Abstract
Previous studies have linked plasma inflammatory markers to elevated fatigue in patients with head and neck cancer (HNC). To identify the molecular mechanisms underlying this association, we conducted promoter-based bioinformatics analyses to determine the relationship between fatigue and specific gene expression profiles associated with inflammation in human papillomavirus (HPV)-related and -unrelated HNC patients undergoing treatment. Patients with newly diagnosed HNC without distant metastasis were assessed at baseline (pre-radiotherapy) and one-month post-radiotherapy. Fatigue was measured by the Multidimensional Fatigue Inventory. Genome-wide gene expression profiles were collected from peripheral blood mononuclear cells (PBMC). Promoter-based bioinformatics analyses were employed to identify transcription control pathways underlying transcriptomic correlates of fatigue in the sample as a whole and in HPV-related and HPV-unrelated HNC patients separately. In transcriptome profiling analyses of PBMC from 44 patients, TELiS bioinformatics analyses linked fatigue to increased nuclear factor-kappa B (NF-kB) transcriptional activity and decreased interferon regulatory factor family (IRF) transcription factor activity. Patients with HPV-related HNC showed lower levels of fatigue-related gene expression profile compared to HPV-unrelated HNC. Fatigue in HNC patients undergoing treatment is associated with gene expression profiles consistent with the conserved transcriptional response to adversity (CTRA) characterized by increased proinflammatory and decreased anti-antiviral transcriptional activity. Interestingly, this CTRA response was mitigated in patients with HPV-related HNC and may explain the lower level of fatigue they experience relative to HPV-unrelated HNC.
Fatigue, one of the most frequently reported and distressing cancer symptoms,1 is prognostic for poor pathologic tumor response2 and survival,3–5 including in patients with head and neck cancer (HNC).3,4 Indeed, an increase in baseline fatigue of 10 points (out of 100) is associated with a 17% reduction in survival in HNC patients.4 A rise in the incidence of HNC, due to the increased incidence of human papillomavirus (HPV) infection, has been noted, and by 2020 HPV-positive HNCs will likely be the most common HPV-associated cancer in the US.6 HNC patients typically receive radiotherapy (RT) and experience high rates of fatigue compared to other cancer patients.7 Most commonly used intensity-modulated RT (IMRT), targeting tumors with higher doses while avoiding normal structures, causes even higher fatigue compared to conventional RT for HNC.8 Our previous work in HNC patients has shown that RT-induced fatigue is consistently ranked among the top three most common and severe symptoms and is significantly related to multiple other treatment-related symptoms (e.g. mucositis and dry mouth).9 However, the management of fatigue is challenging, and no Food and Drug Administration (FDA)-approved pharmacological agent reliably prevents or treats this symptom.10 Understanding the biological mechanisms of cancer-related fatigue may help improve the management of this distressing symptom.
One biological mechanism that may contribute to fatigue is systemic inflammation. Our previous work has identified a positive association between fatigue and peripheral inflammatory cytokines and their receptors in HNC patients. This association exists not only before and during the acute phase of cancer treatment (within one-month of the completion of radiotherapy or concurrent chemoradiotherapy),11 but also at three-month after completion of cancer treatment.12 Interestingly, HPV status appears to play a significant role in this association. Patients with HPV-related tumors experience lower levels of fatigue and peripheral inflammatory biomarkers before and at three months post radiotherapy compared to those with HPV-unrelated tumors, who exhibited high and persistent levels of fatigue throughout the study.12 Although these findings indicate a relationship between inflammation and fatigue that is related to HPV status, the molecular mechanisms of this association remains unknown.
We hypothesize that activation of a previously identified conserved transcriptional response to adversity (CTRA) 13,14 may potentially contribute to inflammation and fatigue in HNC patients during treatment. In the CTRA, neural and endocrine alterations induce a pro-inflammatory/anti-antiviral skew in the circulating leukocyte transcriptome when individuals face prolonged exposure to adversity, such as traumatic stress from a cancer diagnosis or cancer treatment. These pro-inflammatory and anti-antiviral signals in the periphery may influence CNS functions and behavioral processes,13,14 which then may be associated with symptoms like fatigue.13 Although a small cross-sectional study found increased expression of transcripts with response elements for nuclear factor-kappa B (NF-kB) in fatigued cancer survivors,15 the pro-inflammatory/anti-antiviral skew reflective of the CTRA has not been studied in fatigued cancer patients. Thus, in the current longitudinal study, we hypothesized that intra-individual increases in fatigue in HNC patients would be associated with leukocyte activation of the CTRA, characterized by increased activity of the pro-inflammatory transcription factor NF-kB and decreased activity of the antiviral interferon regulatory factor (IRF) family. Given our previous finding of lower fatigue and inflammation in patients with HPV-related tumors, we also hypothesized this CTRA response would be mitigated in HPV-related group.
Methods:
This longitudinal study followed HNC patients at baseline, which was after cancer diagnosis and prior to radiotherapy, and one-month after the completion of radiotherapy. Radiotherapy (or concurrent chemoradiotherapy) normally lasted for 1.5–2 months. Surgery occurred approximately 1 month before radiotherapy (or chemoradiotherapy) and therefore before the baseline assessment. The study was approved by Emory University Institutional Review Board, and all patients provided informed consent.
Sample: patients were enrolled at the Radiation Oncology Clinics at Emory University Clinic and Emory Midtown Hospital from 2013 −2016. Patients were eligible for the study if they were ≥ 21 years of age, diagnosed with squamous cell carcinoma of the head and neck region, no distant metastasis, no evidence of uncontrolled major organ disease. Patients were excluded from the study if they had simultaneous cancer other than in the head and neck region, previous cancer but disease free for < 3 years, positive pregnancy test, and a major psychiatric disorder or inability to understand English. Patients were also excluded if they had conditions that confounded the relationship between fatigue and inflammation, such as hepatitis B or C or regular use of immunosuppressive medications (e.g. oral glucocorticoids) within 6 months of study entry. Over-the-counter anti-inflammatory medications and antidepressants were not exclusionary.
Research staff reviewed patients’ electronic medical record to determine their eligibility. All eligible patients were consented before the start of the radiotherapy. Data were collected at both pre-and post-radiotherapy. All questionnaires were collected at clinic sites, and blood samples were collected by a phlebotomist or certificated nurse on the same day as the questionnaires.
Demographic and clinical characteristics were collected through patient-reported questionnaires or chart review. Demographic characteristics included age, sex, race (white vs. other), marital status (married vs. other), tobacco use (current/history vs. not), and alcohol use (current/history vs. not). Clinical characteristics included body mass index (BMI), antidepressant use (yes vs. no), nonsteroidal anti-inflammatory drug use (yes vs. no), comorbidities (Charlson Comorbidity Index), primary cancer site (oropharynx vs. others), cancer stage (TNM: I-III vs. IV), HPV status (HPV-related vs. -unrelated), treatment types (radiation alone vs. radiation with surgery vs. concurrent chemoradiation vs. concurrent chemoradiation with surgery), radiation dose, chemotherapy regimen (Cisplatin vs. Carboplatin/Paclitaxel vs. others) and feeding tubes (yes vs. no). Patient HPV status was determined based on pathology reports of the tumor tissue before treatment. According to current practice, p16 or HPV positive were counted as HPV-related; otherwise they were counted as HPV-unrelated.
Fatigue, as the major outcome variable, was measured using the Multidimensional Fatigue Inventory (MFI) 20-item questionnaire. MFI includes five dimensions of fatigue: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity.17 The appropriate responses were reversed, and all items were summed for an overall fatigue score (ranging from 20–100) per patient. Higher score indicates higher fatigue. This questionnaire has been used in our previous studies on HNC and breast cancer.11
Whole blood was collected into EDTA tubes for PBMC isolation. The time of collection was not standardized due to variability in subject availability. Total RNA was isolated from PBMCs according to manufacturer’s protocol (Qiagen RNeasy Mini Kit: Qiagen; Valencia, CA). RNA integrity was determined initially by 260/280 =1.9–2.1 and by scanning with an Agilent 2100 Bioanalyzer using the RNA 6000 Nano LabChip. Isolated RNA was kept at −80 C until microarray analysis. The Emory Integrated Genomics Core analyzed RNA samples for gene expression using Clariom S Assay for human (Applied Biosystems; San Diego, CA). Raw probe intensities were corrected for background levels and normalized by the quantile normalization algorithm using GenomeStudio software from Illumina.
Statistical analyses: Descriptive statistics (mean, standard deviation (SD), N, percentage) were used to characterize the sample population. In all hypothesis testing, statistical significance was based on a 2-tailed p < .05. For the primary hypothesis involving CTRA-related transcription factor activation, we first identified all genes showing > 1.2-fold difference in expression over a 4-SD range of continuous variation in fatigue scores (i.e., over the 95% range of normal variation from 2 SD below the average score to 2 SD above the average) in standard linear model analyses of log2-transformed gene expression values that also controlled for age, sex, race/ethnicity, BMI, smoking history, and heavy alcohol consumption history. HPV status was analyzed as a 1/0 score (HPV-related vs HPV-unrelated) to identify genes showing >1.2-fold differential expression after controlling for the samecovariates. Because the goal of this study was to test a priori hypotheses regarding specificCTRA-related transcription factor activity, we made no attempt to assess statistical significance at the level of individual gene transcripts; instead all genes that showed a maximum likelihood point estimate of association > 1.2-fold served as input into higher-order bioinformatics analyses using TELiS promoter sequence analysis18 to test 2 a priori-specified hypotheses regarding activity of CTRA-related transcription control pathways: 1) the positive CTRA component of inflammation (NF-kB, as indicated by differential expression of genes bearing transcription factor-binding motifs [TFMBs] matching the TRANSFAC position-specific weight matrix V$NFKAPPAB65_01), and 2) the inverse CTRA component of interferon response factors (IRFs, as indicated by V$IRF1_01). TELiS analyses were conducted using 9 different parametric combinations of promoter DNA sequence length (−300, −600, and −1000 to +200 nucleotides surrounding the RefSeq-designated transcription start site) and TFBM detection stringency (TRANSFAC mat_sim values of .80, .90, and .95).18 Log2-transformed TFBM ratios (comparing prevalence in promoters of up-vs. down-regulated genes) were averaged across the 9 parametric combinations and tested for statistical significance using standard errors derived from bootstrap resampling of linear model residual vectors (i.e., controlling for potential correlation across genes). Additionally, sensitivity analyses controlling baseline fatigue, and major demographic/clinical characteristics were conducted accordingly.
Results:
Demographic and clinical characteristics:
Selected demographics and clinical characteristics are shown in Table 1 for 44 patients. The sampled population was approximately 59 years old, majority male, and majority of white race. Fifty-one percent of the sample population had previously smoked, and 41% had previously consumed alcohol. Most of the diagnosed cancers were stage IV. Overall, 61% were in the oropharynx and 57% were HPV-related. Fatigue increased from 48 ± 17.69 at baseline to 53 ± 16.19 at one-month post-treatment, very similar to our published studies.11,12 Fatigue levels at both time points were also much higher than the general healthy adult population with an average MFI-20 score of 37.19 Furthermore, baseline fatigue was higher in patients who were single (p=0.004), using an antidepressant (p=0.037), and with a history of smoking (p=0.009). Patients with HPV-related tumors were more likely to be married (p=0.016) and without a history of smoking (p=0.003), compared to those with HPV-unrelated tumors.
Table 1.
Baseline demographic and clinical characteristics of the participants (N=44)
| Variables | Mean ± SD or N (%) |
|
|---|---|---|
| Age (years) | 59.05 ± 10.39 | |
| Gender | Male | 30 (68) |
| Female | 14 (32) | |
| Race | White | 38 (86) |
| Non-White | 6 (14) | |
| Marital statusa | Married | 32 (73) |
| Unmarried | 12 (27) | |
| History of tobacco use | No | 21 (48) |
| Yes | 23 (52) | |
| History of alcohol use | No | 26 (59) |
| Yes | 18 (41) | |
| BMI | 26.77 ± 4.24 | |
| Comorbiditiesb | 0 | 35 (80) |
| 1 | 6 (14) | |
| 2 | 3 (6) | |
| Antidepressants | No | 37 (84) |
| Yes | 7 (16) | |
| NSAIDs | No | 36 (82) |
| Yes | 8 (18) | |
| Cancer site | Oropharynx | 27 (61) |
| Other | 17 (39) | |
| Stage | ≤ III | 11 (25) |
| IV | 33 (75) | |
| HPV status | HPV related | 25 (57) |
| HPV unrelated | 19 (43) | |
| Treatment | IMRT | 3 (7) |
| IMRT + Surgery | 7 (16) | |
| IMRT + Chemo | 21 (48) | |
| IMRT + Chemo + Surgery | 13 (29) | |
| Chemotherapy | Cisplatin | 22 (65) |
| Carboplatin/Paclitaxel | 8 (24) | |
| Other | 4 (11) | |
| Radiation dose | 66.89 ± 4.45 | |
| Feeding tubes | No | 19 (43) |
| Yes | 25 (57) | |
Note. BMI = Body Mass Index, HPV = Human papillomavirus, IMRT = Intensity-Modulated Radiation Therapy, NSAIDs = Nonsteroidal anti-inflammatory drugs, SD = Standard deviation.
Married includes patients married or living as married; Unmarried includes patients single, separated, divorced, or widowed.
Comorbidities was assessed using the Charlson Comorbidity Index excluding tumor.
Fatigue and PBMC gene regulation
To assess the relationship between fatigue and CTRA-related gene regulation, we identified all genes showing > 1.2-fold differential expression in PBMC as a function of continuous individual differences in fatigue (measured at both baseline and follow-up, and controlling for age, sex, race/ethnicity, BMI, smoking history, and heavy alcohol consumption) and conducted TELiS promoter-based bioinformatics analyses to determine whether these empirical transcriptome differences reflected increased activity of the NF-kB family of pro-inflammatory transcription factors and/or decreased activity of the IRF family of antiviral transcription factors. Results (Figure 1a) supported both hypotheses, linking fatigue to greater activity of NF-kB (log2 ratio of TFBMs in up-vs down-regulated genes: mean 1.45 ± SE 0.45, p = 001) and less activity of IRF family factors (−1.25 ± 0.48, p = .010).
Figure 1. Bioinformatic analysis of transcription factor activity.
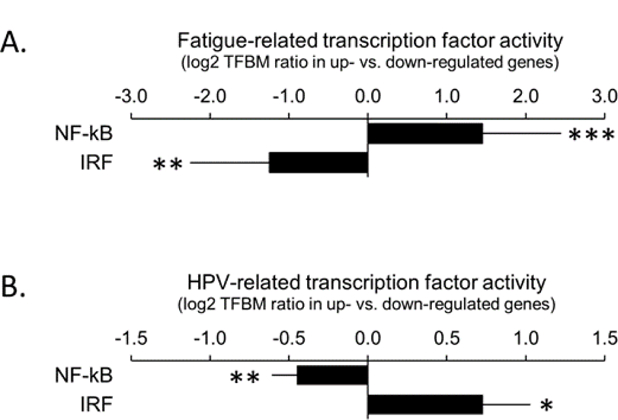
A.) Prevalence of NF-kB and IRF transcription factor-binding motifs(TFBMs) in promoters of genes up-regulated in association with cancer-related fatigue vs.down-regulated. Data represent log2 ratios (null value=1.0-fold=0.0 log2)averaged over 9 combinations of promoter length and TFBM detection stringency. B.) Prevalence of NF-kB and IRF TFBMs in promoters of genes up-regulated in HPV-related HNC vs. down-regulated ***p<.001,**p<.01,*p<.05
To verify the robustness of results, we conducted 5 additional sensitivity analyses. The first analysis additionally controlled for variations in chemotherapy regimen (Cisplatin vs. Carboplatin/Paclitaxel or others) and again linked fatigue to up-regulated NF-kB activity (1.00 ± 0.38, p = .009) and a near-significant down-regulation of IRF activity (−1.02 ± 0.54, p = .055). The second sensitivity analysis additionally controlled for use of anti-inflammatory agents (NSAIDs and Corticosteroids included as separate variables) and again yielded similar results (NF-kB: 1.51 ± 0.50, p = .003; IRF: −1.08 ± 0.61, p = .076). The third sensitivity analysis controlled for baseline individual differences in fatigue by assessing the relationship between within-individual change over time in fatigue (i.e., change = follow-up – baseline) and within-individual change in gene expression. Results of this repeated measures analysis linked greater increases in fatigue to greater increases in NF-kB activity (1.23 ± 0.36, p < .001) and greater decreases in IRF activity (−1.06 ± 0.35, p = .003). The fourth sensitivity analysis again related intra-individual change in fatigue to intra-individual change in gene expression, but additionally controlled for individual differences in age, sex, race/ethnicity, BMI, smoking history, and heavy alcohol consumption history. Results again linked increased fatigue to increased NF-kB activity (1.23 ± 0.36, p < .001) and decreased IRF activity (−1.06 ± 0.35, p = .003). The final sensitivity analysis examined the relationship between fatigue and gene expression at baseline. Despite the limited power available from this subset analysis, results continued to link fatigue to decreased IRF activity (−0.93 ± 0.47, p = .048), although results for NF-kB no longer reached significance (0.04 ± 0.41, p = .932).
HPV status and PBMC gene regulation
As indicated in our previous publication, patients with HPV-related tumors experience lower average levels of fatigue and lower levels of circulating inflammatory proteins than those with HPV-unrelated tumors.12 To understand the potential molecular mechanisms underlying these differences in circulating inflammatory biomarkers, we identified all genes showing > 1.2-fold differential expression in PBMC as a function of HPV status (measured at both baseline and follow-up, and controlling for age, sex, race/ethnicity) and conducted TELiS promoter-based bioinformatics analyses to determine whether these empirical transcriptome differences reflected decreased activity of the NF-kB family of pro-inflammatory transcription factors and/or increased activity of the IRF family of antiviral transcription factors in HPV+ patients. Results (Figure 1b) supported both hypotheses, with HPV-related cases showing lower activity of NF-kB (log2 average TFBM ratio: −0.45 ± 0.16, p = .005) and higher activity of IRF family factors (0.73 ± 0.30, p = .017) relative to HPV-unrelated cases. Similar results emerged in sensitivity analyses that additionally controlled for variations in chemotherapy regimen, with HPV-related cases again showing down-regulated NF-kB activity (−0.40 ± 0.16, p = .012) and up-regulated IRF activity (0.72 ± 0.34, p = .033). Similar results also emerged in analyses of baseline samples alone, with HPV-related cases again showing down-regulated NF-kB activity (−0.47 ± 0.18, p =.009) although IRF differences no longer reached significance (0.15 ± 0.33, p = .646). (No sensitivity analyses of individual change over time were possible because HPV status does not change.)
Discussion:
The findings of this study link cancer-related fatigue in HNC patients to increased activity of pro-inflammatory NF-kB family transcription factors and decreased activity of innate antiviral IRF family transcription factors in PBMC. In addition, HPV-related HNCs were found to show lower expression of these patterns than did HPV-unrelated HNCs, suggesting a potential molecular mechanism for the lower levels of fatigue reported by patients with HPV-related HNC. These findings shed light on the molecular mechanisms of fatigue in HNC patients, and they provide new insight into lower levels of fatigue and inflammation in patients with HPV-related cancers.
The observed pattern of transcription factor activity is consistent with previous studies of the CTRA13,14 implicating increased activity of NF-kB and decreased activity of IRF family factors in mediating this chronic stress-related transcriptome alteration in PBMC. The observed elevation in NF-kB activity not only is consistent with published studies on persistent fatigue in breast cancer survivors,15 but also extends our understanding to patients receiving active cancer treatment. NF-kB is a critical regulator involved in pro-inflammatory signaling pathways and stress-induced responses.20 In patients with cancer, many factors may potentially contribute to NF-kB activation, including ionizing radiation, chemotherapy agents, and cytokines, as well as the psychological stress associated with cancer diagnosis and treatment. Activated NF-kB translocates into the nucleus and binds to TFBMs in target gene promoters or enhancers.21 The subsequent gene induction enhances production of proinflammatory markers like IL-1, IL-6, and TNF-α,20 which have been linked to cancer-related fatigue.11,22 Furthermore, activated NF-kB has been linked to cancer development and progression, which may also explain the reason that fatigue at pre-or post-radiotherapy is prognostic of patients’ pathologic tumor response2 and survival, including in HNC patients.4
Consistent with the CTRA, fatigued patients also showed decreased activity of IRF family transcription factors involved in innate antiviral responses. IRFs are key to regulating transcription of interferons, which are responsive to the presence of pathogens, such as viruses and tumor cells, and thereby heighten the body’s antiviral and antitumor defenses.23 Earlier studies on social adversity, such as loneliness and experiencing cancer diagnosis, have shown similar down-regulation of genes involved in innate antiviral responses, particularly type I interferons.24 Our finding further supports that this down-regulated antiviral responses is connected with cancer-related fatigue.
Interestingly, patients with HPV-related tumors showed significantly less pronounced expression of the pro-inflammatory/anti-antiviral transcriptome shift associated with fatigue than did those with HPV-unrelated tumors. The findings held despite control for covariates including sex, which is important because HPV-related individuals appear to be disproportionately male in our sample, and they at least have Y chromosome-linked genes up-regulated in the presence of HPV.25 Our finding of significantly increased IRF activity in patients with HPV-related tumors could potentially stem from innate antiviral responses to persistent HPV infection. Our finding of decreased expression of the pro-inflammatory transcriptomes support our previous findings that patients with HPV-related tumors experience lower fatigue and lower inflammation as represented by lower levels of peripheral inflammatory markers. However, it is unclear why patients with these virally-related tumors had less inflammation.
One reason that HPV-related patients may have had lower inflammation than HPV-unrelated patients might be related to HPV infection itself. During the early phase of HPV infection, innate immune system responses have been shown to contribute to a pro-inflammatory environment.27 However, HPV evades immune-surveillance by generating an anti-inflammatory microenvironment, which then helps to establish a persistent HPV infection.28 This anti-inflammatory response may extend to peripheral compartments and be linked to lower peripheral inflammation. Indeed, activation of the IRF signaling system is known to cross-inhibit NF-kB activity14,28, providing a biological mechanism through which HPV-related tumors could reciprocally down-regulate pro-inflammatory pathways. Future studies examining more directly the mechanisms of lower inflammation in HPV-related group are warranted.
Conclusion:
Previous studies have linked cancer-related fatigue to elevated protein markers of inflammation, but the molecular mechanisms underlying this association remain poorly understood. Herein, we show that fatigue in HNC patients is associated with elevated activity of the pro-inflammatory transcription factor, NF-kB, and decreased activity of the antiviral IRF transcription factor family. These effects are attenuated in HPV-related HNC, and such patients also show lower levels of fatigue. The identification of the molecular mechanisms has further supported the hypothesized association between fatigue and inflammation, which suggests anti-inflammatory avenues for the management of fatigue. The findings on mitigated CTRA responses in patients with HPV-related tumors have also provided evidence on potential, differential management strategies of fatigue in patients with different HPV status. Limitations of this study include a relatively small sample size and a data analytic strategy that precludes drawing causal conclusions (e.g., it is conceivable that fatigue might induce distress, which leads to CTRA activation, rather than vice versa). However, the subjects’ data were collected at both pre-and post-treatment time points, which provides a complete picture during the treatment and more valid findings for the association (particularly analyses of intra-individual change over time, which controls for baseline individual differences). We also note that some of the associations observed in primary analyses of all available data failed to reach significance in analyses focusing on the subset of baseline samples alone (i.e., excluding data from the follow-up time points). This is not surprising, given that the baseline-only analyses involve substantially fewer observations, and thus have more limited statistical power to detect biological relationships. Nonetheless, larger studies are needed to replicate the findings, particularly the findings relevant to the HPV-related group while controlling for potential confounders. Larger samples will also be needed to determine whether similar effects emerge in different subgroups of participants (e.g., those exposed to anti-inflammatory agents vs. not). This study did not measure risk factor exposures during follow-up (e.g., continued exposure to smoking, alcohol, or poor diet), so it remains to be determined how such continued exposure patterns might contribute to the risk of disease progression and/or CTRA trajectories. Long-term follow-up studies are warranted to examine their implications for symptom burden and survivorship across the cancer care continuum.
Highlights:
Fatigue was associated with increased nuclear factor-kappa B transcriptional activity and decreased interferon regulatory factor family transcriptional activity in head and neck cancer (HNC) patients.
This fatigue-related gene expression profile was consistent with the conserved transcriptional response to adversity (CTRA).
This fatigue-related CTRA response was mitigated in patients with human papillomavirus-related HNCs.
Acknowledgements:
The authors appreciate the support from Emory University School of Nursing, School of Medicine, and Winship Cancer Institute.
Funding:
The study was supported by NIH/NINR K99/R00NR014587, NIH/NINR R01NR015783, NIH/NCI P30CA138292 and Oncology Nursing Society Foundation.
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Janaki MG, Kadam AR, Mukesh S, et al. Magnitude of fatigue in cancer patients receiving radiotherapy and its short term effect on quality of life. J Cancer Res Ther 2010;6(1):22–26. [DOI] [PubMed] [Google Scholar]
- 2.Park HC, Janjan NA, Mendoza TR, et al. Temporal patterns of fatigue predict pathologic response in patients treated with preoperative chemoradiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys 2009;75(3):775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackerstaff AH, Rasch CR, Balm AJ, et al. Five-year quality of life results of the randomized clinical phase III (RADPLAT) trial, comparing concomitant intra-arterial versus intravenous chemoradiotherapy in locally advanced head and neck cancer. Head Neck 2012;34(7):974–980. [DOI] [PubMed] [Google Scholar]
- 4.Fang FM, Liu YT, Tang Y, Wang CJ, Ko SF. Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer 2004;100(2):425–432. [DOI] [PubMed] [Google Scholar]
- 5.Montazeri A Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes 2009;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29(32):4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hickok JT, Morrow GR, Roscoe JA, Mustian K, Okunieff P. Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J Pain Symptom Manage 2005;30(5):433–442. [DOI] [PubMed] [Google Scholar]
- 8.Gulliford SL, Miah AB, Brennan S, et al. Dosimetric explanations of fatigue in head and neck radiotherapy: An analysis from the PARSPORT Phase III trial. Radiother Oncol 2012;104(2):205–212. [DOI] [PubMed] [Google Scholar]
- 9.Xiao C, Hanlon A, Zhang Q, et al. Symptom clusters in patients with head and neck cancer receiving concurrent chemoradiotherapy. Oral oncology 2013;49(4):360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. Cochrane database of systematic reviews (Online) 2010(7):CD006704. [DOI] [PMC free article] [PubMed]
- 11.Xiao C, Beitler JJ, Higgins KA, et al. Fatigue is associated with inflammation in patients with head and neck cancer before and after intensity-modulated radiation therapy. Brain Behav Immun 2016;52:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao C, Beitler JJ, Higgins KA, et al. Associations among human papillomavirus, inflammation, and fatigue in patients with head and neck cancer. Cancer 2018. [DOI] [PMC free article] [PubMed]
- 13.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature reviews Immunology 2011;11(9):625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole SW. Social Regulation of Human Gene Expression: Mechanisms and Implications for Public Health. American Journal of Public Health 2013;103(Suppl 1):S84–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun 2011;25(1):147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 17.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 1995;39(3):315–325. [DOI] [PubMed] [Google Scholar]
- 18.Cole SW, Yan W, Galic Z, Arevalo J, Zack JA. Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics (Oxford, England) 2005;21(6):803–810. [DOI] [PubMed] [Google Scholar]
- 19.Lin J- MS, Brimmer DJ, Maloney EM, Nyarko E, BeLue R, Reeves WC. Further validation of the Multidimensional Fatigue Inventory in a US adult population sample. Population Health Metrics 2009;7:18–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nature reviews Immunology 2005;5(10):749–759. [DOI] [PubMed] [Google Scholar]
- 21.Wan F, Lenardo MJ. The nuclear signaling of NF-kappaB: current knowledge, new insights, and future perspectives. Cell Res 2010;20(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol 2008;26(6):971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist 2001;6(1):34–55. [DOI] [PubMed] [Google Scholar]
- 24.Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome biology 2007;8(9):R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kido T, Lau YF. Roles of the Y chromosome genes in human cancers. Asian journal of andrology 2015;17(3):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Taneja V, Vassallo R. Cigarette Smoking and Inflammation: Cellular and Molecular Mechanisms. Journal of dental research 2012;91(2):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boccardo E, Lepique AP, Villa LL. The role of inflammation in HPV carcinogenesis. Carcinogenesis 2010;31(11):1905–1912. [DOI] [PubMed] [Google Scholar]
- 28.Amador-Molina A, Hernández-Valencia JF, Lamoyi E, Contreras-Paredes A, Lizano M. Role of Innate Immunity against Human Papillomavirus (HPV) Infections and Effect of Adjuvants in Promoting Specific Immune Response. Viruses 2013;5(11):2624–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]


