Abstract
Resting-state functional MRI (rs-fMRI) is one of the most prevalent brain functional imaging modalities. Previous rs-fMRI studies have mainly focused on adults and elderly subjects. Recently, infant rs-fMRI studies have become an area of active research. After a decade of gap filling studies, many facets of the brain functional development from early infancy to toddler has been uncovered. However, infant rs-fMRI is still in its infancy. The image analysis tools for neonates and young infants can be quite different from those for adults. From data analysis to result interpretation, more questions and issues have been raised, and new hypotheses have been formed. With the anticipated availability of unprecedented high-resolution rs-fMRI and dedicated analysis pipelines from the Baby Connectome Project, it is important now to revisit previous findings and hypotheses, discuss and comment existing issues and problems, and make a “to-do-list” for the future studies. This review article aims to comprehensively review a decade of the findings, unveiling hidden jewels of the fields of developmental neuroscience and neuroimage computing. Emphases will be given to early infancy, particularly the first few years of life. In this review, an end-toend summary, from infant rs-fMRI experimental design to data processing, and from the development of individual functional systems to large-scale brain functional networks, is provided. A comprehensive summary of the rs-fMRI findings in developmental patterns is highlighted. Furthermore, an extensive summary of the neurodevelopmental disorders and the effects of other hazardous factors is provided. Finally, future research trends focusing on emerging dynamic functional connectivity and state-of-the-art functional connectome analysis are summarized. In next decade, early infant rs-fMRI and developmental connectome study could be one of the shining research topics.
Keywords: Resting state, functional MRI, infant, neonate, toddler, children, development, functional connectivity, brain network, connectome, graph-theoretical analysis, autism, dynamic functional connectivity, baby connectome project
Introduction
Functional MRI (fMRI) measures blood-oxygen-level-dependent (BOLD) signals, an indirect in vivo indicator of neural activity (Ogawa et al., 1990). fMRI is a non-invasive brain functional imaging technique that probes brain function with relatively high spatial and temporal resolutions compared to other techniques (Logothetis, 2008). Brain functional studies have a long history of task-related experimental design and have substantially advanced our understanding of how the human brain works. Like the task-related fMRI, rs-fMRI is also a powerful tool to probe brain functions. BOLD signals during the resting state may reflect “spontaneous neural activity,” rather than the contextual, “task-evoked” activity (Biswal, 2012). The differences between rs-fMRI and task-related fMRI findings on development are not trivial but reflect substantially distinct neuromechanisms. The spontaneous neuronal activity is believed to relate to “intrinsic” (naturally existing) human brain functional organizations supported by the underlying structural connectivity substrates (Fox and Raichle, 2007; Fransson, 2006; Lowe, 2012; Snyder and Raichle, 2012). It is believed that spontaneous discharge of neuron groups spread to other neuron groups via axonal connections of the white matter, forming a large-scale, coordinated BOLD fluctuation pattern, or FC (Damoiseaux and Greicius, 2009; Friston, 2011; van den Heuvel and Hulshoff Pol, 2010). The spatial patterns of FC resemble the brain activation patterns of various task paradigms and are called functional brain networks or resting-state networks (RSNs) (Rosazza and Minati, 2011; Smith et al., 2009). The hypothetic roles of the spontaneous neural activity and RSNs include preparing for task execution (Smith et al., 2009), continuously monitoring external and internal environments (Fransson, 2005), reflecting underlying anatomical connectivity patterns (Honey et al., 2009), balancing between the excitatory and inhibitory interactions (Menon, 2011), mind wandering (Fox et al., 2015), memory consolidation (Buckner and Vincent, 2007), and so on. A “dark energy” metaphor concludes that a tremendous portion of energy spent in the resting state is biologically meaningful and could be the key to higher-level cognition and the emergence of consciousness (Raichle, 2006; Zhang and Raichle, 2010). Together, rs-fMRI has become one of the most important techniques for neuroscience studies.
Rs-fMRI is also important for developmental neuroscience studies (Di Martino et al., 2014a; Dosenbach et al., 2010; Gao et al., 2016; Grayson and Fair, 2017; Keunen et al., 2017; Menon, 2013; Power et al., 2010; Richmond et al., 2016; Smyser et al., 2016a; Smyser and Neil, 2015; Vertes and Bullmore, 2015). While task-related fMRI has been widely adopted to investigate activations in older children, adolescents, and adults for probing brain functional development, for younger children, including neonates, infants, and toddlers, rs-fMRI is still an indispensable tool by avoiding the need of performing specific tasks, which are clearly difficult for young children to comply. Various task-free paradigms have been used in rs-fMRI-based neurodevelopmental studies (Redcay et al., 2007). These protocols include eye-opened with a fixation on a screen, eye-opened with a black screen, and eye-closed but awake for older children. In contrast, for infants and neonates, eye-closed during natural sleeping (Fransson et al., 2009) or sedation (Kiviniemi et al., 2003), and eye-opened during passive movie watching or story listening have been employed (Emerson et al., 2015). Among them, imaging during natural sleeping has been widely used in the recent neonate and infant studies, while passive movies watching or story listening is still useful for young children studies (Howell et al., 2018).
Usually, with rs-fMRI, FC between any pair of brain regions can be used for the developmental study, while the RSNs can also be extracted to obtain system-level brain functional measurements. Similar to the dramatically increased brain size in the first years of life (Li et al., 2018), brain functional development as reflected by the changes of FC and RSNs could be profound, reflecting rapid development of behavioral and cognitive functions at these ages (Gao et al., 2015a). The derived FC and RSNs can be compared with those of another cohort at later ages (e.g., adults) via a cross-sectional type of analysis, or compared with those of the same subjects in later developmental stages as a longitudinal study.
Rs-fMRI for infant brain development remains an emerging area. In the last decade (from 2008 to 2017), we have witnessed a rapid growth of publications reporting results on characterizing early brain functional development using rs-fMRI, most of which were published within the last 5 years. However, infant brain rs-fMRI studies are relatively fewer than that of adult, adolescent, and geriatric rs-fMRI studies. For example, searching for rs-fMRI studies published in the last two years led to more than 2000 papers; however, less than 100 papers were found if adding “infant” in the search keywords. Of these 100, many are not actually dedicated to infants. Such shortage of rs-fMRI reports for early brain functional development is likely due to factors such as 1) structural imaging is relatively faster than fMRI, with the latter usually requiring hundreds of volumes, 2) acquiring fMRI data during a resting state is difficult for the very young age groups, 3) the importance of brain functional development has long been ignored, and 4) the existing imaging protocols and image analysis methods are mainly based on the adult cohorts and cannot be easily adopted and applied to neonates/infants.
Thanks to the recent advancement of imaging techniques and data processing methods, the BCP project (also see Big Data on Early Development) is one of the largest data-sharing projects focusing on neonatal and infancy cohorts (Howell et al., 2018). With the anticipated availability of rs-fMRI data, future infant rs-fMRI study will be booming. This review article will be necessary to summarize current findings of probing early growth trajectories of the FC and RSNs at different scales using rs-fMRI, discuss pros and cons of achieving a promising pipeline, and attempt to conclude the current findings from the systems neuroscience viewpoint. We will also discuss previously formed neuroscience hypotheses and provide methodological suggestions for future studies. We acknowledge that, there are several excellent review papers on the FC and FC-based findings of the developing infant brains (Cao et al., 2016; Gao et al., 2016; Grayson and Fair, 2017; Hoff et al., 2013; Keunen et al., 2017; Mongerson et al., 2017; Power et al., 2010; Smyser et al., 2011; Uddin et al., 2010). Therefore, a Review of Reviews is provided in Supplementary Materials to highlight key points discussed in these previously published review articles. With rapid technical advancements, our understanding of early brain functional development is deepening. As a result, it is clearly difficult for a review article to cover all aspects. For example, both Cao et al. (2016) and Grayson and Fair (2017) focused on the large-scale brain FC networks and their changes in the whole lifespan rather than the early life specifically. Mongerson et al. (2017) focused on methodological issues about independent component analysis (ICA, a multivariate analysis method for data-driven brain network analysis based on blind source separation) for infant FC analysis. Gao et al. (2016) discussed infant brain plastics and alterations with genetic and environmental influences. During preparation of our review article, two additional review papers were published (Keunen et al., 2017; Ouyang et al., 2017), focusing on the spatial emergence of different RSNs and short-range FC (FC between the closely located brain regions), respectively.
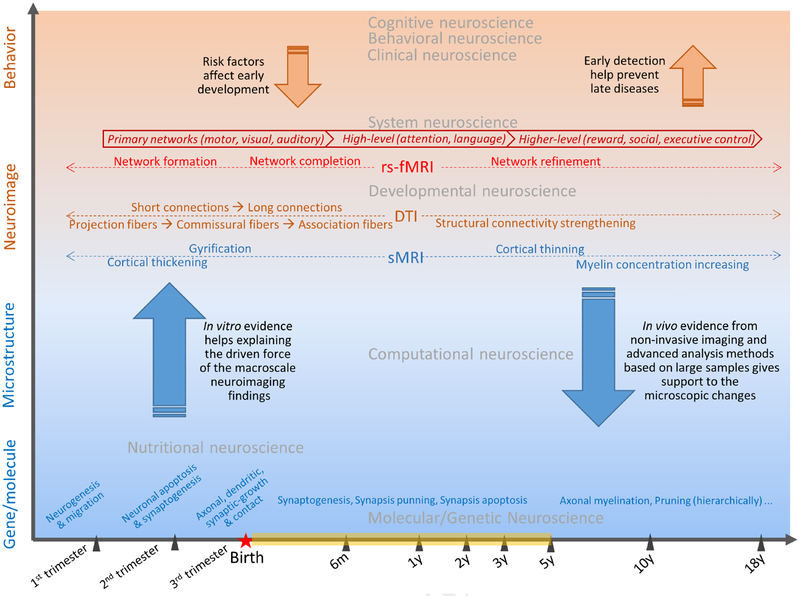
To differentiate from the previously published review papers, the current survey will focus on the early brain functional development as revealed by rs-fMRI from a very young age population spanning from neonates to young children. Theme 1 of this review will give the most up-to-date coverage of this research field. Theme 2 will summarize the consistent findings from previous studies and provide potential hypotheses to be tested in the near future. Theme 3 will provide the most interesting research trends for future studies. In the wake of the emerging big-data sharing projects, the most practical goal of this review is to facilitate the formulation of an rs-fMRI data processing pipeline. Figure 1 shows the big picture of rs-fMRI-based neonate/infant study in the context of different spatial and temporal scales.
Figure 1.
A big picture of rs-fMRI-based early brain developmental study in the context of temporal (horizontal axis) and spatial dimensions (vertical axis) with different scales.
In the following sections, we will review the current rs-fMRI studies focusing on different age groups with different experimental designs. Since there are fundamental differences between infant and adult rsfMRI, we detail the infant rs-fMRI preprocessing procedures with a specific focus on the differences from those in adult studies. A brief summary of the previous post-processing methods used for neonates and infants will be provided. Methods that are not well described in previous review papers will be highlighted. A summary of the developmental trajectories of several functional systems from the systems neuroscience point of view will be provided. We will also briefly review the current efforts on the investigation of the relation between rs-fMRI studies and those based on other imaging modalities. We will review the rs-fMRI findings in neurodevelopmental disorders and other risk populations. Finally, limitations and outstanding issues.
Subjects and rs-fMRI experimental designs
Most previous rs-fMRI studies focused on the school-aged children and older subjects (Dosenbach et al., 2010; Fair et al., 2007; Gu et al., 2015; Sole-Padulles et al., 2016). For a review, please see Vertes and Bullmore (2015). The use of rs-fMRI for neonatal brain studies only emerged in the past 10 years. After the first rs-fMRI study (Biswal et al., 1995), the first neonatal rs-fMRI study was published only one decade ago based on term-equivalent preterm infants (Fransson et al., 2007). The first healthy, term infant rs-fMRI study was conducted one year later (Liu et al., 2008) based on eleven 12-month-old infants. Longitudinal rs-fMRI studies of infants have an even shorter history. The first longitudinal rs-fMRI study on preterm infants with a decent sample size focused on the period between 26 weeks of postmenstrual age (PMA) and term-equivalent age (Smyser et al., 2010). In another similar but pseudo-longitudinal study from 29 weeks PMA to term-equivalent age, the spatiotemporal development of functional networks was reported (Doria et al., 2010). Lin et al. (2008) conducted the first longitudinal rs-fMRI study for healthy term neonates from birth to 2 years of age with a 3-time-point design (2-week-, 1-year-, and 2-year-old) focusing on the primary functional systems, followed by Gao et al. (2009) reporting the maturation processes of the default mode network (DMN).
Several unique characteristics associated with young children could have profound implications for the experimental design of rs-fMRI. These factors include imaging in different states of consciousness states, a smaller brain size, faster respiratory and cardiac rates and, sometimes, the presence of severe motion artifacts. Therefore, experimental designs will need to minimize these confounding factors. More detailed discussion of these factors is provided below.
Consciousness states
For preterm neonates, imaging studies during natural sleep or under anesthesia or sedation have been conducted (Kiviniemi et al., 2000; Kiviniemi et al., 2003). For healthy neonates, natural sleep is the most widely used approach for resting-state fMRI (Fransson et al., 2009; Lin et al., 2008; Liu et al., 2008). However, caution should be taken when interpreting results since FC and RSNs can be modulated by the differences in the consciousness states: sleep stages, anesthesia, or wakefulness state (Graham et al., 2015; Liang et al., 2015).
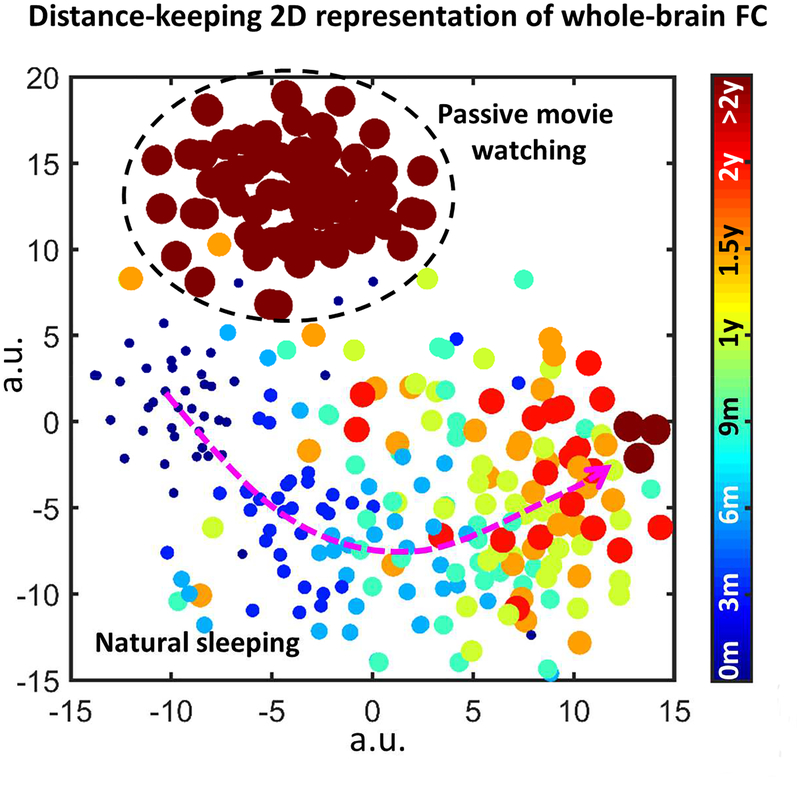
For children older than 3 years old, it becomes more difficult to conduct rs-fMRI during natural sleep. Passive movie watching or story listening could be a good option to keep subjects still and concentrated (Emerson et al., 2015). However, the utilization of these passive tasks could again affect the spatial configurations of the functional networks due to active engagement of visual/auditory stimulus. As shown in Figure 2 (unpublished data), 2D embedding of the whole-brain FC across ages and subject’s statuses (sleeping vs. movie watching) shows clearly distinct clusters (indicating different FC patterns). A recent study reported by Mitra et al. (2017) found that the infant FC networks during natural sleep were more similar to those of the adults during slow-wave sleeping than wakefulness, demonstrating reduced FC in the DMN. Only by comparing the same sleeping state (but not between infant sleep and adult wakefulness) could they reveal significant developmental patterns of fading thalamic earliness in terms of the BOLD signal propagation (Mitra et al., 2017).
Figure 2.
Influence of the age and scanning status on the whole-brain functional connectivity (FC). The result is from unpublished data, where longitudinal rs-fMRI images were obtained from subjects at the ages of 2 weeks, 3 months, 6 months, 9 months, 12 months, 18 months, 24 months, 3 years, 4 years, 5 years and 6 years old, enrolled in the “Multi-visit Advanced Pediatric brain imaging study for characterizing structural and functional development (MAP Study)”. Whole-brain FC is calculated based on Pearson’s correlation between every pair of 268 brain regions (Shen et al., 2013). Rs-fMRI were obtained during natural sleep for the scans at 24 months old or earlier, and during passive movie watching for the scans at 3 years old or later. T-Distributed Stochastic Neighbor Embedding (t-SNE) was used to represent the distribution of the whole-brain FC patterns in a 2D plane, where each dot represents one subject of a certain age, and their distance in the 2D plane is proportional to the high-dimensional Euclidean distance between the 268 × 268 FC features. Different colors and dot sizes indicate different ages. Dark red indicates the FC patterns during passive movie watching, while others indicate the FC patterns during natural sleeping. Clear clustering pattern can be identified, indicating systematic differences between the functional connectome in different states and at different ages.
For longitudinal studies focusing on brain functional development during the first years of life, e.g., 0–5 years of age, the experimental settings may not be easily kept identical between newborns and the 5 years old. If different strategies are used for the same subject at different ages, the interpretation of FC trajectories from the same subjects would at least respect the systematic differences caused by the utilization of different experimental protocols.
Spatial and temporal resolution
Given inherently small brain size of infants, high-spatial-resolution fMRI has been considered. HCP project acquires 2-mm isotropic high-resolution rs-fMRI data at 3-T and 1.6-mm isotropic rs-fMRI at 7-T. However, since an infant brain is about 1/3 of an adult’s brain, the voxel dimension should be scaled to of the 3-mm voxel dimension that is commonly used in adult rs-fMRI studies (Nooner et al., 2012) to ensure an equivalent partial volume effect, which is about 2 mm isotropic. Therefore, at least 2-mm isotropic voxel size should be used for infant studies. The imaging protocol of the BCP (see Big Data on Early Development) would be sufficient.
A high temporal resolution is also critically important for conducting rs-fMRI for infants since their respiratory and heart rates are faster than that of adults and show significant age-dependent patterns (Bar-Haim et al., 2000). To remove the artifacts, a high temporal resolution will be necessary. In addition, the high temporal resolution is needed when dFC (dynamic FC) analysis (calculating time-varying FC instead of stationary FC) or the FC in high-frequency bands are of interest. Multiband echo-planar imaging (EPI) sequence that used in HCP (Glasser et al., 2013) should be adopted if hardware allows.
Motion artifacts
Among all the factors, head motion is the leading cause of failure of obtaining usable images (Fair et al., 2007). A systematic study conducted by Yerys et al. (2009) reports that the failure rate can be as high as 20–40% for 4–6 years old subjects. A recent sleeping-state infant fMRI study indicated that 35% (4-month-old) and 57% (9-month-old) failure rates could be encountered (Damaraju et al., 2014b). Foam cushions between headphone and head coil are necessary, but more cushioning could be necessary for neonates and infants due to the smaller head size. Leg cushion under the knees and weighted sandbag on the feet have also been suggested (Yerys et al., 2009). It is helpful having an adult inside the MRI room with young children during imaging to keep them calm. Training in the mock scanner is helpful (Raschle et al., 2012; Yerys et al., 2009), but could be difficult for children younger than 3 years old. For infants, imaging should be carried out after feeding and changing to a dry diaper to maximize the success of sleep scans. It has been suggested to wait until sound sleep is established to reduce the risk of interruption due to wakefulness (Raschle et al., 2012).
Choosing a proper scanning time could be also important for obtaining useable rs-fMRI data. While there is a general consensus that the acquisition time for rs-fMRI should be as long as possible, preferably around 20 min per HCP protocol (Smith et al., 2013), it is unlikely that infants/toddlers can keep still for such a long time. A shorter scanning time (e.g., 5-min) at each run with multiple runs in each session is thus recommended (Raschle et al., 2012). In addition, Birn et al. (2013) suggested that an rs-fMRI scan of 5–13 min should be sufficient to obtain reliable FC results, so as Van Dijk et al. (2010). However, it remains unclear how many runs of rs-fMRI will be sufficient for infant studies. Additional investigations are needed.
Before the experiment, the recruitment sample size should be adjusted due to the high failure rate in the neonate and infant studies (Yerys et al., 2009). Generally, 20–40% more subjects should be on the recruitment plan, depending on the age. The younger the subjects are, the more sample size should be planned.
Head motion has been shown to correlate with subject’s neurobiological traits, with reduced distance FC in the DMN areas for the subjects with greater head motion or weaker impulsiveness (Kong et al., 2014; Zeng et al., 2014). Therefore, deliberately choosing subjects with a small degree of head motion may result in the creation of an unwanted subgroup with systematic differences compared to the population and thus deteriorate the quality of the study.
Preprocessing methods and pipelines for infant rs-fMRI
Preprocessing of infant rs-fMRI data requires special considerations. Directly adopting adult rs-fMRI preprocessing pipelines could lead to severe methodological issues. Several previously published review articles have separately discussed a few methodological problems for preprocessing pipelines, such as head motion, image distortion, lacking age-specific atlas, ethical considerations, and others (Graham et al., 2015; Smyser and Neil, 2015). In this section, we will review several important issues, such as atlas and registration. In addition, we will propose an HCP-style pipeline for infant rs-fMRI preprocessing.
Infant rs-fMRI registration should be guided by anatomical image (e.g., T1-weighted image) registration. Compared to the adult brains, the neonatal brain is smaller, with less gyrification and folding (Li et al., 2018). The neonatal brain also exhibits different contrasts in early months of life (i.e., before 6 months old, the T1 contrast is inverted compared to the adult’s; during 6–9 months old, the T1 image has an “isointense” appearance). These unique features associated with neonates pose challenges for intensity-based registration to the adult T1 template. Labeled image-based registration is helpful, however, the conventional segmentation methods (i.e., FAST in FSL1 and Unified Segmentation or New Segment in SPM2) are often infeasible. An infant dedicated registration algorithm and template are necessary. In addition, a longitudinal registration algorithm (within-subject registration across different ages, followed by inter-subject registration can also be helpful (Zhang et al., 2017b, c; Zhang et al., 2017d)). Finally, standard brain atlases for neonates and infants are of the great interest in the previous studies. For more details, please see the review paper in the same issue (Li et al., 2018).
Infant brain templates and atlases
Although well-accepted infant brain atlas still lacks, several infant templates have been proposed and will be discussed below.
A preterm template deriving from preterm subjects at the PMA of 28 to 44 weeks was made available by Serag et al. using group-wise registration, which can be used for fetus and neonates studies (Serag et al., 2012). The same team further published their neonatal brain atlas consisting of 107 brain regions that are consistently shown in the adult’s brain (Blesa et al., 2016). They also published a T1-, T2-and diffusion metrics’ (e.g., fractional anisotropy (FA) and mean diffusivity (MD)) templates for registration and atlas propagation, forming longitudinal templates including more (nine) age time points with 2 weeks intervals (Blesa et al., 2016). Similarly, the “Imperial College London neonatal brain probabilistic atlas” was derived from preterm neonates from 29 to 44 gestational weeks. It includes T1 images, three different brain tissue probability maps, plus subcortical grey matter and brain stem probability maps (Kuklisova-Murgasova et al., 2011). The “ALBERTs” atlas was constructed using 14 preterm infants scanned at term age, with another version of this atlas generated based on 5 term neonates. The advantage of this template is that 50 brain regions were manually delineated for each subject and fused to form group-consistent brain parcellations. The T1 and T2 templates were created by inter-subject registration and the second round of registration using group-averaged first-round registered images (Gousias et al., 2012; Gousias et al., 2013).
The “Cincinnati 9–15-month-old template” was derived from a group of infants aged between 9 – 15 months old using a complex, multi-pass, and iterative segmentation algorithm (Altaye et al. (2008). This template includes T1 images and three tissue probability maps that are compatible with SPM. Thus, they could be more easily applied based on the widely used preprocessing toolboxes (e.g., SPM or FSL).
A “UNC 0–1–2-year-old infant brain atlas” (Shi et al., 2011) is available and has been widely used. It consists of a set of brain atlases, including the average T1-weighted brain (intensity) images with and without skull and scalp, brain-tissue hard-segmentation maps (white matter, grey matter and cerebrospinal fluid (CSF)), tissue probability maps of the three tissues, and an automated anatomical labeling (AAL)-based ROI parcellation map. This atlas was based on fuzzy segmentation and atlas-based segmentation from 95 subjects. However, more fine-grained ages (such as 3- and 6-month-old) are not included in this atlas. Moreover, the atlas is not in the standard space and thus a further registration to the MNI space is required.
Sanchez et al. (2012) released a set of publicly available multi-age-group templates3 from 2 weeks to 4 years old based on 13 different age cohorts with relatively large sample size (from an NIH MRI study of normal brain development (NIHPD) with both longitudinal and cross-sectional datasets). Among all available templates, this template likely covers the widest age range. However, this template was constructed from the subjects with preterm birth or growth delay. In 2016, they further widened the age range and provided more age-specific templates with 3-month intervals through 1-year-old, and with 6 months intervals through 19.5 years (Richards et al., 2016). However, like its previous version, this wide-age-range atlas was mainly based on subjects with diseases or risks of diseases and did not use longitudinal datasets. Another publicly available neonate brain atlas is the JHU Neonate Brain Atlas (and Neonate Brain Multi Atlas) (Oishi et al., 2011), built based on 25 term neonates and consisting of T1, T2, and DTI images. This atlas also provides a single-subject-based template, with Talairach’s atlas-based 122-ROI brain parcellation atlas. However, only neonate templates/atlases are available.
Since most of these templates (except the NIHPD-based templates) cover only part of the early brain developmental stages, more templates spanning a large age range, providing more accurate (longitudinal) registration and segmentation, and including brain parcellations obtained from infant population rather than simply adopting from the adult populations are needed. The newly developed brain atlases should also have temporally consistent patterns in geometric, morphometric, and ROIs. In addition, they should be in NIfTI format for easily integrating with the widely used toolboxes.
Infant brain registration
Until now, there has been no well-accepted and easy-to-implement infant rs-fMRI preprocessing pipeline. Previous studies have used existing toolboxes such as FSL to conduct “ordinary” preprocessing including slice timing correction, head motion correction, spatial smoothing, temporal linear trend removing, temporal band-pass filtering, and nuisance signals regression (Gao et al., 2013; Zhang et al., 2017c). All of these steps and parameter configurations are similar to those for adult studies. Among them, however, spatial registration should be carefully considered for infant studies.
Early studies used infant T2-weighted templates for direct rs-fMRI (also T2-weighted images) registration. For example, the template provided by Dehaene-Lambertz et al. (2002) was used to conduct registration based on SPM’s intensity-based registration (Fransson et al., 2007). Several studies directly used the UNC 0–1–2-year-old infant brain atlas (Shi et al., 2011) as the target images with commonly-used adult-brain registration algorithms or toolboxes. After registration was completed for each of the three age groups, the parcellation provided by the atlas can be applied for ROI-wise BOLD signal extraction. In several recent studies, each subject at a specific age group can be registered to the group-mean “template” for the corresponding age group using an attribute-based algorithm like HAMMER (Shen and Davatzikos, 2002). Some other studies used a simpler algorithm in FSL (i.e., FLIRT+FNIRT) by nonlinearly registering each subject’s T1 images at certain ages to a “longitudinal template” made by a single-subject scanned at different ages (Alcauter et al., 2014; Gao et al., 2015a; Gao et al., 2015b; Gao et al., 2014). The longitudinal templates are then registered to the MNI standard space by using 4D registration algorithm (Gao et al., 2013), such as 4D HAMMER (Shen and Davatzikos, 2004). With this strategy, any seed coordinates defined in the stereotaxic space can be projected back to individual rsfMRI data for seed-based FC analysis. The main advantage of such an approach is its simplicity and the straightforwardness. There are other alternative methods. Cao et al. (2017a) used neonatal subjects’ co-registered T2-weighted images as guidance to nonlinearly register the rs-fMRI data to a preterm neonate’s T2 template based on SPM. They further generated a customized, data-specific template by averaging all registered brain images and then conducted another round of nonlinear registration to improve registration accuracy. Damaraju et al. (2014b) used the FSL framework (mainly FNIRT) by selecting one subject as the target and registering other subjects’ brains to the target. All of the registered brain images were further averaged to form a group- and age-specific template for the 4 months old. Affine transformation was then used to register the newly generated 4-month-old template to the existing 9-month-old Cincinnati template (Altaye et al., 2008). Some recent studies even directly used FSL nonlinear registration with a neonate T1 template (He and Parikh, 2016; Toulmin et al., 2015).
In summary, the commonly used strategy is to generate or use age-specific templates and conduct nonlinear registration based on the intensity images. Choosing both FSL and SPM with the specified template(s) and conducting multi-stage deformation field combination/transformation seem to be a feasible and easy way.
To further increase spatial registration accuracy, one can use the labeled image (generated from segmentation) and conduct registration to a labeled template. A more accurate, learning-based brain segmentation algorithm using multimodal images and/or longitudinal images is proposed and a greatly improved registration performance has been reported based on the label maps (Wei et al., 2017). In addition, state-of-the-art registration algorithms, such as a multi-stage, group-wise 4D longitudinal registration (Dong et al., 2017) and deep-learning-based deformable image registration (Cao et al., 2017c), could achieve better registration accuracy. Moreover, surface-based registration using accurate longitudinally-consistent 4D level-set segmentation (Wang et al., 2014) and topology-preserving deformable algorithm (Li et al., 2012; Li et al., 2015a) could further improve surface registration performance. For details, please see the review paper in the same issue (Li et al., 2018). These steps are highly recommended for future rs-fMRI studies, especially those focusing on more detailed structures (e.g., within-thalamus FC), which require more accurate registration.
Motion correction
As extensively discussed above, head motion is another important factor to consider in infant rs-fMRI studies. Even during natural sleep, some subjects may still have large head motions, e.g., > 5mm or > 5 degrees, as shown by Cao et al. (2017a). Only discarding subjects with excessive cumulative head motion as done in traditional SPM-based studies is inadequate. Framewise head motion should also be considered and the “bad” frames should be corrected or at least removed before FC analysis (Power et al., 2012; Van Dijk et al., 2012). The criteria for excluding framewise head motion-affected data vary from study to study and the specific criterion of “bad” frame identification remains lacking for infant rs-fMRI studies. For example, Cao et al. (2017a) used mean frame-wise displacement across the entire scanning time for subject exclusion, while Gao et al. (2014) used single-time-point frame-wise displacement (frame-wise displacement > 0.2 mm) and global signal changes (> 0.3%) to remove the frames with head motions. It has been shown that micro-head motion could alter functional network measurements, e.g., abnormally decreased FC in the DMN and central executive network (CEN), and increased local FC and bilateral motor-area FC (Van Dijk et al., 2012). If this is the case, several early development studies could bear a high risk of under-estimation of the FC in higher-level cognitive function-related networks (DMN and CEN) and over-estimation of the sensorimotor network FC (Fransson et al., 2009). Furthermore, lacking well-accepted criteria to determine “bad” frames could reduce reliability and reproducibility of various rsfMRI metrics (Yan et al., 2013). Simple temporal band-pass filtering and global signal removal are unable to reduce such influence (Power et al., 2014). Data censoring is not recommended as it could destroy the temporal continuity of the data, which will affect dynamic FC analysis (Hutchison et al., 2013). To solve this issue, creating noise-free new 4D volumes by replacing “bad” frames with new interpolated volumes has been suggested (Hutchison et al., 2013). Alternatively, wavelet-based rs-fMRI time series denoising (de-spiking) (Patel and Bullmore, 2016), as well as ICA-based structured artifact removal, e.g., ICA-FIX (Salimi-Khorshidi et al., 2014) or ICA-AROMA (Pruim et al., 2015) seems to be better than simple interpolation and could be used in the future for infant rs-fMRI motion artifact removal.
Infant rs-fMRI data processing pipeline
The unprecedented success of the HCP and its state-of-the-art, “HCP-style” rs-fMRI preprocessing pipeline can be considered as a starting point for an optimal preprocessing pipeline for infant rs-fMRI, especially for the data (from BCP) with high temporal and spatial resolutions. Specifically, the preprocessing procedures for the infant rs-fMRI data from BCP are proposed to be consistent with the HCP-style (Glasser et al., 2016b): 1) avoiding multiple interpolations of rs-fMRI data to minimize interpolation-related BOLD signal alterations by combining different transformation and deformation fields into a single deformation field with one-step resampling; 2) integrating both surface-based and volumetric registration by either representing cortical vertices and subcortical voxels in a common grayordinates system, or using surface-guided volumetric registration in a unified framework; 3) using better brain parcellation atlas either as suggested by the HCP4 (Glasser et al., 2016a) or based on age-specific functional parcellations; 4) increasing the precision of data analysis by preprocessing data in each subject’s native space (Glasser et al., 2016a); and 5) systematic ICA-based structured noise removal.
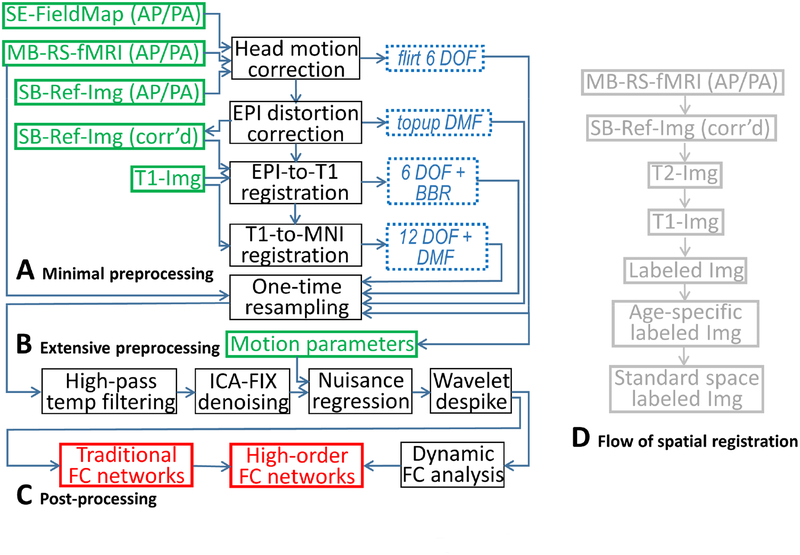
A tentative HCP-style infant rs-fMRI processing pipeline is shown in Figure 3. Specifically, it includes three modules: minimal preprocessing (Figure 3A), extensive preprocessing (Figure 3B) and post-processing for resting-state FC metric calculations (Figure 3C). For the minimal preprocessing, after head motion correction and EPI distortion correction, the corrected single-band reference image is linearly registered to the corresponding T2-weighted images using boundary-based registration. The T2 image is aligned to the corresponding T1-weighted image. Both T1 and T2 images are used to conduct learning-based segmentation based on iBEAT v2.05 (to be released), which generates labeled images (grey matter, white matter, and cerebrospinal fluid). Then, the labeled images of the same subject at different ages can be longitudinally registered using a toolbox, such as 4D HAMMER (skipping this step if the subject has only one scan). Next, the aligned labeled images from multiple subjects within a specific age range are registered to the corresponding age-specific labeled atlas, and the latter is further registered to the labeled atlas in the standard space (i.e., MNI space). All of the deformation fields and translation matrices can be combined and applied to the raw 4D rs-fMRI data, followed by one-step spatial resampling to each volume of the rs-fMRI. Of note, there could be more than one strategy and/or toolbox to conduct spatial registration, such as surface-based registration; the bottom line is using the labeled image and combining all deformation flows (Figure 3D) with one-step resampling to the original rs-fMRI data.
Figure 3.
The proposed full-automated infant rs-fMRI processing pipeline. The pipeline is recommended to process BCP rs-fMRI data, which uses an HCP-style rs-fMRI protocol with high spatial and temporal resolutions. There are three modules in the pipeline: minimal preprocessing (A), extensive preprocessing (B), and post-processing (C). During minimal preprocessing, the flow of spatial registration can be combined into a single deformation field and directly used to warp raw infant rs-fMRI data (D). DOF: degree of freedom; DMF: deformation field; SE: spin echo; AP/PA: phase-encoding direction from anterior to posterior and that with the opposite direction; MB: multiband; SB: single-band (traditional EPI); Ref-Img: single-band, single volume EPI data used as a reference during the spatial registration; BBR: boundary-based registration; ICA-FIX, FLIRT, and TOPUP are all the functions in FSL; Labeled Img: segmented anatomical image with each voxel labeled as grey matter, white matter, or cerebrospinal fluid. See main text for details.
During the extensive preprocessing, after high-pass temporal filtering, ICA-FIX in FSL (Salimi-Khorshidi et al., 2014) can then be applied to each subject’s minimally preprocessed rs-fMRI data for extensive artifact removal. The conventional nuisance regression analysis can be also conducted including the motion parameters. Head motion effects can be further reduced by applying wavelet-based time series de-spiking (Patel and Bullmore, 2016; Patel et al., 2014). Other preprocessing steps including band-pass filtering, global signal regression, and adaptive spatial smoothing are optional (Gao et al., 2015b). The aforementioned infant rs-fMRI analysis has been scripted and pipelined (to be released) based on the modified HCP pipeline6. In a recently published article by the dHCP group (Makropoulos et al., 2017), a similar fully automated minimal processing pipeline7 was also proposed, along with 4D spatiotemporal atlases for 28-to-45 gestational weeks.
After preprocessing, different brain parcellation atlases can be adopted to generate regionally averaged signals for subsequent FC and dFC analyses, which can be the HCP-proposed multimodal imaging-based atlases, or the age-specific functional atlases derived from infant rs-fMRI using clustering analysis or group ICA (GICA, a method for brain network detection based on a group of rs-fMRI data). For the coming BCP data dissemination, the raw rs-fMRI data, minimally preprocessed data, extensively preprocessed data, and the ROI time series data can be included to facilitate future brain functional development studies.
Post-processing of infant rs-fMRI data
There are generally activity- and co-activity-based post-processing for rs-fMRI. The first category mainly includes the BOLD signal fluctuation-based metrics such as Amplitude of Low-Frequency Fluctuations (ALFF) and fractional ALFF (fALFF), which calculated raw and standardized BOLD fluctuation amplitude at a specific frequency band (usually 0.01–0.08 Hz) (Zang et al., 2007). An early study on preterm vs. term infants revealed increased BOLD fluctuations in the low-frequency band at the basal ganglia for subjects at 36-month-old but not at 18-month-old (Damaraju et al., 2010). A more recent study (Long et al., 2017) demonstrated that ALFF in the frontoparietal areas and precuneus increases linearly with age, while those in the sensorimotor, visual and auditory cortices, as well as the inferior medial temporal lobe, show age-related linear reductions from 2 to 6 years of age. However, the results from within-subject longitudinal studies remain largely variable. In a recent longitudinal study on the BOLD signal fluctuation frequency and power, Alcauter et al. (2015b) showed a significant global and network-wise increase in the major BOLD fluctuation frequency in the first year of life, with the peak fluctuation power in the sensorimotor and visual networks correlating with later behavioral scores.
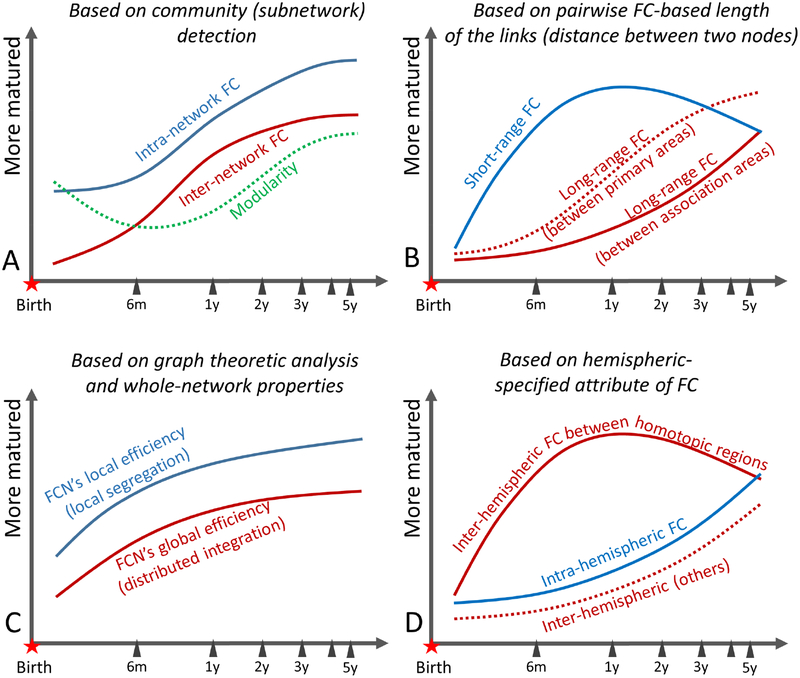
Another type of post-processing analysis is the co-activity-based FC. Previous review papers have covered several widely used FC metrics, such as seed-based correlation, local FC or Regional Homogeneity (ReHo, measuring the temporal synchronization between each voxel and its first-degree neighboring voxels) (Long et al., 2017), pairwise inter-regional FC and the analysis of the constructed complex brain functional networks (or functional connectome) with graph analysis (Gao et al., 2011), ICA (Gao et al., 2015b), and inter-hemisphere homotopic FC (characterizing cross-hemisphere FC between mirroring voxels) (Zuo et al., 2010). For example, Thomason et al. (2013) investigated inter-hemispheric FC in fetuses and found that a half of brain areas have prominent inter-hemispheric FC during the second and third trimesters of pregnancy, which become stronger with increased gestational age. As one of the earliest results, Hagmann et al. (2010) found that, for 2-to-30 years old subjects, the averaged nodal degree and network efficiency increased, while local clustering coefficient (reflecting local efficiency) decreased with age. They also found that, although small-worldness index was significantly higher compared to a random network throughout the development, it decreased with age, which could be attributed to the reduced local efficiency. The structural connectivity network modularity (reflecting functional segregation) was found to decrease with age, with stable compactness within each module but increased inter-modular connections (Hagmann et al., 2010). More importantly, the structural-functional-connectivity association increased in adolescents (> 13 years old) when compared to young subjects (< 4 years old). The brain functional network focusing on the first two years of life, however, suggests both increased local efficiency and global efficiency (Gao et al., 2011). More studies using complex network analysis are needed in the future, such as betweenness centrality and rich club; please see more discussion in Di Martino et al. (2014a). ICA is one of the most commonly used RSN detection methods and has been used since 2007 for neonates with both individual ICA and GICA (Fransson et al., 2007), where five components were reported (with three involving the primary networks and two covering the parietal and frontal areas, respectively). Please refer to the previous review papers for the development studies with these traditional FC analyses (Cao et al., 2016; Gao et al., 2016; Menon, 2013; Sporns, 2013; Zuo et al., 2017).
Aside from traditional stationary FC or static FC (calculated based on the entire rs-fMRI time series by assuming no changes for FC during the entire scan), dFC is an emerging area of research (Hutchison et al., 2013) and should be applied to infant rs-fMRI studies to characterize developmental “chronnectome” (see Research Trends). “High-order” FC network can be built based on traditional FC networks and dFC networks (Zhang et al., 2017a) and could be used to provide useful supplementary information to the traditional developmental studies based on “low-order” FC (based on BOLD time series synchronization), see Research Trends. Since there are relatively few dFC- and high-order FC-based infants studies, we mainly focus on other FC methods that have been already used for infant studies, including FC-based brain functional parcellation and inter-network connectivity.
Inter-network connectivity
A preterm vs. term study in children at 3 years old revealed reduced inter-network FC (Damaraju et al., 2010). The authors hypothesized that the weak inter-network FC could be more vulnerable to the environmental risks, which could be potentially more helpful for detecting early developmental disorders. The negative functional association between the DMN and the task-positive networks (including the attention networks and the CEN) has been consistently observed and well accepted as a normative pattern indicating a suppressive or competing relationship between large-scale networks (Menon, 2013). Limited attention resources could cause an anti-correlation between the inner environment-orientated DMN and the exterior environment-orientated task-positive networks. The reduced anti-correlation could indicate an imbalanced brain, leading to mental disorders. The interaction between DMN and the dorsal attention network (DAN) in the first years of life has been investigated (Gao et al., 2013), where the anti-correlation observed in adults was found to be absent in the neonates, becoming apparent in 1 year old, and even more enhanced in 2 years old. Such a two-way network-level connectivity can be further extended by decomposing the task-positive networks into two functional networks, i.e., CEN and salience network (SN). The multi-way network-level relationship among all these networks has significant cognitive and behavioral implications, and their developmental trajectories could be an interesting topic in the future.
Functional parcellation
Functional parcellation based on region- or voxel-wise FC profiles could be an attractive method for developmental neuroscience studies. It could reveal functional segregation in the whole brain or even within the same brain region, thus uncovering developmental changes in the intrinsic functional organization. Focusing on a specific brain region, Alcauter et al. parcellated the thalamus (2014) and insula (2015a) (Figure 4A, B), while Zhang et al. (2017c) parcellated the high-level function-related medial prefrontal cortex (Figure 4C). Apart from more integrated thalamus in neonates, other two regions were found to be functionally segregated in neonates. From the methodological viewpoint, only not long-range FC, but also short-range FC can be used for better functional parcellation. This is because that less matured long-range FC in the early life could reduce the specificity of the functional segmentation. An example can be found in Wu et al. (2017) for local-FC-based hippocampus subfield segregation. Finally, if longitudinal data is available, better functional parcellation can potentially be achieved by considering temporal smoothness constraint, as the functional segregation is supposed to be gradually changed (Yan et al., 2017).
Figure 4.
The functional connectivity (FC)-based parcellation of the brain regions and their developmental changes. (A) The functional parcellation result in the thalamus (different colors indicate different thalamocortical FC). Adapted from Alcauter et al. Journal of Neuroscience 2014 (Alcauter et al., 2014), modified with permission. (B) The functional parcellation result in the insular lobe. Adapted from Alcauter et al. Cerebral Cortex 2015 (Alcauter et al., 2015a), modified with permission. (C) The functional parcellation results in the medial prefrontal cortex based on independent component analysis, with different colors indicating different sub-regions (three of them are respectively connected with the CEN, SN, and DMN). Adapted from Zhang et al. Connectomics in NeuroImaging (CNI) 2017 (Zhang et al., 2017c), modified with permission. SM: sensorimotor network; SN: salience network; MV: medial visual network; DMN: default mode network; CEN: central executive network; m: month.
Developmental patterns of different brain functional networks
In early studies characterizing early brain functional development, Pearson’s correlation and individual ICA were used to examine the FC of the three primary functional systems in infants and young children (Kiviniemi et al., 2000; Kiviniemi et al., 2003). Later studies using both GICA and seed-based correlation also consistently reported the three primary functional networks in neonates; see recent review papers (Grayson and Fair, 2017; Keunen et al., 2017). Primary functions mediated by the visual, sensorimotor, and auditory networks are mainly for domain-specific information processing.
The sensorimotor system is of importance for neonates and infants as it interacts with the outside world via receiving sensory inputs and exerting coordinated motor movement. Therefore, the sensorimotor network serves as the first test bed to verify the existence of the functional networks in neonates (Liu et al., 2008). It has been demonstrated that the three primary networks are slightly lateralized (Liu et al., 2008), rather than the symmetric patterns observed in the adults, possibly due to the immature interhemispheric white matter myelination in the early years of life. The visual system is another important functional network for humans because it processes visual information from outside world and is consistently found to develop first (Lin et al., 2008). In addition, the visual processing is also important for the maturation processes of other functional networks, such as attention networks, since attention requires accurate and prompt visual information processing. A healthy visual network is thus critical to ensure healthy higher-level functional development. Congenitally blind studies have shown an extensive plasticity throughout the brain (Liu et al., 2007), but how the visual deprivation affects early brain functional development requires further study (Dale et al., 2017).
High-level cognitive functions include language, attention, memory, social interaction, emotion, reward, self-cognition, and so on. Compared to the primary functional networks, these high-level networks are less studied, with inconsistent findings reported. Although early studies found that these higher-order networks are quite immature when compared to the adult networks (Gao et al., 2015b), other recent studies have implicated that these networks may have already emerged even in the neonates or even earlier, and that the subsequent development of the high-level networks is just spatial refinement. See recent review papers (Grayson and Fair, 2017; Keunen et al., 2017) for the excellent summary.
Similar to the primary networks, the high-level networks seem also highly vulnerable to early environmental exposure, especially the factors related to high-level functions. A “social brain” is recently proposed to be correlated with early maternal touch in a five-year-old rs-fMRI study focusing on the FC between temporal association areas and the dorsomedial prefrontal cortex (Brauer et al., 2016). In a recent rs-fMRI study on early brain FC development based on the subjects between 2 and 6 years old, although some regions exhibited local FC increases (as measured by ReHo), decreases of FC in other brain regions were also observed. The regions with increased ReHo are mainly located in the frontal and parietal association areas (i.e., included in the CEN) (Long et al., 2017). In the same study, the global FC, as revealed by eigenvector centrality, shows a different developmental pattern when compared with the local FC, where the age-related increase in FC is mainly in the superior temporal gyrus and cingulate cortex, with the latter mediating various higher-order cognitive functions. Interestingly, some regions in the visual association areas (included in the high-level visual network) in both dorsal and ventral visual pathways have divergent developmental trajectories of the local (increasing along development) and global FC (decreasing along development).
Language development is one of the most prominent behavioral milestones in the first two years of life. Emerson et al. (2016) investigated longitudinal changes of the inter-hemispheric FC in the language-related areas and found an interesting inverted U-shape developmental pattern. An increased hemispheric FC symmetry for both Broca’s and Wernicke’s areas is observed at age one, and reaches its peak at about 1 year old, followed by increasing FC asymmetry, similar to the adults’ pattern at age two. More interestingly, the trajectory of language-related FC between the Broca’s area and its counterpart during the first postnatal year could predict later language performance measured at 4 years old. In addition to interhemispheric homotopic FC, within-language network FC has a log-linear developmental pattern, indicating continuous optimization of the language network with increasing functional integration. The authors further proposed a hypothetic model that the interhemispheric FC is unselectively becoming stronger in the early infancy but is later selectively becoming asymmetric, probably due to the use-dependent FC strengthening within the traditional left-sided language networks.
The DMN has long been an important topic in the rs-fMRI community. The first age-equivalent neonate DMN study was based on anesthetized preterm infants, where the DMN was found to be divided into the anterior and posterior parts (Fransson et al., 2007). Such an incomplete neonatal DMN was further validated by another study with the full-term neonates (Fransson et al., 2009). The first natural sleeping DMN study was conducted by Gao et al. (2009) with a cross-section design, where the DMN at 2 weeks, 1 year and 2 years old were compared among different ages. The general finding is that the DMN has a weaker FC in the neonatal stage, but qualitatively including both anterior and posterior parts of the DMN. The quantitative DMN completion analysis indicated that the DMN is growing more and more similar to its adult form. While the FC between the posterior cingulate cortex (PCC) and the medial prefrontal cortex (MPFC) was found to be already significant at birth, their FC strength might linearly increase from neonate to adulthood. Recently, a “prototype” of the DMN was detected in utero (Seshamani et al., 2016). While these studies use similar ICA-based methods, whether the DMN is split or largely complete in the neonatal stage is still under debate. Another difference regarding the neonatal DMN between Fransson et al. (2009) and Gao et al. (2009) is that the latter study revealed the medial-lateral DMN subdivisions rather than the anterior-posterior subdivisions. Taken together, the developmental trajectory of the DMN and its different parts are quite complex. However, it is generally accepted that the DMN becomes topologically similar to that observed in adults at 1-year-old (Gao et al., 2015a).
In addition to the DMN, there are two other high-level RSNs: CEN (covering the frontoparietal association areas) and SN (encompassing the insular and anterior cingular area). Both of them were found to closely interact with the DMN (Menon, 2011). Previous studies have shown that these three high-level RSNs are not fully developed until age two (Gao et al., 2013; Smyser and Neil, 2015). Specifically, Gao et al. (2015a) conducted a longitudinal study of the SN and CEN within the first year of life. They found that both networks are still in their premature forms by 1 year old, with less specific spatial pattern and less involvement of the dorsolateral prefrontal cortices for the SN, and less completed frontal part of the CEN. The anterior insula has been further identified to have a weaker within-SN FC and inter-network FC in children compared to adults, leading to a hypothesis that weaker FC in the SN could be more vulnerable to neuropathological attacks (Menon, 2013). Alcauter et al. (2015a) found a consistent anterior-posterior insular functional parcellation, indicating that the functional divergence in the insular area has been largely formed at birth, but with their respective long-range FC yet complete. They further found a more rapid SN-related FC increase in the first year than the second year of life.
The subcortical region undergoes a significant development during the first year of life (Alcauter et al., 2014; Toulmin et al., 2015). Despite the homogeneous thalamocortical FC at birth, the thalamocortical FC to the primary areas was found to be well matured (Alcauter et al., 2014). Thalamus-to-SN FC emerges at the neonate and expended in the first year of life, while thalamus-to-DMN FC emerges at 1 year old and expended in the year two (Alcauter et al., 2014). In particular, early thalamus-to-SN FC was found to predict later working memory ability (Alcauter et al., 2014). Focusing on the thalamocortical FC, Toulmin et al. (2015) found similar results from the term-equivalent preterm infants.
In addition to intra-network FC, inter-network FC is another important topic. A series of investigations on the developmental inter-network FC during early brain development have been conducted (Gao et al., 2015b; Gao et al., 2013). In addition to anti-correlation between the DMN and DAN, a more comprehensive whole-brain network-wise FC analysis among multiple functional networks was also conducted (see summary in Gao et al. (2016)). Gao et al. (2015b) further proposed that, with growth, inter-network FC will decrease and intra-network FC will increase. However, the results from whole-brain ROI-based pairwise correlation indicate different developing patterns with inter-network FC increased with age (Damaraju et al., 2014b; Zhang et al., 2017c).
In large-scale RSN studies, an interesting question is – based on data-driven algorithms like ICA, how many functional networks can be detected at birth and later ages? By comparing the early RSNs with adult brain networks, one can quantify maturity index based on the topological resemblance between the early RSNs and adult RSNs. Moreover, the total number of RSNs detected from the infants could provide another indicator of functional maturity. The first ICA study on preterm children imaged at a full-term equivalent age only detected five RSNs, with the high-level RSNs exhibit prominent differences compared with the adults’ (Fransson et al., 2007). Gao et al. (2015b) found the largest number of RSNs with thorough 0–1–2-year-old RSN detection. Future study based on high-resolution, well quality controlled and noise reduced rs-fMRI data could probably detect more RSNs due to increased spatial specificity and image SNR. In addition, study with varying total numbers of independent components should be carried out to exclude “model order effect” (Huang et al., 2016; Lu et al., 2017). Results from such an analysis could be used to generate age-specific functional parcellation atlas of the early brain.
Relation between functional and structural connectivity and other imaging modalities
Brain developmental studies can also be accomplished using DTI (Tymofiyeva et al., 2014) and structural MRI (Giedd and Rapoport, 2010). Integrating these imaging modalities with rs-fMRI could provide a better understanding of brain development. However, studies along this direction are scarce. Hagmann et al. (2010) conducted the first functional-structural connectivity association study in the early life. Although structural connectivity generally matures earlier than FC (Vertes and Bullmore, 2015), some studies have provided different results. For example, a study with young children showed that the FC between the PCC and the medial temporal lobes of the DMN is similar to that of adults while the anatomical connectivity is still weak (Supekar et al., 2010). In addition, previous studies have shown the possibility of using structural connectivity (estimated from DTI) to predict FC with a computational model (Honey et al., 2009). Whether such results can be replicated in neonates and infants should be investigated. Such prediction accuracy could be interpreted as anatomical constraints to the FC, an interesting topic of the developmental neuroscience.
The rs-fMRI-based early brain developmental findings should be compared with those obtained from EEG and magnetoencephalography (MEG). An EEG study on premature infants identified temporal theta activity, a robust neural biomarker, indicating the emerging development of the temporal region during auditory stimulus (Routier et al., 2017). Although EEG offers relatively poor spatial information, it is more convenient for the study of early brain development with superior temporal resolution. For example, Jones et al. (2003) showed that regulating action based on feedback inherent to detect errors in childhood has been developed in 1 year old with different amplitude and latencies, indicating a simpler version of conflict detection using a different strategy. Similarly, near-infrared spectroscopy (NIRS) with high-density optical tomography could be more suitable for neonate and infant studies, because it is easy to operate and less sensitive to head motion (Ferradal et al., 2016; Graham et al., 2015; Smyser and Neil, 2015; Zhang et al., 2011; Zhang et al., 2010). These different functional imaging techniques could effectively supplement the rs-fMRI studies.
Neurodevelopmental disorders and other diseases or hazardous factors
Brain development is a prolonged process. Early disruptive events may cause life-long impacts, such as neuropathological diseases, mental and behavioral disorders during late childhood and later stages. It is quite prevalent (11–15%) to develop psychopathological disorders in the childhood and adolescence, and 36.7% of the participants had at least one psychiatric disorder (Costello et al., 2003). Studies on the neuropsychiatric disorders have proposed a “miswired brain” theory from the developmental viewpoint (Di Martino et al., 2014a; Uddin et al., 2013), where the miswired brain during early development could affect the normative developmental trajectories (Di Martino et al., 2014a). For example, attention deficit hyperactivity disorder (ADHD) could be associated with delayed brain connectome development, while autism spectrum disorder (ASD) has been related to premature but then delayed brain connectome development (Uddin et al., 2013). Finally, preterm birth is usually linked to an overall delayed brain connectome development (Chang et al., 2016). With the brain connectome as features, machine learning could help automatically differentiate atypical from typical development (Levman and Takahashi, 2015a, b; Smyser et al., 2016a).
The utilization of rs-fMRI for early diagnosis of ASD could be a highly promising area of research. Currently, ASD detection studies are still largely based on the data at late childhood from ABIDE-I (Di Martino et al., 2014b) or ABIDE-II8 (Di Martino et al., 2017). Exciting results have started showing that ASD can be now be diagnosed in early childhood and early infancy (Mevel and Fransson, 2016; Uddin et al., 2013). According to the well-accepted hypothesis, ASD-type brain functional connectome may develop abnormally, with hyper-connectivity more likely to occur at young ages, followed by hypo-connectivity later in life (Uddin et al., 2013). Nevertheless, while increasing evidence suggests that ASD symptoms can start at 24 months of age, there are still very few studies on ASD diagnosis at 0–5 years old (Ozonoff et al., 2010) or earlier (Dinstein et al., 2011). Hazlett et al. (2017) found that brain anatomical feature can be used an early (between 6 and 12 months of age) sign of ASD. Later on, Emerson et al. (2017) investigated whether there exist putative FC-based early indicators of ASD with a longitudinal, prospective study on infants with high familial risks of ASD. They successfully (with 92.7% averaged accuracy) identified clinically best-estimated ASD subjects based on 6-month-old brain FC patterns using machine learning. Their finding was further validated by significant correlations between 6-month-old FC and 24-month-old social behavioral, language, motor development and repetitive behavior scores. Furthermore, from the subjects who were diagnosed with ASD one and a half years later, many links were found to have already been significantly weakened at 6 months of age. Independent validations using new datasets are urgently needed to test this classification model. The discrepancy between early ASD studies showing hypo-connectivity (Dinstein et al., 2011; Emerson et al., 2017) and hyper-connectivity in studies in older children (Uddin et al., 2013) requires further studies, especially long-term follow-up, to delineate developmental trajectories of ASD abnormal FC from early infancy to adolescence.
Perinatal high-risk factors and their potential influence in the early brain development are one of the most prominent topics. Among all the influencing factors, early exposure to the environment (preterm delivery) has gained increasing attention (Kwon et al., 2015; Kwon et al., 2014; Scheinost et al., 2016; Scheinost et al., 2015). Due to early exposure, the brain FC development in the preterm infants at term age could be quite different compared to term infants, which also has a long-term effect, such as aberrant cognitive abilities at later ages (Ball et al., 2015). A pattern analysis study using whole-brain FC networks reported a significant FC pattern difference between preterm neonates at term-equivalent ages and term neonates, indicating the early exposure could unselectively alter FC throughout the brain (Smyser et al., 2016b). Other studies, however, suggested that early exposure could selectively affect the formation of macroscale thalamocortical FC, especially the FCs between the thalamus and motor cortex, as well as those between thalamus and brainstem connections (Smyser et al., 2010).
Non-Central Nervous System (CNS) diseases could also alter brain FC in the newborns. A neonatal congenital heart disease study found that hub or rich-club nodes had reduced FC in full-term newborns with neonatal congenital heart disease when compared to the healthy term controls (De Asis-Cruz et al., 2018). It also indicates that such a pathological attack only targets local hub regions in the subcortical regions and brain stem, as well as the thalamocortical connections. A study on childhood drowning-induced anoxic brain injury also found that cognitive function-related brain functional networks were largely preserved while the motor network was disrupted if the injury occurred at 4 years old or younger (Ishaque et al., 2017).
Research trends
Big data on early development
The developing HCP (dHCP)9 is a six-year (2014–2020) collaborative, large-scale, €15 million European project led by King’s College London, Imperial College London, and Oxford University and managed by Connectome Coordination Facility (CCF). The main goal of dHCP is to provide a 4D developing connectome from fetus to birth (prenatal–neonatal stages). The subjects will be “well-phenotyped” (including imaging, clinical and behavioral information) and “well-genotyped”. The dHCP proposed to collect multimodal MRI data (including rs-fMRI) from around 1500 well-characterized fetuses and newborns and has reached 600 neonatal scans by May 2018. The first data release only included 40 representative neonates’ structural MRI, DTI, and rs-fMRI (15 min, 2300 volumes, 9× accelerated multiband EPI, TR = 392 ms, and 2.15 mm isotropic voxel size), as well as the preprocessed data based on the HCP-style preprocessing. Since the preparation of this review paper, no rs-fMRI article based on or detailing the dHCP has been published yet, except for a conference abstract showing the preliminary rsfMRI data processing (Fitzgibbon et al., 2017). In addition to healthy fetuses and neonates, dHCP will also focus on high-risk subgroups of the subjects.
Another recently launched NIH-funded HCP-style brain development project is a $4 million infant HCP project from the United States’ side, a.k.a. Baby Connectome Project (BCP)10. This project is carried out by University of North Carolina at Chapel Hill (UNC) and University of Minnesota (UMN). See details on the protocol design in the same issue (Howell et al., 2018). BCP focuses on acquiring image data and conducting comprehensive behavioral/cognitive assessments from 500 typically developing children during the first five years of life. Of the 500 children, 285 subjects will undergo longitudinal imaging (4–6 visits at different ages). The imaging protocol follows the HCP style with modifications tailored to the unique characteristics of the specific age group. Similar to the HCP data, for rs-fMRI acquisition, different phase-encoding directions (anterior-to-posterior (AP), and posterior-to-anterior (PA)) are used, generating two rs-fMRI data sets in each visit (Figure 5). Children younger than 3 years of age are imaged during natural sleep while older subjects (> 3 years old) are imaged during passive movie watching during rs-fMRI acquisition. This unprecedented infant brain data will deepen our understanding of the development of human brain connectome, establish links between brain connectome and behavioral development. With future establishment and sharing of the BCP dataset, the issue of a small sample size could be overcome, reliable and robust conclusions can be generated, and the validity of the developmental changes can be ensured.
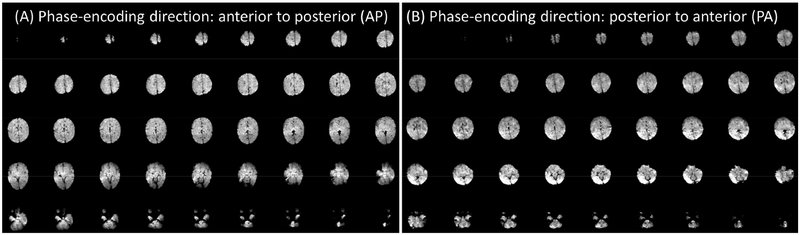
Figure 5.
Raw resting-state fMRI data from one exemplary subject aged 18 days (at the neonate stage). Different phase encoding scans (AP and PA) are acquired for future distortion correction.
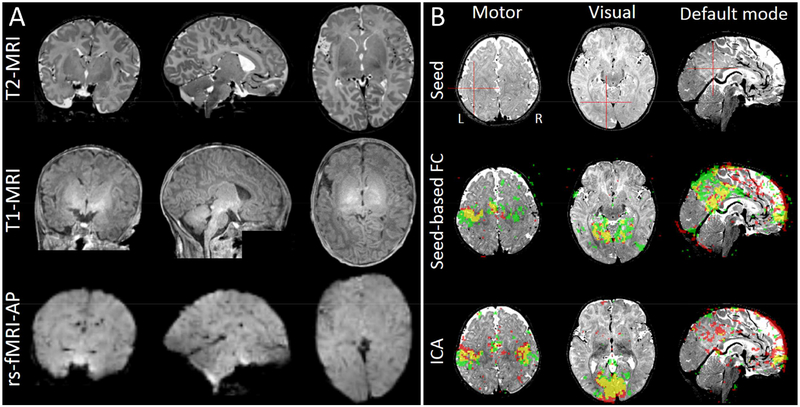
BCP has been continuously collecting data (Howell et al., 2018). The imaging quality of the rs-fMRI is guaranteed in terms of intensity homogeneity, signal loss, and spatial details. Multimodality imaging results from the same neonate (Figure 6, left) shows good correspondence across different modalities and satisfactory imaging quality. A preliminary seed-based correlation result (seeds were manually located at the left hand knot, left lingual gyrus, and left posterior cingulate cortex) is shown from a subject (aged 190 days). Individual ICA results (number of components = 40, results were integrated based on 40 times ICA with different initial values) reveal a putative primary motor network, a primary visual network and the DMN (Figure 6, right). Despite limited preprocessing (head motion correction and band-pass filtering (0.01–0.1 Hz)), the results from AP and PA rs-fMRI data are significantly overlapped. More complex HCP-style data processing for the BCP data (based on the pipeline shown in Figure 3) is ongoing and the raw and preprocessed BCP data will be released. Until now, there is no other neonate or infant brain dataset that is publicly available except a small number of pre-released dHCP data.
Figure 6.
Multimodal imaging data from BCP. (A) Raw T1-weighted, T2-weighted and rs-fMRI data (phase encoding direction: AP) from the same neonate with a chronological age of 18 days as Figure 5. (B) Functional connectivity results from another infant with a chronological age of 190 days (~ 6 months old). Both seed-based correlation (with the seeds put at the left primary motor, left primary visual and posterior cingulate cortex for sensorimotor, visual and the default mode networks, FC maps threshold: r > 0.4) and independent component analysis (ICA, threshold: z > 2) results were shown. Red areas indicate the results from the multiband rs-fMRI data with AP phase encoding direction. Green areas represent the results from the multiband rs-fMRI data with PA phase encoding direction. Yellow areas show their overlap. Of note, ICA results in two sensorimotor networks in both hemispheres in separate components and they were merged together to form the complete sensorimotor network.
Theoretical models of early brain functional development
A better understanding of how the brain undergoes dynamic re-organization during early infancy could facilitate the identification of hazardous factors leading to negative impacts on early brain development as well as early detection of atypical development. An intuitive “stability landscape” model was previously proposed by Knudsen to explain how the brain connections become stabilized and how this process is affected by early experience during sensitive periods (Knudsen, 2004). Such a model assumes that brain connectivity is unstable during early infancy and exposures to a healthy environment will strengthen proper connections while weakening the inappropriate ones. On the other hand, atypical experiences (e.g., preterm, visual deprivation) could drive brain connectivity towards abnormal patterns temporarily or permanently. In lieu of this model, it is highly plausible that the young brain will undergo functional reorganization in response to environmental exposures during early infancy since the white matter constraint has not been well established. As the brain continues to mature, white matter constraints are strengthened, leading to a more matured and stable functional network.
Similarly, Menon (2013) proposed that weak connections could be more easily altered. For example, the salient network (SN), which mediates emotional processing, has been shown to exhibit weak FC with other RSNs, these connections could be more affected by neuropathological attacks in younger ages. This may explain why mood disorders are common in the young population. Fransson et al. (2011) proposed that brain regions and connections for higher-order cognitive functions mature later, thus are more vulnerable to environmental and experience effects (Gao et al., 2016).
The definitions of FC instability and vulnerability are not yet proposed. Recent advances in dynamic FC (dFC) could provide a potential means to assess FC instability and vulnerability (Allen et al., 2014; Calhoun et al., 2014; Chen et al., 2017b; Hutchison et al., 2013). The variability of the dFC during the entire scanning time could measure the stability of FC (Hutchison et al., 2013). It is highly plausible that the dFC could be more variable in neonates compared to that at later ages. Other studies suggested that the time-varying topology of the brain functional networks and their quantitative metrics could also help explain the functional flexibility and brain adaptation (Chai et al., 2017; de Pasquale et al., 2017). With the advancement of analysis tools, dFC could become one of the important tools to shed light on early brain functional development.
Prediction and early detection
One of the major topics of research in early brain functional development is to determine if the FC obtained during early infancy can predict cognitive and/or behavioral outcome in later ages. Several studies have employed baseline FC in early ages to predict the subjects’ learning ability (Zhang et al., 2017c), language performance (Emerson et al., 2016), and other higher-level cognitive performance at later ages (Alcauter et al., 2014). In a preliminary study, Zhang et al. (2017c) used the 1-month-old FC among the medial prefrontal subregions of 25 infants to predict early learning composite score at 4 years old. They achieved an area under the ROC (receiver operating characteristic) curve of 0.72 using support vector machine (SVM) with leave-one-out cross-validation. Their results indicate that, although high-level cognitive-related brain regions are less developed in neonates, the FC patterns during early infancy could provide a means to predict later cognitive performance.
Early identification of risk populations from typically developing subjects has profound clinical implications (Ball et al., 2015; Kwon et al., 2014). In particular, one of the main strategic plans for research of the National Institutes of Mental Health (NIMH) is to “Chart Mental Illness Trajectories to Determine When, Where, and How to intervene (Objective 2)”. It has been suggested that mental illness has a continuous spectrum and long developing trajectories. Therefore, it is plausible that subtle changes in brain function can begin years earlier than the emergence of the clinical symptoms. FC could potentially be a sensitive biomarker of atypical early brain functional alterations prior to the presentations of clinical symptoms. Emerson et al. demonstrated that FC measured at 6 months old can potentially predict infants who received a research clinical best estimate diagnosis of ASD at 24 months of age with a high accuracy (Emerson et al., 2017), demonstrating the potential clinical utility of FC in neurodevelopmental disorders.
Both prediction and early detection could benefit from multivariate analyses. Techniques such as pattern recognition, machine learning, and deep learning are powerful tools to integrate relevant features (e.g., multiple FC links, brain network properties) to potentially achieve better classification performance. A detailed review of the utilization of these approaches for disease diagnosis has been provided by Levman and Takahashi (Levman and Takahashi, 2015a, b). Below, we will focus on better feature extraction from the infant rs-fMRI and provide comments on future works using multivariate analyses. First, effort should be made in developing approaches capable of achieving accurate brain functional abnormalities at the earliest stage, such as preclinical and even presymptomatic stages. Second, it is critically important to develop robust and reliable rs-fMRI analysis methods to mitigate imaging artifacts and noise. Third, additional resting-state FC metrics beyond the traditional metrics are needed to improve the sensitivity of early detection of subtle abnormalities. For example, the recently proposed “high-order FC (HOFC)” has been demonstrated capable of capturing higher-level (more complex) brain functional organization, which appears highly sensitive for early brain disease detection (Chen et al., 2016a; Chen et al., 2017a; Chen et al., 2016b; Zhang et al., 2016; Zhang et al., 2017a; Zhang et al., 2017e) and early prognosis (Liu et al., 2016), see Figure 7 for different types of the HOFC metrics. Another promising resting-state metric is dFC, or “chronnectome” (Abrol et al., 2016; Calhoun et al., 2014; Chen et al., 2017b; Zhu et al., 2016). Based on dFC, the flexibility of functional network affiliations (Li et al., 2015b; Liu and Duyn, 2013), the statuses of the brain functional networks and their dwelling time (Damaraju et al., 2014a), occurring frequency (Abrol et al., 2017), and transition probability (Allen et al., 2014) could be extracted and adopted as informative features for early detection. Finally, with more longitudinal FC becoming available, the development of 4D prediction methods that jointly consider the longitudinal information to achieve better classification performance are greatly needed (Meng et al., 2016; Rekik et al., 2017).
Figure 7.
The diagram of traditional FC or low-order FC (LOFC, A), and different metrics of high-order FC (HOFC, B-D). Topographical profile-based HOFC (tHOFC) is illustrated in (B) and its variant, associated HOFC (aHOFC), is illustrated in (C). For simplicity, only a few regions are used to demonstrate the LOFC and the HOFCs. The LOFC profiles of each region in (B) involve five brain regions. Different line width indicates different connectivity strength. The black lines indicate LOFC, the blue curves represent tHOFC, and the red curve depicts aHOFC. The calculation of dynamic FC-based HOFC (dHOFC) is illustrated in (D). With a further correlation of dynamic FC time series, dHOFC geometrically increases the amount of information compared to the LOFC network. An n×n LOFC matrix generates a larger n×(n−1) × n×(n−1) dLOFC matrix. After calculating the dynamic FC for two pairs of brain regions (i and l, and j and k, respectively), two dynamic FC time series are further correlated to produce one dHOFC value among the four regions. The figure is adopted from Zhang et al. 2017 Front Neurosci (Zhang et al., 2017a).
Genetic vs. environmental effects on brain functional network development
To the best of our knowledge, there is only one rs-fMRI study reporting results on genetic and environmental effects on brain functional networks during the first years of life (Gao et al., 2014). To disentangle the contributions from the two sources, Gao et al. (2014) used a large group of typically developing infants including singletons, dizygotic twins, and monozygotic twins and investigated their FC development during the first two years of life. Low individual variability in the FC at the primary functional systems whereas higher individual FC variability in higher-order functional regions was found at birth. Such a difference in individual FC variability persisted throughout the first two years of development. The relationship between genetic effect and inter-subject variability was found to be spatially and temporally complex. They further observed that genetic control was related to increased subject variability in some brain regions but such a relation was inverted in other brain regions or at a different age. Recently, a multi-site, multi-country collaboration project, namely ENIGMA Consortium11, has been carried out focusing on the genetic effect of brain networks in different scales (Thompson et al., 2016). This large-scale and high profile study is likely to further advance our understanding of how genes and environment jointly and/or individually shape early brain development. In future, more twin studies should be carried out to investigate such dynamic processes during the early development, in addition to the studies from childhood to adulthood.
Early brain functional development charts
Characterizing normative developmental trajectories of functional connectome in the early life using a large group of subjects with a careful sampling strategy is essential to detect early development abnormalities (Kessler et al., 2016). Before the availability of imaging-based developmental charts, fetus head biparietal diameter (BPD) and infant’s head circumference charts have long served as pivotal developmental metrics. Recently, charting the FC networks has been proposed to identify early attention impairment in youth (Kessler et al., 2016). Using rs-fMRI data from more than 500 youths, Kessler et al. (2016) calculated a set of cross-subject co-varied components in the functional connectome. Subsequently, expressive scores on each co-varied component from each subject can be jointly used to form a normative growth curve. Based on the growth curves of different components, a “maturation deviation score” can be calculated, which has been showed to correlate with the subjects’ attention performance. In addition, their results suggested a pattern of “shallow maturation” for ADHD children, where their developmental trajectory is in parallel with that of normal subjects but with lower completeness. Kaufmann et al. proposed a different approach to chart normal development using the individual distinctiveness of functional connectome. Specifically, they calculated the identification ability of a particular subject from a group of subjects with a similar age solely based on their functional connectome (Kaufmann et al., 2017). They found that healthy children’s functional connectome grew apart (with increasing difference in functional connectome among different children), especially for higher-level cognition-related functional systems (e.g., the DMN); they also discovered that the functional connectome in the children with different mental disorders (such as ADHD and depression) also grew more and more distinct. To date, there is no FC-based brain maturity indexing or brain age prediction for infants, but a promising preliminary result of age prediction has shown the promising feasibility (Figure 2).
Functional gradients and the relation to the developmental order
Brain FC networks are organized in a hierarchical fashion (Margulies et al., 2016). In a recently proposed “gradient of connectivity” theory, two types of gradients from the organization of the brain functional connectome have been suggested: one spanning different sensory inputs (from V1, sensorimotor, and to A1), and the other following different functional information processing levels (from unimodal, heteromodal and to para-limbic/limbic/DMN) (Margulies et al., 2016). This model indicates that the topographical organization of the brain cortex could facilitate efficient information processing, with unimodal inputs further processed at the heteromodal regions and then integrated at the DMN (Figure 8A–D). The above findings based on adult rs-fMRI could have intriguing indications on the studies of brain functional network development, since the maturing order of different brain functional networks seems also following such gradients, i.e., from posterior to anterior, from inferior to superior, from medial to the lateral areas, as well as from primary functional systems to the high-level cognition-related networks (Gao et al., 2015a). Of all the primary brain functional networks, the visual area seems to develop earlier and mature faster (becoming topologically consistent with that observed in adults while sensorimotor networks continue to split into unilateral halves) (Doria et al., 2010). Could such “maturation orders” reflect the aforementioned FC gradients as described in this model? To answer this question, future brain functional development studies may consider this “functional gradient” method.
Figure 8.
State-of-the-art analysis methods that are promising in future brain functional development study. (A-D) Functional gradients that resemble the developmental order, adapted from Margulies et al. PNAS 2016 (Margulies et al., 2016), modified with permission. (E-F) The multi-layer network constructed based on multiple frequency-specific networks, adapted from De Domenico et al. Front Neurosci 2016 (De Domenico et al., 2016). (G-H) The typical sliding window-based dynamic FC time series and the temporal network analysis, adapted from Thompson et al. Network Neuroscience 2017 (Thompson et al., 2017). (I) Different across-layer link types: full connected and neighboring connection, adapted from Mucha et al. Science 2010 (Mucha et al., 2010). (J-K) Two different network topological organizations, adapted from Bassett et al. PLoS Comput Biol (Bassett et al., 2013), modified with permission.
Dynamic FC, multilayer network analysis, and temporal network analysis
It is widely accepted that the dFC is not pure noise but reflecting nontrivial brain adaptation to the changing environment (Calhoun et al., 2014; Hutchison et al., 2013). While a few pioneering studies have investigated dFC in children (Chai et al., 2017), dFC studies in neonates and early infants remain scant. Yet, dFC may provide great insights into early brain functional development that could be not available if using static FC. In particular, young brains could be more plastic and flexible due to fewer myelinated axons and less structural connectivity constraints. For example, less matured connections or regions may be temporally unstable, while the weaker inter-network connections in the early ages may lead to less flexibility of brain regions in participating across multiple brain functional systems (connecting more within functional systems instead of between functional systems).
One of the most widely used analysis approaches for dFC is sliding window correlation, which calculates FC for each overlapping or non-overlapping small temporal segments of rs-fMRI. Various postprocessing methods can be used to quantify time-varying connectome. Among them, clustering and other data decomposition methods have been commonly used to identify brain “statuses”. Subsequently, occurrence frequency and dwelling time for each status, as well as the transition probabilities between any two statuses could be calculated (Calhoun et al., 2014). Alternatively, the link-wise variability of dFC time series can also be a flexibility metric (Chen et al., 2017b). To further investigate temporal associations among the dFC times series, dynamics-based HOFC (dHOFC) can be used (Chen et al., 2016a), see Prediction and Early Detection and Figure 7D. In dHOFC, the sliding-window-derived dFC time series between a pair of brain regions is further used to calculate the temporal correlation with another dFC time series between another pair of brain regions, thus characterizing the temporal associations of the FC dynamics, a higher-order, more complex functional association measurement (Chen et al., 2016a; Chen et al., 2017a; Liu et al., 2016). All of the above dFC-based metrics could potentially provide complementary information to the traditional static FC studies.
In addition to the aforementioned approaches, two advanced data analysis methods, multilayer network analysis, and temporal network analysis warrant further discussion.
The multilayer network analysis approach connects the nodes across different time windows forming a multilayer network where each layer represents a transient brain FC network. This is in great contrast to the traditional network analysis approaches, which mainly focus on single-layer networks. Mucha et al. (2010) proposed and outlined how the multilayer network analysis approach enables network-based modularity detection from a social network, where additional cross-layer edges were added to link different network layers, forming a supra-adjacency matrix (Figure 8I). Subsequently, a multilayer generalized Louvain (GenLouvain12) algorithm or other network analysis methods (Battiston et al., 2017; De Domenico, 2017; Stanley et al., 2016) can be used to detect the network modules. The inter-layer edges serve as a constraint, making the network topology of each transient FC network more temporally consistent (Bassett et al., 2011), thus more suitable for dFC (small time scale) or early developmental (large time scale) studies. Specifically, the cross-layer edges can be added to connect corresponding nodes between two neighboring network layers for dFC, different ages in longitudinal developmental studies, or across all layers (fully connected) for multi-subject analysis in cross-sectional static FC study to ensure inter-subject consistency. Finally, the multilayers of networks can also be formed by calculating frequency-specific FC networks with different frequency bands (De Domenico, 2017) (Figure 8E–F), or by calculating connectivity networks from different imaging modalities for cross-modularity integration (Battiston et al., 2017) between the functional and structural connectivity networks. Temporal network analysis has been recently proposed to rigorously analyze dFC networks with network properties defined in a spatiotemporal way (Thompson et al., 2017; Thompson and Fransson, 2016) (Figure 8G, H), such as dynamic community (Bassett et al., 2013) (Figure 8J, K), temporal betweenness, closeness centrality, and reachability. This method allows investigation of information flow along a short time period (Thompson et al., 2017).
Findings in developmental patterns and trajectories during the first five years of life
In this section, we will summarize the developmental patterns and trajectories in the first five years of life that have been widely reported. Since this area of research is still at its early “infancy,” interpretations of these results should consider the aforementioned limitations associated with rs-fMRI. These developmental patterns and trajectories are depicted in Figure 9.
Figure 9.
Consistently suggested functional developmental patterns or trajectories for various FC and FC network (FCN) metrics from birth to 5 years old (see main text for more details). Subplot (A) is for developmental trajectories of intra- and inter-network FC, with that of FC network modularity. Subplot (B) depicts short- and long-range FC between primary and higher-level association regions. Subplot (C) is for FCN properties, including local and global efficiency, and both of the developmental trajectories fall in the small-world zone. Subplot (D) depicts the trajectories of inter- and intra-hemispheric FC. For inter-hemispheric FC, both FC between homotopic regions and between non-homotopic regions are plotted.
The large-scale functional networks (RSNs) could have been largely formed (even though in the prototypes for some of them) after the third trimester (Doria et al., 2010; Jakab et al., 2014; Schopf et al., 2012; Seshamani et al., 2016). At birth, most of them can be identified, although the connection strengths may be weaker than that in adults, and the topologies may not be completely consistent with that observed in adults, especially for the DMN (Doria et al., 2010; Fransson et al., 2009; Fransson et al., 2007; Gao et al., 2015a; Gao et al., 2009).
Different functional systems have distinct developmental trajectories. The maturation trajectories could follow such orders: from the primary functional systems, the temporal and parietal association cortices (e.g., those mediate primary language and spatial attention), and then to the higher cognitive functional systems (e.g., social cognition and executive control) (Gao et al., 2015a; Gao et al., 2015b; Lin et al., 2008; Liu et al., 2008; Ouyang et al., 2017). Spatially, the maturing patterns start from posterior to anterior regions, from inferior to superior regions, and from medial to lateral regions (Gao et al., 2015a). Most higher-order association cortices and the projections from them to the subcortical areas could have protracted maturation processes (Zhang et al., 2017b).
With the exception of homotopic FCs in the primary functional systems, long-term FCs are generally weak and more vulnerable in neonatal brains, possibly due to unmyelinated white matter (Deoni et al., 2011; Keunen et al., 2017). Short-range FCs dominate where spatially proximal regions tend to be densely connected (Di Martino et al., 2014a). With development, gradual and myelination processes could lead to strengthened long-range FCs (Fair et al., 2009; Power et al., 2010; Smyser et al., 2011; Vertes and Bullmore, 2015; Vogel et al., 2010; Zuo et al., 2017), leading to weaker spatial constraint (Zuo et al., 2017). In contrast, pruning processes could selectively weaken some connections (mostly short-range FCs) (Collin and van den Heuvel, 2013; Hagmann et al., 2010; Hoff et al., 2013; Vertes and Bullmore, 2015) (Figure 9B, D).
The modular structure is largely preserved throughout early brain development (Power et al., 2010; Vertes and Bullmore, 2015), but could have different spatial configurations at different ages. At birth, the modules likely involve anatomically proximal regions, followed by limited (not extensive) reconfigurations of modular structures by rewiring during early infancy and eventually in toddlers (Di Martino et al., 2014a). As a result, functional hubs could also shift from primary regions to higher-level association regions (Fransson et al., 2011).
Intra- and inter-network FC reorganization could both increases during early brain development, but with a different pace or slope (Figure 9A) (Damaraju et al., 2014b). The brain becomes more functionally specified (Gao et al., 2015b) but also with a small number of strong inter-modular connections (Damaraju et al., 2014b) for functional integration (i.e., locally segregated and globally integrated, Figure 9C) (Cao et al., 2017b; Dosenbach et al., 2010; Menon, 2013).
Global efficiency of the large-scale whole-brain FC network could increase during the first years of life and maintains its level with only slightly increase from childhood to adulthood (Gao et al., 2011; Hagmann et al., 2010). The whole-brain FC network’s local efficiency could probably follow a similar trend (Figure 9C) (Cao et al., 2017b; Gao et al., 2011) since increasing functional segregation could be reflected by increasing local efficiency (Cohen and D’Esposito, 2016). Rewiring could make the brain functional network changing from one efficient scheme to another (more) efficient scheme, with small-worldness guaranteed all the time (Power et al., 2010). It should be mentioned that network-level efficiency development for the structural connectivity networks could be different from FC networks (Cao et al., 2016; De Domenico et al., 2016), possibly with opposite findings (Tymofiyeva et al., 2014; Vertes and Bullmore, 2015).
The brain network topology exhibits local segregation with global integration consistent with the known small world topology immediately after birth (Gao et al., 2011), indicating an efficient brain at birth. At 1 year old, the brain functional topology becomes more region-based, without evidence of substantial, long-distance connections whereas connectivity becomes more evenly distributed, with increased long-distance connections in year two (Zhang et al., 2017b).
Remaining issues
While we have extensively discussed many aspects of rs-fMRI in early brain development, many issues remain. First, early brain rs-fMRI processing pipelines are different in different studies, making it difficult to integrate of results from different studies. A unified, well accepted, end-to-end neonate/infant rs-fMRI analysis pipeline similar to the HCP pipeline is greatly needed. Second, age-specific 4D brain templates and atlases at every 3 months (even every 1 month for the first year of life) during the first years of life are the key to develop a pipeline dedicated to pediatric subjects. Third, considering the aforementioned limitations associated with rs-fMRI, test-retest reliability or reproducibility studies, particularly for early brain functional network development, are urgently needed (Zuo et al., 2012; Zuo et al., 2017). Fourth, previous infant rs-fMRI-based brain FC network studies have largely adopted parameters and settings from that used in adults, such as ROI definitions, module assignment, FC matrix binarization, and so on. As a result, biases may be introduced. Fifth, how to combine longitudinal results from natural sleep to passive movie watching data in the same subjects but at different ages remains to be an active area of research. In particular, most of the studies with children younger than 2–3 years of age were typically done during natural sleep while studies with older children (> 3 years old) have been done during the awake state. Finally, the results based on pseudo-longitudinal data should be carefully interpreted, as the differences could come from other confounding factors, rather than development itself.
Supplementary Material
Supplementary Table 1. Review of reviews.
Scope and synopsis.
Early brain development in human beings is a fascinating yet largely unknown research field. Previous developmental and cognitive neuroscience studies mainly focused on the “maturing” process of children from school ages to mid-adulthood. Comparing to these studies, brain development during early infancy remains largely elusive, especially on how brain functional connectivity (FC) and functional networks emerge and develop at the first years of life. Many attempts have been made to fill this gap and probe the developmental processes of young brains by using functional magnetic resonance imaging (fMRI). Since it is unlikely that children will corporate to perform specific tasks, previous studies used either task-free paradigms, a.k.a. the “resting state,” or passive task-related paradigms. In this review, we summarized major infant developmental findings based on resting-state fMRI (rs-fMRI) by providing a comprehensive survey of early brain functional maturation (i.e., under 5 years old).
Despite great interests, rs-fMRI-based brain functional developmental studies in the first years of life are still in its infancy. With a thorough literature review in PubMed between 2008 and 2017, less than 250 papers were found to document the rs-fMRI studies on the first years of life. Of which, studies specifically dedicated to the use of rs-fMRI for early brain development are even fewer. Dozens of review papers (mainly published in recent years) provide the trending of abstracting well-accepted models, forming reasonable hypotheses, and standardizing processing methods (see Review of Reviews in Supplementary Materials). However, considerable growth in the field of early brain development remains ahead of us. Recently, exciting news has been released on the ongoing big-data projects, including developing Human Connectome Project (dHCP) and Baby Connectome Project (BCP). These projects can significantly boost both methodological development and hypothesis generations. Therefore, this review paper focuses on the pressing issues and opportunities previously omitted, including 1) development of brain FC networks in the first five years of life, 2) the newly emerging rs-fMRI analysis methods that have been used or can be useful for these studies, 3) large-scale data-sharing projects, and 4) current issues and future trends. Importantly, we aimed to integrate the existing findings to the best of our knowledge, to form a big picture of the field of early brain development and to generate hypotheses for future studies. Particularly, we will emphasize an HCP-style data processing pipeline for infant rs-fMRI analyses. Results of recent advances in the brain functional “connectome” and “dynamic functional connectivity (dFC)” will be highlighted. Finally, it is one of our goals that a unified infant rs-fMRI pilot processing pipeline can be formulated through this review article.
Acknowledgment
This study was supported in part by NIH grants (MH100217, MH108914, MH107815, and EB022880). This work utilizes approaches developed by an NIH grant (1U01MH110274) and the efforts of the UNC/UMN Baby Connectome Project (BCP) Consortium. This work also uses the data from “Multi-visit Advanced Pediatric brain imaging study for characterizing structural and functional development (MAP Study)”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors claim no conflict of interest.
References
- Abrol A, Chaze C, Damaraju E, Calhoun VD, 2016. The chronnectome: Evaluating replicability of dynamic connectivity patterns in 7500 resting fMRI datasets. Conf Proc IEEE Eng Med Biol Soc 2016, 5571–5574. [DOI] [PubMed] [Google Scholar]
- Abrol A, Damaraju E, Miller RL, Stephen JM, Claus ED, Mayer AR, Calhoun VD, 2017. Replicability of time-varying connectivity patterns in large resting state fMRI samples. Neuroimage 163, 160–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Keith Smith J, Gilmore JH, Gao W, 2015a. Consistent anterior-posterior segregation of the insula during the first 2 years of life. Cereb Cortex 25, 1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Smith JK, Goldman BD, Reznick JS, Gilmore JH, Gao W, 2015b. Frequency of spontaneous BOLD signal shifts during infancy and correlates with cognitive performance. Dev Cogn Neurosci 12, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, Gilmore JH, Gao W, 2014. Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci 34, 9067–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD, 2014. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24, 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altaye M, Holland SK, Wilke M, Gaser C, 2008. Infant brain probability templates for MRI segmentation and normalization. Neuroimage 43, 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Pazderova L, Chew A, Tusor N, Merchant N, Arichi T, Allsop JM, Cowan FM, Edwards AD, Counsell SJ, 2015. Thalamocortical Connectivity Predicts Cognition in Children Born Preterm. Cereb Cortex 25, 4310–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ, Fox NA, 2000. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Dev Psychobiol 37, 44–56. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST, 2011. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci U S A 108, 7641–7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Rombach MP, Porter MA, Mucha PJ, Grafton ST, 2013. Task-based core-periphery organization of human brain dynamics. PLoS Comput Biol 9, e1003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battiston F, Nicosia V, Chavez M, Latora V, 2017. Multilayer motif analysis of brain networks. Chaos 27, 047404. [DOI] [PubMed] [Google Scholar]
- Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, Nair VA, Meyerand ME, Prabhakaran V, 2013. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage 83, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS, 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34, 537–541. [DOI] [PubMed] [Google Scholar]
- Biswal BB, 2012. Resting state fMRI: a personal history. Neuroimage 62, 938–944. [DOI] [PubMed] [Google Scholar]
- Blesa M, Serag A, Wilkinson AG, Anblagan D, Telford EJ, Pataky R, Sparrow SA, Macnaught G, Semple SI, Bastin ME, Boardman JP, 2016. Parcellation of the Healthy Neonatal Brain into 107 Regions Using Atlas Propagation through Intermediate Time Points in Childhood. Front Neurosci 10, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer J, Xiao Y, Poulain T, Friederici AD, Schirmer A, 2016. Frequency of Maternal Touch Predicts Resting Activity and Connectivity of the Developing Social Brain. Cereb Cortex 26, 3544–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Vincent JL, 2007. Unrest at rest: default activity and spontaneous network correlations. Neuroimage 37, 1091–1096; discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Miller R, Pearlson G, Adali T, 2014. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron 84, 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, He Y, Dai Z, Liao X, Jeon T, Ouyang M, Chalak L, Bi Y, Rollins N, Dong Q, Huang H, 2017a. Early Development of Functional Network Segregation Revealed by Connectomic Analysis of the Preterm Human Brain. Cereb Cortex 27, 1949–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Huang H, He Y, 2017b. Developmental Connectomics from Infancy through Early Childhood. Trends Neurosci 40, 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Huang H, Peng Y, Dong Q, He Y, 2016. Toward Developmental Connectomics of the Human Brain. Front Neuroanat 10, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Yang J, Zhang J, Nie D, Kim MJ, Wang Q, Shen D, 2017c. Deformable Image Registration based on Similarity-Steered CNN Regression. Med Image Comput Comput Assist Interv 10433, 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai LR, Khambhati AN, Ciric R, Moore T, Gur RC, Gur RE, Satterthwaite TD, Bassett DS, 2017. Evolution of brain network dynamics in neurodevelopment. Network Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Akazawa K, Yamakawa R, Hayama S, Buchthal S, Alicata D, Andres T, Castillo D, Oishi K, Skranes J, Ernst T, 2016. Delayed early developmental trajectories of white matter tracts of functional pathways in preterm-born infants: Longitudinal diffusion tensor imaging data. Data Brief 6, 1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang H, Gao Y, Wee CY, Li G, Shen D, 2016a. High-order resting-state functional connectivity network for MCI classification. Hum Brain Mapp 37, 3282–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang H, Lee SW, Shen D, 2017a. Hierarchical High-Order Functional Connectivity Networks and Selective Feature Fusion for MCI Classification. Neuroinformatics 15, 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang H, Shen D, 2016b. Ensemble Hierarchical High-Order Functional Connectivity Networks for MCI Classification. Med Image Comput Comput Assist Interv 9901, 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang H, Zhang L, Shen C, Lee SW, Shen D, 2017b. Extraction of dynamic functional connectivity from brain grey matter and white matter for MCI classification. Hum Brain Mapp 38, 5019–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, D’Esposito M, 2016. The Segregation and Integration of Distinct Brain Networks and Their Relationship to Cognition. J Neurosci 36, 12083–12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G, van den Heuvel MP, 2013. The ontogeny of the human connectome: development and dynamic changes of brain connectivity across the life span. Neuroscientist 19, 616–628. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A, 2003. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry 60, 837–844. [DOI] [PubMed] [Google Scholar]
- Dale N, Sakkalou E, O’Reilly M, Springall C, De Haan M, Salt A, 2017. Functional vision and cognition in infants with congenital disorders of the peripheral visual system. Dev Med Child Neurol 59, 725–731. [DOI] [PubMed] [Google Scholar]
- Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, Mueller BA, Pearlson GD, Potkin SG, Preda A, Turner JA, Vaidya JG, van Erp TG, Calhoun VD, 2014a. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin 5, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Caprihan A, Lowe JR, Allen EA, Calhoun VD, Phillips JP, 2014b. Functional connectivity in the developing brain: a longitudinal study from 4 to 9months of age. Neuroimage 84, 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Phillips JR, Lowe JR, Ohls R, Calhoun VD, Caprihan A, 2010. Resting-state functional connectivity differences in premature children. Front Syst Neurosci 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD, 2009. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct 213, 525–533. [DOI] [PubMed] [Google Scholar]
- De Asis-Cruz J, Donofrio MT, Vezina G, Limperopoulos C, 2018. Aberrant brain functional connectivity in newborns with congenital heart disease before cardiac surgery. Neuroimage Clin 17, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Domenico M, 2017. Multilayer modeling and analysis of human brain networks. Gigascience 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Domenico M, Sasai S, Arenas A, 2016. Mapping Multiplex Hubs in Human Functional Brain Networks. Front Neurosci 10, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Corbetta M, Betti V, Della Penna S, 2017. Cortical cores in network dynamics. Neuroimage. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L, 2002. Functional neuroimaging of speech perception in infants. Science 298, 2013–2015. [DOI] [PubMed] [Google Scholar]
- Deoni SC, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Williams SC, Murphy DG, 2011. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci 31, 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Fair DA, Kelly C, Satterthwaite TD, Castellanos FX, Thomason ME, Craddock RC, Luna B, Leventhal BL, Zuo XN, Milham MP, 2014a. Unraveling the miswired connectome: a developmental perspective. Neuron 83, 1335–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, O’Connor D, Chen B, Alaerts K, Anderson JS, Assaf M, Balsters JH, Baxter L, Beggiato A, Bernaerts S, Blanken LM, Bookheimer SY, Braden BB, Byrge L, Castellanos FX, Dapretto M, Delorme R, Fair DA, Fishman I, Fitzgerald J, Gallagher L, Keehn RJ, Kennedy DP, Lainhart JE, Luna B, Mostofsky SH, Muller RA, Nebel MB, Nigg JT, O’Hearn K, Solomon M, Toro R, Vaidya CJ, Wenderoth N, White T, Craddock RC, Lord C, Leventhal B, Milham MP, 2017. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci Data 4, 170010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K, Anderson JS, Assaf M, Bookheimer SY, Dapretto M, Deen B, Delmonte S, Dinstein I, Ertl-Wagner B, Fair DA, Gallagher L, Kennedy DP, Keown CL, Keysers C, Lainhart JE, Lord C, Luna B, Menon V, Minshew NJ, Monk CS, Mueller S, Muller RA, Nebel MB, Nigg JT, O’Hearn K, Pelphrey KA, Peltier SJ, Rudie JD, Sunaert S, Thioux M, Tyszka JM, Uddin LQ, Verhoeven JS, Wenderoth N, Wiggins JL, Mostofsky SH, Milham MP, 2014b. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry 19, 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, Courchesne E, 2011. Disrupted neural synchronization in toddlers with autism. Neuron 70, 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong P, Cao X, Zhang J, Kim M, Wu G, Shen D, 2017. Efficient Groupwise Registration for Brain MRI by Fast Initialization. Mach Learn Med Imaging 10541, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, Counsell SJ, Murgasova M, Aljabar P, Nunes RG, Larkman DJ, Rees G, Edwards AD, 2010. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci U S A 107, 20015–20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR Jr., Barch DM, Petersen SE, Schlaggar BL, 2010. Prediction of individual brain maturity using fMRI. Science 329, 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RW, Adams C, Nishino T, Hazlett HC, Wolff JJ, Zwaigenbaum L, Constantino JN, Shen MD, Swanson MR, Elison JT, Kandala S, Estes AM, Botteron KN, Collins L, Dager SR, Evans AC, Gerig G, Gu H, McKinstry RC, Paterson S, Schultz RT, Styner M, Schlaggar BL, Pruett JR Jr., Piven J, 2017. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RW, Gao W, Lin W, 2016. Longitudinal Study of the Emerging Functional Connectivity Asymmetry of Primary Language Regions during Infancy. J Neurosci 36, 10883–10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RW, Short SJ, Lin W, Gilmore JH, Gao W, 2015. Network-Level Connectivity Dynamics of Movie Watching in 6-Year-Old Children. Front Hum Neurosci 9, 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE, 2009. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol 5, e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL, 2007. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A 104, 13507–13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferradal SL, Liao SM, Eggebrecht AT, Shimony JS, Inder TE, Culver JP, Smyser CD, 2016. Functional Imaging of the Developing Brain at the Bedside Using Diffuse Optical Tomography. Cereb Cortex 26, 1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbon S, Andersson J, Harrison S, Robinson E, Bozek J, Makropoulos A, Bastiani M, Griffanti L, Wright R, Schuh A, Hughes E, O’Muircheartaigh J, Gomes A, Allsop J, Steinweg J, Tusor N, Wurie J, Bueno-Conde J, Abaei M, Price A, Cordero-Grande L, Hutter J, Beckmann CF, Hajnal JV, Rueckert D, Edwards AD, Smith SM, Jenkinson M, Duff EP, 2017. The developing Human Connectome Project automated functional pre-processing pipeline for neonates. Annual Conference of Organization of Human Brain Mapping (OHBM), Vancouver, Canada. [Google Scholar]
- Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K, 2015. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage 111, 611–621. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME, 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8, 700–711. [DOI] [PubMed] [Google Scholar]
- Fransson P, 2005. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp 26, 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, 2006. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44, 2836–2845. [DOI] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, Lagercrantz H, 2011. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex 21, 145–154. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Engstrom M, Hallberg B, Mosskin M, Aden U, Lagercrantz H, Blennow M, 2009. Spontaneous brain activity in the newborn brain during natural sleep--an fMRI study in infants born at full term. Pediatr Res 66, 301–305. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Aden U, 2007. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A 104, 15531–15536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, 2011. Functional and effective connectivity: a review. Brain Connect 1, 13–36. [DOI] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Elton A, Hernandez-Castillo CR, Smith JK, Ramirez J, Lin W, 2015a. Functional Network Development During the First Year: Relative Sequence and Socioeconomic Correlations. Cereb Cortex 25, 2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Smith JK, Gilmore JH, Lin W, 2015b. Development of human brain cortical network architecture during infancy. Brain Struct Funct 220, 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Elton A, Zhu H, Alcauter S, Smith JK, Gilmore JH, Lin W, 2014. Intersubject variability of and genetic effects on the brain’s functional connectivity during infancy. J Neurosci 34, 11288–11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Giovanello KS, Smith JK, Shen D, Zhu H, Lin W, 2011. Temporal and spatial evolution of brain network topology during the first two years of life. PLoS One 6, e25278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Shen D, Smith JK, Zhu H, Lin W, 2013. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb Cortex 23, 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W, Grewen K, Gilmore JH, 2016. Functional Connectivity of the Infant Human Brain: Plastic and Modifiable. Neuroscientist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, Lin W, 2009. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci U S A 106, 6790–6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, 2010. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron 67, 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC, 2016a. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Smith SM, Marcus DS, Andersson JL, Auerbach EJ, Behrens TE, Coalson TS, Harms MP, Jenkinson M, Moeller S, Robinson EC, Sotiropoulos SN, Xu J, Yacoub E, Ugurbil K, Van Essen DC, 2016b. The Human Connectome Project’s neuroimaging approach. Nat Neurosci 19, 1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M, 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousias IS, Edwards AD, Rutherford MA, Counsell SJ, Hajnal JV, Rueckert D, Hammers A, 2012. Magnetic resonance imaging of the newborn brain: manual segmentation of labelled atlases in term-born and preterm infants. Neuroimage 62, 1499–1509. [DOI] [PubMed] [Google Scholar]
- Gousias IS, Hammers A, Counsell SJ, Srinivasan L, Rutherford MA, Heckemann RA, Hajnal JV, Rueckert D, Edwards AD, 2013. Magnetic resonance imaging of the newborn brain: automatic segmentation of brain images into 50 anatomical regions. PLoS One 8, e59990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Pfeifer JH, Fisher PA, Lin W, Gao W, Fair DA, 2015. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev Cogn Neurosci 12, 12–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DS, Fair DA, 2017. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Satterthwaite TD, Medaglia JD, Yang M, Gur RE, Gur RC, Bassett DS, 2015. Emergence of system roles in normative neurodevelopment. Proc Natl Acad Sci U S A 112, 13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Sporns O, Madan N, Cammoun L, Pienaar R, Wedeen VJ, Meuli R, Thiran JP, Grant PE, 2010. White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci U S A 107, 19067–19072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KN, Collins DL, Constantino JN, Dager SR, Estes AM, Evans AC, Fonov VS, Gerig G, Kostopoulos P, McKinstry RC, Pandey J, Paterson S, Pruett JR, Schultz RT, Shaw DW, Zwaigenbaum L, Piven J, 2017. Early brain development in infants at high risk for autism spectrum disorder. Nature 542, 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Parikh NA, 2016. Brain functional network connectivity development in very preterm infants: The first six months. Early Hum Dev 98, 29–35. [DOI] [PubMed] [Google Scholar]
- Hoff GE, Van den Heuvel MP, Benders MJ, Kersbergen KJ, De Vries LS, 2013. On development of functional brain connectivity in the young brain. Front Hum Neurosci 7, 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P, 2009. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A 106, 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BR, Styner M, Gao W, Yap PT, Wang L, Baluyot K, Yacoub E, Jamison K, Potts T, Salzwedel AP, Li G, Gilmore JH, Piven J, Smith JK, Shen D, Ugurbil K, Zhu H, Lin W, Elison JT, 2018. The UNC/UMN Baby Connectome Project (BCP): An Overview of the Study Design and Protocol Development. Neuroimage In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Ding Z, Mao D, Yuan J, Zhu F, Chen S, Xu Y, Lou L, Feng X, Qi L, Qiu W, Zhang H, Zang YF, 2016. PreSurgMapp: a MATLAB Toolbox for Presurgical Mapping of Eloquent Functional Areas Based on Task-Related and Resting-State Functional MRI. Neuroinformatics 14, 421–438. [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J, Handwerker DA, Keilholz S, Kiviniemi V, Leopold DA, de Pasquale F, Sporns O, Walter M, Chang C, 2013. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80, 360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaque M, Manning JH, Woolsey MD, Franklin CG, Tullis EW, Beckmann CF, Fox PT, 2017. Functional integrity in children with anoxic brain injury from drowning. Hum Brain Mapp 38, 4813–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab A, Schwartz E, Kasprian G, Gruber GM, Prayer D, Schopf V, Langs G, 2014. Fetal functional imaging portrays heterogeneous development of emerging human brain networks. Front Hum Neurosci 8, 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LB, Rothbart MK, Posner MI, 2003. Executive attention in preschool children Development of executive attention in preschool children. Dev Sci 6, 498–504. [Google Scholar]
- Kaufmann T, Alnaes D, Doan NT, Brandt CL, Andreassen OA, Westlye LT, 2017. Delayed stabilization and individualization in connectome development are related to psychiatric disorders. Nat Neurosci 20, 513–515. [DOI] [PubMed] [Google Scholar]
- Kessler D, Angstadt M, Sripada C, 2016. Growth Charting of Brain Connectivity Networks and the Identification of Attention Impairment in Youth. JAMA Psychiatry 73, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keunen K, Counsell SJ, Benders MJ, 2017. The emergence of functional architecture during early brain development. Neuroimage. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Jauhiainen J, Tervonen O, Paakko E, Oikarinen J, Vainionpaa V, Rantala H, Biswal B, 2000. Slow vasomotor fluctuation in fMRI of anesthetized child brain. Magn Reson Med 44, 373–378. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Kantola JH, Jauhiainen J, Hyvarinen A, Tervonen O, 2003. Independent component analysis of nondeterministic fMRI signal sources. Neuroimage 19, 253–260. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, 2004. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci 16, 1412–1425. [DOI] [PubMed] [Google Scholar]
- Kong XZ, Zhen Z, Li X, Lu HH, Wang R, Liu L, He Y, Zang Y, Liu J, 2014. Individual differences in impulsivity predict head motion during magnetic resonance imaging. PLoS One 9, e104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklisova-Murgasova M, Aljabar P, Srinivasan L, Counsell SJ, Doria V, Serag A, Gousias IS, Boardman JP, Rutherford MA, Edwards AD, Hajnal JV, Rueckert D, 2011. A dynamic 4D probabilistic atlas of the developing brain. Neuroimage 54, 2750–2763. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Scheinost D, Lacadie C, Sze G, Schneider KC, Dai F, Constable RT, Ment LR, 2015. Adaptive mechanisms of developing brain: cerebral lateralization in the prematurely-born. Neuroimage 108, 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SH, Vasung L, Ment LR, Huppi PS, 2014. The role of neuroimaging in predicting neurodevelopmental outcomes of preterm neonates. Clin Perinatol 41, 257–283. [DOI] [PubMed] [Google Scholar]
- Levman J, Takahashi E, 2015a. Multivariate analyses applied to fetal, neonatal and pediatric MRI of neurodevelopmental disorders. Neuroimage Clin 9, 532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levman J, Takahashi E, 2015b. Multivariate Analyses Applied to Healthy Neurodevelopment in Fetal, Neonatal, and Pediatric MRI. Front Neuroanat 9, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Nie J, Wu G, Wang Y, Shen D, 2012. Consistent reconstruction of cortical surfaces from longitudinal brain MR images. Neuroimage 59, 3805–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang L, Shi F, Gilmore JH, Lin W, Shen D, 2015a. Construction of 4D high-definition cortical surface atlases of infants: Methods and applications. Med Image Anal 25, 22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang L, Yap P-T, Wang F, Wu Z, Meng Y, Dong P, Kim J, Shi F, Rekik I, Lin W, Shen D, 2018. Computational Neuroanatomy of Baby Brains: A Review. Neuroimage In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zang YF, Zhang H, 2015b. Exploring Dynamic Brain Functional Networks Using Continuous “State-Related” Functional MRI. Biomed Res Int 2015, 824710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, Zhang H, Xu Y, Jia W, Zang Y, Li K, 2015. Disruption of cortical integration during midazolam-induced light sedation. Hum Brain Mapp 36, 4247–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Zhu Q, Gao W, Chen Y, Toh CH, Styner M, Gerig G, Smith JK, Biswal B, Gilmore JH, 2008. Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am J Neuroradiol 29, 1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang H, Rekik I, Chen X, Wang Q, Shen D, 2016. Outcome prediction for patients with high-grade cliomas from brain functional and structural networks. Medical Image Computing and Computer Assisted Intervention (MICCAI 2016), Athens, Greece. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WC, Flax JF, Guise KG, Sukul V, Benasich AA, 2008. Functional connectivity of the sensorimotor area in naturally sleeping infants. Brain Res 1223, 42–49. [DOI] [PubMed] [Google Scholar]
- Liu X, Duyn JH, 2013. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc Natl Acad Sci U S A 110, 4392–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yu C, Liang M, Li J, Tian L, Zhou Y, Qin W, Li K, Jiang T, 2007. Whole brain functional connectivity in the early blind. Brain 130, 2085–2096. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, 2008. What we can do and what we cannot do with fMRI. Nature 453, 869–878. [DOI] [PubMed] [Google Scholar]
- Long X, Benischek A, Dewey D, Lebel C, 2017. Age-related functional brain changes in young children. Neuroimage 155, 322–330. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, 2012. The emergence of doing “nothing” as a viable paradigm design. Neuroimage 62, 1146–1151. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhang H, Hameed NUF, Zhang J, Yuan S, Qiu T, Shen D, Wu J, 2017. An automated method for identifying an independent component analysis-based language-related resting-state network in brain tumor subjects for surgical planning. Sci Rep 7, 13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makropoulos A, Robinson EC, Schuh A, Wright R, Fitzgibbon S, Bozek J, Counsell SJ, Steinweg J, Passerat-Palmbach J, Lenz G, Mortari F, Tenev T, Duff EP, Bastiani M, Cordero-Grande L, Hughes E, Tusor N, Tournier J-D, Hutter J, Price AN, Murgasova M, Kelly C, Rutherford M, Smith SM, Edwards AD, Hajnal JV, Jenkinson M, Rueckert D, 2017. The Developing Human Connectome Project: a Minimal Processing Pipeline for Neonatal Cortical Surface Reconstruction. bioRxiv. http://dx.doi.org/10.1101/125526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, Bezgin G, Eickhoff SB, Castellanos FX, Petrides M, Jefferies E, Smallwood J, 2016. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci U S A 113, 12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Li G, Gao Y, Lin W, Shen D, 2016. Learning-based subject-specific estimation of dynamic maps of cortical morphology at missing time points in longitudinal infant studies. Hum Brain Mapp 37, 4129–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15, 483–506. [DOI] [PubMed] [Google Scholar]
- Menon V, 2013. Developmental pathways to functional brain networks: emerging principles. Trends Cogn Sci 17, 627–640. [DOI] [PubMed] [Google Scholar]
- Mevel K, Fransson P, 2016. The functional brain connectome of the child and autism spectrum disorders. Acta Paediatr 105, 1024–1035. [DOI] [PubMed] [Google Scholar]
- Mitra A, Snyder AZ, Tagliazucchi E, Laufs H, Elison J, Emerson RW, Shen MD, Wolff JJ, Botteron KN, Dager S, Estes AM, Evans A, Gerig G, Hazlett HC, Paterson SJ, Schultz RT, Styner MA, Zwaigenbaum L, Schlaggar BL, Piven J, Pruett JR Jr., Raichle M, 2017. Resting-state fMRI in sleeping infants more closely resembles adult sleep than adult wakefulness. PLoS One 12, e0188122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongerson CRL, Jennings RW, Borsook D, Becerra L, Bajic D, 2017. Resting-State Functional Connectivity in the Infant Brain: Methods, Pitfalls, and Potentiality. Front Pediatr 5, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha PJ, Richardson T, Macon K, Porter MA, Onnela JP, 2010. Community structure in time-dependent, multiscale, and multiplex networks. Science 328, 876–878. [DOI] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, Panek LJ, Brown S, Zavitz ST, Li Q, Sikka S, Gutman D, Bangaru S, Schlachter RT, Kamiel SM, Anwar AR, Hinz CM, Kaplan MS, Rachlin AB, Adelsberg S, Cheung B, Khanuja R, Yan C, Craddock CC, Calhoun V, Courtney W, King M, Wood D, Cox CL, Kelly AM, Di Martino A, Petkova E, Reiss PT, Duan N, Thomsen D, Biswal B, Coffey B, Hoptman MJ, Javitt DC, Pomara N, Sidtis JJ, Koplewicz HS, Castellanos FX, Leventhal BL, Milham MP, 2012. The NKI-Rockland Sample: A Model for Accelerating the Pace of Discovery Science in Psychiatry. Front Neurosci 6, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW, 1990. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 87, 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Mori S, Donohue PK, Ernst T, Anderson L, Buchthal S, Faria A, Jiang H, Li X, Miller MI, van Zijl PC, Chang L, 2011. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage 56, 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Kang H, Detre JA, Roberts TPL, Huang H, 2017. Short-range connections in the developmental connectome during typical and atypical brain maturation. Neurosci Biobehav Rev 83, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, Rogers SJ, Rozga A, Sangha S, Sigman M, Steinfeld MB, Young GS, 2010. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry 49, 256–266 e251–252. [PMC free article] [PubMed] [Google Scholar]
- Patel AX, Bullmore ET, 2016. A wavelet-based estimator of the degrees of freedom in denoised fMRI time series for probabilistic testing of functional connectivity and brain graphs. Neuroimage 142, 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AX, Kundu P, Rubinov M, Jones PS, Vertes PE, Ersche KD, Suckling J, Bullmore ET, 2014. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. Neuroimage 95, 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE, 2010. The development of human functional brain networks. Neuron 67, 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE, 2014. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF, 2015. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112, 267–277. [DOI] [PubMed] [Google Scholar]
- Raichle ME, 2006. Neuroscience. The brain’s dark energy. Science 314, 1249–1250. [PubMed] [Google Scholar]
- Raschle N, Zuk J, Ortiz-Mantilla S, Sliva DD, Franceschi A, Grant PE, Benasich AA, Gaab N, 2012. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann N Y Acad Sci 1252, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Kennedy DP, Courchesne E, 2007. fMRI during natural sleep as a method to study brain function during early childhood. Neuroimage 38, 696–707. [DOI] [PubMed] [Google Scholar]
- Rekik I, Li G, Yap PT, Chen G, Lin W, Shen D, 2017. Joint prediction of longitudinal development of cortical surfaces and white matter fibers from neonatal MRI. Neuroimage 152, 411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE, Sanchez C, Phillips-Meek M, Xie W, 2016. A database of age-appropriate average MRI templates. Neuroimage 124, 1254–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond S, Johnson KA, Seal ML, Allen NB, Whittle S, 2016. Development of brain networks and relevance of environmental and genetic factors: A systematic review. Neurosci Biobehav Rev 71, 215–239. [DOI] [PubMed] [Google Scholar]
- Rosazza C, Minati L, 2011. Resting-state brain networks: literature review and clinical applications. Neurol Sci 32, 773–785. [DOI] [PubMed] [Google Scholar]
- Routier L, Mahmoudzadeh M, Panzani M, Azizollahi H, Goudjil S, Kongolo G, Wallois F, 2017. Plasticity of neonatal neuronal networks in very premature infants: Source localization of temporal theta activity, the first endogenous neural biomarker, in temporoparietal areas. Hum Brain Mapp 38, 2345–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM, 2014. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 90, 449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CE, Richards JE, Almli CR, 2012. Neurodevelopmental MRI brain templates for children from 2 weeks to 4 years of age. Dev Psychobiol 54, 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Kwon SH, Shen X, Lacadie C, Schneider KC, Dai F, Ment LR, Constable RT, 2016. Preterm birth alters neonatal, functional rich club organization. Brain Struct Funct 221, 3211–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Lacadie C, Vohr BR, Schneider KC, Papademetris X, Constable RT, Ment LR, 2015. Cerebral Lateralization is Protective in the Very Prematurely Born. Cereb Cortex 25, 1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf V, Kasprian G, Schwindt J, Kollndorfer K, Prayer D, 2012. Visualization of resting-state networks in utero. Ultrasound Obstet Gynecol 39, 487–488. [DOI] [PubMed] [Google Scholar]
- Serag A, Aljabar P, Ball G, Counsell SJ, Boardman JP, Rutherford MA, Edwards AD, Hajnal JV, Rueckert D, 2012. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. Neuroimage 59, 2255–2265. [DOI] [PubMed] [Google Scholar]
- Seshamani S, Blazejewska AI, McKown S, Caucutt J, Dighe M, Gatenby C, Studholme C, 2016. Detecting default mode networks in utero by integrated 4D fMRI reconstruction and analysis. Hum Brain Mapp 37, 4158–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Davatzikos C, 2002. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging 21, 1421–1439. [DOI] [PubMed] [Google Scholar]
- Shen D, Davatzikos C, 2004. Measuring temporal morphological changes robustly in brain MR images via 4-dimensional template warping. Neuroimage 21, 1508–1517. [DOI] [PubMed] [Google Scholar]
- Shen X, Tokoglu F, Papademetris X, Constable RT, 2013. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage 82, 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yap PT, Wu G, Jia H, Gilmore JH, Lin W, Shen D, 2011. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One 6, e18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, Duff E, Feinberg DA, Griffanti L, Harms MP, Kelly M, Laumann T, Miller KL, Moeller S, Petersen S, Power J, Salimi-Khorshidi G, Snyder AZ, Vu AT, Woolrich MW, Xu J, Yacoub E, Ugurbil K, Van Essen DC, Glasser MF, 2013. Resting-state fMRI in the Human Connectome Project. Neuroimage 80, 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF, 2009. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A 106, 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Dosenbach NU, Smyser TA, Snyder AZ, Rogers CE, Inder TE, Schlaggar BL, Neil JJ, 2016a. Prediction of brain maturity in infants using machine-learning algorithms. Neuroimage 136, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ, 2010. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex 20, 2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Neil JJ, 2015. Use of resting-state functional MRI to study brain development and injury in neonates. Semin Perinatol 39, 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Neil JJ, 2011. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. Neuroimage 56, 1437–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, Neil JJ, 2016b. Resting-State Network Complexity and Magnitude Are Reduced in Prematurely Born Infants. Cereb Cortex 26, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AZ, Raichle ME, 2012. A brief history of the resting state: the Washington University perspective. Neuroimage 62, 902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole-Padulles C, Castro-Fornieles J, de la Serna E, Calvo R, Baeza I, Moya J, Lazaro L, Rosa M, Bargallo N, Sugranyes G, 2016. Intrinsic connectivity networks from childhood to late adolescence: Effects of age and sex. Dev Cogn Neurosci 17, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, 2013. Structure and function of complex brain networks. Dialogues Clin Neurosci 15, 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley N, Shai S, Taylor D, Mucha PJ, 2016. Clustering network layers with the strata multilayer stochastic block model. IEEE Trans Netw Sci Eng 3, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V, 2010. Development of functional and structural connectivity within the default mode network in young children. Neuroimage 52, 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Dassanayake MT, Shen S, Katkuri Y, Alexis M, Anderson AL, Yeo L, Mody S, Hernandez-Andrade E, Hassan SS, Studholme C, Jeong JW, Romero R, 2013. Cross-hemispheric functional connectivity in the human fetal brain. Sci Transl Med 5, 173ra124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hibar DP, Stein JL, Prasad G, Jahanshad N, 2016. Genetics of the Connectome and the ENIGMA Project In: Kennedy H, Van Essen DC, Christen Y (Eds.), Micro-, Meso- and Macro-Connectomics of the Brain, Cham (CH). [Google Scholar]
- Thompson WH, Brantefors P, Fransson P, 2017. From static to temporal network theory: Applications to functional brain connectivity. Network Neuroscience 1, 69–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WH, Fransson P, 2016. Bursty properties revealed in large-scale brain networks with a point-based method for dynamic functional connectivity. Sci Rep 6, 39156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmin H, Beckmann CF, O’Muircheartaigh J, Ball G, Nongena P, Makropoulos A, Ederies A, Counsell SJ, Kennea N, Arichi T, Tusor N, Rutherford MA, Azzopardi D, Gonzalez-Cinca N, Hajnal JV, Edwards AD, 2015. Specialization and integration of functional thalamocortical connectivity in the human infant. Proc Natl Acad Sci U S A 112, 6485–6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymofiyeva O, Hess CP, Xu D, Barkovich AJ, 2014. Structural MRI connectome in development: challenges of the changing brain. Br J Radiol 87, 20140086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V, 2010. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci 4, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V, 2013. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci 7, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE, 2010. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 20, 519–534. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL, 2010. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103, 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL, 2012. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59, 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes PE, Bullmore ET, 2015. Annual research review: Growth connectomics--the organization and reorganization of brain networks during normal and abnormal development. J Child Psychol Psychiatry 56, 299–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AC, Power JD, Petersen SE, Schlaggar BL, 2010. Development of the brain’s functional network architecture. Neuropsychol Rev 20, 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shi F, Gao Y, Li G, Gilmore JH, Lin W, Shen D, 2014. Integration of sparse multi-modality representation and anatomical constraint for isointense infant brain MR image segmentation. Neuroimage 89, 152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Cao X, Wang Z, Gao Y, Hu S, Wang L, Wu G, Shen D, 2017. Learning-based deformable registration for infant MRI by integrating random forest with auto-context model. Med Phys 44, 6289–6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Gao Y, Shi F, Ma G, Jewells V, Shen D, 2017. Segmenting hippocampal subfields from 3T MRI with multi-modality images. Med Image Anal 43, 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP, 2013. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76, 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Meng Y, Li G, Lin W, Zhao D, Shen D, 2017. Longitudinally-Consistent Parcellation of Infant Population Cortical Surfaces Based on Functional Connectivity In: Wang Q, Shi Y, Suk H, Suzuki K (Eds.), Machine Learning in Medical Imaging (MLMI 2017). Springer, Cham, Quebec City, QC, Canada, pp. 194–202. [Google Scholar]
- Yerys BE, Jankowski KF, Shook D, Rosenberger LR, Barnes KA, Berl MM, Ritzl EK, Vanmeter J, Vaidya CJ, Gaillard WD, 2009. The fMRI success rate of children and adolescents: typical development, epilepsy, attention deficit/hyperactivity disorder, and autism spectrum disorders. Hum Brain Mapp 30, 3426–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF, 2007. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29, 83–91. [DOI] [PubMed] [Google Scholar]
- Zeng LL, Wang D, Fox MD, Sabuncu M, Hu D, Ge M, Buckner RL, Liu H, 2014. Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci U S A 111, 6058–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Raichle ME, 2010. Disease and the brain’s dark energy. Nat Rev Neurol 6, 15–28. [DOI] [PubMed] [Google Scholar]
- Zhang H, Chen X, Shi F, Li G, Kim M, Giannakopoulos P, Haller S, Shen D, 2016. Topographical Information-Based High-Order Functional Connectivity and Its Application in Abnormality Detection for Mild Cognitive Impairment. J Alzheimers Dis 54, 1095–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Chen X, Zhang Y, Shen D, 2017a. Test-Retest Reliability of “High-Order” Functional Connectivity in Young Healthy Adults. Front Neurosci 11, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Duan L, Zhang YJ, Lu CM, Liu H, Zhu CZ, 2011. Test-retest assessment of independent component analysis-derived resting-state functional connectivity based on functional near-infrared spectroscopy. Neuroimage 55, 607–615. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yin W, Lin W, Shen D, 2017b. The Developing Triple Networks in Infants from 2-Week-Old to 2-Year-Old: A Longitudinal Study. OHBM 2017, Vancouver, Canada. [Google Scholar]
- Zhang H, Yin W, Lin W, Shen D, 2017c. Early Brain Functional Segregation and Integration Predict Later Cognitive Performance In: Wu G, Laurienti P, Bonilha L, Munsell B (Eds.), Connectomics in NeuroImaging (CNI2017). Springer, Quebec, Canada. [Google Scholar]
- Zhang H, Yin W, Meng Y, Lin W, Shen D, 2017d. Functional and Structural Developments of Medial Frontal Subdivisions in First 2 Years of Life. OHBM 2017, Vancouver, Canada. [Google Scholar]
- Zhang H, Zhang YJ, Lu CM, Ma SY, Zang YF, Zhu CZ, 2010. Functional connectivity as revealed by independent component analysis of resting-state fNIRS measurements. Neuroimage 51, 1150–1161. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Chen X, Lee SW, Shen D, 2017e. Hybrid High-order Functional Connectivity Networks Using Resting-state Functional MRI for Mild Cognitive Impairment Diagnosis. Sci Rep 7, 6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhu X, Zhang H, Gao W, Shen D, Wu G, 2016. Reveal Consistent Spatial-Temporal Patterns from Dynamic Functional Connectivity for Autism Spectrum Disorder Identification. Med Image Comput Comput Assist Interv 9900, 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O, Milham MP, 2012. Network centrality in the human functional connectome. Cereb Cortex 22, 1862–1875. [DOI] [PubMed] [Google Scholar]
- Zuo XN, He Y, Betzel RF, Colcombe S, Sporns O, Milham MP, 2017. Human Connectomics across the Life Span. Trends Cogn Sci 21, 32–45. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang YF, Castellanos FX, Milham MP, 2010. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 30, 15034–15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Review of reviews.