-Vu et al., 2008). Our findings also emphasized individual variations and state dependency of cerebellar sub-regions and their relationship with awakenings. While at the group level, sADC showed SLEEP related increases in cerebellar clusters, these clusters behaved independently of awakenings. Thus, sleep might have influenced ADCs predominantly in a subset of cerebellar regions, whereas awakenings targeted the whole cerebellar grey matter ADC, or the ADCs of other cerebellar sub-regions, being strongest for fADC.
The left temporal pole and the left parahippocampus showed changes for sADC and fADC albeit in the opposite direction (i.e., increases and decreases for SLEEP respectively). We had not a priori expected the temporal pole to be more sensitive to the effects of sleep on ADC. However two recent studies reporting an association between cortical thinning in temporal pole and hours of sleep in the elderly (Spira et al., 2016) and with obstructive sleep apnea (OSA) (Macey et al., 2018) suggest that this brain region might be particularly susceptible to the effects of sleep. The decreases in ADC in parahippocampus were also not expected and might reflect its involvement in verbal encoding, which is sensitive to SD (Jonelis et al., 2012). Interestingly, delta-ADCs in these two regions also exhibited correlations with Stage-3 sleep (again in opposite directions), which might reflect the modulatory role of slow wave sleep on sleep related diffusion and/or sleep related neural activity in these regions during slow wave sleep. Finally, as observed for the left cerebellar region, the globus pallidum delta-fADC correlated with awakenings, suggesting that sleep might also influence diffusivity in deep brain regions of the brain.
Our findings are preliminary and, while not conclusive, are consistent with the presence of region-specific glymphatic system in the human brain that as in the rodent brain operates predominantly during sleep. In this respect it adds to recent clinical studies that have provided indirect evidence of its presence; including findings of 1) increases in amyloid beta in brain and in CSF after one night of SD (Ooms et al., 2014; Shokri-Kojori et al., 2018), 2) increases in CSF Aβ following disruption of slow-wave sleep (Ju et al., 2017), 3) association between poor sleep quality and Aβ accumulation in brain (Spira et al., 2013), 4) peri-vascular and parenchymal CSF transport of paramagnetic contrast agents into the brain (Eide and Ringstad, 2018; Ringstad et al., 2017) similar to those observed in rodents (Iliff et al., 2013; Ringstad et al., 2017), and 5) presence of lymphatic vessels in the meninges (Absinta et al., 2017) which, in rodents serve as a drainage for the glymphatic system (Aspelund et al., 2015). Future studies will shed more light on the potential relationship between sleep-driven diffusion, its strength and timing during sleep, and its role in amyloid clearance in the human brain.
Limitations of the study
Individual variability:
Our study indicated that sleep patterns were highly variable across participants. Future studies that preselect participants that are good sleepers might increase the ability to detect ADC changes associated with sleep.
ADC Measures:
To assess ADC we used a novel approach where we computed three diffusion directions (x, y, and z) with b-values of 50, 300, and 1000 s/mm2, which allowed us to calculate Brownian motion, and to model a slow and a fast ADC, and allowed us to dissociate temporal derivatives of diffusion. However, the significance of these distinct temporal ADC measures is unclear. Also, the ADC measure themselves might have lacked sensitivity for detecting 40% changes in ISF considering that it is only 20% of total brain parenchyma. Alternatively, increasing b-values to >2000 s/mm2, might have allowed for the extraction of additional diffusion parameters that might have been more sensitive to tracking ISF changes and such studies could be pursued in the future.
Lack of EEG measures during SLEEP:
Unfortunately, due to the technical difficulties related to combining EEG and DWI, we were not able to associate ADC changes to particular sleep stages. Thus we could not measure sleep stages while participants slept in the scanner and could not quantify the presence of slow wave sleep, which has been linked with amyloid beta clearance (Ju et al., 2017; Xie et al., 2013). In the future, development of compatible electrodes will enable simultaneous MRI and EEG recording when using the gradients and rapidly switching radio pulses needed for diffusion imaging and allow assessing the dynamics of changes in diffusivity as a function of sleep stages.
Sleeping in the scanner:
While slow-wave sleep (or, deep sleep) is an important sleep stage frequently seen in the first part of the diurnal sleep compared to the second part of the night, it might not be easy to achieve deep sleep in the scanner due to scanner noise, narrow bed and it being a novel environment. Though we minimized sound effects via ear-plugs this might have been insufficient to filter out the diffusion scanning gradient noise. Thus some individuals might have achieved only the early stages of sleep (i.e., Stage-1 and Stage-2) whereas others might have been able to reach slow-wave sleep. In addition, optimal glymphatic clearance appears to be associated with the lateral position (Lee et al., 2015), whereas our participants had to lie supine in the scanner, which might have hindered glymphatic clearance in some brain regions. The position of the cerebellum within the cranium in the supine position might have facilitated glymphatic clearance and account for it having the largest sADC changes. Future studies using rodents might tease apart these possibilities.
Short-term sleep recording:
We recorded only 90 minutes of sleep, so our findings are pertinent only to the initial sleep period and further studies are needed to determine if ADC changes at later times in the sleep period.
Sleep Profiler measures:
The Sleep Profiler detects only awakenings that are 30s or longer and while unlikely we cannot rule out the possibility that the observed associations between SLEEP-induced changes in ADC and awakenings might have differed have we been able to record shorter awakenings (2–15 seconds).
Visual Task:
In our study we used a visual cue to track arousal rather than EEG and thus we might have classified time periods as sleep when participants closed their eyes but were actually awake. However the recordings of eye motion under the eye-lid indicated that in most instances when eyes were closed in the SLEEP scan they showed slow eye movements consistent with sleep onset. Similarly, the fact that very few participants showed rapid eye movements and when they did they were of short duration indicate that sleep onset REM (SOREM) was not a confound in our study.
Potential effect of SD:
We measured ADC during sleep following a night of SD to facilitate their falling asleep in the scanner and while we did not observe differences in ADC or CSF between the A-SD and the A-RW conditions we cannot completely rule out that our findings reflect an interaction between SD and SLEEP effects.
Time differences in fMRI scans between studies:
STUDY #1 scans were approximately 80 minutes earlier than STUDY #2 scans and though unlikely we cannot rule out the possibility that this time difference might have affected our measures.
In summary, we document significant increases in CSF volumes with sleep and also changes in ADC that tended to show increase in slow diffusivity in cerebellum and left temporal pole during SLEEP state, and increases in fast diffusivity in subcortical regions, parahippocampus, insula and ventral cerebellum in the AWAKE state. The association of the ADC measures with sleep quality indicates that sleep drives these changes since they were not observed in the AWAKE state after SD.
Supplementary Material
Acknowledgements
We would like to thank Lori Talagala for her enormous assistance in the data collection and preparation phase. We also thank Okan Irfanoglu and Amritha Nayak for their help in diffusion data processing. We also thank Peter Manza for his suggestions in figure preparation and discussion of the findings. In addition, we thank Hendrik Mandelkow for his help in setting up the infrared camera and providing support for the camera software and analysis, and David Dinges for valuable suggestions on how to monitor that participants were asleep. We also thank Karen Torres for her administrative assistance, and Minoo McFarland and Tom Lionetti for their amazing support in participant admissions and clinical assistance. This work was accomplished with support from the National Institute on Alcohol Abuse and Alcoholism (Y1AA-3009).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG, 2018. The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol 135, 387–407. [DOI] [PubMed] [Google Scholar]
- Abe Y, Tsurugizawa T, Le Bihan D, 2017a. Water diffusion closely reveals neural activity status in rat brain loci affected by anesthesia. PLoS Biol 15, e2001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe Y, Van Nguyen K, Tsurugizawa T, Ciobanu L, Le Bihan D, 2017b. Modulation of water diffusion by activation-induced neural cell swelling in Aplysia Californica. Sci Rep 7, 6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, Reich DS, 2017. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC, 2001. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med Imaging 20, 1131–1139. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW, 1991. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex 1, 103–116. [DOI] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K, 2015. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H, Hedlund LW, Johnson GA, 1992. Mechanism of detection of acute cerebral ischemia in rats by diffusion-weighted magnetic resonance microscopy. Stroke 23, 746–754. [DOI] [PubMed] [Google Scholar]
- Bernardi G, Cecchetti L, Siclari F, Buchmann A, Yu X, Handjaras G, Bellesi M, Ricciardi E, Kecskemeti SR, Riedner BA, Alexander AL, Benca RM, Ghilardi MF, Pietrini P, Cirelli C, Tononi G, 2016. Sleep reverts changes in human gray and white matter caused by wake-dependent training. Neuroimage 129, 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ, 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Oddo S, Sugarman MC, Akbari Y, LaFerla FM, 2005. Age- and region-dependent alterations in Abeta-degrading enzymes: implications for Abeta-induced disorders. Neurobiol Aging 26, 645–654. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas AL, Duyckaerts C, 2017. Alzheimer disease. Handb Clin Neurol 145, 325–337. [DOI] [PubMed] [Google Scholar]
- Calderon-Garcidueñas AL, Duyckaerts C, 2018. Chapter 23 - Alzheimer disease. In: Kovacs GG, Alafuzoff I (Eds.), Handb Clin Neurol Elsevier, pp. 325–337. [DOI] [PubMed] [Google Scholar]
- Canto CB, Onuki Y, Bruinsma B, van der Werf YD, De Zeeuw CI, 2017. The Sleeping Cerebellum. Trends in Neurosciences 40, 309–323. [DOI] [PubMed] [Google Scholar]
- Cedernaes J, Osorio RS, Varga AW, Kam K, Schioth HB, Benedict C, 2017. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer’s disease. Sleep Med Rev 31, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, Gais S, Rauchs G, Sterpenich V, Vandewalle G, Carrier J, Moonen G, Balteau E, Degueldre C, Luxen A, Phillips C, Maquet P, 2008. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci U S A 105, 15160–15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D, Ulatowski J, Eleff S, Izuta M, Mori S, Shungu D, van Zijl PC, 1994. Rapid monitoring of changes in water diffusion coefficients during reversible ischemia in cat and rat brain. Magnetic Resonance in Medicine 31, 454–460. [DOI] [PubMed] [Google Scholar]
- de Crespigny AJ, Rother J, Beaulieu C, Moseley ME, Hoehn M, 1999. Rapid monitoring of diffusion, DC potential, and blood oxygenation changes during global ischemia. Effects of hypoglycemia, hyperglycemia, and TTX. Stroke 30, 2212–2222. [DOI] [PubMed] [Google Scholar]
- Decanniere C, Eleff S, Davis D, van Zijl PC, 1995. Correlation of rapid changes in the average water diffusion constant and the concentrations of lactate and ATP breakdown products during global ischemia in cat brain. Magnetic Resonance in Medicine 34, 343–352. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- Eide PK, Ringstad G, 2018. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: A glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab, 271678X18760974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvsashagen T, Norbom LB, Pedersen PO, Quraishi SH, Bjornerud A, Malt UF, Groote IR, Westlye LT, 2015. Widespread changes in white matter microstructure after a day of waking and sleep deprivation. Plos One 10, e0127351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvsashagen T, Zak N, Norbom LB, Pedersen PO, Quraishi SH, Bjornerud A, Alnaes D, Doan NT, Malt UF, Groote IR, Westlye LT, 2017. Evidence for cortical structural plasticity in humans after a day of waking and sleep deprivation. Neuroimage 156, 214–223. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM, 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Gakuba C, Gaberel T, Goursaud S, Bourges J, Di Palma C, Quenault A, de Lizarrondo SM, Vivien D, Gauberti M, 2018. General Anesthesia Inhibits the Activity of the “Glymphatic System”. Theranostics 8, 710–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Dahnke R, 2016. CAT- A Computational Anatomy Toolbox for the Analysis of Structural MRI Data, Organization for Human Brain Mapping, Geneva, Switzerland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M, Consortium WU-MH, 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A, 2002. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic Resonance in Medicine 47, 1202–1210. [DOI] [PubMed] [Google Scholar]
- Harris NG, Zilkha E, Houseman J, Symms MR, Obrenovitch TP, Williams SR, 2000. The relationship between the apparent diffusion coefficient measured by magnetic resonance imaging, anoxic depolarization, and glutamate efflux during experimental cerebral ischemia. J Cereb Blood Flow Metab 20, 28–36. [DOI] [PubMed] [Google Scholar]
- Hoddevik EH, Khan FH, Rahmani S, Ottersen OP, Boldt HB, Amiry-Moghaddam M, 2017. Factors determining the density of AQP4 water channel molecules at the brain-blood interface. Brain Struct Funct 222, 1753–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H, 2013. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. Journal of Clinical Investigation 123, 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M, 2012. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Science Translational Medicine 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonelis MB, Drummond SP, Salamat JS, McKenna BS, Ancoli-Israel S, Bondi MW, 2012. Age-Related Influences of Prior Sleep on Brain Activation during Verbal Encoding. Front Neurol 3, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, Fagan AM, Mignot E, Zempel JM, Claassen J, Holtzman DM, 2017. Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain 140, 2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, 2015. ppcor: An R Package for a Fast Calculation to Semi-partial Correlation Coefficients. Commun Stat Appl Methods 22, 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M, 2014. Impairment of paravascular clearance pathways in the aging brain. Annals of Neurology 76, 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M, 1988. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168, 497–505. [DOI] [PubMed] [Google Scholar]
- Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, Logan J, Nedergaard M, Benveniste H, 2015. The Effect of Body Posture on Brain Glymphatic Transport. J Neurosci 35, 11034–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Haris N, Kumar R, Thomas MA, Woo MA, Harper RM, 2018. Obstructive sleep apnea and cortical thickness in females and males. Plos One 13, e0193854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFall JR, Maki JH, Johnson GA, Hedlund L, Benveniste H, Copher G, 1991. Diffusion/microcirculation MRI in the rat brain. Magnetic Resonance in Medicine 19, 305–310. [DOI] [PubMed] [Google Scholar]
- Moeller S, Yacoub E, Olman C, Auerbach E, Strupp J, Harel N, Uğurbil K, 2010. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magnetic Resonance in Medicine 63, 1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugler JP, Brookeman JR, 1990. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magnetic Resonance in Medicine 15, 152–157. [DOI] [PubMed] [Google Scholar]
- Mulkern RV, Gudbjartsson H, Westin CF, Zengingonul HP, Gartner W, Guttmann CR, Robertson RL, Kyriakos W, Schwartz R, Holtzman D, Jolesz FA, Maier SE, 1999. Multi-component apparent diffusion coefficients in human brain. NMR Biomed 12, 51–62. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Goldman SA, 2016. Brain Drain. Sci Am 314, 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C, Sykova E, 1998. Extracellular space structure revealed by diffusion analysis. Trends in Neurosciences 21, 207–215. [DOI] [PubMed] [Google Scholar]
- Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA, 2014. Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol 71, 971–977. [DOI] [PubMed] [Google Scholar]
- Osorio RS, Ayappa I, Mantua J, Gumb T, Varga A, Mooney AM, Burschtin OE, Taxin Z, During E, Spector N, Biagioni M, Pirraglia E, Lau H, Zetterberg H, Blennow K, Lu SE, Mosconi L, Glodzik L, Rapoport DM, de Leon MJ, 2014. Interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer’s disease in cognitively normal elderly individuals. Neurobiol Aging 35, 1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Sarlls JE, Nevo U, Basser PJ, Horkay F, 2009. Polyvinylprrolidone (PVP) Water Solutions as Isotropic Phantoms for Diffusion MRI Studies. Proc. of the XVII ISMRM, Honolulu, p. p. 1414. [Google Scholar]
- Pierpaoli C, Walker L, Irfanoglu MO, Barnett A, Basser P, Chang LC, Koay C, Pajevic S, Rohde G, Sarlls J, Wu M, 2010. TORTOISE: an integrated software package for processing of diffusion MRI data. ISMRM 18th annual meeting, Stockholm, Sweden. [Google Scholar]
- Popovic D, Khoo M, Westbrook P, 2014. Automatic scoring of sleep stages and cortical arousals using two electrodes on the forehead: validation in healthy adults. Journal of Sleep Research 23, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullens P, Bladt P, Sijbers J, Maas AI, Parizel PM, 2017. Technical Note: A safe, cheap, and easy-to-use isotropic diffusion MRI phantom for clinical and multicenter studies. Med Phys 44, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Ratner V, Gao Y, Lee H, Elkin R, Nedergaard M, Benveniste H, Tannenbaum A, 2017. Cerebrospinal and interstitial fluid transport via the glymphatic pathway modeled by optimal mass transport. Neuroimage 152, 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RCoreTeam, 2013. R: A language and environment for statistical computing R Foundation for Statistical Computing; Vienna, Austria. [Google Scholar]
- Ringstad G, Vatnehol SAS, Eide PK, 2017. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140, 2691–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehy JV, Ackerman JJH, Neil JJ, 2002. Evidence that both fast and slow water ADC components arise from intracellular space. Magnetic Resonance in Medicine 48, 765–770. [DOI] [PubMed] [Google Scholar]
- Sevick RJ, Kanda F, Mintorovitch J, Arieff AI, Kucharczyk J, Tsuruda JS, Norman D, Moseley ME, 1992. Cytotoxic brain edema: assessment with diffusion-weighted MR imaging. Radiology 185, 687–690. [DOI] [PubMed] [Google Scholar]
- Sharma RA, Varga AW, Bubu OM, Pirraglia E, Kam K, Parekh A, Wohlleber M, Miller MD, Andrade A, Lewis C, Tweardy S, Buj M, Yau PL, Sadda R, Mosconi L, Li Y, Butler T, Glodzik L, Fieremans E, Babb JS, Blennow K, Zetterberg H, Lu SE, Badia SG, Romero S, Rosenzweig I, Gosselin N, Jean-Louis G, Rapoport DM, de Leon MJ, Ayappa I, Osorio RS, 2018. Obstructive Sleep Apnea Severity Affects Amyloid Burden in Cognitively Normal Elderly. A Longitudinal Study. Am J Respir Crit Care Med 197, 933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri-Kojori E, Wang G-J, Wiers CE, Demiral SB, Guo M, Kim SW, Lindgren E, Ramirez V, Zehra A, Freeman C, Miller G, Manza P, Srivastava T, De Santi S, Tomasi D, Benveniste H, Volkow ND, 2018. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proceedings of the National Academy of Sciences [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Williams DS, Koretsky AP, 1997. Evidence for the exchange of arterial spin-labeled water with tissue water in rat brain from diffusion-sensitized measurements of perfusion. Magnetic Resonance in Medicine 38, 232–237. [DOI] [PubMed] [Google Scholar]
- Sizonenko SV, Camm EJ, Garbow JR, Maier SE, Inder TE, Williams CE, Neil JJ, Huppi PS, 2007. Developmental changes and injury induced disruption of the radial organization of the cortex in the immature rat brain revealed by in vivo diffusion tensor MRI. Cereb Cortex 17, 2609–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotak CH, 2004. Nuclear magnetic resonance (NMR) measurement of the apparent diffusion coefficient (ADC) of tissue water and its relationship to cell volume changes in pathological states. Neurochem Int 45, 569–582. [DOI] [PubMed] [Google Scholar]
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM, 2013. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol 70, 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Gonzalez CE, Venkatraman VK, Wu MN, Pacheco J, Simonsick EM, Ferrucci L, Resnick SM, 2016. Sleep Duration and Subsequent Cortical Thinning in Cognitively Normal Older Adults. Sleep 39, 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stejskal EO, Tanner JE, 1965. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. Journal of Chemical Physics 42, 288-+. [Google Scholar]
- Sykova E, 2004. Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience 129, 861–876. [DOI] [PubMed] [Google Scholar]
- van Gelderen P, de Vleeschouwer MH, DesPres D, Pekar J, van Zijl PC, Moonen CT, 1994. Water diffusion and acute stroke. Magnetic Resonance in Medicine 31, 154–163. [DOI] [PubMed] [Google Scholar]
- Verheul HB, Balazs R, Berkelbach van der Sprenkel JW, Tulleken CA, Nicolay K, Tamminga KS, van Lookeren Campagne M, 1994. Comparison of diffusion-weighted MRI with changes in cell volume in a rat model of brain injury. NMR Biomed 7, 96–100. [DOI] [PubMed] [Google Scholar]
- Vorisek I, Sykova E, 1997. Evolution of anisotropic diffusion in the developing rat corpus callosum. Journal of Neurophysiology 78, 912–919. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang HY, Xu QW, Chen MJ, Liao YH, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M, 2013. Sleep Drives Metabolite Clearance from the Adult Brain. Science 342, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.