Abstract
B cell activating factor from the TNF family (BAFF) is implicated in not only the physiology of normal B cells, but also the pathophysiology of aggressive B cells related to malignant and autoimmune diseases. Autophagy plays a crucial role in balancing the beneficial and detrimental effects of immunity and inflammation. However, little is known about whether and how excessive BAFF mediates autophagy contributing to B-cell proliferation and survival. Here, we show that excessive human soluble BAFF (hsBAFF) inhibited autophagy with a concomitant reduction of LC3-II in normal and B-lymphoid (Raji) cells. Knockdown of LC3 not only potentiated hsBAFF inhibition of autophagy, but also attenuated hsBAFF activation of Akt/mTOR pathway, thereby diminishing hsBAFF-induced B-cell proliferation/viability. Further, we found that hsBAFF inhibition of autophagy was Akt/mTOR-dependent. This is supported by the findings that hsBAFF increased mTORC1-mediated phosphorylation of ULK1 (Ser757); Akt inhibitor X, mTORC1 inhibitor rapamycin, mTORC1/2 inhibitor PP242, expression of dominant negative Akt, or knockdown of mTOR attenuated hsBAFF-induced phosphorylation of ULK1, decrease of LC3-II level, and increase of cell proliferation/viability. Chelating intracellular free Ca2+ ([Ca2+]i) with BAPTA/AM or preventing [Ca2+]i elevation using EGTA or 2-APB profoundly blocked hsBAFF-induced activation of Akt/mTOR, phosphorylation of ULK1 and decrease of LC3-II, as well as increase of cell proliferation/viability. Similar effects were observed in the cells where CaMKII was inhibited by KN93 or knocked down by CaMKII shRNA. Collectively, these results indicate that hsBAFF inhibits autophagy promoting cell proliferation and survival through activating Ca2+-CaMKII-dependent Akt/mTOR signaling pathway in normal and neoplastic B-lymphoid cells. Our findings suggest that manipulation of intracellular Ca2+ level or CaMKII, Akt, or mTOR activity to promote autophagy may be exploited for prevention of excessive BAFF-induced aggressive B lymphocyte disorders and autoimmune diseases.
Keywords: BAFF, Autophagy, Akt, mTOR, Calcium ion, B cells
1. Introduction
The B-cell activating factor from the TNF family (BAFF, also known as BLyS, TALL-1, THANK, and zTNF4) is a type II cytokine that exists in both membrane bound and soluble forms, and plays a pivotal role in B-cell development, survival, proliferation, maturation, and homeostasis [1; 2; 3; 4; 5]. Excessive endogenous and transgenic BAFF in mice prolongs the life span and increases the population of peripheral B lymphocytes, resulting in elevated secretion of superfluous autoantibodies [6]. BAFF is likely involved in the pathogenesis of a number of autoimmune diseases [7]. BAFF overexpression has been reportedly associated with the breakdown of B cell tolerance and autoantibody production. Serum BAFF concentrations are increased in systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), Sjögren’s syndrome (SS), autoimmune hepatitis, primary biliary cirrhosis, and Wegener’s granulomatosis [8; 9; 10; 11]. Therefore, high levels of BAFF, which contribute to aggressive or neoplastic B-cell disorders, have been thought to actually play a central role in the pathophysiology of autoimmune diseases [12; 13].
Macroautophagy, often called autophagy, is a highly conserved process in which cellular components (e.g. damaged cell organelles and unused macromolecules) are orderly degraded by lysosomes or vacuoles and recycled [14]. Autophagy has been regarded as an adaptive response to stress, promoting survival, but it can also promote cell death under certain conditions [14]. So, autophagy is a double-edged sword.
A series of ATG genes have been reported to be related to autophagosome initiation and formation, among which the microtubule-associated protein 1 light chain 3 (LC3), a mammalian homologue of the yeast protein Atg8, has been regarded as a specific biochemical marker for autophagy [15; 16]. LC3 exists in two molecular forms with LC3-I and LC3-II. LC3-I is the unconjugated form in the cytosol, whereas LC3-II is the phosphatidylethanolamine-conjugated form that is recruited to autophagosomal membranes [16; 17]. The cellular level of LC3-II directly correlates with the number of autophagosomes formed [16; 17]. Thus, the level of LC3-II or GFP-LC3-II in cells is widely used as a marker for monitoring the status of autophagy. Recent studies have demonstrated that autophagy plays a crucial role in balancing the beneficial and detrimental effects of immunity and inflammation [18]. However, the role of autophagy in BAFF-stimulated B-cell proliferation and survival has not been addressed.
Mammalian or mechanistic target of rapamycin (mTOR), a serine/threonine protein kinase, is a central controller for cell proliferation/growth, survival and autophagy [19]. mTOR lies downstream of phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt/PKB), so activated Akt may positively regulate mTOR, leading to increased phosphorylation of ribosomal p70S6 kinase (S6K1) and eukaryotic initiation factor 4E binding protein 1 (4E-BP1), two best characterized downstream effector molecules of mTOR [19]. Under nutrient sufficiency, high mTOR activity prevents Unc51-like kinase 1 (ULK1) activation contributing to inhibition of autophagy by phosphorylating ULK1 (Ser757) [20]. Studies have shown that mTOR is required for the maturation and differentiation of multiple immune cell lineages [21; 22], highlighting that mTOR is a promising therapeutic target in multiple lymphoid malignancies [23]. Calcium ion (Ca2+) is a ubiquitous intracellular signal responsible for cell proliferation/growth, differentiation, and survival of various cell types in the immune system [24; 25]. The intracellular free Ca2+ ([Ca2+]i) elevation may activate Akt/mTOR signaling pathway [26; 27]. Calcium/calmodulin-dependent protein kinase II (CaMKII) is activated in the presence of Ca2+ and calmodulin (CaM) [28; 29; 30]. In addition, Ca2+ is regarded as an important regulator of autophagy [31]. Our group has recently demonstrated that excessive human soluble BAFF (hsBAFF) promotes proliferation and survival in cultured B lymphocytes via Ca2+/CaMKII-mediated activation of Akt/mTOR pathway [32]. This prompted us to study whether BAFF may mediate autophagy contributing to B-cell proliferation and survival by Ca2+/CaMKII-Akt/mTOR signaling.
Here, we show that excessive hsBAFF inhibited autophagy, promoting cell proliferation and survival via increasing ULK1 (Ser757) phosphorylation and decreasing LC3-II protein level, which was through activating Ca2+-CaMKII-dependent Akt/mTOR signaling pathway in normal and neoplastic B-lymphoid cells. Our findings suggest that manipulation of intracellular Ca2+ level or CaMKII, Akt, or mTOR activity to promote autophagy may be exploited for prevention of excessive BAFF-induced aggressive B lymphocyte disorders and autoimmune diseases.
2. Materials and methods
2.1. Materials
Anti-CD19 magnetic fluorobeads-B was purchased from One Lambda (Canoga Park, CA, USA). Refolded human soluble BAFF (hsBAFF) was a recombinant form of the extracellular domain of the BAFF produced in Escherichia coli from this group [33]. RPMI 1640 medium was from Gibco (Rockville, MD, USA). Fetal bovine serum (FBS) was supplied by Hyclone (Logan, UT, USA). CellTiter 96®AQueous One Solution Cell Proliferation Assay kit was from Promega (Madison, WI, USA). Enhanced chemiluminescence solution was from Sciben Biotech Company (Nanjing, China). Akt inhibitor X and PP242 were supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA). 1,2-bis(o-aminophenoxy) ethane-N,N,N’,N’-tetraacetic acid tetra(acetoxymethyl)ester (BAPTA/AM) and 2-aminoethoxydiphenyl borane (2-APB) were purchased from Calbiochem (San Diego, CA, USA), whereas ethylene glycol tetra-acetic acid (EGTA) and monodansylcadaverine (MDC) were from Sigma (St. Louis, MO, USA). KN93 and rapamycin were from ALEXIS (San Diego, CA, USA). The following antibodies were used: LC3, phospho-mTOR (Ser2448), mTOR, HA (Sigma), phospho-CaMKII (Thr286), phospho-Akt (Ser473), phospho-S6K1 (Thr389), phospho-4EBP1 (Thr70), 4E-BP1 (Cell Signaling Technology, Beverly, MA, USA), phospho-ULK1 (Ser757), ULK1 (Sciben Biotech Company), Akt, CaMKII, S6K1, β-actin (Santa Cruz Biotechnology), goat anti-rabbit IgG-horseradish peroxidase (HRP), goat anti-mouse IgG-HRP, and rabbit anti-goat IgG-HRP (Pierce, Rockford, IL, USA). Other chemicals were purchased from local commercial sources and were of analytical grade.
2.2. Cell Culture
Raji cell line (American Type Culture Collection, Manassas, VA, USA) was maintained in RPMI 1640 medium supplemented with 10 % FBS, 100 U/ml penicillin, 100 U/ml streptomycin and incubated at 37°C in a humidified incubator containing 5 % CO2. Because of the replicative nature and cost-effectiveness, the cell line is widely used as B-cell models, so they were employed in this study. To verify the data obtained from Raji cells, primary B cells were also used in this study. For this, normal mouse B lymphocytes were purified from fresh splenic cells of healthy mice using anti-CD19 magnetic fluorobeads-B and cultured as described previously [32]. All procedures used in this study were approved by the Institutional Animal Care and Use Committee, and were in compliance with the guidelines set forth by the Guide for the Care and Use of Laboratory Animals.
2.3. Recombinant adenoviral constructs and infection of cells
Recombinant adenovirus encoding HA-tagged dominant negative Akt (Ad-dn-Akt, T308A/S473A) was generously provided from Dr. Kenneth Walsh (Boston University, Boston, MA), and the control adenovirus expressing the green fluorescent protein (GFP) (Ad-GFP) was described previously [34]. Adenovirus expressing GFP-LC3 fusion protein (Ad-GFP-LC3) was purchased from Sciben Biotech Company (Nanjing, China). For experiments, Raji cells were grown in the growth medium and infected with the individual adenovirus for 24 h at 5 of multiplicity of infection (MOI = 5). Subsequently, cells were used for experiments. Ad-GFP served as a control. Expression of HA-tagged dn-Akt was detected by Western blotting with antibodies to HA.
2.4. Lentiviral shRNA cloning, production and infection
Lentiviral shRNAs to mTOR, CaMKII and GFP (for control) were generated and used as described [35; 36]. To generate lentiviral shRNA to LC3, oligonucleotides containing the target sequences were synthesized, annealed and inserted into FSIPPW lentiviral vector [37] via the EcoR1/BamH1 restriction enzyme site. Oligonucleiotides used were: LC3 sense:
5’-AATTCCCGGTCTATGCCTCCCAGGAGACTGCAAGAGAGTCTCCTGGGAGGCATAGACCTTTTTG-3’, anti-sense:
5’-GATCCAAAAAGGTCTATGCCTCCCAGGAGACTCTCTTGCAGTCTCCTGGGAGGCATAGACCGGG-3’. To produce lentiviral particles, above constructs were co-transfected together with pMD2G and psPAX2 (Addgene, Cambridge, MA, USA) to 293TD cells using MegaTran 1.0 reagent (OriGene Technologies, Rockville, MD, USA). Each virus-containing medium was collected 48 h and 60 h post-transfection, respectively. For use, Raji cells, when grown to about 70% confluence, were infected with above lentivirus-containing medium in the presence of 8 μg/ml polybrene for 12 h twice at an interval of 6 h. Uninfected cells were eliminated by exposure to 2 μg/ml puromycin for 48 h before use. After 5 days of culture, cells were used for experiments.
2.5. Cell proliferation and viability assay
Purified mouse B lymphocytes and/or Raji cells, or Raji cells infected with Ad-dn-Akt and Ad-GFP, respectively, or Raji cells infected with lentiviral shRNAs to LC3, mTOR, CaMKII and GFP, respectively, were seeded in 24-well plates (3×105 cells/well, for cell proliferation assay) or 96-well plates (3×104 cells/well, for cell viability assay) under standard culture conditions and kept overnight at 37°C humidified incubator with 5 % CO2. The next day, cells were treated with/without 0–5 μg/ml hsBAFF for 48 h, with/without 2.5 μg/ml hsBAFF for 0–48 h, or with/without 2.5 μg/ml hsBAFF for 48 h following pre-incubation with/without rapamycin (100 ng/ml) for 2 h and/or with/without Akt inhibitor X (20 μM), PP242 (1 μM), BAPTA/AM (20 μM), EGTA (100 μM), 2-APB (100 μM) or KN93 (10 μM) for 1 h with 3–6 replicates of each treatment. Subsequently, the proliferation and the viability of the cells were assessed using a Coulter Counter (Beckman Coulter, Fullerton, CA, USA) and a Victor X3 Light Plate Reader (PerkinElmer, Waltham, MA, USA), respectively, as described previously [38].
2.6. MDC-labeled autophagic vacuoles
Intracellular autophagic status was monitored by the incorporation of the autofluorescent drug MDC, a specific autophagolysosome marker, as described [39; 40]. In brief, Raji cells, or Raji cells infected with lentiviral shRNA to LC3 or GFP, respectively, were seeded at a density of 2 × 106 cells per well in a 6-well plate under standard culture conditions and kept overnight at 37°C humidified incubator with 5% CO2. The next day, cells were incubated with hsBAFF (0, 1 and/or 2.5 μg/ml) for 12, 24 and/or 48 h. Subsequently, the cells were labeled with 0.05 mM MDC in PBS for 10 min at 37°C, and then washed 3 times with PBS, followed by cell imaging under a fluorescence microscopy (Leica DMi8, Wetzlar, Germany) equipped with a digital camera. At least five independent fields per well were photographed. The number of MDC-labeled autophagic vacuoles per cell was counted.
2.7. GFP-LC3 assay
Raji cells and Raji cells infected with lentiviral shRNA to CaMKII, or GFP, respectively, were infected with Ad-GFP-LC3 and seeded at a density of 2 × 106 cells/well in a 6-well plate under standard culture conditions and kept overnight at 37°C humidified incubator with 5% CO2. The next day, cells were treated with hsBAFF (0, 1 and/or 2.5 μg/ml) for 12, 24 and/or 48 h. Subsequently, the cells were washed 3 times with PBS, followed by imaging and counting the numbers of LC3 puncta per cell as described above.
2.8. Western blot analysis
After treatments, cells were briefly washed with cold PBS, lysed on ice in the radioimmunoprecipitation assay buffer, and then subjected to Western blotting as described previously [35].
2.9. Statistical analysis
All data were presented as mean ± SEM. Student’s t-test for non-paired replicates was used to identify statistically significant differences between treatment means. Group variability and interaction were compared using either one-way or two-way ANOVA followed by Bonferroni’s post-tests to compare replicate means. The criterion for the statistical significance was P < 0.05.
3. Results
3.1. hsBAFF reduces autophagosome formation with a concomitant downregulation of LC3-II in B cells
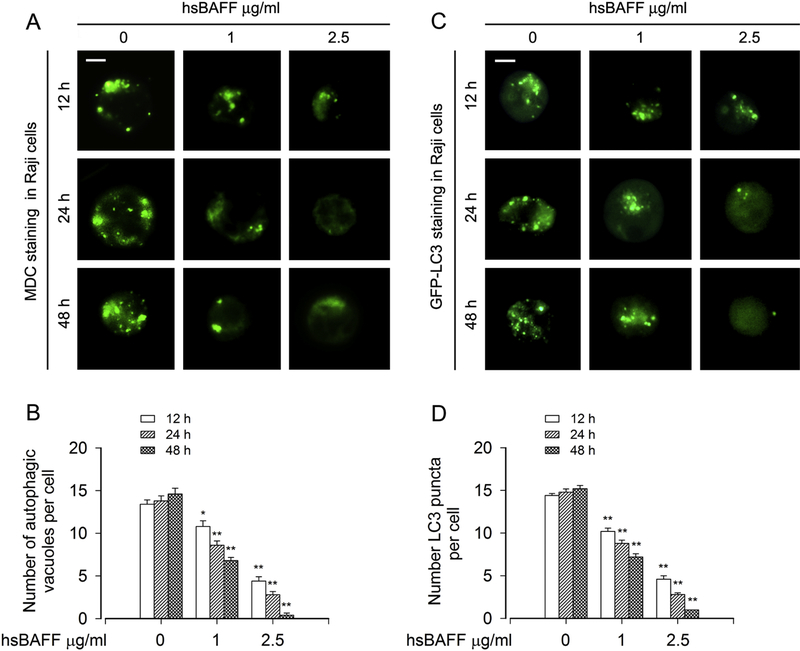
To investigate autophagic status in hsBAFF-stimulated B cells, the autofluorescent drug MDC, a specific autophagolysosome marker [39; 40], was employed. We observed that the accumulation of MDC in autophagic vacuoles (in green) was significantly suppressed by hsBAFF dose- and time-dependently in Raji cells, as evidenced by gradually reduced number of MDC-labeled autophagic vacuoles per cell (Fig. 1A and B). To corroborate the finding, we extended the studies by analyzing autophagic vacuoles with GFP-LC3 localization. Imaged and quantified results showed that when Raji cells, infected with Ad-GFP-LC3, were treated with hsBAFF (0, 1 and 2.5 μg/ml) for 12, 24 or 48 h, the number of LC3 puncta per cell markedly decreased compared to that of the vehicle-treated cells in a concentration- and time-dependent fashion (Fig. 1C and D).
Fig. 1. hsBAFF reduces autophagosome formation in B cells.
Raji cells, or Raji cells infected with Ad-GFP-LC3 were stimulated with hsBAFF (0, 1 and 2.5 μg/ml) for 12, 24 and 48 h, respectively. (A and B) The cells were labeled with MDC (a specific autophagolysosome marker). Then, the MDC-labeled autophagic vacuoles (in green) were photographed (A) and quantified (B) as described in “Materials and Methods”. (C and D) Shown are representative GFP-LC3 fluorescence images (in green) (C) and quantified number (D) for GFP-LC3 puncta in the cells. Scale bar: 2 μm. All quantified data were expressed as mean ± SEM (n = 5). Using one-way ANOVA, *P < 0.05, **P < 0.01, difference vs 0 μg/ml hsBAFF group.
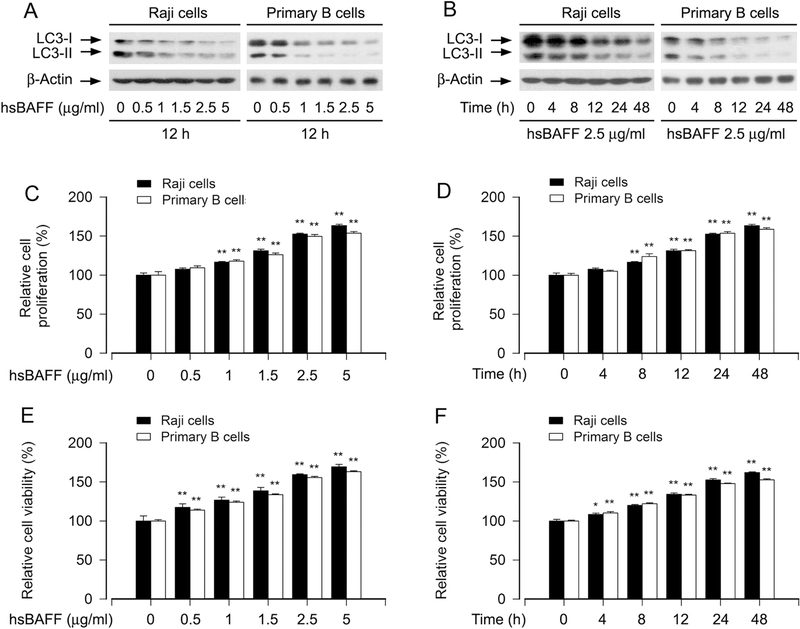
In addition, our Western blot analysis also showed that hsBAFF treatment reduced the protein levels of both LC3-I and LC3-II in a concentration- and time-dependent manner in Raji cells and primary B cells (Fig. 2A and B). However, in contrast to the above findings, hsBAFF substantially stimulated B-cell proliferation and survival dose- and time-dependently (Fig. 2C-F), as evaluated by cell counting and MTS assay. Since LC3-II is essential for autophagosome formation [16; 17], the above results indicate that hsBAFF reduces autophagosome formation with a concomitant downregulation of LC3-II in B cells, which contributes to increased B-cell proliferation and viability.
Fig. 2. hsBAFF downregulates the protein levels of LC3-II with a concomitant increase of cell proliferation and viability in B cells.
Raji cells and purified mouse splenic B lymphocytes were stimulated with hsBAFF (0–5 μg/ml) for 12 h and 48 h, respectively, or with 2.5 μg/ml hsBAFF for indicated time. (A and B) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-actin as a loading control. Similar results were observed in at least three independent experiments. (C and D) The cell proliferation was evaluated by cell counting. (E and F) The cell viability was determined by the MTS assay. All quantified data were expressed as mean ± SEM (n = 5). Using one-way ANOVA, *P < 0.05, **P < 0.01, difference vs 0 μg/ml hsBAFF group or 0 h group.
3.2. LC3-II exerts an essential role for hsBAFF-induced decrease of autophagosome formation and increase of proliferation/viability in B cells
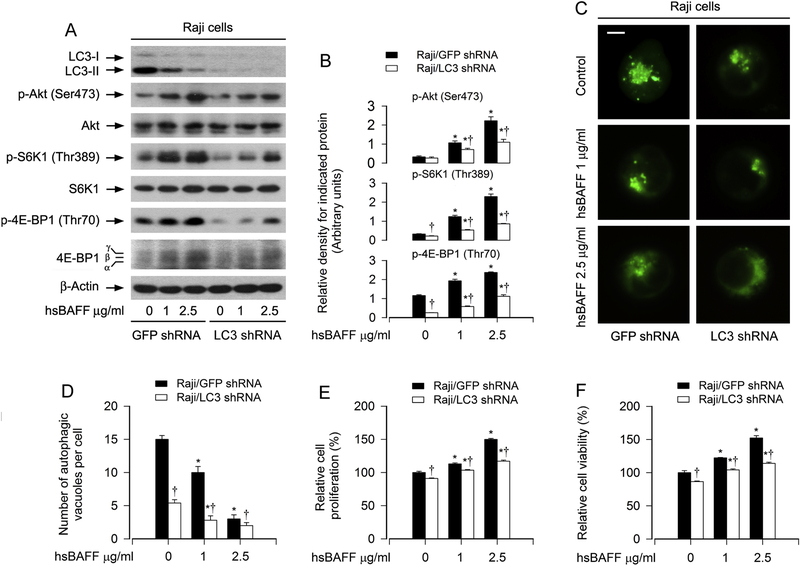
LC3-II, a phosphatidylethanolamine-conjugated form of LC3, is an essential protein for autophagosome formation [16; 17; 41; 42]. Based on the fact that hsBAFF-promoted B-cell proliferation/viability is connected to suppressed autophagy, we therefore proposed that silencing LC3 to prevent autophagy might enhance hsBAFF-stimulated B-cell proliferation/viability. To test this, Raji cells, infected with lentiviral shRNA to LC3 and GFP, respectively, were treated with hsBAFF (0, 1, or 2.5 μg/ml) for 12 h or 48 h. As shown in Fig. 3A, lentiviral shRNA to LC3, but not GFP, silenced the protein expression of LC3-I/II by ~ 90% in Raji cells, as detected by Western blotting. Knockdown of LC3 potentiated hsBAFF-suppressed MDC accumulation (Fig. 3C and D). However, we found that silencing LC3 significantly attenuated the basal and/or hsBAFF-induced phosphorylation of Akt, S6K1 and 4E-BP1 (Fig. 3A and B), and also abated hsBAFF-stimulated cell proliferation/viability (Fig. 3E and F) in the cells. Collectively, our results support the notion that LC3-II plays a critical role in hsBAFF-induced decrease of autophagosome formation and increase of proliferation/viability in B cells, and that depletion of LC3-II attenuated the stimulatory effect of hsBAFF on the Akt/mTOR pathway.
Fig. 3. LC3-II exerts an essential role for hsBAFF-induced decrease of autophagosome formation and increase of proliferation/viability in B cells.
Raji cells, infected with lentiviral shRNA to LC3 or GFP (as control), respectively, were stimulated with hsBAFF (0, 1, and 2.5 μg/ml) for 12 h (for Western blotting and MDC staining) or 48 h (for cell proliferation/viability assay). (A) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (B) The relative densities for p-Akt (Ser473) to Akt, p-S6K1 (Thr389) to S6K1, and p-4E-BP1 (Thr70) to β-actin were semi-quantified using NIH image J. (C and D) Shown are representative fluorescence images (in green) (C) and quantified number (D) for MDC-labeled autophagic vacuoles in the cells. Scale bar: 2 μm. (E) The cell proliferation was evaluated by cell counting. (F) The cell viability was determined by the MTS assay. All quantified data were expressed as mean ± SE (n = 5). Using one-way ANOVA or Student’s t-test, *P < 0.05, difference vs 0 μg/ml hsBAFF group; †P < 0.05, LC3 shRNA group vs GFP shRNA group.
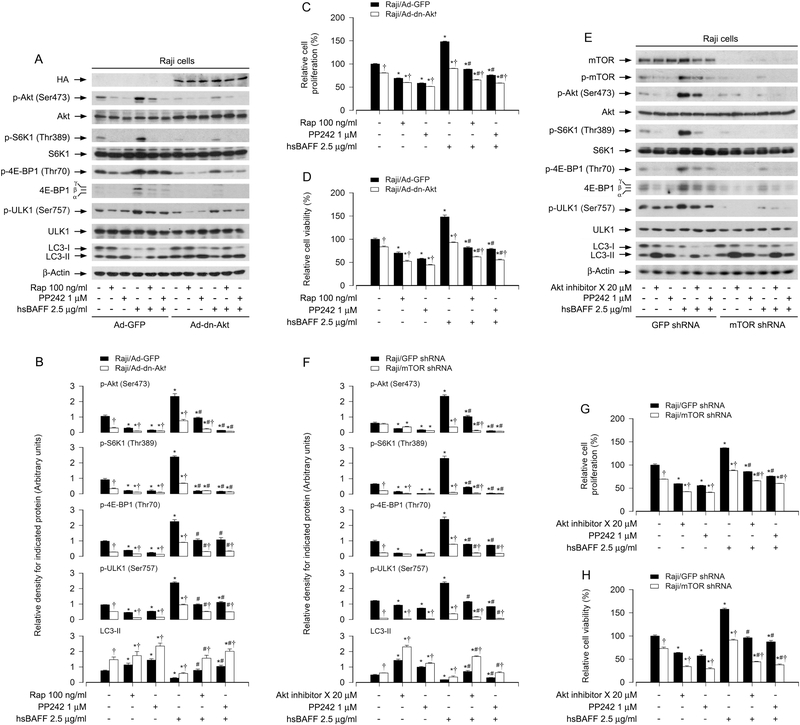
3.3. hsBAFF inhibits autophagy promoting B-cell proliferation/viability via activating the Akt/mTOR signaling pathway
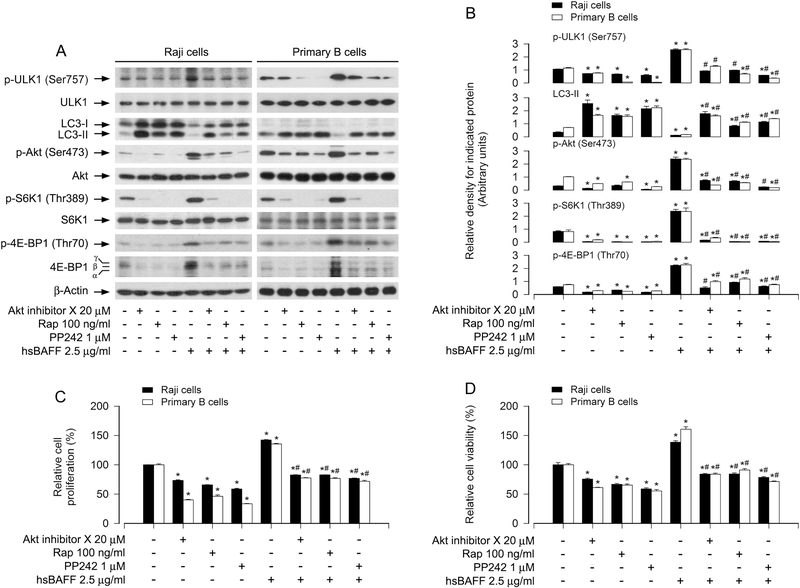
It is known that in response to nutrients or growth factors, mTORC1 is activated, which phosphorylates ULK1 (Ser757) and inhibits ULK1 kinase activity, thereby blocking autophagosome formation and eventually autophagy [19, 20]. Our previous study has demonstrated that hsBAFF promotes proliferation and survival in cultured B lymphocytes via activation of Akt/mTOR pathway [32]. So, we hypothesized that hsBAFF inhibits autophagy and increases B-cell proliferation/viability by increasing Akt/mTOR-mediated phosphorylation of ULK1 (Ser757). To this end, we studied whether inhibition of Akt or mTOR attenuates hsBAFF-induced increase of p-ULK1 and decrease of LC3-II in B cells. Raji cells and primary B cells were pretreated with/without mTORC1 inhibitor rapamycin (100 ng/ml) for 2 h, or mTORC1/2 inhibitor PP242 (1 μM) or Akt inhibitor X (20 μM) for 1 h, then stimulated with/without hsBAFF (2.5 μg/ml) for 12 h or 48 h, followed by Western blotting. As expected, rapamycin inhibited p-S6K1 and p-4E-BP1, and Akt inhibitor X inhibited p-Akt, whereas PP242 inhibited both p-S6K1/p-4E-BP1 and p-Akt, regardless of the presence or absence of hsBAFF. hsBAFF-induced upregulation of p-ULK1 and downregulation of LC3-II were markedly reversed by these inhibitors in the cells (Fig. 4A and B). Here, in primary B cells, because the basal level of LC3-I was too low, hsBAFF did not further reduce LC3-I expression apparently, but was able to downregulate the LC3-II level clearly (Fig. 4A and B), further supporting that the level of LC3-II determines the autophagosome formation. Consistently, the basal or hsBAFF-stimulated cell proliferation and viability were also reduced by these kinase inhibitors (Fig. 4C and D).
Fig. 4. Pharmacological inhibition of the Akt/mTOR pathway attenuates hsBAFF-induced downregulation of LC3-II and increase of proliferation/viability in B cells.
Raji cells and purified mouse splenic B lymphocytes were pretreated with/without mTORC1 inhibitor rapamycin (Rap) (100 ng/ml) for 2 h, or mTORC1/2 inhibitor PP242 (1 μM) or Akt inhibitor X (20 μM) for 1 h, then stimulated with/without hsBAFF (2.5 μg/ml) for 12 h (for Western blotting) or 48 h (for cell proliferation/viability assay). (A) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (B) The relative densities for p-ULK1 (Ser757) to ULK1, p-Akt (Ser473) to Akt, p-S6K1 (Thr389) to S6K1, and LC3-II, p-4E-BP1 (Thr70) to β-actin were semi-quantified using NIH image J. (C) The cell proliferation was evaluated by cell counting. (D) The cell viability was determined by the MTS assay. All quantified data were expressed as mean ± SE (n = 5). Using one-way ANOVA, *P < 0.05, difference vs 0 μg/ml hsBAFF group; #P < 0.05, difference vs 2.5 μg/ml hsBAFF group.
To gain more insights into the effects of Akt and mTOR activity on hsBAFF’s suppressing autophagy and subsequently promoting B-cell proliferation/viability, genetic approaches were taken. Firstly, Raji cells were infected with Ad-dn-Akt or Ad-GFP (as control), and then pretreated with/without rapamycin (100 ng/ml) for 2 h or PP242 (1 μM) for 1 h, followed by stimulation with/without hsBAFF (2.5 μg/ml) for 12 or 48 h. As expected, a high level of HA-tagged dn-Akt was seen in Ad-dn-Akt-infected cells, but not in Ad-GFP-infected cells (as control) (Fig. 5A). Ectopic expression of dn-Akt profoundly blocked hsBAFF-triggered phosphorylation of Akt, S6K1, 4E-BP1 and ULK1, decrease of LC3-II, as well as increase of cell proliferation/viability (Fig. 5A-D). Interestingly, treatment with rapamycin or PP242 was able to potentiate the inhibitory effects of dn-Akt (Fig. 5A-D). Similarly, silencing mTOR also dramatically blocked the phosphorylation of Akt, mTOR, S6K1, 4E-BP1 and ULK1 in the cells stimulated with/without hsBAFF (Fig. 5E and F). Of importance, depleting mTOR conferred high resistance to hsBAFF-decreased LC3-II expression (Fig. 5E and F) and coincidently attenuated hsBAFF-stimulated B-cell proliferation/viability (Fig. 5G and H). Moreover, treatment with Akt inhibitor X or PP242 also reinforced the inhibitory effects of mTOR silencing (Fig. 5E-H). Taken together, our findings underscore that hsBAFF inhibits autophagy via activating Akt/mTOR signaling pathway, leading to increased B-cell proliferation and survival.
Fig. 5. Ectopic expression of dominant negative Akt or downregulation of mTOR attenuates hsBAFF-induced decrease of LC3-II and increase of cell proliferation/viability in B cells.
Raji cells, infected with Ad-dn-Akt or Ad-GFP (as control), or infected with lentiviral shRNA to mTOR or GFP (as control), respectively, were pretreated with/without rapamycin (Rap) (100 ng/ml) for 2 h, or Akt inhibitor X (20 μM) or PP242(1 μM) for 1 h, then stimulated with/without hsBAFF (2.5 μg/ml) for 12 h (for Western blotting) or 48 h (for cell proliferation/viability assay). (A and E) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (B and F) The relative densities for p-Akt (Ser473) to Akt, p-S6K1 (Thr389) to S6K1, p-ULK1 (Ser757) to ULK1, and p-4E-BP1 (Thr70), LC3-II to β-actin were semi-quantified using NIH image J. (C and G) The cell proliferation was evaluated by cell counting. (D and H) The cell viability was determined by the MTS assay. All quantified data were expressed as mean ± SE (n = 5). Using one-way ANOVA or Student’s t-test, *P < 0.05, difference vs 0 μg/ml hsBAFF group; #P < 0.05, difference vs 2.5 μg/ml hsBAFF group; †P < 0.05, Ad-dn-Akt group vs Ad-GFP group or mTOR shRNA group vs GFP shRNA group.
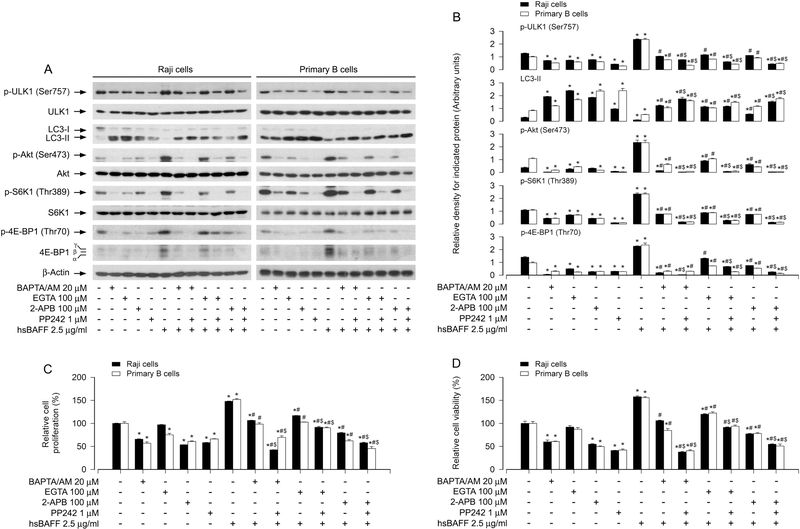
3.4. hsBAFF activates the Akt/mTOR pathway, suppressing autophagy in B cells, in Ca2+-dependent manner
Our recent studies have demonstrated that hsBAFF promotes B-cell proliferation and survival by elevating intracellular calcium ([Ca2+]i) level [32; 43]. We therefore sought to validate whether hsBAFF activates the Akt/mTOR pathway leading to autophagy suppression and B-cell proliferation/viability by Ca2+-dependent mechanism. For this, Raji cells and primary B cells were pretreated with/without BAPTA/AM (20 μM), an intracellular Ca2+ chelator, EGTA (100 μM), an extracellular Ca2+ chelator, 2-APB (100 μM), an inhibitor for both inositol 1,4,5-trisphosphate (IP3) receptors and the Ca2+ release activated Ca2+ (CRAC) channels, and/or PP242 (1 μM) for 1 h, followed by stimulation with/without hsBAFF (2.5 μg/ml) for 12 h or 48 h. We observed that chelating [Ca2+]i with BAPTA/AM or preventing [Ca2+]i elevation using EGTA or 2-APB drastically blocked hsBAFF-induced phosphorylation of Akt, S6K1, 4E-BP1 and ULK1 (Fig. 6A and B), decrease of LC3-II (Fig. 6A and B), as well as increase of cell proliferation/viability in the cells (Fig. 6C and D); and the effects were further strengthened by co-treatment with PP242 (Fig. 6A-D). The results imply that hsBAFF-induced extracellular Ca2+ influx and ER Ca2+ release should be involved in hsBAFF-activated Akt/mTOR pathway, thereby mediating autophagy suppression contributing to B-cell proliferation/survival.
Fig. 6. hsBAFF activates Akt/mTOR pathway, inhibiting autophagy and increasing cell proliferation/viability in B cells in Ca2+-dependent manner.
Raji cells and purified mouse splenic B lymphocytes were pretreated with/without BAPTA/AM (20 μM), EGTA (100 μM), 2-APB (100 μM) or/and PP242 (1 μM) for 1 h, then stimulated with/without hsBAFF (2.5 μg/ml) for 12 h (for Western blotting) or 48 h (for cell proliferation/viability assay). (A) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (B) The relative densities for p-ULK1 (Ser757) to ULK1, p-Akt (Ser473) to Akt, p-S6K1 (Thr389) to S6K1, and LC3-II, p-4E-BP1 (Thr70) to β-actin were semi-quantified using NIH image J. (C) The cell proliferation was evaluated by cell counting. (D) The cell viability was determined by the MTS assay. All quantified data were expressed as mean ± SE (n = 5). Using one-way or two-way ANOVA, *P < 0.05, difference vs 0 μg/ml hsBAFF group; #P < 0.05, difference vs 2.5 μg/ml hsBAFF group; $P < 0.05, difference vs BAPTA/AM/hsBAFF group, EGTA/hsBAFF group or 2-APB/hsBAFF group.
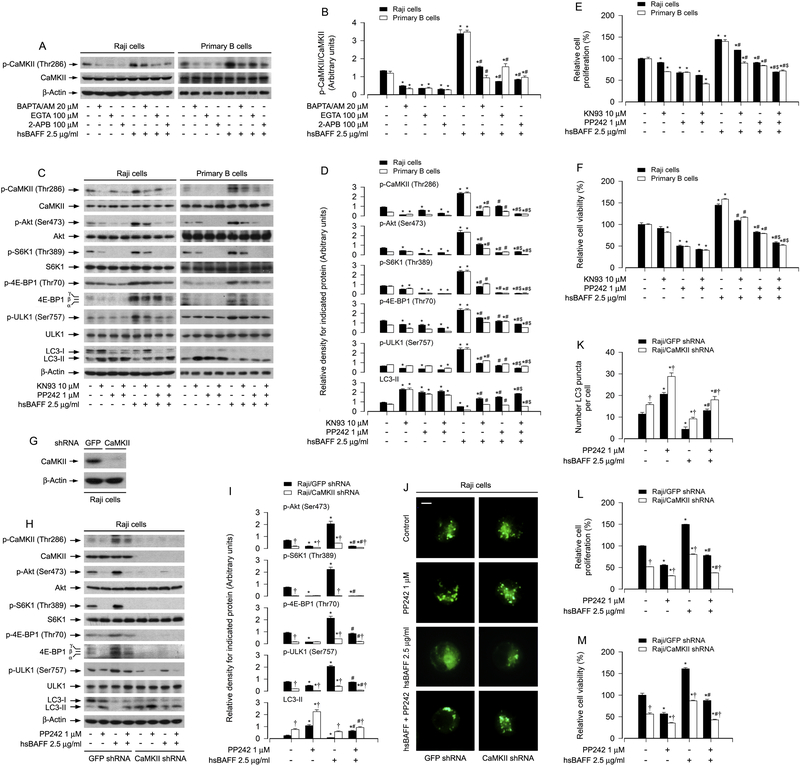
3.5. hsBAFF inhibits autophagy by stimulating CaMKII-dependent activation of Akt/mTOR pathway in B cells
CaMKII is a general integrator of Ca2+ signaling [28; 29]. Our group has demonstrated that hsBAFF-induced phosphorylation of CaMKII is attributed to [Ca2+]i elevation in cultured B lymphocytes [32]. In line with this, here we further observed that pretreatment with BAPTA/AM, EGTA or 2-APB remarkably attenuated the basal and hsBAFF-induced p-CaMKII (Thr286) in Raji cells and primary B cells (Fig. 7A and B). Next, we tested whether the effects of hsBAFF on the Akt/mTOR pathway contributing to autophagy suppression in B cells are through activating CaMKII. For this, Raji cells and primary B cells were treated with/without hsBAFF (2.5μg/ml) for 12 h or 48 h following pretreatment with/without CaMKII inhibitor KN93 (10 μM). The results showed that the basal and hsBAFF-induced p-CaMKII was significantly attenuated by KN93; and KN93 also markedly inhibited the basal and hsBAFF-induced expression of p-Akt, p-S6K1 p-4E-BP1 and p-ULK1 (Fig. 7C and D), as well as B-cell proliferation/viability (Fig. 7E and F). However, interestingly, KN93 elevated the basal and hsBAFF-inhibited LC3-II expression (Fig. 7C and D). Moreover, of note, co-treatment with KN93 and PP242 inhibited hsBAFF-induced p-CaMKII more robustly than treatment with KN93 or PP242 alone in Raji cells and primary B cells (Fig. 7C and D). Similarly, hsBAFF-induced phosphorylation of Akt, S6K1, 4E-BP1 and ULK1 as well as decrease of LC3-II were reversed by co-treatment with KN93/PP242 more potently than by treatment with KN93 or PP242 alone in the cells (Fig. 7C and D). In line with this, the combination of KN93 and PP242 more vigorously inhibited cell proliferation/viability than KN93 or PP242 alone in the cells in response to hsBAFF (Fig. 7E and F).
Fig. 7. hsBAFF activates Akt/mTOR pathway, inhibiting autophagy and increasing cell proliferation/viability by stimulating [Ca2+]i-dependent CaMKII phosphorylation in B cells.
Raji cells and purified mouse splenic B lymphocytes, or Raji cell infected with lentiviral shRNA to CaMKII or GFP (as control), were pretreated with/without BAPTA/AM (20 μM), EGTA (100 μM) or 2-APB (100 μM) for 1 h, or with/without KN93 (10 μM) or/and PP242 (1 μM) for 1 h, then stimulated with/withoutt hsBAFF (2.5 μg/ml) for 12 h (for Western blotting and GFP-LC3 assay) or 48 h (for cell proliferation/viability assay). (A, C, G and H) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (B, D and I) The relative densities for p-Akt (Ser473) to Akt, p-S6K1 (Thr389) to S6K1, p-ULK1 (Ser757) to ULK1, p-CaMKII (Thr286) to CaMKII, and p-4E-BP1 (Thr70), LC3-II to β-actin were semi-quantified using NIH image J. (E and L) The cell proliferation was evaluated by cell counting. (F and M) The cell viability was determined by the MTS assay. (J and K) The fluorescence imaging (in green) (J) and quantified number (K) for LC3 puncta in the cells was shown by GFP-LC3 assay. All quantified data were expressed as mean ± SE (n = 5). Using one-way or two-way ANOVA or Student’s t-test, *P < 0.05, difference vs 0 μg/ml hsBAFF group; #P < 0.05, difference vs 2.5 μg/ml hsBAFF group; $P < 0.05, difference vs KN93/hsBAFF group or PP242/hsBAFF group; †P < 0.05, CaMKII shRNA group vs GFP shRNA group.
To further corroborate the role of CaMKII in hsBAFF-activated Akt/mTOR pathway leading to autophagy inhibition, CaMKII was knocked down. As shown in Fig. 7G, CaMKII protein level was downregulated by ~90% in shRNA CaMKII-infected Raji cells compared to shRNA GFP-infected Raji cells. Silencing CaMKII obviously attenuated the basal and hsBAFF-induced phosphorylation of CaMKII, Akt, S6K1, 4E-BP1 and ULK1 (Fig. 7H and I). Correspondingly, depleting CaMKII upregulated the basal LC3-II level and conferred partial resistance to hsBAFF-induced decrease of LC3-II, which was further potentiated by PP242 treatment (Fig. 7H and I). Of importance, down-regulation of CaMKII significantly elevated the number of LC3 puncta per cell under the basal condition; and attenuated hsBAFF-induced decrease of LC3 puncta per cell (Fig. 7J and K). Concurrently, silencing CaMKII strongly inhibited hsBAFF-stimulated B-cell proliferation/viability (Fig. 7L and M). All of these effects were strengthened by PP242 (Fig. H-M). Collectively, these data demonstrate that hsBAFF inhibits autophagy promoting cell proliferation/viability by Ca2+-CaMKII-dependent activation of Akt/mTOR pathway in B cells.
4. Discussion
Numerous lines of evidence have shown that the cytokine BAFF plays a pivotal role in the development and homeostasis of normal B lymphocytes, as well as the growth and survival of neoplastic B-lymphoid cells [1; 2; 3; 4; 5]. Excessive or increased BAFF concentrations in serum are deemed to be involved in the pathogenesis of autoimmune diseases, including SLE, RA, SS, autoimmune hepatitis, primary biliary cirrhosis, and Wegener’s granulomatosis, etc. [7; 8; 9; 10; 11]. Therefore, it is of great importance to elucidate the molecular mechanism of BAFF-dependent B-cell proliferation and survival, in order to find a novel strategy to combat against excessive BAFF-associated autoimmune diseases and other aggressive/neoplastic B-cell disorders. Autophagy is an important and evolutionarily conserved cellular digestion process, which removes damaged macromolecules and organelles or recycle macromolecules in response to nutrient and environmental stress within the lysosomes or vacuoles in eukaryotic cells [44; 45]. Studies have demonstrated that dysregulation of autophagy contributes to autoimmune diseases [44; 46]. In this study, we discovered that hsBAFF inhibited autophagy, resulting in increased proliferation and viability in Raji cells and primary B cells. Mechanistically, hsBAFF suppressed autophagy by activating Ca2+-CaMKII-dependent Akt/mTOR signaling pathway.
It is well-known that LC3-II is a phosphatidylethanolamine-conjugated form of LC3, which binds to autophagosomes and directly correlates with the number of autophagosomes formed [16; 17]. Having found that hsBAFF inhibited autophagy by reducing autophagosome formation in Raji cells and primary B cells, we further studied whether this is by downregulating the expression of LC3-II. Our results showed that treatment with hsBAFF did reduce the levels of LC3-II, along with LC3-I, in the cells (Fig. 2). Silencing LC3 potentiated the effect of hsBAFF on autophagy inhibition, as evidenced by the decreased number of MDC-labeled autophagic vacuoles (Fig. 3). However, to our surprise, knockdown of LC3 did not strengthen hsBAFF-stimulated B-cell proliferation and survival, but markedly diminished the events. Especially, we found that hsBAFF-elevated p-Akt, p-S6K1 and p-4E-BP1 were substantially attenuated by depletion of LC3 in B cells (Fig. 3). These results imply that knockdown of LC3 not only intensifies hsBAFF-induced inhibition of autophagy, but also attenuates hsBAFF-induced activation of Akt/mTOR pathway, thereby reducing hsBAFF-induced B-cell proliferation/viability. Atg 5 is one of the critical enzymes responsible for production of the lipid modified form of LC3, i.e. LC3-II [16; 17]. It has been reported that deletion of Atg5 completely inhibits autophagy [47] and Atg5 plays an essential role in B lymphocyte development [48]. During this research, we observed that knockdown of Atg5 also inhibited autophagy in B cells. Whether hsBAFF downregulates Atg5 expression remains to be determined. Nevertheless, these data support the idea that hsBAFF downregulates the expression of LC3-II, thereby inhibiting autophagy and promoting B-cell proliferation and survival. In another word, certain level of LC3-II is critical for the effects of hsBAFF on autophagy and proliferation/viability in B cells.
mTOR is a downstream effector molecule of Akt, and negatively regulates autophagy partly by suppressing autophagosome formation [19]. The ULK1 (the orthologous yeast Atg1) forms a complex with ATG13 and FIP200, and is a key regulator in autophagy initiation [49; 50; 51; 52], and links cellular nutrient and energy status to downstream events in autophagy [53]. It is known that mTORC1 inhibits ULK1 activation by phosphorylating ULK1 (Ser757) and disrupt ULK1 complex formation, thereby suppressing autophagy [20]. Our recent studies have demonstrated that Akt/mTOR signaling pathway is involved in hsBAFF-stimulated B-cell proliferation and survival [32]. In the current study, treatment with hsBAFF elevated p-Akt, p-S6K1, p-4E-BP1 and p-ULK1, reduced LC3-II level, and increased cell proliferation/viability in the cells, which was remarkably attenuated by Akt inhibitor X or mTOR inhibitor (rapamycin and PP242) (Fig. 4). Similarly, expression of dominant negative Akt or down-regulation of mTOR also attenuated hsBAFF-induced phosphorylation of ULK1, decrease of LC3-II expression and increase of cell proliferation/viability in the cells (Fig. 5). Collectively, our results strongly support that hsBAFF suppresses autophagy via increasing p-ULK1 (Ser757) mediated by the Akt/mTOR signaling pathway.
Ca2+ is a ubiquitous intracellular signal responsible for numerous cellular events, such as proliferation/growth, differentiation, and survival in various immune cells [24; 25]. Our recent studies have described that hsBAFF activates mTOR pathway promoting proliferation and survival in cultured B lymphocytes via Ca2+ signaling [32]. This drove us to test whether hsBAFF-activated Akt/mTOR pathway mediates autophagy suppression in B cells in Ca2+-dependent manner. As predicted, pretreatment with BAPTA/AM (20 μM), an intracellular Ca2+ chelator, EGTA (100 μM), an extracellular Ca2+ chelator, or 2-APB (100 μM), an inhibitor for both inositol 1,4,5-trisphosphate (IP3) receptors and the Ca2+ release activated Ca2+ (CRAC) channels, did obviously attenuate hsBAFF-induced activation of Akt/mTOR, suppression of autophagy, and increase of cell proliferation/survival in normal and Raji B-lymphoid cells; and these effects were further strengthened by co-treatment with PP242 (Fig. 6). The results underline that hsBAFF-induced extracellular Ca2+ influx and ER Ca2+ release result in elevated [Ca2+]i level, which activates Akt/mTOR pathway, leading to suppressed autophagy and increased cell proliferation/viability in normal and Raji B-lymphoid cells.
CaMKII, a ubiquitously expressed serine/threonine protein kinase, has been reported to regulate the development and activity of many different cell types including immune cells [28; 29; 30; 54; 55; 56; 57]. Since CaMKII acts as a general integrator of Ca2+ signaling, we asked whether hsBAFF-activated Akt/mTOR pathway inhibits autophagy by stimulating Ca2+-dependent CaMKII. Our results showed that KN93 dramatically blocked hsBAFF-induced phosphorylation of Akt, S6K1 p-4E-BP1 and p-ULK1, decrease of LCI-3-II, and increase of proliferation/viability in Raji cells and primary B cells; and the effects were further potentiated by co-treatment with PP242 (Fig. 7C-F). Similar results were seen in the cells treated with lentiviral shRNA to CaMKII (Fig. 7G-M). These results strongly support that hsBAFF-elevated [Ca2+]i-dependent CaMKII phosphorylation activates Akt/mTOR-mediated phosphorylation of ULK1 and consequential suppression of autophagy, thereby promoting B-cell proliferation and survival.
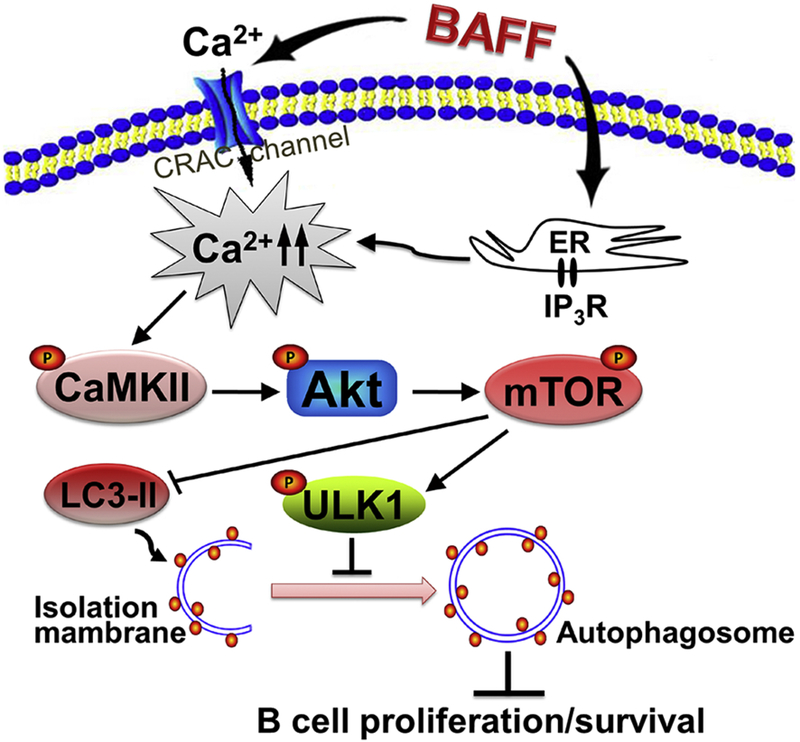
In summary, here we identify that excessive hsBAFF promotes cell proliferation and survival by inhibiting autophagy in normal and neoplastic B-lymphoid cells. Mechanistically, BAFF inhibits autophagy by increasing ULK1 phosphorylation (Ser757) and decreasing LC3-II protein expression in B cells, which is through activating the Ca2+-CaMKII-Akt-mTOR signaling pathway (Fig. 8). Our findings suggest that manipulation of intracellular Ca2+ level or CaMKII, Akt, or mTOR activity to promote autophagy may be exploited for prevention of excessive BAFF-induced aggressive B lymphocyte disorders and autoimmune diseases.
Fig. 8. Graphical model showing how hsBAFF inhibits autophagy, promoting cell proliferation and survival in B cells.
hsBAFF inhibits autophagy, promoting cell proliferation and survival, by increasing ULK1 phosphorylation (Ser757) and decreasing LC3-II protein expression in normal and neoplastic B-lymphoid cells. This is through activating the Ca2+-CaMKII-Akt-mTOR signaling pathway. Manipulation of intracellular Ca2+ level or CaMKII, Akt, or mTOR activity to promote autophagy may be exploited for prevention of excessive BAFF-induced aggressive B-cell disorders.
Highlights.
-
●
hsBAFF promotes B-cell proliferation and survival by inhibiting autophagy.
-
●
Downregulation of LC3 protein level is critical for hsBAFF inhibition in B cells.
-
●
hsBAFF-activated Akt/mTOR signaling represses autophagy via phosphorylating ULK1 (Ser757) in B cells.
-
●
hsBAFF inhibits autophagy by activating Ca2+-CaMKII-dependent Akt/mTOR signaling pathway in B cells.
Acknowledgements
This work was supported in part by the grants from National Natural Science Foundation of China (No.31172083; LC), NIH (CA115414; SH), Project for the Priority Academic Program Development of Jiangsu Higher Education Institutions of China (PAPD-14KJB180010; LC), and American Cancer Society (RSG-08–135-01-CNE; SH).
Abbreviations:
- 2-APB
2-aminoethoxydiphenyl borate
- 4E-BP1
eukaryotic initiation factor 4E binding protein 1
- Akt
protein kinase B (PKB)
- ATG
autophagy-related
- BAFF
B-cell activating factor of the TNF family
- BAPTA/AM
1,2-bis(o-aminophenoxy) ethane-N,N,N’,N’-tetraacetic acid tetra(acetoxymethyl) ester
- BLyS
B lymphocyte stimulator
- Ca2+
calcium ion
- CaM
calmodulin
- CaMKII
calcium/calmodulin-dependent protein kinase II
- CRAC
Ca2+-release activated Ca2+
- EGTA
ethylene glycol tetra-acetic acid
- FBS
fetal bovine serum; GFP, green fluorescent protein
- LC3
microtubule-associated protein 1 light chain 3
- MAPK
mitogen-activated protein kinase
- MDC
monodansylcadaverine
- mTOR
mammalian target of rapamycin
- PBS
phosphate buffered saline
- PI3K
phosphatidylinositol 3′-kinase
- RA
rheumatoid arthritis
- S6K1
S6 kinase 1
- SLE
systemic lupus erythematosus
- SS
Sjögren’s syndrome
- TALL-1
TNF and apoptosis ligand-related leukocyte-expressed ligand1
- THANK
TNF homologue that activates apoptosis, nuclear factor κB, and c-Jun NH2-terminal kinase
- ULK1
Unc51-like kinase 1
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fairfax KA, Tsantikos E, Figgett WA, Vincent FB, Quah PS, LePage M, Hibbs ML, Mackay F, BAFF-driven autoimmunity requires CD19 expression. J. Autoimmun 62 (2015) 1–10. [DOI] [PubMed] [Google Scholar]
- [2].Henley T, Kovesdi D, Turner M, B-cell responses to B-cell activation factor of the TNF family (BAFF) are impaired in the absence of PI3K delta. Eur. J. Immunol 38 (2008) 3543–3548. [DOI] [PubMed] [Google Scholar]
- [3].Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM, BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 285 (1999) 260–263. [DOI] [PubMed] [Google Scholar]
- [4].Mueller CG, Boix C, Kwan WH, Daussy C, Fournier E, Fridman WH, Molina TJ, Critical role of monocytes to support normal B cell and diffuse large B cell lymphoma survival and proliferation. J. Leukoc. Biol 82 (2007) 567–575. [DOI] [PubMed] [Google Scholar]
- [5].Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J, BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med 189 (1999) 1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moisini I, Davidson A, BAFF: a local and systemic target in autoimmune diseases. Clin. Exp. Immunol 158 (2009) 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lahiri A, Pochard P, Le Pottier L, Tobon GJ, Bendaoud B, Youinou P, Pers JO, The complexity of the BAFF TNF-family members: implications for autoimmunity. J. Autoimmun 39 (2012) 189–198. [DOI] [PubMed] [Google Scholar]
- [8].Binard A, Le Pottier L, Saraux A, Devauchelle-Pensec V, Pers JO, Youinou P, Does the BAFF dysregulation play a major role in the pathogenesis of systemic lupus erythematosus? J. Autoimmun 30 (2008) 63–67. [DOI] [PubMed] [Google Scholar]
- [9].Krumbholz M, Specks U, Wick M, Kalled SL, Jenne D, Meinl E, BAFF is elevated in serum of patients with Wegener’s granulomatosis. J. Autoimmun 25 (2005) 298–302. [DOI] [PubMed] [Google Scholar]
- [10].Migita K, Ilyassova B, Kovzel EF, Nersesov A, Abiru S, Maeda Y, Komori A, Ito M, Yano K, Yatsuhashi H, Shimoda S, Ishibashi H, Nakamura M, Serum BAFF and APRIL levels in patients with PBC. Clin. Immunol 134 (2010) 217–225. [DOI] [PubMed] [Google Scholar]
- [11].Pillai S, Mattoo H, Cariappa A, B cells and autoimmunity. Curr. Opin. Immunol 23 (2011) 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cornec D, Devauchelle-Pensec V, Tobon GJ, Pers JO, Jousse-Joulin S, Saraux A, B cells in Sjogren’s syndrome: from pathophysiology to diagnosis and treatment. J. Autoimmun 39 (2012) 161–167. [DOI] [PubMed] [Google Scholar]
- [13].Ramanujam M, Davidson A, BAFF blockade for systemic lupus erythematosus: will the promise be fulfilled? Immunol. Rev 223 (2008) 156–174. [DOI] [PubMed] [Google Scholar]
- [14].Botbol Y, Guerrero-Ros I, Macian F, Key roles of autophagy in regulating T-cell function. Eur. J. Immunol 46 (2016) 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Feng Y, Yao Z, Klionsky DJ, How to control self-digestion: transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell. Biol 25 (2015) 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ni HM, Bockus A, Wozniak AL, Jones K, Weinman S, Yin XM, Ding WX, Dissecting the dynamic turnover of GFP-LC3 in the autolysosome. Autophagy 7 (2011) 188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T, LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19 (2000) 5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Levine B, Mizushima N, Virgin HW, Autophagy in immunity and inflammation. Nature 469 (2011) 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Laplante M, Sabatini DM, mTOR signaling in growth control and disease. Cell 149 (2012) 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim J, Kundu M, Viollet B, Guan KL, AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol 13 (2011) 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang S, Readinger JA, DuBois W, Janka-Junttila M, Robinson R, Pruitt M, Bliskovsky V, Wu JZ, Sakakibara K, Patel J, Parent CA, Tessarollo L, Schwartzberg PL, Mock BA, Constitutive reductions in mTOR alter cell size, immune cell development, and antibody production. Blood 117 (2011) 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhong XP, Shin J, Gorentla BK, O’Brien T, Srivatsan S, Xu L, Chen Y, Xie D, Pan H, Receptor signaling in immune cell development and function. Immunol. Res 49 (2011) 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yun S, Vincelette ND, Knorr KL, Almada LL, Schneider PA, Peterson KL, Flatten KS, Dai H, Pratz KW, Hess AD, Smith BD, Karp JE, Hendrickson AE, Fernandez-Zapico ME, Kaufmann SH, 4EBP1/c-MYC/PUMA and NF-kappaB/EGR1/BIM pathways underlie cytotoxicity of mTOR dual inhibitors in malignant lymphoid cells. Blood 127 (2016) 2711–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wen L, Chen SJ, Zhang W, Ma HW, Zhang SQ, Chen L, hsBAFF regulates proliferation and response in cultured CD4+ T lymphocytes by upregulation of intracellular free Ca2+ homeostasis. Cytokine 53 (2011) 215–222. [DOI] [PubMed] [Google Scholar]
- [25].Wilson C, Munoz-Palma E, Henriquez DR, Palmisano I, Nunez MT, Di Giovanni S, Gonzalez-Billault C, A Feed-Forward Mechanism Involving the NOX Complex and RyR-Mediated Ca2+ Release During Axonal Specification. J. Neurosci 36 (2016) 11107–11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cheng A, Wang S, Yang D, Xiao R, Mattson MP, Calmodulin mediates brain-derived neurotrophic factor cell survival signaling upstream of Akt kinase in embryonic neocortical neurons. J. Biol. Chem 278 (2003) 7591–7599. [DOI] [PubMed] [Google Scholar]
- [27].Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G, Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 7 (2008) 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Colbran RJ, Brown AM, Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr. Opin. Neurobiol 14 (2004) 318–327. [DOI] [PubMed] [Google Scholar]
- [29].Liu Y, Templeton DM, Cadmium activates CaMK-II and initiates CaMK-II-dependent apoptosis in mesangial cells. FEBS Lett. 581 (2007) 1481–1486. [DOI] [PubMed] [Google Scholar]
- [30].Swulius MT, Waxham MN, Ca2+/calmodulin-dependent protein kinases. Cell. Mol. Life Sci. 65 (2008) 2637–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].East DA, Campanella M, Ca2+ in quality control: an unresolved riddle critical to autophagy and mitophagy. Autophagy 9 (2013) 1710–1719. [DOI] [PubMed] [Google Scholar]
- [32].Ke Z, Liang D, Zeng Q, Ren Q, Ma H, Gui L, Chen S, Guo M, Xu Y, Gao W, Zhang S, Chen L, hsBAFF promotes proliferation and survival in cultured B lymphocytes via calcium signaling activation of mTOR pathway. Cytokine 62 (2013) 310–321. [DOI] [PubMed] [Google Scholar]
- [33].Cao P, Mei JJ, Diao ZY, Zhang S, Expression, refolding, and characterization of human soluble BAFF synthesized in Escherichia coli. Protein Expr. Purif 41 (2005) 199–206. [DOI] [PubMed] [Google Scholar]
- [34].Liu L, Luo Y, Chen L, Shen T, Xu B, Chen W, Zhou H, Han X, Huang S, Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity. J. Biol. Chem 285 (2010) 38362–38373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen L, Liu L, Luo Y, Huang S, MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. J. Neurochem 105 (2008) 251–261. [DOI] [PubMed] [Google Scholar]
- [36].Chen S, Xu Y, Xu B, Guo M, Zhang Z, Liu L, Ma H, Chen Z, Luo Y, Huang S, Chen L, CaMKII is involved in cadmium activation of MAPK and mTOR pathways leading to neuronal cell death. J. Neurochem 119 (2011) 1108–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K, Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 19 (2005) 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zeng Q, Zhang H, Qin J, Xu Z, Gui L, Liu B, Liu C, Xu C, Liu W, Zhang S, Huang S, Chen L, Rapamycin inhibits BAFF-stimulated cell proliferation and survival by suppressing mTOR-mediated PP2A-Erk1/2 signaling pathway in normal and neoplastic B-lymphoid cells. Cell. Mol. Life Sci 72 (2015) 4867–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Munafo DB, Colombo MI, A novel assay to study autophagy: regulation of autophagosome vacuole size by amino acid deprivation. J. Cell Sci 114 (2001) 3619–3629. [DOI] [PubMed] [Google Scholar]
- [40].Biederbick A, Kern HF, Elsasser HP, Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur. J. Cell Biol 66 (1995) 3–14. [PubMed] [Google Scholar]
- [41].Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y, A protein conjugation system essential for autophagy. Nature 395 (1998) 395–398. [DOI] [PubMed] [Google Scholar]
- [42].Tanida I, Ueno T, Kominami E, LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol 36 (2004) 2503–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liang D, Zeng Q, Xu Z, Zhang H, Gui L, Xu C, Chen S, Zhang S, Huang S, Chen L, BAFF activates Erk1/2 promoting cell proliferation and survival by Ca2+-CaMKII-dependent inhibition of PP2A in normal and neoplastic B-lymphoid cells. Biochem. Pharmacol 87 (2014) 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Levine B, Klionsky DJ, Autophagy wins the 2016 Nobel Prize in Physiology or Medicine: Breakthroughs in baker’s yeast fuel advances in biomedical research. Proc. Natl. Acad. Sci. U S A 114 (2017) 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Song S, Tan J, Miao Y, Li M, Zhang Q, Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J. Cell. Physiol 232 (2017) 2977–2984. [DOI] [PubMed] [Google Scholar]
- [46].Wu DJ, Adamopoulos IE, Autophagy and autoimmunity. Clin. Immunol 176 (2017) 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ma T, Li J, Xu Y, Yu C, Xu T, Wang H, Liu K, Cao N, Nie BM, Zhu SY, Xu S, Li K, Wei WG, Wu Y, Guan KL, Ding S, Atg5-independent autophagy regulates mitochondrial clearance and is essential for iPSC reprogramming. Nat. Cell Biol 17 (2015) 1379–1387. [DOI] [PubMed] [Google Scholar]
- [48].Miller BC, Zhao Z, Stephenson LM, Cadwell K, Pua HH, Lee HK, Mizushima NN, Iwasaki A, He YW, Swat W, Virgin H.W.t., The autophagy gene ATG5 plays an essential role in B lymphocyte development. Autophagy 4 (2008) 309–314. [DOI] [PubMed] [Google Scholar]
- [49].Chan EY, Tooze SA, Evolution of Atg1 function and regulation. Autophagy 5 (2009) 758–765. [DOI] [PubMed] [Google Scholar]
- [50].Chang YY, and Neufeld TP, An Atg1/Atg13 complex with multiple roles in TOR-mediated autophagy regulation. Mol. Biol. Cell 20 (2009) 2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kabeya Y, Kamada Y, Baba M, Takikawa H, Sasaki M, Ohsumi Y, Atg17 functions in cooperation with Atg1 and Atg13 in yeast autophagy. Mol. Biol. Cell 16 (2005) 2544–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mizushima N, The role of the Atg1/ULK1 complex in autophagy regulation. Curr. Opin. Cell Biol 22 (2010) 132–139. [DOI] [PubMed] [Google Scholar]
- [53].Lin MG, and Hurley JH, Structure and function of the ULK1 complex in autophagy. Curr. Opin. Cell Biol 39 (2016) 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bui JD, Calbo S, Hayden-Martinez K, Kane LP, Gardner P, Hedrick SM, A role for CaMKII in T cell memory. Cell 100 (2000) 457–467. [DOI] [PubMed] [Google Scholar]
- [55].Lin MY, Zal T, Ch’en IL, Gascoigne NR, Hedrick SM, A pivotal role for the multifunctional calcium/calmodulin-dependent protein kinase II in T cells: from activation to unresponsiveness. J. Immunol 174 (2005) 5583–5592. [DOI] [PubMed] [Google Scholar]
- [56].Si J, and Collins SJ, Activated Ca2+/calmodulin-dependent protein kinase IIgamma is a critical regulator of myeloid leukemia cell proliferation. Cancer Res. 68 (2008) 3733–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wayman GA, Tokumitsu H, Davare MA, Soderling TR, Analysis of CaM-kinase signaling in cells. Cell Calcium 50 (2011) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]