Abstract
Purpose
Gadolinium-based contrast agents (GBCA) for MRI are generally administrated in direct relationship to body weight. Instead, we propose a model for GBCA dosing on the basis of blood volume. The new method was tested by exploring the associations between MRI T1 mapping indices and weight in the multi-ethnic study of atherosclerosis (MESA).
Methods
Empirically derived methods based on sex and body habitus were used to calculate blood volumes. GBCA dose (in ml) in blood (in L) was calculated as the injected volume divided by the blood volume (i.e DBV). Of the 1219 participants with cardiac MRI T1 mapping, 845 studies had standard dose of 0.15 mmol/kg (cohort 1), and 166 studies had 30 ml GBCA regardless of weight (cohort 2). We also created a specific cohort with similar DBV (N=357, cohort 3).
Results
Post contrast blood relaxation rate R1blood and DBV were significantly correlated (R=0.641, P<0.001). R1blood was significantly associated to weight in cohort 1 and 2 but the correlation coefficient was positive for cohort 1 and negative for cohort 2, indicating GBCA overdosing in cohort 1 and underdosing in cohort 2 in heavy relative to lean subjects. R1blood was not associated to weight in cohort 3. Simulated results demonstrated less contrast should be administrated for heavy subjects compared to the conventional weight-based dose.
Conclusion
GBCA dosing on the basis of blood volume could improve the efficacy and safety of contrast enhanced MRI studies. This method could be implemented to standardize dose and augment precision in study comparisons.
Keywords: MR imaging, T1 mapping, blood volume, GBCA
Introduction
Gadolinium-based contrast agents (GBCA) are widely used in MRI examinations to improve diagnostic accuracy. In the United States, the approved labels of all current extracellular GBCA indicate that gadolinium dose should be administered in direct proportion to body weight. Since measurement of blood volume is not readily accessible, body weight is used as a surrogate measure. This assumes a linear relationship between body weight and blood volume. However, blood volume per kilogram of body weight decreases non-linearly with increasing weight (1). Furthermore, blood volume also depends on sex, age, and/or height (2). This indicates that GBCA dosing based on body weight, without considering blood volume, might lead to under or overdosing of GBCA to patients.
With the advent of MRI T1 mapping techniques (3), quantitative determination of a range of parameters, including native T1, post gadolinium T1 or extracellular volume fraction (ECV) have been used to study myocardial structure in epidemiological studies (4), predict prognosis of cardiovascular diseases (5), or define cut-points for the presence or absence of interstitial fibrosis (6, 7). T1 mapping uses GBCA to access post contrast T1 times and ECV. Improper dosing might lead to biased results which hamper the application of T1 mapping.
We propose a new MRI gadolinium dosing method based on blood volume. Compared to the conventional weight-based GBCA dosing method, our new method considers blood volume as the main dose determinant parameter, despite the fact that GBCA distributes beyond the vascular space into the extracellular space of most organs and tissues in the body. The new method was tested by exploring the associations between T1 mapping indices and weight in the multi-ethnic study of atherosclerosis (MESA). We hypothesize that results are more biased with standardization of the GBCA concentration for body weight than for blood volume. Therefore, we simulated the ideal GBCA dose based on blood volume calculations from the MESA study.
Methods
Calculation of GBCA concentration in blood
There are several methods available for blood volume calculations. One of the most popular methods uses the Nadler equations (8):
| [1] |
Where h=body height in meters, w=body weight in kilograms, BV=Blood volume in liters. The GBCA dose per ml of blood volume (DBV) is calculated as,
| [2] |
Likewise, GBCA concentration in the plasma (DPV) can also be calculated as
| [3] |
Where plasma volume = blood volume × (1-Hematocrit).
We can also derive the blood volume based on the lean body mass method (LBM). LBM has been proven to be suitable for normalization of blood volumes independent of sex and age (age 2 and above) (9, 10).
| [4] |
Where LBM (men) = 0.407 × w + 26.7 × h − 19.2, and LBM (women) = 0.252 × w + 47.3 × h − 48.3. The aim is to ensure similar DBV values in all studies. We used both approaches for blood volume estimation to derive the GBCA volume in ml versus body weight for similar GBCA blood concentration.
MRI T1 mapping and the MESA study
Study subjects were evaluated in the Multi-Ethnic Study of Atherosclerosis (MESA). The cohort included four ethnicities (white, African-American, Chinese, and Hispanic) of men and women recruited from six field centers (Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minnesota). In 2010, MESA participants were examined for the ten-year follow up (fifth examination). 1334 participants consented to receive MRI contrast agent and had T1 mapping. 1219 participants (49% men, 67.27±8.62 years) with negative late gadolinium enhancement (LGE) from MRI were included in the final analysis. Institutional review boards at each of the six field centers approved the study protocols and all attendees at the examinations provided written informed consent.
MESA myocardial T1 mapping and cardiac MRI methods have been described previously (4). Briefly, 1.5T MRI systems were used and GBCA (Magnevist, Bayer Healthcare Pharmaceuticals, NJ, USA) was injected intravenously. T1 mapping indices including pre and post-contrast T1 times at 12 minutes, and ECV were assessed using a single-breath hold modified Look-Locker inversion recovery (MOLLI) sequence.
Statistical analysis
Continuous variables were expressed as mean±SD. Univariate linear regression was used to explore the relationship between DBV or DPV and T1 mapping indices. Of the 1219 participants, 845 studies had GBCA standard dose of 0.15 mmol/kg (cohort 1), and 166 studies had GBCA injection volume of 30 ml regardless of weight (cohort 2). We selected participants with 4.8<= DBV<=5.2 (N=357) from entire cohort to create a special cohort with similar GBCA blood concentration (cohort 3). For each cohort, linear regression was used to determine the relationship between ECV, post contrast myocardial T1 (T1myo), and the reciprocal of blood T1 (i.e. blood relaxation rate, R1blood) obtained from left-ventricular cavity, as well as body weight. Regression models were examined as follows: Model 1 – unadjusted. Model 2 – adjusted for age sex, and race. Model 3 – age, sex, race, height, HDL, LDL, Triglycerides, systolic blood pressure (SBP), diastolic blood pressure (DBP), smoking, heart rate, diabetes, hypertension medication, serum creatinine. All analyses were performed using SPSS (version 23.0, IBM Inc. Chicago, IL) and significance was declared as P<0.05.
Results
T1 mapping indices and GBCA concentration in blood
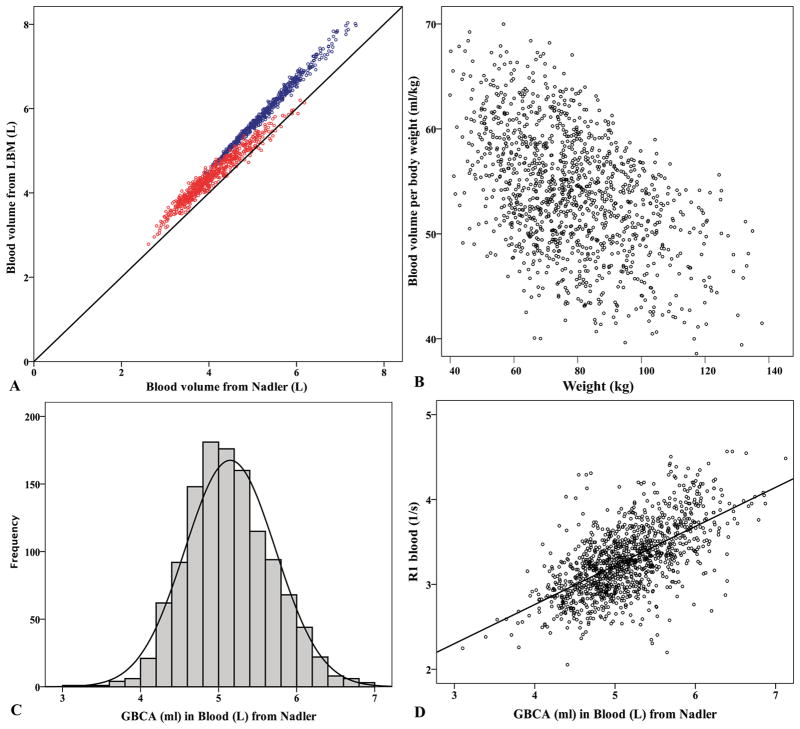
From the entire cohort of 1219 MESA participants, blood volumes estimated from Nadler equations were systematically lower than those from LBM (Figure 1a). However, given that the Nadler and LBM methods were highly correlated (R=0.984, P<0.001), we present results using the Nadler equations only (unless otherwise specified), for simplicity. Figure 1b shows the scattered plot of blood volume per kilogram of body weight versus body weight. Figure 1c demonstrates the DBV (dose per L of blood volume) distributions with mean DBV=5.15±0.58 ml/L. ECV, T1myo, and R1blood were all significantly correlated to both DBV and DPV [dose per L of plasma volume (all P<0.001)]. However, the correlations for R1blood were stronger for DBV than for DPV (R=0.641 and 0.445, respectively). The relationship of R1blood to DBV is plotted in Figure 1d.
Figure 1. Characteristics of blood volumes derived from body habitus and GBCA concentrations in the MESA T1 mapping study (N=1219).
(a) Relationship of blood volume estimated from lean body mass (LBM) and Nadler equations (R=0.984). Blue circles for men and red circles for women. Solid line represents the identity. (b) Scattered plot of blood volume per kilogram of body weight versus weight. This implies weight-based GBCA dose might overdose heavier individuals. (c) Histogram of GBCA concentration (ml) per liter of blood (i.e. DBV) based on Nadler equations for blood volume estimation. (d) Post contrast R1blood is proportional to DBV (R=0.641, P<0.001).
T1 mapping indices and weight
Table 1 displays the regression coefficients B and the P values for relationships between T1 mapping indices and body weight. T1myo and R1blood were significantly (P<0.001) associated with weight in all models for both cohort 1 (with 0.15 mmol/kg dose) and 2 (with 30 ml fixed dose) but the correlations were in opposite directions: the correlation coefficient was positive for cohort 1 and negative for cohort 2. Moreover, there was no significant association between T1myo or R1blood and body weight for cohort 3 (with constant DBV) in any of the statistical models. The relationships between ECV and weight were heterogeneous. Considering the fully adjusted model, ECV was not related to weight in cohort 1 (P=0.21), it was significant in cohort 2 (P=0.003), and of borderline significance in cohort 3 (P=0.056).
Table 1.
Relationship of ECV, T1myo and R1blood to weight (kg) in three different cohorts. Values are B(P).
| Cohort | ECV (%) | T1myo (ms) | R1blood (s−1) | |
|---|---|---|---|---|
| Unadjusted | 1 | −0.026 (<0.001) | −0.482 (<0.001) | 0.007 (<0.001) |
| 2 | 0.031 (0.21) | 1.711 (<0.001) | −0.02 (<0.001) | |
| 3 | −0.033 (<0.001) | 0.004 (0.95) | 0.002 (0.18) | |
|
| ||||
| Adjusted for age, sex, race | 1 | −0.015 (0.03) | −0.791 (<0.001) | 0.009 (<0.001) |
| 2 | 0.051 (0.028) | 1.469 (<0.001) | −0.018 (<0.001) | |
| 3 | 0.009 (0.43) | −0.119 (0.37) | 0.0002 (0.4) | |
|
| ||||
| Fully adjusted* | 1 | −0.009 (0.21) | −0.815 (<0.001) | 0.011 (<0.001) |
| 2 | 0.07 (0.003) | 1.156 (<0.001) | −0.015 (<0.001) | |
| 3 | 0.029 (0.056) | 0.189 (0.23) | −0.002 (0.16) | |
Cohort 1 – Studies with GBCA dose 0.15mmol/kg (N=845). Cohort 2 – Studies with GBCA dose 30ml (N=166). Cohort 3 – Studies with 4.8<= DBV<=5.2 (N=357).
Fully adjusted: age, race, height, HDL, LDL, Triglycerides, SBP, DBP, smoking, heart rate, diabetes, hypertension medication, serum creatinine.
GBCA dosing based on blood volume
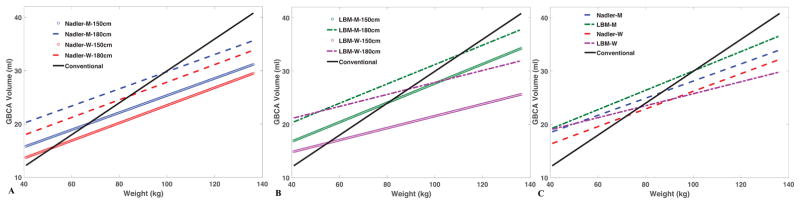
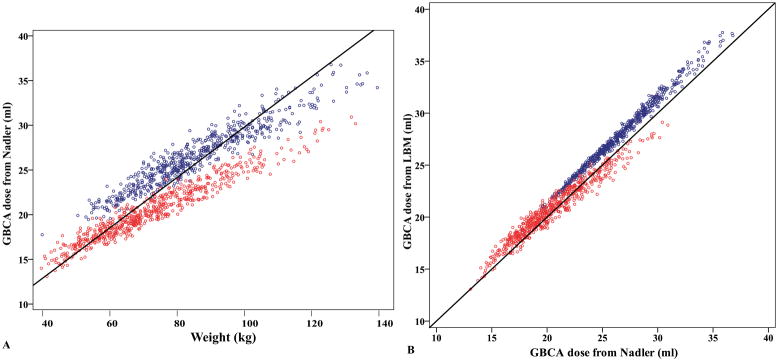
For a given DBV, we calculated the GBCA dose in ml versus weight for MESA participants of different height. Figure 2 shows the simulated results based on two different methods (i.e. Nadler equations and LBM) for blood volume estimations. DBV=5 ml/L was used in Figure 2a, and 4.7 ml/L was used in Figure 2b to maintain similar doses for both methods. These numbers were the average DBV in each method from MESA study. It should be noted that the choice of DBV could offset the regression line in the design of GBCA dosing protocol. Figure 2c compares the dose using these two methods for 1.7m men and women. Compared to the conventional weight-based dose (solid black line in Figure 2), the standardized DBV dosing gives less contrast for heavy subjects and more contrast for lean subjects. Assuming DBV=5 ml/L, we retrospectively calculated the desired GBCA dose for all MESA participants (Figure 3a). The magnitude of over-dosing was 11.4% (men) and 16.4% (women) in extreme obese (122kg, 175cm, corresponding to BMI=40 kg/m2) and 23% (men) and 12% (women) under-dosing in lean (61kg, 175cm, corresponding to BMI=20 kg/m2) subjects. Using DBV=4.7 ml/L from LBM, the desire dose is linearly correlated to that from Nadler equations (Figure 3b).
Figure 2. Blood volume based GBCA dosing.
Simulated relationships of GBCA volume and weight to maintain equal concentration of GBCA in blood of men and women of different height. Blood volume estimation based on Nadler equations (a) and lean body mass (LBM) (b). Comparison of both methods for men and women of similar height (1.7m) (c). Black line represents the relationship for the conventional body weight dosing method (in MESA that dose was 0.15 mmol/kg).
Figure 3. Retrospectively calculated GBCA dose on basis on blood volume for MESA participants (Blue circles: men. Red circles: women).
(a) Simulated GBCA dosing based on blood volume, assuming 5 ml of GBCA per liter blood (i.e. DBV=5 ml/L estimated from Nadler equations). Less contrast is used for heavy subjects compared to the conventional dosing of 0.15 mmol/kg (black line). (b) Using DBV=4.7 ml/L for blood volume from LBM, the desire dose is linearly correlated to that from Nadler equations. Black line represents the identity.
Discussion
We have proposed a new method for MRI GBCA dosing based on blood volume. We validated this concept in a large multi-ethnic population encompassing a wide body size range and proved that only when blood GBCA concentration was standardized among participants, T1 mapping indices (post contrast myocardial T1 and ECV) could be obtained without bias. Gai et al (11) showed that weight-based GBCA dosing could lead to erroneous conclusions in post contrast myocardial T1. We further proved that not only T1myo but also ECV, which is less sensitive to dose than other T1 parameters, could also be biased if GBCA concentration was not standardized relative to blood volume. The dependence of ECV on Gadolinium dose is less realized due to normalization for blood T1 in its calculation. This assumes that the change of pre and post contrast R1 of myocardium and blood is proportional regardless of GBCA concentration. Miller et al (12) examined the effect of contrast agent dose on T1 indices. In their study, thirty healthy volunteers were spit into 3 age and sex matched groups and each group received GBCA of 0.1, 0.15, and 0.2 mmol/kg respectively. There was no difference in the precontrast myocardial and blood T1 between groups. As expected, post contrast T1 shortened significantly as GBCA dose increased, but blood T1 shortened more than myocardial T1. This resulted in ECV decrease with increasing GBCA dose in their study.
In our study, the relationships of ECV and T1myo to weight, conventional weight-based or fixed 30 ml dosing methods led to opposite conclusions. The results demonstrated overdosing (for conventional) and underdosing (for fixed 30 ml) for overweight relative to lean participants. Such disagreement can be explained by both the blood volume per body weight decrease with increased body weight (Figure 1b), and by the positive correlation between post contrast R1blood and GBCA concentration (Figure 1d). Both ECV and T1myo failed to show associations with weight after adjusting for risk factors in the special cohort with constant blood GBCA dose. The role of obesity in heart failure is controversial because it is difficult to separate obesity from its accompanying comorbidities (13).
To improve the efficacy of quantitative MRI T1 mapping, most researchers have carefully monitored and maintained experimental parameters including contrast medium type and dose, injection method, field strength, image acquisition time and scheme, in addition to post processing algorithms. Since all of these factors affect the observed longitudinal relaxation rates, calibration or correction for these experimental parameters has been proposed to mitigate discrepancies between studies (11, 14). However, even in well-conducted research, intrinsic problem underlying the weight-based GBCA dosing method cannot be circumvented because blood or plasma volume is not linearly related to body weight. Without contrast, blood T1 depends on several physiological conditions such as hematocrit and oxygenation fraction (15). After contrast administration, the paramagnetic ion Gd+3 interacts directly with the surrounding protons and shortens their nuclei relaxation times. The degree of T1 reduction is directly related to the amount of injected gadolinium and to its pharmacokinetic properties within the biologic system. Most GBCAs are extracellular space markers. After peripheral intravenous injection, contrast circulation is mainly regulated by the cardiovascular system with rapid redistribution of extracellular agents from the vascular into the interstitial space in different organs. A contrast bolus of such an agent is therefore diluted in the blood and extracellular space as it is dispersed to the organs, and is then excreted via glomerular filtration by the kidneys. Since relaxation times in tissue and blood are closely related, evaluating pathology in tissues by relaxation times either T1myo or ECV may be misleading without considering the GBCA concentration.
The bolus-injection single-compartment model was employed in our study, in which it is assumed that the contrast mixes throughout its volume of distribution (the single compartment) and achieves dynamic equilibrium in its steady-state phase. It is worth noting that DBV=5 ml/L used in the construction of Figure 3 was the calculated initial concentration based on the MESA protocol. As the contrast washes in and out of the tissue through the circulatory system, its concentration changes dynamically. However, our results indicated that this initial GBCA concentration correlated strongly to the post contrast blood relaxation rate even after 12 minutes of administration. This might imply that GBCA blood concentration out-weights other physiological factors in the determination of tissue relaxation times in part because of how rapidly it diffuses in and out of the extracellular space, resulting for practical purposes, in a single large volume of distribution. Since R1blood affects the myocardial T1 directly, it is important to control the initial GBCA blood concentration to enhance reproducibility of serial clinical examinations and for research purposes.
Several empirically derived blood volume estimation methods based on body habitus and sex have been proposed (8–10, 16). We used the Nadler and lean body mass equations to calculate blood volume. Although blood volumes from these two methods were extremely correlated, the subsequently derived dosing curves were not the same due to different underlying algorithms underlying the two different blood volume estimation methods. In this regard, the desired dosing based on either Nadler or LBM equations might differ but, given that they are highly correlated (Figure 3b), we do not expect significant discrepancies by choosing either method. In addition to the model used for blood volume estimation, to apply our proposed dosing method, it is crucial to determine the desired DBV based on the study cohort and application. For example, for cardiac MRI, double dose GBCA is usually used. Assuming the average weight and estimated blood volume in a given cohort are 70 kg and 5 liters respectively, for most of 0.5 Molar GBCA, 28 ml should be used (i.e. double dose) which results in DBV=5.6 ml/L. GBCA injected volume can then be calculated by multiplication of this desired DBV by blood volume of each participant to ensure that equal GBCA concentration in the study.
Blood volume calculation based on body weight and height has been criticized when body composition deviates significantly from standard (1). Since we have established the relationship between DBV and R1blood, the relationship could be employed as another empirical method for blood volume measurement. Blood volume measured from radioactive tracers (17, 18) would be desirable to provide genuine dosing but it is impractical in clinical research as well as in most clinical studies. If GBCA could be used as the “dye” in blood, similar to the radioactive tracer in the true blood measurement, the pitfall of body-size dependence could potentially be alleviated and only one equation is necessary for all age and gender. It will remain to be established how robust this new method will be when validated in future studies. An assessment in special populations such as heart failure or anemia patients who have abnormal blood volume would also be helpful.
Limitations
There are several limitations in our study. We validated the concept of GBCA dosing on the basis of blood volume by correlating T1 mapping indices with weight because weight is currently the main determinant of dose for conventional administration methods. However, our findings need independent confirmation and correlative measures particularly of total extracellular space as opposed to blood volume as used here. The T1 mapping methods have been rapidly developed in the MRI field (18). Our hypothesis-generating results require validations of serial studies from different T1 mapping techniques. More research is also needed to investigate other sources known to affect blood relaxation rate other than GBCA concentration such as perfusion of the interstitial space, flow rate, body temperature, oxygen content, and biological factors (anemia or iron-overload in extreme cases). Our model used for simulation is a bolus single compartment model which assumes that gadolinium concentration is rapidly distributed and uniform in the body at all times. While this is a reasonable approximation, sophisticated physiological models should be considered when certain patient characteristics, such as age, body composition, function of organs or comorbidity, and medications could alter the pharmacokinetic parameters of GBCA. It should be noted that our results were only validated in the MESA cardiac MRI study of asymptomatic middle aged and older individuals. Therefore, further studies using our proposed methodology in patients with different types of pathologic conditions will be of interest before widespread clinical application is undertaken. We used all participants to increase statistical power and to prevent selection bias. This resulted in an imbalance in the number of subjects between cohorts which could affect the results.
Conclusions
On the basis of detailed analyses of myocardial and blood T1 measured obtained as part of the MESA MRI study, we propose important modifications to GBCA dosing from conventional weight-based to blood volume-based method to improve the efficacy of quantitative MRI applications. The model used for blood volume estimation might affect the dosing strategy. This method could potentially be implemented beyond T1 mapping for all MRI GBCA examinations to standardize comparisons between different clinical and clinical research studies.
Acknowledgments
The authors thank all investigators, staff, and participants of the MESA Study for their valuable contributions. A full list of participating MESA Investigators and institutions can be found at http://www.mesa-nhlbi.org. This research was supported by contracts N01-HC-95159 through N01-HC-95168 from the National Heart, Lung, and Blood Institute. This manuscript has been reviewed by the MESA Investigators for scientific content and consistency of data interpretation with previous MESA publications and significant comments have been incorporated prior to submission for publication.
Abbreviations
- GBCA
Gadolinium-based contrast agent
- MESA
Multi-ethnic study of atherosclerosis
- MRI
Magnetic resonance imaging
- DBV
GBCA dose in ml in the blood volume in L
- DPV
GBCA dose in ml in the plasma volume in L
- ECV
Extracellular volume fraction
- LGE
Late gadolinium enhancement
- LBM
Lean body mass
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
References
- 1.Feldschuh J, Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation. 1977;56(4 Pt 1):605–12. doi: 10.1161/01.cir.56.4.605. [DOI] [PubMed] [Google Scholar]
- 2.Feldschuh J, Katz S. The importance of correct norms in blood volume measurement. Am J Med Sci. 2007;334(1):41–6. doi: 10.1097/MAJ.0b013e318063c707. [DOI] [PubMed] [Google Scholar]
- 3.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52(1):141–6. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 4.Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S, Nacif M, Bluemke DA, Lima JAC. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;62(14):1280–7. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youn JC, Hong YJ, Lee HJ, Han K, Shim CY, Hong GR, Suh YJ, Hur J, Kim YJ, Choi BW, Kang SM. Contrast-enhanced T1 mapping-based extracellular volume fraction independently predicts clinical outcome in patients with non-ischemic dilated cardiomyopathy: a prospective cohort study. European radiology. 2017;27(9):3924–33. doi: 10.1007/s00330-017-4817-9. [DOI] [PubMed] [Google Scholar]
- 6.Hinojar R, Varma N, Child N, Goodman B, Jabbour A, Yu CY, Gebker R, Doltra A, Kelle S, Khan S, Rogers T, Arroyo Ucar E, Cummins C, Carr-White G, Nagel E, Puntmann VO. T1 Mapping in Discrimination of Hypertrophic Phenotypes: Hypertensive Heart Disease and Hypertrophic Cardiomyopathy: Findings From the International T1 Multicenter Cardiovascular Magnetic Resonance Study. Circ Cardiovasc Imaging. 2015;8(12) doi: 10.1161/CIRCIMAGING.115.003285. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Borlotti A, Viliani D, Jerosch-Herold M, Alkhalil M, De Maria GL, Fahrni G, Dawkins S, Wijesurendra R, Francis J, Ferreira V, Piechnik S, Robson MD, Banning A, Choudhury R, Neubauer S, Channon K, Kharbanda R, Dall’Armellina E. CMR Native T1 Mapping Allows Differentiation of Reversible Versus Irreversible Myocardial Damage in ST-Segment-Elevation Myocardial Infarction: An OxAMI Study (Oxford Acute Myocardial Infarction) Circ Cardiovasc Imaging. 2017;10(8) doi: 10.1161/CIRCIMAGING.116.005986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–32. [PubMed] [Google Scholar]
- 9.Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol. 1984;247(4 Pt 2):F632–6. doi: 10.1152/ajprenal.1984.247.4.F632. [DOI] [PubMed] [Google Scholar]
- 10.Raes A, Van Aken S, Craen M, Donckerwolcke R, Vande Walle J. A reference frame for blood volume in children and adolescents. BMC Pediatr. 2006;6:3. doi: 10.1186/1471-2431-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gai ND, Sandfort V, Liu S, Lima JA, Bluemke DA. Dose correction for post-contrast T1 mapping of the heart: the MESA study. Int J Cardiovasc Imaging. 2016;32(2):271–9. doi: 10.1007/s10554-015-0754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller CA, Naish JH, Bishop P, Coutts G, Clark D, Zhao S, Ray SG, Yonan N, Williams SG, Flett AS, Moon JC, Greiser A, Parker GJ, Schmitt M. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging. 2013;6(3):373–83. doi: 10.1161/CIRCIMAGING.112.000192. [DOI] [PubMed] [Google Scholar]
- 13.Nagarajan V, Kohan L, Holland E, Keeley EC, Mazimba S. Obesity paradox in heart failure: a heavy matter. ESC Heart Fail. 2016;3(4):227–34. doi: 10.1002/ehf2.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gai N, Turkbey EB, Nazarian S, van der Geest RJ, Liu CY, Lima JA, Bluemke DA. T1 mapping of the gadolinium-enhanced myocardium: adjustment for factors affecting interpatient comparison. Magn Reson Med. 2011;65(5):1407–15. doi: 10.1002/mrm.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Grgac K, Huang A, Yadav N, Qin Q, van Zijl PC. Quantitative theory for the longitudinal relaxation time of blood water. Magn Reson Med. 2016;76(1):270–81. doi: 10.1002/mrm.25875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemmens HJ, Bernstein DP, Brodsky JB. Estimating blood volume in obese and morbidly obese patients. Obes Surg. 2006;16(6):773–6. doi: 10.1381/096089206777346673. [DOI] [PubMed] [Google Scholar]
- 17.Manzone TA, Dam HQ, Soltis D, Sagar VV. Blood volume analysis: a new technique and new clinical interest reinvigorate a classic study. J Nucl Med Technol. 2007;35(2):55–63. doi: 10.2967/jnmt.106.035972. quiz 77, 9. [DOI] [PubMed] [Google Scholar]
- 18.Recommended methods for measurement of red-cell and plasma volume: International Committee for Standardization in Haematology. J Nucl Med. 1980;21(8):793–800. [PubMed] [Google Scholar]
- 19.Roujol S, Weingartner S, Foppa M, Chow K, Kawaji K, Ngo LH, Kellman P, Manning WJ, Thompson RB, Nezafat R. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology. 2014;272(3):683–9. doi: 10.1148/radiol.14140296. [DOI] [PMC free article] [PubMed] [Google Scholar]