Abstract
Psychological stress is known to have profound effects on immune function and to promote inflammatory conditions. Elevated circulating levels of cytokines associated with stress are known to increase the risk to several diseases, but little is known about this mechanism. This study assessed the role of T cells on cytokine levels after exposure to stress in the learned helplessness paradigm. Adoptive transfer of CD4+ T cells into Rag2−/− mice did not change cytokine levels to stress while CD8+ T cells resulted in an increase in TNF-α, IL-6 and IFN-γ in stressed Rag2−/− mice. Moreover, depletion of CD8+ T cells in WT mice abolished these cytokine responses to stress. Corticosterone and behavioral stress responsiveness was impaired in Rag2−/− mice reconstituted with CD8+ T cells. Notably, depletion of these cells in WT mice had no effect on behavior or corticosterone levels. Exposure to stress did not change the expression of canonical markers of T cell activation including CD62L and CD44 or modified intracellular cytokine content, suggesting that they are not the main producers of circulating cytokines in response to stress. These results show that CD8+ T cells promote TNF-α, IL-6 and IFN-γ responses to stress, possibly by stimulating non-lymphoid cells.
1. Introduction
Clinical and basic research has shown that psychological stress has profound effects on immune function, which varies depending on the type, intensity and duration of the stressor [1]. Stress-induced immune modulation, mediated primarily by the stress hormones corticosterone (CORT) and adrenaline, is part of the physiological mechanism of adaptation to environmental challenges [2]. Of importance for human health, prolonged and repeated exposure to moderate to high levels of stress has been shown to be a critical factor leading to pathophysiology in almost all aspects of human diseases, including susceptibility and progression to infection [3], cancer [4], autoimmune [5], cardiovascular [6] and metabolic diseases, as well as psychiatric illnesses [7, 8]. A hallmark of the immunological response to acute psychological stress is a transient increase in circulating levels of cytokines in both humans and laboratory animals [9, 10]. It is believed that maladaptation during repeated stress exposure results in the consolidation of these increases creating an inflammatory milieu caused by the negative effects of stress on immune function. Indeed, elevated basal circulating levels of cytokines and increased cytokine gene expression in the brain have been extensively documented in people suffering from stress related disorders, such as depression, anxiety and posttraumatic stress disorder [11, 12]. Among the cytokines shown to be responsive to psychological stress, IL-6, TNF-α and IL-1β are the most consistently reported across studies, stressors and species [10, 13]. Moreover, IL-2, 4 and 10 and IFN-γ have also been implicated in the stress response, but with varied consistency depending on the stressor. Nevertheless, the origin, mechanisms and resolution of cytokine responses to stress remain largely to be defined.
Recent research in mice and humans has shown that stress results in the mobilization and activation of several innate immune cells, including monocytes and macrophages [14–17], all of which constitute a potential source of cytokines in response to stress. These studies confirm earlier observations proposing that stress suppresses acquired immunity while potentiating innate immunity [18, 19]. However, a role for the adaptive immune system on cytokine responses to stress has also been documented. Depletion of CD4+ Tregs enhances cytokine responses to immobilization stress [20] while adoptive transfer of lymphocytes from stressed mice into Rag2−/− mice reduces cytokine responses elicited by social defeat stress [21]. These studies indicate that T cells may provide important modulatory functions on cytokine production in response to psychological stress. In support of this possibility, several studies, including work from our laboratory, have shown a beneficial role for CD4+ T cells in stress responsiveness [22, 23], hippocampal dependent memory [24, 25] and emotional behavior [26]. Moreover, studies also document a detrimental role for differentiated Th17 CD4+ cells during stress exposure [27], and for CD8+ T cells in models of stress-induced vascular inflammation in hypertension [28–30]. Nevertheless, there is a paucity of studies examining the role of T cells on cytokines in response to stress, thus, the present study evaluated the role of CD4+ and CD8+ T cells on circulating and brain cytokine responses to repeated exposure to electric foot shocks in the learned helplessness (LH) paradigm along with corticosterone and behavioral responses. Because basal circulating levels of cytokines are used as biomarkers in clinical research [6, 11, 12, 31], cytokine measurements were performed under basal conditions, 24 h after stress sessions.
2. Material and methods
2.1. Animals and treatments
All mice were obtained from Taconic (Rensselaer, NY, USA) and allowed to acclimate to our animal facility for at least one week before starting with any procedure. Age matched mice were randomly assigned to either LH or control non-stressed groups. Lymphopenic Rag2−/− mice were reconstituted by adoptive transfer with 8–10 million naive CD4+ (n = 23) or CD8+ (n = 21) T cells in a 1:1 donor to recipient transfer from age-matched WT mice. Donor cells were prepared as previously described [32] using the EasySep CD4+ or CD8+ purification kits (Stem Cell Technologies, Vancouver, BC, Canada) following manufacturer’s guidelines. Control Rag2−/− mice (n = 18) received PBS only. The mice were left undisturbed for three weeks before starting the LH paradigm. Wild type (WT) C57BL/6 male mice were administered either anti-CD8 (α- CD8) neutralizing antibodies (n = 11) or isotype control anti-IgG (n =12) (0.5 mg in 200 μl sterile saline; BioXCell, Lebanon, NH, USA) via intraperitoneal injection 72 h prior to beginning the LH paradigm. Mice were euthanized 24 h after completing behavioral tests via isoflurane overdose; plasma was collected by cardiac puncture. All procedures were carried out under approved IACUC protocols and institutional guidelines at the University of Maryland School of Medicine.
2.2. Learned helplessness paradigm
The protocol employed was based on the model established by Shanks and Anisman for C57BL/6 mice [33]. Briefly, it consists of an inescapable stress session in which mice were placed on one side of a shuttle box with an electrified grid floor (Coulbourn Instruments; Whitehall, PA, USA) where they received 360 footshocks (2 s duration at 240 μA) with an 8 s inter-trial interval (ITI). Mice were then tested 24 h (T1) and 7 days later (T2) in an escapable stress session when they were given the option to avoid the foot-shocks by escaping through the shuttle door. For these sessions, mice underwent 30 foot-shock trials (240 μΑ) for a maximum duration of 24 s with an average ITI of 15 s. Escape behavior was evaluated by the number of escape failures during the last 25 trials.
2.3. Plasma Corticosterone and Cytokine determinations
Plasma CORT levels were determined by radioimmunoassay using the ImmuChem Corticosterone Double Antibody RIA kit (MP Biomedicals; Orangeburg, NY, USA) according to manufacturer’s instruction. Intra assay variation was less than 5%.
Plasma levels of cytokines for IL-1β, IL-2, IL-4, IL-6, IL-10, INF-γ and TNF-α were determined at the University of Maryland Cytokine Core Laboratory using the Luminex Multianalyte System (EMD Millipore; Billerica, MA, USA). The detectable range for measured cytokines was 0.064 – 10000 pg/ml.
2.4. Real-time RT-PCR determination for brain cytokines
Blood was removed from the brain by perfusion with sterile PBS. Dissected brain tissue from the hypothalamic region (Hyp) and the hippocampus (Hipp) was processed for mRNA extraction using TRIzol (Invitrogen, USA), and real-time RT-PCR performed as described previously [34]. The same cytokines assayed in plasma were assessed in the Hyp and Hipp using the primer sets listed in table 1.
Table 1.
Primer set sequences and amplification conditions used in real-time RT-PCR determinations. IL: interleukins; IFN-γ: interferon gamma; TNF-α: tumor necrosis factor alpha; GAPDH: glyceraldehyde-3-phosphate dehydrogenase transcript variant 1; ACTB: actin beta; 18S: ribosomal 18s rRNA
| Gene | Reference Sequence | Forward | Reverse | Anneal Temp (°C) | Extension Temp (°C) |
|---|---|---|---|---|---|
| IL1-ß | NM_008361.3 | GCAGGCAGTATCACTCATTG | CACCAGCAGGTTATCATCATC | 55 | 72 |
| IL6 | NM_031168.1 | AGGAGACTTCACAGAGGATAC | TTCTGCAAGTGCATCATCG | 55 | 72 |
| TNF-a | NM_013693.3 | AAGAGGCACTCCCCAAAA G | CTG GGCCATAGAACTGATGAG | 55 | 68 |
| IFN-γ | NM_008337.3 | CTAGCTCTGAGACAATGA ACG | GCCAGTTCCTCCAGATATCC | 52 | 65 |
| 18S | NR_003278.3 | CCAGTAAGTGCGGGTCAT AAG C | CCATCCAATCGGTAGTAGCGAC | 55 | 72 |
| ACTB | NM_007393.3 | TGGAGAAGATCTGGCACCAC | TGGTACGACCAGAGGCATGC | 55 | 72 |
| GAPDH | NM_001289726.1 | TCCACTCACGGCAAATTCAAC | ATGACCCTTTTGGCTCCACC | 55 | 72 |
2.5. Flow cytometry
Single cell suspensions from the lymph nodes (LNs) and Spleen were processed as described [32]. Antibodies used in this study were: anti-CD3-eFluor 450 (eBioscience, 48–0032-82), anti-CD8-FITC (BD Biosciences, cat #553030), anti-CD8-PerCP-Cy5.5 (BD Biosciences, cat #561109), anti-CD4-APC (BD Biosciences, cat #553051), anti- CD4-PE (BD Biosciences, cat #553730), anti-NK1.1-PerCP-Cy5.5 (BD Biosciences, cat #561111), anti-CD44-APC (BD Biosciences, cat #559250), anti-CD44-PE (BD Biosciences, cat #553133), PE-rat anti mouse CD62L (BD Biosciences, cat #553151; eFluor450-rat anti mouse IFNγ (eBioscience, cat #48–7311-82), PE-rat anti-mouse IL-6 (BD Biosciences, cat #: 562050) and APC-rat anti-mouse TNF-α (BD Biosciences, cat #: 561062). Single-cell suspensions were analyzed on BDTM LSR II flow cytometer (BD Biosciences, San Jose, California) and data was analyzed using FlowJo version 10 software (Tree Star, Ashland, Oregon).
2.6. Cytokine production in cultured T cells
Splenocytes were isolated from mice in control and LH conditions and plated at a concentration of 2 million cells per ml in 24 well plates in complete RPMI with 10% of FBS and penicillin-streptomycin in the presence of IL-2 and incubated at 37° C in 5 % C02 for 24 h. T cells were stimulated with anti mouse CD3 (clone 2C11, BD Pharmingen, 1μg/ml) and anti mouse CD28 (clone 37.51, BD Pharmingen, 4 μg/ml) antibodies and the supernatants collected 24 h later for cytokine determinations.
2.7. Statistical Analysis
Kolmogorov-Smirnov non-parametric analyses were used to compare distributions of results for LH. Two-way ANOVA analyses with Holm-Sidak post hoc test was used to determine effect of stress and CD8 status on CORT and cytokines. A Student’s t test was used to analyze in vitro cytokine results. Correlations were evaluated by Pearson or Spearman tests. Statistical analyses were conducted with GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA). All values are reported as mean ± SEM, with a p value less than 0.05 considered significant.
3. Results
3.1. CD8+ T cells profoundly affect cytokine responses to stress
Cytokine measurements were done at baseline 24 h after stress exposure as most of the studies in humans associate disease risk with baseline levels of cytokines. It was first determined that baseline cytokine levels in WT mice were not elevated with respect to control non-stressed mice after one or two sessions of stress. Consequently, basal circulating and brain cytokine levels were compared among all groups 24 h after the third stress session (T2). Adoptive transfer of naive CD4+ T cells into Rag2−/− mice did not modify cytokine or corticosterone response to stress (Fig. 1). In contrast, CD8+ T cells had profound effects on specific cytokine subsets, including circulating TNF-α, IL-6, and IFN-γ as well as brain IL-1β (Fig. 2). No effects of stress or CD8 treatment were detected for circulating IL-2, 4 and 10 and for any cytokine in the brain, except for IL-1β. Analysis of plasma TNF-α in Rag2−/− mice showed that there were main effects of stress (p = 0.045) and reconstitution (p = 0.0005), with stressed ReconCD8 mice exhibiting the highest levels (Fig. 2C). In WT mice stress resulted in an increase in IgG treated mice that was abolished by depletion of CD8+ T cells (interaction: p = 0.019). Consistent with this, IL-6 was increased in Rag2−/− mice by both reconstitution (p = 0.007) and stress (p = 0.026; Fig. 2D), whereas depletion of CD8+ T cells reduced IL-6 (p = 0.0002; stress and interaction: n.s.). Similarly, IFN-γ was increased in Rag2-/ mice by reconstitution (p = 0.0004) and stress (p = 0.021, interaction: p = 0.010; Fig. 2E) and reduced by CD8+ T cell depletion (p = 0.006; stress and interaction: n.s.). Notably, plasma levels of IL-Ιβ were reduced by stress in both Rag2−/− (p = 0.025) and WT mice (p = 0.005) regardless of CD8 T cell status, with most cases having non-detectable levels (Fig. 2F). However, reconstitution of Rag2−/− mice did increase IL-1β levels in non-stressed conditions (p = 0.014; interaction: p = 0.02). In the brain, expression of IL-1β was measured in the hypothalamus (Hyp) and hippocampus (Hipp) (Fig. 2G-H), two regions that play important roles in hormonal and behavioral stress responses [35]. A main effect of reconstitution in Rag2−/− mice was detected in the Hyp (p = 0.001) and Hipp (p = 0.033), which was driven by stress-induced increases in ReconCD8 mice (Hyp: p = 0.011, interaction: p = 0.026; Hipp, interaction: p = 0.033). Conversely, in WT mice, stress induced increases in expression of IL-1β mRNA in both the Hyp (p < 0.0001) and Hipp (p = 0.004) were blocked by CD8+ T cell depletion (Hyp: p = 0.014, interaction: p = 0.015; Hipp: p = 0.002, interaction: p = 0.001). The results from the two models show that CD8+ T cells promote circulating TNF-α, IL-6, IFN-γ and brain IL-1β mRNA increases in response to repeated stress exposure.
Figure 1.
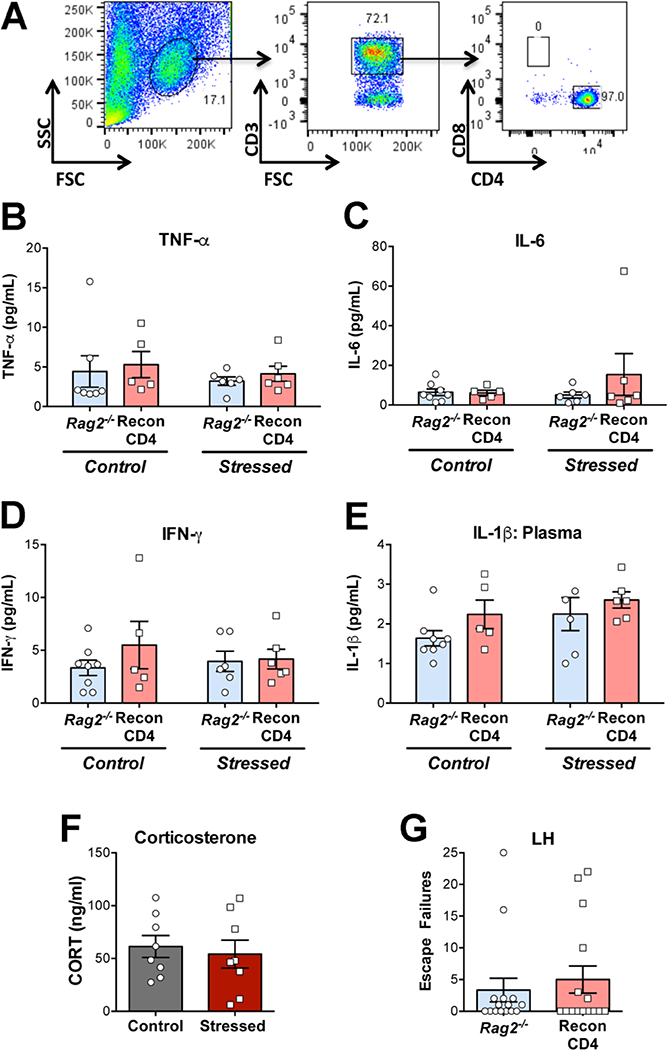
Representative flow cytometry dot plots (A) of cell harvested from the lymph nodes of Rag2−/− mice 3 weeks after reconstitution with 8–10 million naive CD4 cells. B-E: Plasma cytokine concentrations in Rag2−/− mice and Rag2−/− mice reconstituted with 8–10 million CD4+ cells (ReconCD4) in control and 24 h after T2 in the learned helplessness (LH) paradigm. F: Plasma corticosterone (CORT) concentration in ReconCD4 mice in control and 24 h after T2 in the LH. G: Escape failures during T2 in the LH in Rag2−/− and ReconCD4 mice.
Figure 2.
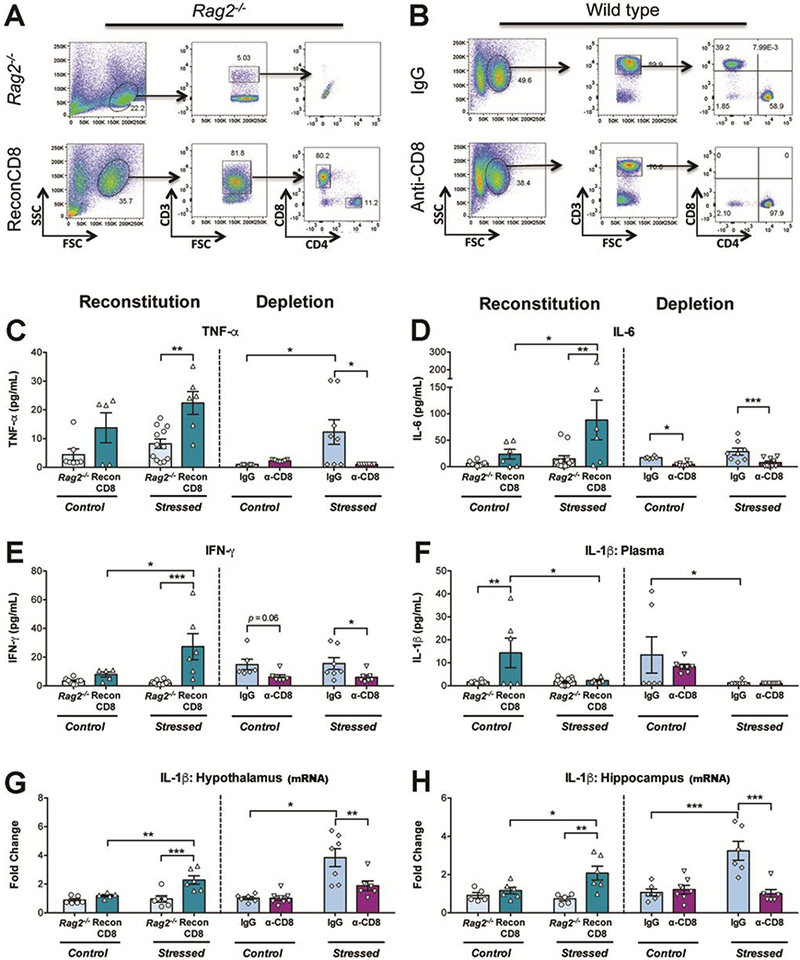
Representative dot plots of cells from the lymph nodes (A) in Rag2−/− mice 3 weeks after adoptive transfer of naive CD8+ T cells (ReconCD8) showing the proportion of CD3+/CD4+ & CD3+/CD8+ T cells. Note a small proportion of CD4+ T cells in ReconCD8. B) Representative dot plots illustrating successful depletion on CD8+ T cells in wild type mice. C-F: Plasma cytokine levels in Rag2−/− and ReconCD8 (left side of each graph) and in WT treated with anti-CD8 neutralizing antibodies (a-CD8) or isotype control (IgG) (right side of each graph) in control handled or 24 h after T2 in the learned helplessness (LH; stressed). C) Tumor-necrosis factor-alpha (TNF-α). D) Interleukin-6 (IL-6). E) Interferon-gamma (IFN-γ). F) Interleukin-1 beta (IL-1β). G-H) IL-1β mRNA expression in the hypothalamus (G) and hippocampus (H) in the same groups stated above. N = 5–12/group. Mean ± SEM. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
Finally, as differences in cytokine responses between stressed Rag2−/− and WT mice were noted, a posteriori comparisons between groups were performed to analyze how a full CD4+ and CD8+ repertoire in WT mice may influence cytokine response to stress. Analyses using one-tailed Student’s t test showed that WT mice mounted a greater cytokine response to stress compared to Rag2−/− mice, with higher levels of peripheral IFN-γ [t (17) = 4.3, p = 0.0002] and central IL-1β expression in the hypothalamus [t (11) = 4.1,p = 0.0009] and hippocampus [t (9) = 4.5,p = 0.0007]. Notably, ReconCD8 mice displayed an exacerbated peripheral cytokine response to stress when compared to WT mice, with both IL-6 and IL-1β levels higher in ReconCD8 mice [t (12) = 1.8,p = 0.04 and t (12) = 2, p = 0.03, respectively]. Taken together these findings further support a role for T cells in cytokine responses to stress and suggest that CD4+ T cells present in WT mice may provide a moderating effect by attenuating the magnitude of the CD8+ influences on cytokine production.
3.2. CORT levels and behavioral performance in stressed mice are minimally affected by CD8+ T cells
Stress resulted in a significant difference in CORT levels between Rag2−/− and ReconCD8 mice (interaction: p = 0.015; Fig 3), however this difference was due to a decrease in stressed Rag2−/− mice with respect to control rather than an increase in ReconCD8, which showed no effect of stress. In contrast, stress exposure in WT mice resulted in the expected increases in basal CORT levels in stressed animals; nevertheless, depletion of CD8+ T cells in WT mice did not prevent stress-induced increases in CORT (stress: p < 0.0001; interaction: p = 0.032, 2-way ANOVA) (Fig. 3A). These results show that CORT levels are modulated by CD8+ T cells in lymphopenic mice, but are not affected by their absence in WT. Likewise, behavioral responses in the LH indicate limited effects of CD8+ T cells. In this paradigm, mice display either active coping (i.e. escaping) or passive coping (i.e. failure to escape, or helplessness) responses during escapable stress resulting in a bimodal distribution of escapers and non-escapers [36]. Reconstitution with CD8+ T cells in Rag2−/− mice resulted in a significant shift in response distribution during T2, with a greater proportion of non-escaper ReconCD8 mice with respect to Rag2−/− (p = 0.038, Kolmogorov-Smirnov test) suggesting that a CD8+ biased environment impairs coping responses to stress after repeated exposure. Nevertheless, depletion of CD8+ T cells resulted in no difference in this distribution between IgG and α-CD8 treated mice (Fig. 3B). As in the case of CORT responses, these results show that a dominant CD8+ environment impairs stress coping behavior in lymphopenic mice, while removing CD8+ T cells does not necessarily improve behavioral stress responsiveness in WT.
Figure 3.
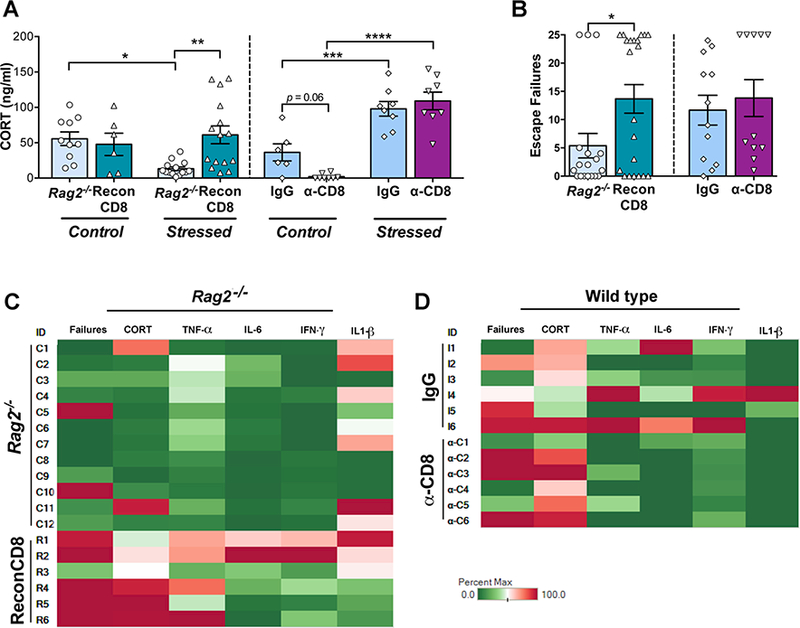
Effect of reconstitution with CD8+ T cells (ReconCD8) in Rag2−/− mice is shown on the left side of each graph; effect of CD8+ T cell depletion (a-CD8) in wild type mice is shown on the right. A) Basal circulating corticosterone (CORT) levels in control and stressed mice 24 h after Test 2 in the learned helplessness (LH) paradigm. B) Effect of CD8+ T cells on escape failures during LH Test 2. C and D) Heat maps generated to illustrate individual responses (rows) to repeated stress exposure in all measures (columns): behavior, CORT, and cytokines (Tableau; Seattle, WA, EISA). The percent max represents an individual animal’s response relative to the highest response within each measure for all animals. Greens represent a lower response, reds represent a high response and pales an average response. Mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p< 0.0001.
3.3. Correlation analyses
Correlation analyses were conducted to further examine the relationship of cytokine levels with CORT and coping responses to stress. In Rag2−/− and ReconCD8 mice there was a positive correlation between TNF-α and escape failures (p = 0.025) and CORT (p = 0.046). IFN-γ also showed a positive correlation with escape failures (p = 0.013) and CORT (p = 0.003). In contrast, there were no correlations between cytokine levels and behavior or CORT in IgG and CD8 depleted mice. Analysis between cytokines in individual animals showed correlations for all groups between TNF-α and IL-6 (Rag2−/−: p = 0.039; WT: p = 0.017) and TNF-α and IFN-γ (Rag2−/−: p = 0.048; WT: p = 0.009). Results for individual stressed animals for which all measures were available are illustrated as heat maps for the reconstitution and depletion experiments (Fig. 3C-D). These correlation analyses further indicate that increases in cytokines are associated with impaired stress responsiveness, but do not directly modulate behavioral and hormonal responses to stress.
3.4. Additional markers of T cell function
CD8+ T cells were analyzed after the LH in cells harvested from the LNs and spleen in ReconCD8 and WT mice and compared with control non-stressed mice. Intracellular IFN-γ content was not different between control and stress conditions in CD8+ T cells from ReconCD8 or WT mice (Fig. 4A-B). Likewise, IL-6 and TNF-α content did not differ between conditions (data not shown). No differences were observed between control and stress conditions in the proportion of naive CD62Lhlgh/CD44lowor effector memory CD62Llow/CD44hlgh CD8 T cells in Recon CD8 and WT and no differences were observed in the proportion of CD4/CD8 cells in WT mice between control and stress conditions (data not shown). Finally, in an independent study, T cell receptor stimulation in cultured splenocytes from WT mice resulted in lower cytokines release compared with non-stressed control (Fig. 4C) indicating that T cells are unlikely to be the source of increased circulating levels of cytokines. These results show that CD8+ T cells are key cellular players promoting inflammatory cytokine responses to repeated acute stress exposure without being the primary producers of cytokines and without changes in canonical markers of activation or differentiation.
Figure 4.
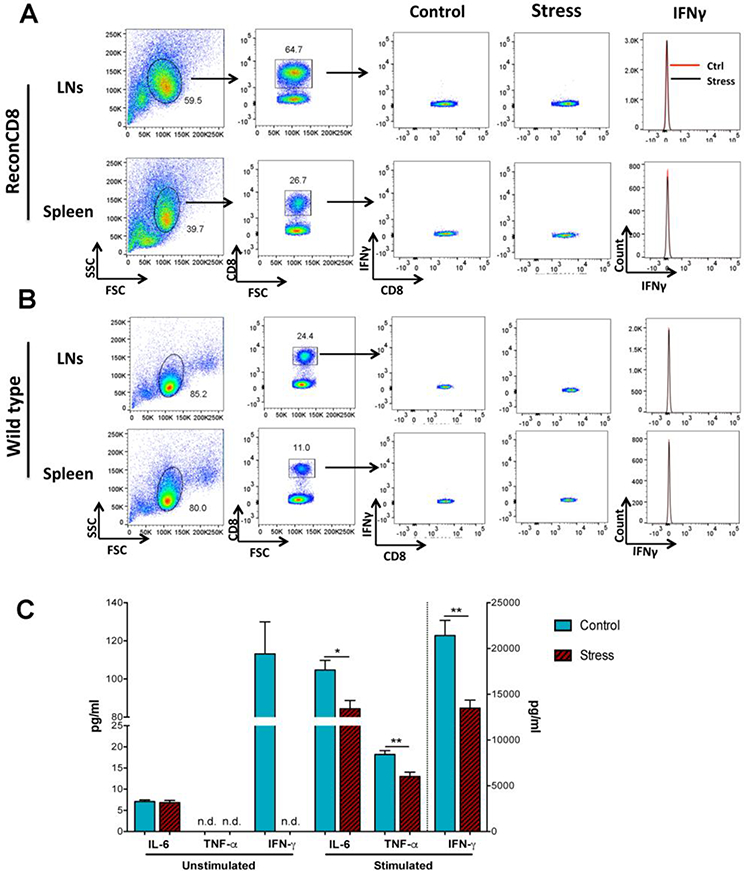
Representative flow cytometry dot plots (A) of CD8+ T cells harvested from the lymph nodes (LNs) and spleen of ReconCD8 (A) and wild type (B) mice showing IFN-γ content in control conditions and 24 h after T2 in the LH. C) Splenocytes were isolated from control and stressed wild type mice and cultured for 24 h and stimulated with anti mouse CD3 (clone 2C11, BD Pharmingen, 1μg/ml) and anti-mouse CD28 (clone 37.51, BD Pharmingen, 4 μg/ml) antibodies and the supernatants collected 24 h later for cytokine determinations. ** p < 0.01.
4. Discussion
The present study using two different mouse models indicate that CD8+ T cells regulate circulating plasma levels of the cytokines TNF-α, IL-6 and IFN-γ and gene expression of IL-1β in the brain in response to stress. Depletion of CD8+ T cells in WT mice 72 h prior to LH completely abolished stress-induced circulating TNF-α, IL-6 and IFN-γ and brain IL-1β increases, while adoptive transfer of naive cells into lymphocyte deficient Rag2−/− mice resulted in enhanced expression of these cytokines 24 h after the last stress session. These effects were observed in the two models independent of changes in classical markers of activation or in the proportions of cells suggesting that the mechanism by which stress stimulates CD8+ T cells may involve non-classical pathways of T cell activation and/or programming. These results provide supportive evidence indicating that CD8+ T cells, in the absence of foreign antigenic stimulation, respond to repeated psychological trauma by inducing the production of cytokines, revealing a pro- inflammatory role for CD8+ T cells in stress responsiveness.
Using the LH paradigm as a stressor, the overall effects of CD8+ T cells on stress responsiveness were consistent towards a pro-inflammatory mechanism. However, adoptive transfer of CD4+ cells had no effects on any parameter studied including cytokines, CORT and behavior. These results are not in line with their reported protective role revealed by other tests of stress responsiveness [26], including work from our group [22, 37]. Nevertheless, as we have previously discussed [22], while the evidence supports a beneficial role of CD4+ cells on stress responsiveness, their effects appear to largely depend on the intensity and type of the stressor, as well as specific aspects of behavior in which these cells may be protective.
In the present study, no evidence for an effect of stress on CD8+ T cell activation or intrinsic changes in cytokine content was observed, and T cells from stressed mice did not produce more cytokines when stimulated compared with non-stressed controls (Fig. 4C). Thus, it is unlikely that they are the major producers in response to stress, but rather it appears that they may influence non-lymphocyte cells to produce these cytokines. Indeed, monocytes and macrophages are known to produce cytokines under stress conditions [14–17], therefore a plausible mechanism is that CD8+ T cells stimulate these cells via specific MHC Class I interactions. This model assumes that Rag2−/− mice may be hyporesponsive to stress-induced cytokine production, not because they don’t have functional lymphocytes that produce these cytokines, but because their innate immune cells are resistant to activation in the absence of MHC Class I interactions with CD8+ T cells. This model would also predict that MHC Class I is necessary for the effects of CD8+ T cells on innate immune cells during stress exposure, which can be tested in future studies in Beta2 microglobulin (B2micro) deficient mice or Rag2−/− x B2micro−/− mice reconstituted with WT CD8+ T cells.
Unexpectedly, circulating levels of IL-1β were reduced by stress and not affected by CD8+ reconstitution or depletion. In contrast, the only cytokine that showed consistent changes in expression in the brain in response to stress was IL-1β, with an effect after both CD8+ reconstitution and depletion methodologies. While the effects of the LH on circulating IL-1β are paradoxical with respect to those reported in humans, they may reflect a particular regulatory point of this cytokine during the progression of stress exposure. The effects of stress on circulating IL-1β reported here require further investigation and are beyond the scope of the present studies. In contrast, the effects in the brain are in line with those reported in the literature and possibly related to cellular processes at the neurovascular interface. Expression of this cytokine in the brain has been shown in varied cell types, but more prominently in microglial and perivascular cells [38]. Recently, lymphocytes and T cells have been shown to circulate in a brain lymphatic system [39], and work from our group has shown that they proliferate in the brain of reconstituted Rag2−/− mice with a significant degree of interaction with the microvasculature during this process [32]. Thus, it is possible that CD8+ T cells increase their interaction with the brain microvasculature in response to stress, stimulating the production of IL-1β by perivascular cells, similar to the model of stress-induced vascular inflammation in hypertension [28].
Of relevance for the present results, expression of IL-1β in the Hyp and Hipp is believed to modulate different aspects of the stress response, including CORT and emotional processing [40]. Increases in CORT and impaired behavioral coping responses in ReconCD8 mice are consistent with increased IL-1β in these regions [40]. Nevertheless, depletion of CD8+ T cells prior to stress, which precluded IL-1β increases in the Hyp and Hipp, did not prevent stress-induced CORT increases or modify behavioral coping responses. This indicates that the effects of CD8+ T cells on CORT and behavior likely involves additional regulatory pathways between the peripheral immune system and the brain or downstream mechanisms such as different adrenal capacity in Rag2−/− due to the constitutive absence of lymphocytes. Thus, it is possible that the effects of CD8+ T cells on CORT and behavior were evidenced in a lymphopenic environment that has not experienced the presence of lymphocytes, but not in a natural setting in which developmental processes result in a different set point between these systems. In this regard, the presence of a small CD4+ population in ReconCD8 mice should be noted (Figure 2A). The proliferative capacity of CD4+ cells in Rag2−/− mice has been reported by several studies [37, 41, 42], and it is possible that they influenced these results. However, a dominant CD8 environment likely accounted for the effects on ReconCD8 mice.
Finally, special focus was devoted to ensuring the general health of these animals during the course of our experiments, and no overt signs of sickness or distress were observed in any group excepting mild piloerection following the inescapable stress session for both WT and Rag2−/− mice, which resolved by T1.
5. Conclusion
The present studies provide consistent evidence indicating that CD8+ T cells are key cellular elements promoting circulating TNF-α, IL-6 and IFN-γ cytokine responses to stress and brain IL-1β expression and suggest that they may influence hormonal and behavioral stress responsiveness through this mechanism.
Highlights.
- Depletion of CD8+ T cells in wild type mice abrogates cytokine responses to repeated foot-shocks
- T cell deficient mice have an attenuated cytokine response to repeated foot-shocks
- T cell deficient mice reconstituted wit CD8 T cells display enhanced cytokine responses to repeated foot-shocks
Acknowledgments
The author’s wish to thank Dr. Amit Golding from the University of Maryland School of Medicine for his thoughtful comments on the manuscript. We also wish to thank Lisa Hester from the Cytokine Core Laboratory and Ferenc Livak from the Flow Cytometry Core Laboratory, Center for Innovative Biomedical Resources (CIBR) of the University of Maryland School of Medicine for their help with cytokine determinations and flow cytometry, and Dana Brady from the Maryland Psychiatric Research Center (MPRC) for her help with corticosterone determinations. Supported by the VA Research Merit Award BX003631 and the National Institute of Mental Health Research Grant R01MH097676 (OppNet) to LHT.
Footnotes
Disclosure
The authors have no financial interests to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dhabhar FS, Effects of stress on immune function: the good, the bad, and the beautiful, Immunol Res 58(2–3) (2014) 193–210. [DOI] [PubMed] [Google Scholar]
- [2].Webster JI, Tonelli L, Sternberg EM, Neuroendocrine regulation of immunity, Annu Rev Immunol 20 (2002) 125–63. [DOI] [PubMed] [Google Scholar]
- [3].Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB, Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk, Proc Natl Acad Sci U S A 109(16) (2012) 5995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Reiche EM, Nunes SO, Morimoto HK, Stress, depression, the immune system, and cancer, Lancet Oncol 5(10) (2004) 617–25. [DOI] [PubMed] [Google Scholar]
- [5].Stojanovich L, Marisavljevich D, Stress as a trigger of autoimmune disease, Autoimmun Rev 7(3) (2008) 209–13. [DOI] [PubMed] [Google Scholar]
- [6].Kivimaki M, Steptoe A, Effects of stress on the development and progression of cardiovascular disease, Nat Rev Cardiol (2017). [DOI] [PubMed] [Google Scholar]
- [7].Miller MM, McEwen BS, Establishing an agenda for translational research on PTSD, Ann N Y Acad Sci 1071 (2006) 294–312. [DOI] [PubMed] [Google Scholar]
- [8].Watson K, Nasca C, Aasly L, McEwen B, Rasgon N, Insulin resistance, an unmasked culprit in depressive disorders: Promises for interventions, Neuropharmacology (2017). [DOI] [PubMed] [Google Scholar]
- [9].Cheng Y, Jope RS, Beurel E, A pre-conditioning stress accelerates increases in mouse plasma inflammatory cytokines induced by stress, BMC Neurosci 16 (2015) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Steptoe A, Hamer M, Chida Y, The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis, Brain Behav Immun 21(7) (2007) 901–12. [DOI] [PubMed] [Google Scholar]
- [11].Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL, A meta-analysis of cytokines in major depression, Biol Psychiatry 67(5) (2010) 446–57. [DOI] [PubMed] [Google Scholar]
- [12].Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctot KL, Carvalho AF, Peripheral cytokine and chemokine alterations in depression: a metaanalysis of 82 studies, Acta Psychiatr Scand (2017). [DOI] [PubMed] [Google Scholar]
- [13].Rohleder N, Aringer M, Boentert M, Role of interleukin-6 in stress, sleep, and fatigue, Ann N Y Acad Sci 1261 (2012) 88–96. [DOI] [PubMed] [Google Scholar]
- [14].Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M, Chronic variable stress activates hematopoietic stem cells, Nat Med 20(7) (2014) 754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Lebonte B, Horn S, Lapidus KA, Stelzhammer V, Wong EH, Bahn S, Krishnan V, Bolanos-Guzman CA, Murrough JW, Merad M, Russo SJ, Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress, Proc Natl Acad Sci U S A 111(45) (2014) 16136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, Ramirez-Chan K, Wang Y, Roeth RM, Sucaldito AD, Sobol CG, Quan N, Sheridan JF, Godbout JP, Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety, Mol Psychiatry (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW, Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis, Proc Natl Acad Sci U S A 110(41) (2013) 16574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fleshner M, Nguyen KT, Cotter CS, Watkins LR, Maier SF, Acute stressor exposure both suppresses acquired immunity and potentiates innate immunity, Am J Physiol 275(3 Pt 2) (1998) R870–8. [DOI] [PubMed] [Google Scholar]
- [19].Shi Y, Devadas S, Greeneltch KM, Yin D, Allan Mufson R, Zhou JN, Stressed to death: implication of lymphocyte apoptosis for psychoneuroimmunology, Brain Behav Immun 17 Suppl 1 (2003) S18–26. [DOI] [PubMed] [Google Scholar]
- [20].Kim SJ, Lee H, Lee G, Oh SJ, Shin MK, Shim I, Bae H, CD4+CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice, PLoS One 7(7) (2012) e42054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brachman RA, Lehmann ML, Maric D, Herkenham M, Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice, J Neurosci 35(4) (2015) 1530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Clark SM, Soroka JA, Song C, Li X, Tonelli LH, CD4(+) T cells confer anxiolytic and antidepressant-like effects, but enhance fear memory processes in Rag2(−/− ) mice, Stress 19(3) (2016) 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Herkenham M, Kigar SL, Contributions of the adaptive immune system to mood regulation: Mechanisms and pathways of neuroimmune interactions, Prog Neuropsychopharmacol Biol Psychiatry 79(Pt A) (2017) 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kipnis J, Gadani S, Derecki NC, Pro-cognitive properties of T cells, Nat Rev Immunol 12(9) (2012) 663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G, CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis, J Immunol 182(7) (2009) 3979–84. [DOI] [PubMed] [Google Scholar]
- [26].Rattazzi L, Piras G, Ono M, Deacon R, Pariante CM, D’Acquisto F, CD4(+) but not CD8(+) T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice, Transl Psychiatry 3 (2013) e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Beurel E, Harrington LE, Jope RS, Inflammatory T helper 17 cells promote depression-like behavior in mice, Biol Psychiatry 73(7) (2013) 622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Marvar PJ, Vinh A, Thabet S, Lob HE, Geem D, Ressler KJ, Harrison DG, T lymphocytes and vascular inflammation contribute to stress-dependent hypertension, Biol Psychiatry 71(9) (2012) 774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mills PJ, Farag NH, Hong S, Kennedy BP, Berry CC, Ziegler MG, Immune cell CD62L and CD11a expression in response to a psychological stressor in human hypertension, Brain Behav Immun 17(4) (2003) 260–7. [DOI] [PubMed] [Google Scholar]
- [30].Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG, Oligoclonal CD8+ T cells play a critical role in the development of hypertension, Hypertension 64(5) (2014) 1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, Salum G, Magalhaes PV, Kapczinski F, Kauer-Sant’Anna M, Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and metaregression, Lancet Psychiatry 2(11) (2015) 1002–12. [DOI] [PubMed] [Google Scholar]
- [32].Song C, Nicholson JD, Clark SM, Li X, Keegan AD, Tonelli LH, Expansion of brain T cells in homeostatic conditions in lymphopenic Rag2−/− mice, Brain Behav Immun (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shanks N, Anisman H, Stressor-provoked behavioral changes in six strains of mice, Behav Neurosci 102(6) (1988) 894–905. [DOI] [PubMed] [Google Scholar]
- [34].Tonelli LH, Katz M, Kovacsics CE, Gould TD, Joppy B, Hoshino A, Hoffman G, Komarow H, Postolache TT, Allergic rhinitis induces anxiety-like behavior and altered social interaction in rodents, Brain Behav Immun 23(6) (2009) 784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lupien SJ, McEwen BS, Gunnar MR, Heim C, Effects of stress throughout the lifespan on the brain, behaviour and cognition, Nat Rev Neurosci 10(6) (2009) 434–45. [DOI] [PubMed] [Google Scholar]
- [36].Chourbaji S, Zacher C, Sanchis-Segura C, Dormann C, Vollmayr B, Gass P, Learned helplessness: validity and reliability of depressive-like states in mice, Brain Res Brain Res Protoc 16(1–3) (2005) 70–8. [DOI] [PubMed] [Google Scholar]
- [37].Clark SM, Vaughn CN, Soroka JA, Li X, Tonelli LH, Neonatal adoptive transfer of lymphocytes rescues social behavior during adolescence in immune deficient mice, Eur J Neurosci (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wong ML, Bongiorno PB, Rettori V, McCann SM, Licinio J, Interleukin (IL) 1beta, IL-1 receptor antagonist, IL-10, and IL-13 gene expression in the central nervous system and anterior pituitary during systemic inflammation: pathophysiological implications, Proc Natl Acad Sci U S A 94(1) (1997) 227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J, Structural and functional features of central nervous system lymphatic vessels, Nature 523(7560) (2015) 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Goshen I, Yirmiya R, Interleukin-1 (IL-1): a central regulator of stress responses, Front Neuroendocrinol 30(1) (2009) 30–45. [DOI] [PubMed] [Google Scholar]
- [41].Min B, Yamane H, Hu-Li J, Paul WE, Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms, J Immunol 174(10) (2005) 6039–44. [DOI] [PubMed] [Google Scholar]
- [42].Song C, Nicholson JD, Clark SM, Li X, Keegan AD, Tonelli LH, Expansion of brain T cells in homeostatic conditions in lymphopenic Rag2(−/−) mice, Brain Behav Immun 57 (2016) 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]


