Abstract
Brain injury sustained during the neonatal period may disrupt development of critical structural and functional connectivity networks leading to subsequent neurodevelopmental impairment in affected children. These networks can be characterized using structural (via diffusion MRI) and functional (via resting state-functional MRI) neuroimaging techniques. Advances in neuroimaging have led to expanded application of these approaches to study term- and prematurely-born infants, providing improved understanding of cerebral development and the deleterious effects of early brain injury. Across both modalities, neuroimaging data are conducive to analyses ranging from characterization of individual white matter tracts and/or resting state networks through advanced ‘connectome-style’ approaches capable of identifying highly connected network hubs and investigating metrics of network topology such as modularity and small-worldness. We begin this review by summarizing the literature detailing structural and functional connectivity findings in healthy term and preterm infants without brain injury during the postnatal period, including discussion of early connectome development. We then detail common forms of brain injury in term- and prematurely-born infants. In this context, we next review the emerging body of literature detailing studies employing diffusion MRI, resting state-functional MRI and other complementary neuroimaging modalities to characterize structural and functional connectivity development in infants with brain injury. We conclude by reviewing technical challenges associated with neonatal neuroimaging, highlighting those most relevant to studying infants with brain injury and emphasizing the need for further targeted study in this high-risk population.
Keywords: structural connectivity, functional connectivity, magnetic resonance imaging, infant, prematurity, brain injury
1. Introduction
1.1. Manuscript Overview
Brain “connectivity” can be viewed at different scales (Sporns, Tononi, & Kotter, 2005). At a microscopic scale, it involves evaluating single neurons and their connections by recording electrical activity and/or using histologic methods. At the mesoscopic scale, it refers to connection patterns among elementary processing units, corresponding to local populations of neurons such as cortical minicolumns. Finally, at the macroscopic scale, it refers to functional subdivisions of cortex (e.g. primary motor cortex) composed of elementary processing units. For the purposes of this review, connectivity will be described at a macroscopic scale, focusing on two complementary MRI methods, diffusion tractography and functional MRI (Hagmann, Cammoun, et al., 2010). Diffusion tractography provides structural information, tracing physical connections between brain regions by following white matter tracts from region to region. Functional MRI, in contrast, identifies cortical areas demonstrating synchronous changes in local blood oxygenation levels, indicating these regions have linked neuronal activity. As detailed herein, the application of these state-of-the-art methods to study infants has improved our comprehension of brain development on two levels. The first is by advancing our understanding of typical brain development from a neuroscience perspective, providing unique insights into early cortical and subcortical gray and white matter development and the emergence of structural and functional connectivity. The second is by better characterizing the deleterious effects of common forms of injury on early brain development, linking local and widespread alterations previously defined through histology and cytopathology with changes in cerebral connectivity now readily identifiable through multimodal neuroimaging.
In this context, we begin this review by providing overviews of the neuroimaging modalities used to study structural and functional connectivity, including a description of connectome-based methods and terminology. Next, we summarize the literature detailing structural and functional connectivity findings in healthy, term-born infants and prematurely-born infants without brain injury on T1- and T2-weighted imaging, including discussion of early development of the structural and functional connectome. We then detail forms of brain injury common in this population, including periventricular leukomalacia, intraventricular hemorrhage and post-hemorrhagic hydrocephalus in prematurely-born infants and hypoxic-ischemic brain injury in term-born infants. Using these data to provide a framework, we then discuss the emerging body of literature detailing results from studies employing diffusion MRI (dMRI), resting state functional connectivity MRI (rs-fMRI) and other complementary neuroimaging modalities to study structural and functional connectivity in infants with these common forms of brain injury. We conclude by reviewing technical issues important for neonatal dMRI and rs-fMRI investigations, including highlighting issues directly relevant to studying infants with brain injury.
1.2. Structural Connectivity
A wealth of information regarding structural connectivity is available through dMRI. Diffusion data are obtained by measuring water diffusion along multiple axes to obtain a three-dimensional mathematical representation of water displacements for each voxel in an image. These displacements are affected by a variety of factors in addition to Brownian thermal motion, including facilitation by active transport and hindrance by the presence of cell membranes. In recognition of the factors influencing water displacements in tissue, tissue water diffusion is referred to as “apparent” diffusion, though we will simply use “diffusion” in the discussion to follow. A number of parameters related to tissue water diffusion can be calculated (Neil, 2008). The most commonly used clinically is the directionally averaged water diffusion coefficient. This parameter decreases within minutes of a brain injury, and images in which contrast is based on diffusion coefficients are useful for early detection of brain injury in the clinical setting. A second parameter available from dMRI is diffusion anisotropy. Anisotropy is a numerical value representing the degree to which water diffusion varies directionally. In ventricular spinal fluid, for example, water diffusion values are equivalent in all directions, and anisotropy values are essentially zero. In white matter, in contrast, water diffusion values are greater parallel to axons than orthogonal to them because water movement orthogonal to axons is hindered by the lipid membranes of myelin. In this case, anisotropy values are high.
The fact that water displacements are greatest parallel to axons in white matter can be used to identify the orientation of white matter fibers in a given voxel. By following this orientation from voxel to voxel, it is possible to follow fiber tracts through the brain, an approach known as streamline tractography (O’Donnell & Westin, 2011). A more sophisticated tractography approach, known as probabilistic tractography, provides an explicit representation of uncertainty in path direction and an estimate of the relative probabilities of directions. Using this method, a pathway can continue even if the probability is low for any single direction. A second useful advantage of a probabilistic algorithm is resistance to noise. It can be difficult to track beyond a noisy voxel using a non-probabilistic algorithm, as it may initiate a meaningless change in path. With a probabilistic algorithm, however, paths that have taken errant routes tend to disperse quickly, so that voxels along these paths are classified with low probability. In contrast, true paths tend to group together, giving a higher probability of connection for voxels on these paths (Behrens et al., 2003). Thus, the probabilistic approach may be more effective for evaluating neonates, for whom anisotropy values typically are low.
Of note, the term “connectivity” has been used a number of ways in the diffusion literature. For example, it has been applied to studies in which tractography is used to identify white matter regions of interest from which diffusion parameters, such as diffusivity and anisotropy, are extracted for analysis. It has also been applied to tract-based spatial statistics studies in which the white matter of the entire brain is identified and the associated diffusion parameters are used for voxel-wise statistical analyses (Smith et al., 2006). While these approaches are valid and useful (Anderson, Cheong, & Thompson, 2015; Counsell, Ball, & Edwards, 2014; Massaro, 2015; van Kooij et al., 2012), we focus the dMRI portion of this manuscript on the emerging body of work on structural connectivity in which tractography is employed to derive whole brain networks which are analyzed using a graph theory approach, in keeping with the idea of a “structural connectome.”
1.3. Functional Connectivity
The second method included in this review is rs-fMRI, which is used to study functional connectivity. rs-fMRI is based upon detecting temporal correlations in infra-slow frequency (<0.1 Hz) fluctuations in blood oxygen level dependent (BOLD) signal that occur during quiet rest (Biswal, Yetkin, Haughton, & Hyde, 1995; Fox et al., 2005; Lowe, Mock, & Sorenson, 1998). These data are used to identify brain regions which demonstrate synchronous, spontaneous neuronal activity and are members of the same resting state network (RSN) (Fox & Raichle, 2007; Smith et al., 2009). These networks may involve both cortical and subcortical gray matter regions. They are also consistent with networks identified in activation studies (i.e., employing positron emission tomography or task-based functional MRI) in that they are often composed of regions that are co-activated by a specific task. The architecture of these RSNs and their interrelationships have been well-characterized in adults and older pediatric populations (Buckner et al., 2009; Smith et al., 2009) and have also been increasingly established in healthy, term-born infants and neonatal clinical populations of interest (Doria et al., 2010; Fransson, Aden, Blennow, & Lagercrantz, 2011; Fransson et al., 2009; Fransson et al., 2007; Gao, Alcauter, Smith, Gilmore, & Lin, 2015; Gao, Gilmore, Shen, et al., 2013; Lin et al., 2008; C. D. Smyser, N. U. Dosenbach, et al., 2016; Smyser et al., 2010; Smyser et al., 2013; C. D. Smyser, A. Z. Snyder, et al., 2016).
In many ways, rs-fMRI is well-suited to study neonatal populations due to its acquisition and analysis methods (Smyser & Neil, 2015). From a single, high-quality data set acquired in minutes, global functional connectivity properties can be investigated. Data can be collected from subjects resting quietly, asleep and/or under anesthesia without the requirement of performing a task or attending to stimulus. Increasing use of rs-fMRI has provided new insights into neurodevelopment, the effects of brain injury and the neurobiological basis of disease, with recent literature demonstrating aberrant functional connectivity patterns underlying common pediatric disorders (Church et al., 2009; Posner, Park, & Wang, 2014; Redcay et al., 2013). As with dMRI, these data are conducive to analyses ranging from straightforward study focused upon within-network measures to more advanced mathematical approaches such as graph theoretical methods and multivariate pattern analysis. The rs-fMRI portion of this manuscript focuses upon studies which, by their nature, include a number of different networks comprising a “functional connectome.”
Importantly, the information provided by rs-fMRI complements that available through other commonly used neuroimaging modalities, including task-based fMRI and dMRI. While tractography provides information on physical connectivity, rs-fMRI identifies areas that are functionally linked, though not necessarily via a monosynaptic pathway, as functional connections may exist between regions with no direct structural connection due to polysynaptic connections. Interestingly, rs-fMRI connectivity measures also show variability within and across scanning sessions (i.e., non-stationarity) (Abrol et al., 2017). Thus, while rs-fMRI results correlate with tractography patterns in general, the relationship between structural and functional connectivity is complex (Honey et al., 2009).
1.4. Brain Connectome
A brain connectome can be represented as a network of interrelations between pairwise ensembles of neuronal elements (nodes), where the associations consist of connections (links) formed by white matter tracts (e.g., a structural connectome derived from dMRI data) or functional associations (e.g., a functional connectome derived from rs-fMRI data). Nodes should ideally represent brain regions with coherent patterns of extrinsic anatomical or functional connections. They should not include heterogeneously connected brain regions, nor should they overlap one another. Links can be considered binary (i.e., present or absent) or weighted (e.g., by the anisotropy of an anatomic tract or the correlation coefficient of a functional connection).
Structural and functional connectivity data conceptualized in this manner lend themselves to graph theory analysis, from which summary parameters can be derived to characterize and quantify aberrant patterns of communication associated with brain injury (Figure 1) (Fischi-Gomez et al., 2016; Griffa, Baumann, Thiran, & Hagmann, 2013; Rubinov & Sporns, 2010; Vertes & Bullmore, 2015). Several of the more commonly used parameters are outlined in Table 1.Notably, network integration reflects the ability of the brain to incorporate information across disparate brain regions (Rubinov & Sporns, 2010). Common examples of network integration measures include global and local efficiency, which quantify the capacity of a brain (or a set of brain regions) to integrate and communicate information. Further, specific regions of the brain which play a particularly central role to communication among brain regions can be thought of as ‘hubs’. These hubs can be characterized as belonging to a ‘rich club’ (Zhou & Mondragon, 2004), which serves as a backbone for efficient communication within the brain and may be particularly vulnerable to disruptions in neurodevelopmental disorders (Rubinov & Sporns, 2010; van den Heuvel & Sporns, 2011). In contrast, network segregation reflects the potential for specialized processing within densely interconnected groups of brain regions. Measures of network segregation, such as clustering coefficient and modularity, have been used to estimate networks of the brain which purportedly reflect functionally distinct cognitive processes (Watts & Strogatz, 1998). Finally, brain networks which are both highly segregated and integrated can be considered to have ‘small-world’ organization (Rubinov & Sporns, 2010).
Figure 1.
Brain connectomes can be analyzed using graph theoretical methods. A) Functional connectivity can be assessed by correlating the BOLD signal between regions of the brain. B) A matrix depicting BOLD correlations between all pairs of brain regions forms the functional connectome. C) Measures of network segregation include modularity and clustering coefficients, which can be used to delineate regions of the brain into functionally distinct brain networks. Metrics of network integration, including node connectedness (orange lines) and measures based on shortest path length between regions (red lines), can be used to characterize communication within and between networks of the brain. Nodes that play a central function in communicating information across networks can be characterized as hubs (black circles) and may belong to a ‘rich club’ (dashed line). Due to heirt importance in network communication, injury to hub regions may result in poorer clinical outcomes than that in brain regions less central to communication across regions (Rubinov & Sporns, 2010).
Table 1.
Graph Theoretical Metrics*
| Category | Parameter | Parameter definition | Comment |
|---|---|---|---|
| Network integration | Characteristic path length | The average shortest path length between all pairs of nodes in the network | Primarily affected by long paths |
| Global efficiency | The average inverse shortest path length | Primarily affected by short paths | |
| Node degree | The number of links connected to a node | ||
| Network segregation | Local efficiency | Characterizes how well information is exchanged by a node’s neighbors when it is removed | Quantifies a network’s resistance to failure on a small scale |
| Clustering coefficient | The number of connections that exist between the nearest neighbors of a node as a proportion of the maximum number of possible connections | Reflects the presence of highly interconnected groups of nodes | |
| Betweenness centrality |
The fraction of all shortest paths in the network that pass through a given node | Nodes with high betweenness centrality participate in many short paths within a network and act as important controls of information flow | |
| Network topology | Hubs | Nodes with high degree | Hubs play a key role in network’s resilience to insult |
| Small-world networks | Networks that are significantly more clustered than random networks, yet have approximately the same characteristic path length as random networks. They combine the presence of functionally specialized (segregated) modules with a robust number of intermodular (integrating) links. | These networks maximize information processing while minimizing wiring costs, support segregated and integrated information processing and present resilience against pathology |
|
| Modularity | Quantifies the ease with which a whole-brain network can be divided into distinct subnetworks | Networks with high modularity have dense connections between nodes within modules but sparse connections between nodes in different modules | |
| Rich-club index | The extent to which well-connected nodes also connect to each other | High rich-club index implies the hubs are well connected and global connectivity is resilient to any one hub being removed |
Adapted from (Rubinov & Sporns, 2010) and (Fischi-Gomez et al., 2016).
2. Structural and Functional Connectivity in Healthy Infants
Increasing numbers of investigations have applied dMRI and rs-fMRI to study infants, beginning during the neonatal period and extending through the first two years of life (Alcauter, Lin, Keith Smith, Gilmore, & Gao, 2013; Ball et al., 2014; Brown et al., 2014; Damaraju et al., 2014; Damaraju et al., 2010; Doria et al., 2010; Fransson et al., 2011; Fransson et al., 2009; Gao et al., 2009; W. Lee et al., 2012; Lin et al., 2008; Perani et al., 2011; Smyser et al., 2010; Smyser et al., 2013; Smyser et al., 2014; Song et al., 2017; van den Heuvel et al., 2015; Vertes & Bullmore, 2015). These inquiries have included healthy, term-born infants and neonatal clinical populations of interest, with acquisition and analysis techniques differing across institutions. Despite this variance in study populations and approaches to assessment of connectivity, consistent patterns have emerged. Subsequently, these data have provided invaluable information regarding the complex interplay of early structural and functional cerebral development and provide a foundation for expanded investigation applying dMRI and rs-fMRI to infants with cerebral injury.
2.1. Maturation of Structural Connectivity
At the simplest level, the maturation of structural connectivity can be seen as maturation of brain systems. It has long been known that primary motor and sensory cortex develops earlier than association cortical areas (Sidman & Rakic, 1982). This is reflected in conventional MR imaging, as the changes in intensity on T1- and T2-weighted imaging associated with myelination are evident first in corticospinal tracts and the thalamostriate pathways of the visual system. Differential white matter maturation is also detectable by dMRI, with anisotropy values increasing earlier (reflecting earlier myelination) in tracts connecting primary motor and sensory cortex when compared with those connecting association areas. In an investigation of the rates of change in cortical anisotropy and associated white matter tracts during the neonatal period, early declines in cortical anisotropy were matched by increases in adjoining white matter anisotropy, indicative of earlier myelination and maturing structural connectivity in these regions (T. A. Smyser et al., 2016).
At a network level, there is evidence that maturation of structural connectivity networks is associated with a change from local, proximity-based connectivity patterns to a more integrated topology supportive of higher cognitive function (Vertes & Bullmore, 2015). As with functional connectivity, structural networks are detectable early in gestation. In an investigation of fetuses studied at 20 weeks’ gestation, preterm infants studied at 35 weeks’ postmenstrual age (PMA) and term-born infants, small-world network organization was prominent as early as 20 weeks’ gestation. During the period from 20 to 40 weeks, network strength and global efficiency increased overall, but more rapidly from 20–35 weeks’ than from 35–40 weeks’ PMA, an observation linked to an increase in major long association white matter fibers (Song et al., 2017). Similarly, a modular architecture of interconnected hubs has been identified in preterm infants as early as 30 weeks’ PMA (Ball et al., 2014; van den Heuvel et al., 2015). From this age through term equivalent, the principal development was a proliferation of connections between core hubs and the remainder of the brain, providing improved efficiency and integration capacity (van den Heuvel & Sporns, 2013). In another study of prematurely-born infants without injury on conventional MRI and normal developmental outcomes at age 18 months, brain networks showed high efficiency and clustering measures across a range of network scales, with the structural connectome becoming more clustered from 27 to 42 weeks’ PMA, leading to a significant increase in its small-world structure (Brown et al., 2014). Thus, as demonstrated across these investigations, much of the structural network architecture required for normal brain function appears to be present by the time of normal birth, though not necessarily in mature form. Importantly, improvements in measures of integration, including global and local efficiency, have been shown to continue from infancy through the first two years of life (Fan et al., 2011) and into childhood (Huang et al., 2015) and adolescence (Khundrakpam et al., 2013). Subsequently, it has been suggested that dynamic changes in structural connectome organization over the lifespan follow an inverted U-shaped pattern, with an increasingly integrated topology during early development (Hagmann, Sporns, et al., 2010; Yap et al., 2011), followed by a plateau lasting for the majority of adulthood, with an increasingly localized topology in late life (Collin & van den Heuvel, 2013).
2.2. Maturation of Functional Connectivity
Across rs-fMRI investigations, multiple canonical RSNs incorporating cortical and subcortical gray matter regions and the cerebellum have been identified during infancy. These include RSNs located in primary motor and sensory cortices (e.g., somatomotor, visual, auditory networks) and those involving association cortices (e.g., default mode, frontoparietal control, dorsal attention networks). Through investigations of preterm infants and complemented by information available from fetal fMRI investigations, early forms of these networks are identifiable as early as 26 weeks’ PMA (Blazejewska et al., 2017; Jakab et al., 2014; Schopf et al., 2014; Thomason et al., 2014; Thomason et al., 2015; Thomason et al., 2017). Many networks initially consist of strong interhemispheric correlations between homotopic counterparts, with intrahemispheric correlations quantifiably weaker. Early thalamocortical connectivity is also evident (Alcauter et al., 2014; Doria et al., 2010; Smyser et al., 2010). The topology of these networks is consistent with results obtained in adult and older pediatric populations, though the relationship of findings between age groups differs based upon network. These similarities (and differences) have been consistently identified across multiple reports, with varied terminology (e.g., ‘immature’, ‘precursor’, ‘proto’) used to describedifferences between infant and adult populations (Doria et al., 2010; Fransson et al., 2009; Fransson et al., 2007; Smyser et al., 2010).
The rate at which correlations within and between RSNs develop differs by network (Doria et al., 2010; Gao, Alcauter, et al., 2014; Smyser et al., 2010; Smyser et al., 2014). It is assumed that early RSN development is dependent upon effective establishment of structural connectivity (Mrzljak, Uylings, Kostovic, & van Eden, 1992; Petanjek, Judas, Kostovic, & Uylings, 2008; Petanjek et al., 2011), and recent reports have suggested RSN development closely reflects known rates of cortical development based upon histology (Gao, Alcauter, et al., 2014; Smyser et al., 2014). Networks located in cortical regions known to mature early and incorporating primary sensory and motor regions are established by term. These networks demonstrate less variability between subjects (Gao, Elton, et al., 2014) and are potentially less susceptible to pathology (Smyser et al., 2014). In contrast, higher-order RSNs, such as the default mode network, are quantifiably weaker or topographically incomplete at term (Doria et al., 2010; Fransson et al., 2009; Fransson et al., 2007; Smyser et al., 2010). These networks mature non-linearly over the first years of life and are centered in association cortices known to mature later, demonstrating greater intersubject variability in spatial and temporal patterns. Relationships between RSNs gradually evolve, with correlation between RSN pairs also assuming adult-like patterns during the first years of life (Gao, Alcauter, et al., 2014; Gao, Gilmore, Alcauter, & Lin, 2013).
These patterns of network development are also reflected across neonatal rs-fMRI investigations using graph theoretical methods. Similar to dMRI findings, small-world network organization has been identified at birth, with RSNs demonstrating increasing local and global efficiency through the first two years of life (Gao et al., 2011). In addition, RSNs identified using modularity and community detection algorithms suggest these networks may exist as fragmented forms of adult networks at younger ages (Eggebrecht et al., 2017), and that homotopic and anterior posterior connectivity between these proto-networks increase with advancing age (Grayson & Fair, 2017). Further, these studies have identified hubs in primary motor and sensory cortices in term neonates, likely reflecting the relative maturity of these primary cortical regions. Other hub candidates located in higher-order association cortices in the frontal and parietal lobes have been less consistently identified at birth (Fransson et al., 2011; Mevel & Fransson, 2016; van den Heuvel & Sporns, 2013). This combination suggests early RSN development may demonstrate network-specific susceptibility to brain injury and/or disruption of key structural processes during critical developmental periods.
3. Common Forms of Brain Injury in Infants
3.1. Brain Injury in Preterm Infants
3.1.1. Periventricular Leukomalacia:
The hallmark of brain injury in preterm infants is white matter injury, or periventricular leukomalacia (PVL). PVL consists of two distinct components: focal necrosis, with loss of all cellular elements dorsal and lateral to the lateral ventricles, and diffuse injury involving pre-oligodendrocytes and marked by astrogliosis and microgliosis (Volpe, Kinney, Jensen, & Rosenberg, 2011). The most severe form, cystic PVL, involves focal macroscopic necroses, often several millimeters in size, which evolve to tissue dissolution and cysts over weeks (Figure 2A). Cystic PVL is now uncommon, with an incidence of ~5%. A moderate form, noncystic PVL, involves focal necrotic lesions 1–2 mm in size which, on tissue dissolution, evolve not to cysts but rather focal glial scars, visible as punctate areas of increased signal intensity on T1-weighted images. This form occurs in ~25% of preterm infants. The least severe form involves a focal necrotic component less than 1 mm in size not visible on structural MRI. All three subtypes include astrogliosis/microgliosis, and, after the initial pre-oligodendrocyte cell death, an excess of oligodendroglial progenitors. Diffuse white matter gliosis without focal necroses has also been described (Martinez-Biarge et al., 2011; J. J. Volpe, 2017). This may appear as diffuse excessive high signal intensity (DEHSI) on conventional MRI (Counsell et al., 2003; Counsell et al., 2006; Hart, Smith, Rigby, Wallis, & Whitby, 2010; Kidokoro, Anderson, Doyle, Neil, & Inder, 2011) and manifest as diffusion anisotropy abnormalities (Huppi et al., 2001). The most common neurologic disorder associated with PVL is spastic diplegia, however, a myriad of other deficits, often emerging later in life and involving cognition, are also found in affected children. These may be a consequence of white matter injury or associated effects on gray matter (Anderson et al., 2017).
Figure 2. Brain injury in term and preterm infants.
Representative coronal T2-weighted and transverse diffusion-weighted MR images illustrating A) cystic periventricular leukomalacia, B) grade IV intraventricular hemorrhage and C) post-hemorrhagic hydrocephalus in prematurely-born infants at term equivalent postmenstrual age. Areas of hemorrhage appear dark and cerebrospinal fluid appears bright on these T2-weighted images. Also demonstrated are D) basal ganglia and E) watershed injury patterns on diffusion-weighted images in term-born infants with hypoxic-ischemic brain injury.
3.1.2. Intraventricular Hemorrhage:
Intraventricular hemorrhage (IVH) is the second predominant form of preterm brain injury. Occurring in up to 23% of very preterm infants (VPT; born at ≤32 weeks gestation), IVH results from a confluence of intravascular, vascular and extravascular factors (J. J. Volpe, 2001; Joseph J. Volpe, 2009). IVH typically occurs in the first 72 hours of life, with infants born earliest at greatest risk (Stoll et al., 2015). IVH occurs following initial bleeding from endothelial-lined vessels into the immature subependymal germinal matrix, with extension into the ventricular system in approximately 80% of cases. IVH is categorized into one of four grades based upon hemorrhage location and severity (Papile, Burstein, Burstein, & Koffler, 1978; J. J. Volpe, 2001). Approximately 15% of infants develop a related parenchymal lesion resulting from hemorrhagic venous infarction adjacent to the lateral ventricle (i.e., grade IV IVH; Figure 2B). These lesions are typically unilateral (Bassan, Benson, et al., 2006; Bassan, Feldman, et al., 2006). Infants with high-grade IVH (i.e., grade III/IV) develop neurodevelopmental disability in greater than 50% of cases, with effects across motor, cognitive, language and social domains (Ira Adams-Chapman, Hansen, Stoll, & Higgins, 2008; Ancel et al., 2006; McCrea & Ment, 2008).
3.1.3. Post-Hemorrhagic Ventricular Dilatation:
Post-hemorrhagic ventricular dilatation (PHVD) occurs in up to 50% of infants who develop IVH, and now represents the most common cause of pediatric hydrocephalus in North America (Figure 2C) (Christian et al., 2016). Typically occurring in the first one to three weeks after IVH, PHVD results from impaired CSF absorption by the arachnoid villi and/or impaired drainage due to cerebral aqueduct obstruction (J. J. Volpe, 2001). Infants requiring neurosurgical intervention for PHVD have post-hemorrhagic hydrocephalus (PHH) and often undergo temporizing and permanent procedures designed to decompress the ventricular system and relieve related cerebral ischemia (J. J. Volpe, 2001).
The outcomes of infants with PHH are among the worst in newborn medicine, with cognitive deficits in >85% and cerebral palsy in 70% of affected infants (I. Adams-Chapman, Hansen, Stoll, Higgins, & Network, 2008). PHH often requires complex, lifelong neurosurgical care and frequent neurosurgical revision surgery (Limbrick et al., 2010; Murphy et al., 2002; Vassilyadi, Tataryn, Shamji, & Ventureyra, 2009).
3.2. Brain Injury in Term Infants
3.2.1. Hypoxic-Ischemic Encephalopathy (HIE):
The forms of brain injury typically sustained by term-born infants differ from those of preterm infants, likely as a consequence of variation in regional tissue vulnerability related to brain maturation. The two most common forms of injury are deep nuclear gray matter injury and watershed injury (Figure 2D-E) (Barkovich et al., 1998; Shalak & Perlman, 2004). Deep nuclear gray matter injury involves the thalamus and basal ganglia, sometimes extending superiorly to include corticospinal tracts and perirolandic cortex and inferiorly to the brainstem. It occurs in 25–80% of cases of HIE and is often associated with severe insult of prolonged duration or a combined partial with profound terminal insult (e.g., placental abruption or uterine rupture) (“Neonatal Encephalopathy and Neurologic Outcome, Second Edition,” 2014). These infants demonstrate hypotonia and poor feeding, and outcome is strongly related to the extent of injury on MRI (Martinez-Biarge et al., 2011; Martinez-Biarge, Diez-Sebastian, Rutherford, & Cowan, 2010). The watershed pattern of injury affects white matter and may extend to involve neuronal necrosis of the overlying cortex in the watershed vascular zones. It occurs in 15–45% of cases of HIE, and is associated with milder, subacute injury (D’Alton et al., 2014). These infants have proximal and axial weakness with seizures during the neonatal period, and may have cognitive impairment with clumsiness and/or spasticity later in life.
4. Relationship Between Injury and Connectivity
Despite the increasingly established body of literature detailing normative patterns of early structural and functional connectivity, much less is known regarding the impact of brain injury on early structural and functional connectivity development. should read "As term and preterm infants commonly demonstrate distinct patterns of injury, studies seeking to establish these effects have been population specific. However, only limited investigation has been performed to date using dMRI and rs-fMRI to characterize structural and functional connectivity in term- and prematurely-born infants with cerebral injury identified on conventional MRI. As outlined below, these investigations have delineated the deleterious effects of forms of brain injury common in these populations. Nevertheless, consistent patterns have emerged, suggesting interrelated local and brain-wide effects of brain injury on structural and functional connectivity development, dependent on injury type, spatial pattern, location and severity (Figures 3–4).
Figure 3. Effect of brain injury on structural connectivity in prematurely-born infants.
Anterior and lateral views of the bilateral corticospinal tracts identified using diffusion tensor tractography in representative individual very preterm infants scanned at term equivalent postmenstrual age with A) grade IV intraventricular hemorrhage (note the asymmetry in tract volume between injured and uninjured hemispheres), B) post-hemorrhagic hydrocephalus and cystic periventricular leukomalacia. Results from D) an uninjured very preterm infant and E) a healthy, term-born infant are provided for comparison. Note the differences in effects on tract development across different forms of brain injury common in this clinical population.
Figure 4. Effect of brain injury on functional connectivity in prematurely-born infants.
Transverse views of the motor resting state network identified using resting state-functional MRI in representative, individual very preterm infants scanned at term equivalent postmenstrual age with A) grade IV intraventricular hemorrhage (note the asymmetric effect dependent upon hemisphere of injury), B) post-hemorrhagic hydrocephalus and C) cystic periventricular leukomalacia. Results from D) an uninjured very preterm infant and E) a healthy, term-born infant are provided for comparison. Note the differences in effects on resting state network development across each form of brain injury common in this clinical population.
When evaluating the effects of injury on outcome based on conventional imaging (T1- and T2- weighted images), structural injury to brain regions of well-defined function and clear input/output pathways show the strongest correlations with outcome. Using preterm birth as an example, PVL includes injury to the motor fibers projecting to the lower extremities and is associated with leg spasticity (spastic diplegia). Similarly, interruption of the geniculostriate pathway and/or injury to primary visual cortex is associated with cortical visual impairment (J. J. Volpe, 2001). However, this is less the case for higher-order cognitive functions involving more distributed brain networks. For example, executive function disorders, common in preterm children, are often associated with widespread, subtle abnormalities of white and gray matter rather than focal lesions (Anderson et al., 2017; Boardman et al., 2010). As such, neurodevelopmental outcomes in these domains may be better predicted with ‘connectome-type’ analyses that include measures of segregation and integration (Fischi-Gomez et al., 2016). However, as noted in (Fischi-Gomez et al., 2016), a direct link between altered integrative pattern and cognitive task performance has not yet been demonstrated.
4.1. Structural Connectivity in Infants with Brain Injury
4.1.1. Effects of Prematurity:
A small number of studies have shown disruption in connectivity (using a network-based approach) in preterm infants at term equivalent age. Research suggests that preterm infants have significant disruptions in both cortical-subcortical and short-distance cortico-cortical connections (Ball et al., 2014; Pandit et al., 2014). However, rich-club organization in preterm infants remains intact, suggesting a fundamental white matter framework is in place early in gestational development (Ball et al., 2014). Despite this, preterm infants demonstrate reduced clustering coefficient, modularity, and small-world index measures compared to term-born children (Tymofiyeva et al., 2013). In a study of 65 infants who underwent MRI between 25 and 45 weeks’ PMA, a core of key connections was not affected by gestational age at birth. However, local connectivity involving thalamus, cerebellum, superior frontal lobe, cingulate gyrus and short-range cortico-cortical connections varied with gestational age and contributed to altered global topology of structural brain networks. The authors concluded this relative preservation of core connections at the expense of local connections supports more effective use of impaired white matter reserve following preterm birth (Batalle et al., 2017).
Findings in older children and adults are consistent with those from infants, further extending our understanding of the effects of preterm birth. In a study of the small-world attributes and rich club organization of 147 preadolescent children whose gestational age ranged from 29 to 42 weeks, higher network efficiency was positively associated with longer gestation. Longer gestation was also correlated with increased local efficiency in the posterior medial cortex, including the precuneus, cuneus and superior parietal regions. Rich club organization was present, and connectivity among rich club members and from rich club regions was positively associated with gestational age (Kim et al., 2014). Further, in a study of 61 preterm children imaged at age six years, average network node degree and strength were reduced in preterm children. In addition, the decomposition of the brain networks into an optimal set of clusters remained substantially different in preterm children, indicating an altered network community structure. Nevertheless, typical small-world, rich-club and modularity characteristics were maintained. As above, these results suggest brain reorganization in preterm infants prioritizes a tight modular structure, thereby maintaining small-world, rich-club and modularity characteristics (Fischi-Gomez et al., 2016). Finally, in a study of rich-club organization and modularity in 51 VPT adults, establishment of global connectivity patterns appeared to be prioritized over peripheral connectivity. The preterm subjects exhibited stronger rich-club architecture than the control subjects, despite possessing a relative paucity of white matter resources. Using a simulated lesion approach, the authors investigated whether putative structural reorganization takes place in the preterm brain in order to compensate for its anatomical constraints. They found connections between the basal ganglia and frontal regions, as well as between subcortical regions, assumed an altered role in the structural connectivity of the VPT brain (Karolis et al., 2016). Overall, many of the alterations in structural networks detectable during the neonatal period persist into adulthood.
4.1.2. Periventricular Leukomalacia:
There has been a single study of network characteristics of prematurely-born children with PVL (Table 2). Reorganization of frontal–striatal and frontal– limbic pathways was detected in a study of 16 preterm children with PVL and cerebral palsy in comparison to 75 healthy controls imaged during childhood (Ceschin, Lee, Schmithorst, & Panigrahy, 2015). There were also abnormalities of the posterior portions of visual-related white matter tracts, corresponding to reduced areas of nodal efficiency in the parietal-occipital regions. With regards to small-world architecture, preterm infants with PVL showed a tendency towards less short-range connectivity as well as differences in the number and spatial localization of long-range connections in the frontal, temporal and subcortical areas. Long-range connections appeared more numerous and clustered in the preterm cerebral palsy group in these regions compared with the parietal–occipital region. In contrast, long-range connections were more uniformly distributed in the term-born group. Further, nodal efficiency was reduced. Overall, these findings suggest a selective parietal-occipital regional vulnerability to injury in PVL.
Table 2.
Structural connectome studies of aberrant connectivity
| Author | Age | Sample Size | Finding |
|---|---|---|---|
| Ceschin et al., 2015 | Children | 16 preterm+PVL, 75 TC | PVL associated with reduced global efficiency and reduced clustering coefficient and local and nodal efficiency in parietaloccipital regions |
| Tymofiyeva et al., 2012 | Infants | 17 HIE | Trend level negative correlation between clustering coefficient and neuromotor deficits at 6 months |
TC, term-born control; PVL, periventricular leukomalacia; HIE, hypoxic ischemic encephalopathy
4.1.3. Hypoxic-Ischemic Encephalopathy:
Studies reporting changes in the structural connectome in association with brain injury in the context of neonatal encephalopathy are also limited (Table 2). In a proof-of-principle study of 17 infants with perinatal HIE who were imaged during the newborn period, a trend toward declining global brain network integration and segregation was associated with increasing neuromotor deficit scores at age six months, thereby suggesting a connection between network structure and outcome in this population (Tymofiyeva et al., 2012).
4.2. Functional Connectivity in Infants with Brain Injury
4.2.1. Effects of Prematurity:
Driven by the deleterious effects of prematurity on cerebral development identified using other neuroimaging modalities (Ball et al., 2014; Inder, Neil, Yoder, & Rees, 2005), many studies have focused upon the effects of preterm birth on RSN development. Early reports demonstrated similar RSN topography between term and VPT infants scanned at term equivalent PMA (Doria et al., 2010; Fransson et al., 2007; Smyser et al., 2010). However, quantitative measures have demonstrated network-specific differences in intrinsic brain activity between these populations (Ball et al., 2016; C. D. Smyser, N. U. Dosenbach, et al., 2016; Smyser et al., 2010; Smyser et al., 2014). Consistent with structural connectivity studies, prematurity leads to RSN-specific reductions in network magnitude (i.e., correlation coefficients of BOLD signal amplitude fluctuations within a given network) and complexity. These differences are also evident when performing comparisons using advanced mathematical techniques, such as graph theoretical analysis and dimensionality estimation, with these techniques demonstrating greater sensitivity than other methods for delineating individual and group differences within and between RSNs (Ball et al., 2015; Cao et al., 2017; C. D. Smyser, N. U. Dosenbach, et al., 2016). These disruptions persist into early childhood (Damaraju et al., 2010), adolescence (Constable et al., 2013; Myers et al., 2010) and early adulthood (White et al., 2014). However, their long-term effects on neurodevelopmental outcomes remains incompletely investigated.
4.2.2. Intraventricular Hemorrhage and Periventricular Leukomalacia:
In preterm infants, the effects of common forms of white matter injury on RSN development have been characterized across a limited number of targeted investigations (Table 3). Our group studied a cohort of 14 VPT infants with moderate-severe periventricular hemorrhagic infarction at term equivalent age (Smyser et al., 2013). Canonical RSNs with bilateral connectivity were identifiable in each subject, however network architecture was affected in a manner dependent upon injury severity and location (Figure 4A). Reductions were most prominent in regions adjacent to the injury site and in infants with more severe injury. Infants with IVH and PHH demonstrated the most prominent effects (Figure 4B). Further, results in these infants differed from those obtained from local matched cohorts which included VPT infants without brain injury and healthy, term-born infants (Figure 4D-E). Interestingly, in some cases infants with PHH can demonstrate increases in structural and functional connectivity measures across studies performed in the week immediately before and after ventriculoperitoneal shunt placement (Figure 5). We have also demonstrated that cystic PVL impacts RSN development. Similar to infants with high-grade IVH, RSNs were identifiable in 11 infants with PVL studied at term equivalent age, though these networks demonstrated reduced within network connectivity with effects modulated by injury severity (Figure 4C). In contrast, connectivity strength within motor, visual, and auditory networks was similar between infants with and without white matter injury. Most recently, utilizing methods previously applied in older infants and toddlers (Eggebrecht et al., 2017), we have investigated the effects of high-grade white matter injury on brain-wide RSN architecture (i.e., the functional connectome). These individual subject results demonstrate brain-wide effects of cerebral injury, with aberrant topology of canonical RSNs and reduced interhemispheric connectivity (Figure 6).
Table 3.
Functional connectome studies of aberrant connectivity
| Author | Age | Sample Size | Finding |
|---|---|---|---|
| Smyser et al., 2013 | Infants | 25 preterm, 14 preterm+PHI, 25 TC | Reduced fc in PHI compared to preterm without injury and TC, with reduced fc more prominent in injured hemisphere |
| He et al., 2015 | Infants | 27 preterm+DWMA | Reduced within-network fc in infants with moderate-severe DWMA compared to mild DWMA |
| Arichi et al., 2014 | Infants | 3 preterm, 3 preterm+PHI | Reduced diffusion and functional connectivity between motor and supplementary motor |
| Cai et al., 2017 | Infants | 40 preterm, 22 preterm+PVL, 31 TC | Alterations in thalamus-salience network connectivity in PVL |
| Burton et al., 2009 | Children-adults | 12 preterm+SDCP, 11 TC | Aberrant motor network connectivity in SDCP |
| Lee et al., 2011 | Children-adults | 43 preterm+PVL+SDCP, 20 TC | Reduced motor network connectivity in PVL+SDCP |
| Lee et al., 2017 | Children-adults | 14 preterm+PVL+SDCP, 20 TC | Reduced motor network efficiency in PVL+SDCP |
| Wingert et al., 2010 | Children-adults | 10 SDCP, 10 TC | Reduced cortical activation to sensory task in SDCP |
| Froudist-Walsh et al., 2015 | Adults | 21 preterm, 20 preterm+IVH±PHVD, 46 TC | Altered cortical activation during working memory task in IVH±PHVD |
| Kalpakidou et al., 2014 | Adults | 13 preterm, 17 preterm+IVH, 12 preterm+PHVD, 17 TC | Reduced cortical activation during working memory task in PHVD compared to IVH alone |
| Tusor et al., 2014 | Infants | 15 term+HIE, 23 TC | RSNs unilateral with reduced long-distance connections in HIE |
| Chalia et al., 2016 | Infants | 6 HIE | Changes in blood oxygenation related to EEG burst activity in HIE as measured with DOT |
| Hebden et al., 2002 | Infants | 1 HIE | Blood volume and oxygen saturation reduced in infant with IVH as measured with DOT |
| Singh et al., 2014 | Infants | 1 HIE | Decreased cortical blood volume following seizure as measured with DOT |
| White et al., 2012 | Infants | 3 preterm, 1 preterm+IVH, 1 preterm+stroke, 3 TC | Absent bilateral connectivity in injured hemisphere in preterm infant with occipital stroke as measured with DOT |
| Austin et al., 2006 | Infants | 1 preterm+IVH, 5 preterm | Lower oxygenation in IVH as measured with DOT |
| Gibson et al., 2006 | Infants | 2 preterm+IVH, 4 preterm | Reduced total hemoglobin changes from passive arm movements in IVH as measured with DOT |
| Omidvarnia et al., 2015 | Infants | 6 preterm+IVH, 11 preterm | Reduced electrical coherence in long-range connections in IVH; greater network vulnerability in infancy associated with poorer neurodevelopmental outcome as measured with EEG |
fc, functional connectivity; TC, term-born control; PHI, periventricular hemorrhagic infarction; DWMA, diffuse white matter abnormality; SDCP, spastic diplegic cerebral palsy; PVL, periventricular leukomalacia; IVH, intraventricular hemorrhage; PHVD, post-hemorrhagic ventricular dilatation; RSN, resting state network; HIE, hypoxic ischemic encephalopathy; DOT, diffuse optical tomography; EEG, electroencephalogram
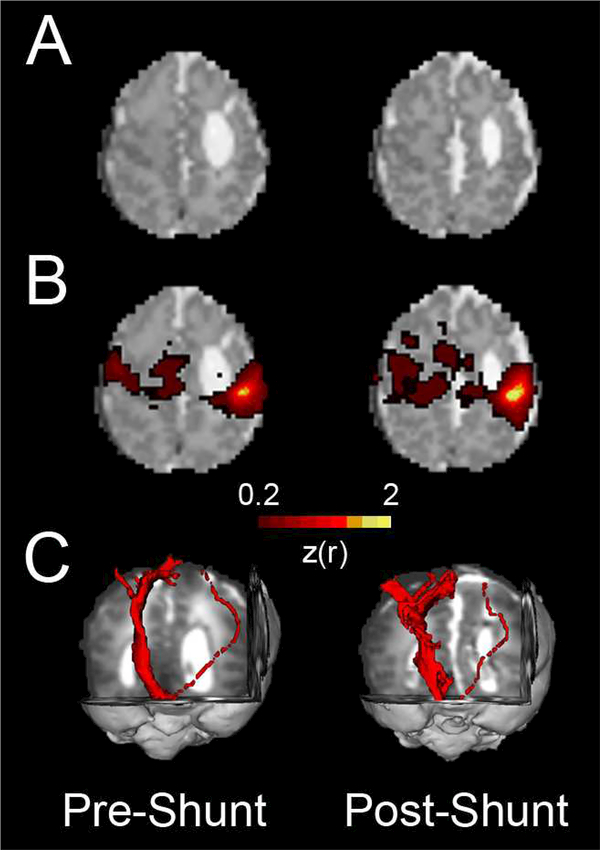
Figure 5. Effect of neurosurgical intervention on structural and functional connectivity in an infant with post-hemorrhagic hydrocephalus.
A) Axial T2-weighted images demonstrating post-hemorrhagic hydrocephalus in the same prematurely-born infant during the week before and after ventriculoperitoneal shunt placement. Cerebrospinal fluid appears bright in these images. B) Motor resting state networks before and after shunt placement. Note the increase in interhemispheric connectivity in post-operative compared to pre-operative results. C) Diffusion tensor tractography showing corticospinal tracts for the same subject. Note the asymmetry between the more injured and less injured hemispheres.
Figure 6. Resting state network architecture characterized using a community detection algorithm in an infant with brain injury.
A) T2-weighted image demonstrating the location and severity of grade IV intraventricular hemorrhage in an individual very preterm infant scanned at term equivalent age. B) Brain networks generated using the Infomap community detection algorithm to cluster rs-fMRI data in the same infant; 200 cortical and subcortical gray matter regions of interest were used for analysis. Displayed are color-coded individual resting state network results on an individual-specific cortical surface identified using a consensus algorithm across an edge density range of 2.5–3.1%. C) The corresponding correlation matrix demonstrating rs-fMRI relationships within and across networks. Note the strong relationships within and across networks based upon anatomic location. Warm colors denote positive correlations and cool colors denote negative correlations.
In addition, Arichi and colleagues studied three prematurely-born infants with unilateral periventricular hemorrhagic infarctions using DTI tractography and task-based and rs-fMRI at term equivalent age (Arichi et al., 2014). This investigation demonstrated marked asymmetry in corticospinal tracts on DTI tractography, with aberrant diffusion measures in the injured hemisphere. On rs-fMRI, interhemispheric connectivity in the motor RSN was predominantly preserved despite pathology, with effects local to the injury manifest as reduced connectivity between the motor and supplementary motor cortices. For task fMRI, positive BOLD responses were seen contralateral to the side of passive motor task, though in one infant the response was only seen in the injured hemisphere. More recently, Cai and colleagues used rs-fMRI to study 22 preterm infants with ≤10 punctate periventricular white matter lesions identified on T1- and T2-weighted images scanned between 32 and 39 weeks PMA (Cai, Wu, Su, Shi, & Gao, 2017). Findings in these infants were compared with those from a matched group of preterm infants without brain injury. Functional connectivity alterations were identified in infants with white matter lesions, most notably between the thalamus and salience network. Similar patterns of reduced connectivity were also observed in a cohort of preterm infants with diffuse white matter injury (He & Parikh, 2015). In this investigation, RSNs were identified in infants with and without diffuse white matter injury, though reductions in within-network connectivity were observed in executive control and frontoparietal networks in the injured group.
Recent reports suggests these effects persist into adulthood. Two investigations of prematurely-born children, adolescents and adults with spastic diplegic cerebral palsy due to PVL (including 12 and 11 subjects, respectively) demonstrated aberrant motor network connectivity in relation to term-born peers that correlated with severity of motor impairment, though the pattern of identified alterations differed between studies (Burton, Dixit, Litkowski, & Wingert, 2009; Lee et al., 2011). Another related investigation demonstrated reductions in motor network efficiency and structure-function coupling in 14 prematurely-born individuals with spastic diplegic cerebral palsy due to PVL compared to controls (D. Lee et al., 2017). An additional study demonstrated reduced cortical activation in response to sensory stimulation in task-based fMRI in 10 children, adolescents and adults with spastic diplegic cerebral palsy (Wingert, Sinclair, Dixit, Damiano, & Burton, 2010). Similar patterns of reduced cortical activation have also been identified using working memory tasks in VPT young adults with a history of perinatal brain injury, including neonatal periventricular hemorrhagic infarction with ventricular dilatation (Froudist-Walsh et al., 2015; Kalpakidou et al., 2014).
4.2.3. Hypoxic-Ischemic Encephalopathy:
For term-born infants, only a single report has characterized the effects of hypoxic-ischemic encephalopathy on RSNs using rs-fMRI. Tusor and colleagues studied a cohort of 15 infants with HIE who were treated with therapeutic hypothermia (Tusor, 2014). Infants underwent sedated MRI scans within five weeks of birth. Conventional MRI demonstrated heterogeneous white and gray matter injury across the cohort. In these infants, canonical RSNs, including the auditory, somatomotor, visual and default mode networks, were identified, though they were more likely to be unilateral and/or localized with more limited interhemispheric and long-range intrahemispheric correlations. RSNs differed across each network in comparison to those from a matched cohort of local healthy, term-born control infants. Within this small cohort, outcomes were mixed, with infants with lower neonatal functional connectivity measures having worse outcomes, with relationships differing across RSNs.
4.3. Alternative imaging modalities for the study of aberrant functional connectivity
Alternative neuroimaging techniques have similarly been adapted and employed to study functional brain connectivity in infants with brain injury. For example, modalities such as magnetoencephalography (MEG) and electroencephalography (EEG) have been used to assess coherence of electrical activity across cortical regions of the brain in children born preterm (Doesburg et al., 2011; Ye, AuCoin-Power, Taylor, & Doesburg, 2016). Though use of bedside monitoring with EEG in the NICU is common in standard clinical practice, few studies have employed this technique to assess cerebral connectivity in this population. In one study comparing 11 preterm infants without brain injury and 6 preterm infants with IVH, reduced long-range cortical correlation amplitude was observed in preterm infants with IVH compared to those without. The authors further assessed the association between graph theoretical measures during the neonatal period and outcomes at age two years, observing that global clustering coefficient was negatively correlated with neurodevelopmental performance measures. This suggested that infants with diffuse impairment in functional brain networks during infancy, as identified and characterized using EEG, are at increased risk for neurodevelopmental impairment during childhood (Omidvarnia, Metsaranta, Lano, & Vanhatalo, 2015).
Optical imaging technologies, such as functional near infrared spectroscopy (fNIRS) and diffuse optical tomography (DOT), have also been used to assess connectivity between cortical structures using hemodynamic contrasts (Hebden et al., 2004; Hintz et al., 2001; White, Liao, Ferradal, Inder, & Culver, 2012). Critically, these portable imaging technologies can be employed for serial imaging of the effects of brain injury on functional connectivity at the bedside in the neonatal intensive care unit (NICU) in neonatal populations unable to travel to the MRI scanner due to clinical instability and/or medical equipment required for therapeutic interventions. For example, several DOT studies have assessed bedside functional connectivity in infants with HIE in the NICU receiving therapeutic hypothermia treatment for neuroprotection (Chalia et al., 2016; Hebden et al., 2002; Singh et al., 2014). These studies demonstrated changes in cortical blood oxygenation levels related to cerebral hemorrhage, high frequency EEG bursts and during seizures identified on EEG. Additionally, DOT has also been used to investigate the deleterious effects of IVH on cerebral oxygenation in preterm infants in the NICU (Austin et al., 2006; Gibson et al., 2006), as well as to demonstrate decreased visual network connectivity in a preterm infant following occipital stroke (White et al., 2012). These portable techniques provide exciting alternative tools enabling lines of novel investigation focused on serial bedside assessment of aberrant brain connectivity related to neonatal brain injury.
5. Technical Challenges of Studying Infants
Neuroimaging studies of infants are technically demanding, presenting unique challenges related to data acquisition and analysis. Principal among these are selection of radiofrequency (RF) coils and acquisition parameters, correction of signal inhomogeneities, atlas registration procedures and subject state and motion during acquisition. These technical areas have received increasing focus, as they have proven to be critical for leveraging advances in scanner technology and pulse sequence development to optimize data quality, maximize signal-to-noise ratio (SNR) and minimize sources of colored noise which can drive differences identified between populations.
5.1. Radiofrequency Coils
Across populations, the size and shape of RF coils utilized for MRI data collection impact image quality. Early studies in infants utilized adult head, surface or knee RF coils (Born et al., 1998; Yamada et al., 1997). This resulted in suboptimal SNR and limited spatial resolution (Souweidane et al., 1999). Subsequently, RF coils designed for neonatal brain imaging were developed and became commercially available. Use of these age-specific coils resulted in 2–3 fold improvements in SNR (Bluml et al., 2004; Erberich, Friedlich, Seri, Nelson, & Bluml, 2003), and their utilization became increasingly common. Recent studies have begun to employ the current generation of RF coil technology (i.e., 32- and 64-channel head/neck coils) to consistently and comfortably acquire data with high spatial and temporal resolution and low motion in neonates (Glasser et al., 2016; Ugurbil et al., 2013).
5.2. Acquisition Parameters
MRI acquisition parameters significantly affect SNR in structural and functional connectivity data, with research suggesting the T2* relaxation time constant for fMRI in neonates is longer than adults, requiring a longer echo time (TE) for optimum contrast-to-noise ratio (Rivkin et al., 2004). Early investigations included noteworthy differences in pulse sequences and spatial resolution (Alcauter et al., 2013; Damaraju et al., 2014; Damaraju et al., 2010; Doria et al., 2010; Fransson et al., 2011; Fransson et al., 2009; Gao et al., 2009; Lee et al., 2012; Lin et al., 2008; Perani et al., 2011; Smyser et al., 2010; Smyser et al., 2013; Smyser et al., 2014). In one of the few evaluations of acquisition parameters in infants, Lin demonstrated comparable measures in the sensorimotor cortex with a short (0.75 seconds) versus long repetition time (TR; 2 seconds) (Lin et al., 2008). However, the field of view in the short TR scan only covered the sensorimotor cortex. Multiband acquisition techniques now allow shorter TRs and full coverage of the head while providing SNR similar to that of standard echo planar (long TR) sequences (Smith-Collins, Luyt, Heep, & Kauppinen, 2015). Current studies now seek to define optimal approaches for translating this most recent iteration of pulse sequences into neonatal scanning protocols. Early investigations demonstrate feasibility and reliability in collecting neonatal dMRI and fMRI data with unparalleled spatial (voxel size ≤2 mm isotropic) and temporal (TR time ≤1 second) resolution in well-tolerated scanning sessions.
5.3. Magnetic Field Inhomogeneity
Image distortion caused by static magnetic field inhomogeneity is a common problem related to the use of fast image acquisition methods. While these effects are less prevalent in infants than adults, they must be corrected, and multiple approaches are available. One is through collection of an additional imaging data set from each subject to generate a map of the field distortions caused by susceptibility effects (Cusack, Brett, & Osswald, 2003; Jezzard & Balaban, 1995). These field maps are used to correct the susceptibility-induced voxel shifts, creating an undistorted image. In a somewhat more efficient approach, an additional image set can be collected in which the phase encode polarity is reversed (Holland, Kuperman, & Dale, 2010). The opposite phase encoding polarity between the two image sets (i.e., stretch versus compression) can be used to create a single undistorted image set. Finally, an average field map derived from subjects from the same clinical population (e.g., neonates) imaged with the same RF coil and scanner can be employed (Gholipour, Kehtarnavaz, Gopinath, Briggs, & Panahi, 2008). This approach is less desirable than subject-specific options, particularly for infants where differences in field inhomogeneities may exist due to injury. However, it can be applied retrospectively when field maps or reversed phase polarity images were not acquired during collection of the original data.
5.4. Subject State and Motion
5.4.1. Subject State:
Motion is a problematic issue in neuroimaging for all populations, including infants, for whom both training prior to scanning and feedback during data collection are not practical. As a result, at some institutions infants receive sedation for MRI scans (Doria et al., 2010; Fransson et al., 2007). While this reduces the amount of motion, it can also alter rs-fMRI results (Greicius et al., 2008; Stamatakis, Adapa, Absalom, & Menon, 2010; Vincent et al., 2007). Subsequently, well-established methods for successfully scanning infants without sedation have been increasingly used across centers (Mathur, Neil, McKinstry, & Inder, 2008). These practices and our understanding of the effects of subject state on rs-fMRI data in infants may further evolve with improved characterization of the arousal state of non-sedated subjects. However, this requires simultaneous EEG data collection during scanning. While the technology and procedures for collecting these data are available, combined fMRI-EEG acquisitions have only recently begun in infants (Arichi et al., 2017; Vanhatalo, Alnajjar, Nguyen, Colditz, & Fransson, 2014).
5.4.2. Subject Motion:
When movement occurs during rs-fMRI data collection, data are affected in a stereotypical manner in which unwanted motion increases local correlations more than distant ones (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). As a result, analysis of data with excessive motion makes connections between anatomically approximate regions seem stronger, while making connections between regions anatomically further apart seem weaker. To complicate matters further, regions laterally oriented to one another tend to undergo greater increases in correlation values than those in other orthogonal orientations (Power, Schlaggar, & Petersen, 2014). Finally, an even more insidious effect of subject motion is related to the finding that head motion correlates with a number of behavioral, demographic and physiological measures, including intelligence, reading ability, weight and psychiatric diagnostic scales (Siegel et al., 2017). Consequently, head motion is a potential source of systematic error (bias) in the measurement of relationships between RSNs and behavior. Fortunately, these effects can be minimized through strategies designed to reduce the effects of motion, with frame censoring (Power, Mitra, et al., 2014) and whole brain regression (i.e., the time course of the average signal intensity within the brain) firmly established as critical components of this process (Ciric et al., 2017; Power, Schlaggar, & Petersen, 2015). Most recently, approaches have included provision of real-time feedback regarding within run movement and data quality to investigators during acquisition (Dosenbach et al., 2017). This can allow data collection in neonates to be extended (or truncated) at the scanner based upon individual subject performance, increasing efficiency and lowering costs.
Motion can also be an issue for dMRI, particularly since the method is intentionally designed to be sensitive to motion on the order of microns. Fortunately, motion effects in diffusion data are conspicuous. In an approach similar to that used for rs-fMRI, dMRI data are frequently obtained with more diffusion encodings than are necessary for the final analysis. Software is then used to detect and reject those diffusion-encoded acquisitions corrupted by motion.
5.5. Image Registration
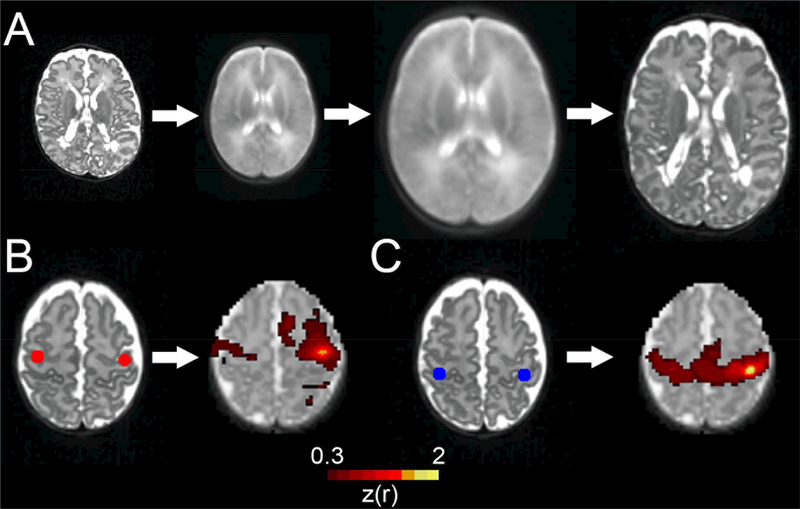
Image registration represents a unique challenge in infants, particularly in those with brain injury. Spatial normalization to a standardized atlas space provides a common framework for comparison of inter- and intra-individual data, which would otherwise be challenging due to variability in anatomy across individuals. However, the size and cortical folding of the brain vary markedly during early brain development, with cerebral injury introducing further heterogeneity. Thus, it is important to use gestational-age specific atlas targets encompassing narrow postmenstrual periods (i.e., 2–4 weeks) for studies of brain structure and function (Figure 7A). Such atlases demonstrate improved image normalization and registration across gray and white matter and cerebrospinal fluid, particularly in infants with brain injury. Increasing numbers of atlases are now available (e.g., www.brain-development.org and sumsdb.wustl.edu). Even with successful atlas registration procedures, it is important to utilize approaches which account for subject-specific alterations in anatomy resulting from brain injury. For example, as a direct or downstream result of cerebral injury, the location of regions of interest for structural and functional connectivity analyses can deviate markedly from those anticipated based upon standard atlas coordinates, altering measured results (Figure 7B). These effects, which can be local and/or brain-wide, can be accounted for with methods accommodating individual differences in anatomy (Figure 7C).
Figure 7. Methodological considerations for connectivity analyses in infants with brain injury.
A) Accurate registration of images and transformation to standard (711–2N) atlas space is feasible for infants with brain injury using age appropriate atlas targets and registration procedures. B) Regions of interest in the bilateral motor cortex identified using standard atlas coordinates with the corresponding correlation map generated using a left motor cortex seed in an infant with cystic periventricular leukomalacia. Note the anterior location of regions of interest in relation to the actual motor cortex bilaterally. C) Individual-specific bilateral motor cortex regions of interest in the same subject accounting for effects of cerebral injury with results from identical correlation analysis. Note the marked qualitative improvement in the motor network result using individual-specific regions of interest. This is also noted on quantitative analysis, with an interhemispheric correlation between regions of interest of 0.35 for the result in B and 0.64 for the result in C.
5.6. Challenges in Infants with Brain Injury
In addition to the general technical issues relevant to the study of infants cited above, there are challenges unique to studying this clinical population. Principal among these is timely recruitment, driven in part by the clinical instability of affected infants and the related stressors of the NICU environment for families. These factors pose challenges for participation and collection of neuroimaging data, requiring research teams with substantive experience working with these clinical populations and in this setting. Scanning sessions in this population must typically be coordinated with the NICU teams in the context of the clinical care plan, mandatorily including careful monitoring by trained personnel including NICU nursing staff and/or physicians. This potential for clinical instability can also necessitate modification and/or truncation of scanning protocols based upon subject tolerance of scanning, making collection of sufficient high-quality data for appropriately powered analyses challenging. Further, following successful collection of high-quality data, substantive technical challenges related to analysis remain. Driven by the differences in anatomy related to the presence of brain injury, analyses should ideally be performed using data specific to each individual infant whenever possible. For example, employing subject-specific as opposed to mean field maps for magnetic field inhomogeneity correction and accurate individual subject-specific regions of interest and/or surface segmentations for registration and localization of rs-fMRI data. This constellation of challenges requires careful attention across all aspects of study, and these factors have undoubtedly contributed to the relative dearth of literature detailing findings in this high-risk population. Nonetheless, the methods necessary to successfully study infants with brain injury are now increasingly established and can be readily implemented at most institutions, with recent advances leveraging improvements in hardware, scanner technology and analysis methods. Importantly, successful application of these techniques enables collection of high-quality data which will provide unparalleled information regarding interrelated effects of brain injury on structural and functional connectivity.
6. Conclusions
Early brain development, in which primary motor and sensory systems develop before higher-order cognitive systems, is reflected in both structural and functional connectivity. Network development during the prenatal and early postnatal periods is characterized by a transition from local, proximity-based connectivity patterns to a more integrated topology supportive of higher cognitive function. Early development of these structural and functional networks is susceptible to the harmful effects of brain injury, dependent upon injury type, timing, spatial pattern, location and severity. The resulting differences in these networks may also be reflected in the association between imaging findings and outcome. The motor and visual impairments that often accompany preterm birth are strongly associated with focal abnormalities on conventional (T1- and T2-weighted) imaging, which may indicate injury to localized areas of function. However, disorders of higher-order cognitive function (e.g., executive function) are more strongly associated with diffuse and often subtle abnormalities on conventional MR imaging. These more widespread abnormalities may be more likely to disrupt the integrated network topology necessary to support higher-order functions. Recent work in adults suggests that individuals have distinct functional network topography which is susceptible to brain injury (Braga & Buckner, 2017; Gordon et al., 2017). Related investigation has demonstrated that individual characteristics, such as intelligence, are associated with intrinsic individual-specific functional connectivity motifs (Finn et al., 2015). Thus, disruptions to the structural and functional connectome described in prematurely-born infants with and without brain injury are highly relevant to their neurodevelopmental outcomes. Similarly, disruption of network architecture following hypoxic-ischemic injury in term-born infants, though less studied, may also directly affect outcomes. However, a direct link between altered structural and functional connectivity patterns and task performance has not yet been demonstrated in these populations. Future research must continue to focus on determining whether such relationships exist and establish their suitability for predicting outcome in infants with brain injury and other high-risk neonatal populations.
Acknowledgements:
This work was supported by the National Institutes of Health (grant numbers R01 MH113570, K02 NS089852, T32 MH100019–02 and UL1 TR000448), Cerebral Palsy International Research Foundation, The Dana Foundation, Child Neurology Foundation and March of Dimes.
The authors would like to thank Jeanette K. Kenley for assistance with figure generation and Tara A. Smyser for useful comments on the manuscript.
Abbreviations
- dMRI
diffusion MRI
- rs-fMRI
resting state functional connectivity MR
- BOLD
blood oxygen level dependent
- RSN
resting state network
- PMA
postmenstrual age
- PVL
periventricular leukomalacia
- DEHSI
diffuse excessive high signal intensity
- IVH
intraventricular hemorrhage
- VPT
very preterm
- PHVD
post-hemorrhagic ventricular dilatation
- PHH
post-hemorrhagic hydrocephalus
- HIE
hypoxic-ischemic encephalopathy
- MEG
magnetoencephalography
- EEG
electroencephalography
- fNIRS
functional near infrared spectroscopy
- DOT
diffuse optical tomography
- NICU
neonatal intensive care unit
- RF
radiofrequency
- SNR
signal-to-noise ratio
- TE
echo time
- TR
repetition time
Footnotes
Competing Interests Statement: All authors have no competing interests and/or relevant conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- Abrol A, Damaraju E, Miller RL, Stephen JM, Claus ED, Mayer AR, & Calhoun VD (2017). Replicability of time-varying connectivity patterns in large resting state fMRI samples. Neuroimage, 163, 160–176. doi:10.1016/j.neuroimage.2017.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams-Chapman I, Hansen NI, Stoll BJ, & Higgins R (2008). Neurodevelopmental Outcome of Extremely Low Birth Weight Infants With Posthemorrhagic Hydrocephalus Requiring Shunt Insertion. Pediatrics, 121(5), e1167–e1177. doi:10.1542/peds.2007-0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R, & Network NR (2008). Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics, 121(5), e1167–1177. doi:10.1542/peds.2007-0423. Epub 2008 Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Keith Smith J, Gilmore JH, & Gao W (2013). Consistent Anterior-Posterior Segregation of the Insula During the First 2 Years of Life. Cereb Cortex. doi:10.1093/cercor/bht312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, … Gao W (2014). Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci, 34(27), 9067–9075. doi:10.1523/JNEUROSCI.0796-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancel PY, Livinec F, Larroque B, Marret S, Arnaud C, Pierrat V, … Group ES (2006). Cerebral palsy among very preterm children in relation to gestational age and neonatal ultrasound abnormalities: the EPIPAGE cohort study. Pediatrics, 117(3), 828–835. doi:10.1542/peds.2005-0091 [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Cheong JL, & Thompson DK (2015). The predictive validity of neonatal MRI for neurodevelopmental outcome in very preterm children. Semin Perinatol, 39(2), 147–158. doi:10.1053/j.semperi.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Treyvaud K, Neil JJ, Cheong JLY, Thompson DK, Lee KJ, … Inder TE (2017). Newborn brain MRI predicts long-term neurodevelopmental impairments in very preterm children. J Pediatr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arichi T, Counsell SJ, Allievi AG, Chew AT, Martinez-Biarge M, Mondi V, … Edwards AD (2014). The effects of hemorrhagic parenchymal infarction on the establishment of sensori-motor structural and functional connectivity in early infancy. Neuroradiology, 56(11), 985–994. doi:10.1007/s00234-014-1412-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arichi T, Whitehead K, Barone G, Pressler R, Padormo F, Edwards AD, & Fabrizi L (2017). Localization of spontaneous bursting neuronal activity in the preterm human brain with simultaneous EEG-fMRI. Elife, 6. doi:10.7554/eLife.27814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin T, Gibson AP, Branco G, Yusof RM, Arridge SR, Meek JH, … Hebden JC (2006). Three dimensional optical imaging of blood volume and oxygenation in the neonatal brain. Neuroimage, 31(4), 1426–1433. doi:10.1016/j.neuroimage.2006.02.038 [DOI] [PubMed] [Google Scholar]
- Ball G, Aljabar P, Arichi T, Tusor N, Cox D, Merchant N, … Counsell SJ (2016). Machine-learning to characterise neonatal functional connectivity in the preterm brain. Neuroimage, 124(Pt A), 267–275. doi:10.1016/j.neuroimage.2015.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Aljabar P, Zebari S, Tusor N, Arichi T, Merchant N, … Counsell SJ (2014). Rich-club organization of the newborn human brain. Proc Natl Acad Sci U S A, 111(20), 7456–7461. doi:10.1073/pnas.1324118111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Pazderova L, Chew A, Tusor N, Merchant N, Arichi T, … Counsell SJ (2015). Thalamocortical Connectivity Predicts Cognition in Children Born Preterm. Cereb Cortex, 25(11), 4310–4318. doi:10.1093/cercor/bhu331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, & Ferriero DM (1998). Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol, 19, 143–149. [PMC free article] [PubMed] [Google Scholar]
- Bassan H, Benson CB, Limperopoulos C, Feldman HA, Ringer SA, Veracruz E, … du Plessis AJ (2006). Ultrasonographic features and severity scoring of periventricular hemorrhagic infarction in relation to risk factors and outcome. Pediatrics, 117(6), 2111–2118. doi:10.1542/peds.2005-1570 [DOI] [PubMed] [Google Scholar]
- Bassan H, Feldman HA, Limperopoulos C, Benson CB, Ringer SA, Veracruz E, … du Plessis AJ (2006). Periventricular hemorrhagic infarction: risk factors and neonatal outcome. Pediatr Neurol, 35(2), 85–92. doi:10.1016/j.pediatrneurol.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Batalle D, Hughes EJ, Zhang H, Tournier JD, Tusor N, Aljabar P, … Counsell SJ (2017). Early development of structural networks and the impact of prematurity on brain connectivity. Neuroimage, 149, 379–392. doi:10.1016/j.neuroimage.2017.01.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, … Matthews PM (2003). Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience, 6, 750–757. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, & Hyde JS (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med, 34, 537–541. [DOI] [PubMed] [Google Scholar]
- Blazejewska AI, Seshamani S, McKown SK, Caucutt JS, Dighe M, Gatenby C, & Studholme C (2017). 3D in utero quantification of T2* relaxation times in human fetal brain tissues for age optimized structural and functional MRI. Magn Reson Med, 78(3), 909–916. doi:10.1002/mrm.26471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluml S, Friedlich P, Erberich S, Wood JC, Seri I, & Nelson MD Jr. (2004). MR imaging of newborns by using an MR-compatible incubator with integrated radiofrequency coils: initial experience. Radiology, 231(2), 594–601. doi:10.1148/radiol.2312030166 [DOI] [PubMed] [Google Scholar]
- Boardman JP, Craven C, Valappil S, Counsell SJ, Dyet LE, Rueckert D, … Edwards AD (2010). A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. Neuroimage, 52(2), 409–414. doi:10.1016/j.neuroimage.2010.04.261 [DOI] [PubMed] [Google Scholar]
- Born P, Leth H, Miranda MJ, Rostrup E, Stensgaard A, Peitersen B, … Lou HC (1998). Visual Activation in Infants and Young Children Studied by Functional Magnetic Resonance Imaging. Pediatr Res, 44(4), 578–583. [DOI] [PubMed] [Google Scholar]
- Braga RM, & Buckner RL (2017). Parallel Interdigitated Distributed Networks within the Individual Estimated by Intrinsic Functional Connectivity. Neuron, 95(2), 457–471 e455. doi:10.1016/j.neuron.2017.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Miller SP, Booth BG, Andrews S, Chau V, Poskitt KJ, & Hamarneh G (2014). Structural network analysis of brain development in young preterm neonates. Neuroimage, 101, 667–680. doi:10.1016/j.neuroimage.2014.07.030 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, … Johnson KA (2009). Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci, 29(6), 1860–1873. doi:10.1523/JNEUROSCI.5062-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Dixit S, Litkowski P, & Wingert JR (2009). Functional connectivity for somatosensory and motor cortex in spastic diplegia. Somatosens Mot Res, 26(4), 90–104. doi:10.3109/08990220903335742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Wu X, Su Z, Shi Y, & Gao JH (2017). Functional thalamocortical connectivity development and alterations in preterm infants during the neonatal period. Neuroscience, 356, 22–34. doi:10.1016/j.neuroscience.2017.05.011 [DOI] [PubMed] [Google Scholar]
- Cao M, He Y, Dai Z, Liao X, Jeon T, Ouyang M, … Huang H (2017). Early Development of Functional Network Segregation Revealed by Connectomic Analysis of the Preterm Human Brain. Cereb Cortex, 27(3), 1949–1963. doi:10.1093/cercor/bhw038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceschin R, Lee VK, Schmithorst V, & Panigrahy A (2015). Regional vulnerability of longitudinal cortical association connectivity: Associated with structural network topology alterations in preterm children with cerebral palsy. Neuroimage Clin, 9, 322–337. doi:10.1016/j.nicl.2015.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalia M, Lee CW, Dempsey LA, Edwards AD, Singh H, Michell AW, … Cooper RJ (2016). Hemodynamic response to burst-suppressed and discontinuous electroencephalography activity in infants with hypoxic ischemic encephalopathy. Neurophotonics, 3(3), 031408. doi:10.1117/1.NPh.3.3.031408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian EA, Jin DL, Attenello F, Wen T, Cen S, Mack WJ, … McComb JG (2016). Trends in hospitalization of preterm infants with intraventricular hemorrhage and hydrocephalus in the United States, 2000–2010. J Neurosurg Pediatr, 17(3), 260–269. doi:10.3171/2015.7.PEDS15140. Epub 2015 Nov 6. [DOI] [PubMed] [Google Scholar]
- Church JA, Fair DA, Dosenbach NU, Cohen AL, Miezin FM, Petersen SE, & Schlaggar BL (2009). Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain, 132(Pt 1), 225–238. doi:10.1093/brain/awn223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, … Satterthwaite TD (2017). Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage, 154, 174–187. doi:10.1016/j.neuroimage.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G, & van den Heuvel MP (2013). The ontogeny of the human connectome: development and dynamic changes of brain connectivity across the life span. Neuroscientist, 19(6), 616–628. doi:10.1177/1073858413503712 [DOI] [PubMed] [Google Scholar]
- Constable RT, Vohr BR, Scheinost D, Benjamin JR, Fulbright RK, Lacadie C, … Ment LR (2013). A left cerebellar pathway mediates language in prematurely-born young adults. Neuroimage, 64, 371–378. doi:10.1016/j.neuroimage.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell SJ, Allsop JM, Harrison MC, Larkman DJ, Kennea NL, Kapellou O, … Rutherford MA (2003). Diffusion-Weighted Imaging of the Brain in Preterm Infants With Focal and Diffuse White Matter Abnormality. Pediatrics, 112(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Ball G, & Edwards AD (2014). New imaging approaches to evaluate newborn brain injury and their role in predicting developmental disorders. Current Opinion in Neurology, 27(2), 168–175. doi:10.1097/wco.0000000000000073 [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Shen Y, Boardman JP, Larkman DJ, Kapellou O, Ward P, … Rutherford MA (2006). Axial and Radial Diffusivity in Preterm Infants Who Have Diffuse White Matter Changes on Magnetic Resonance Imaging at Term-Equivalent Age. Pediatrics, 117(2), 376–386. doi:10.1542/peds.2005-0820 [DOI] [PubMed] [Google Scholar]
- Cusack R, Brett M, & Osswald K (2003). An Evaluation of the Use of Magnetic Field Maps to Undistort Echo-Planar Images. Neuroimage, 18(1), 127–142. doi:10.1006/nimg.2002.1281 [DOI] [PubMed] [Google Scholar]
- Damaraju E, Caprihan A, Lowe JR, Allen EA, Calhoun VD, & Phillips JP (2014). Functional connectivity in the developing brain: a longitudinal study from 4 to 9months of age. Neuroimage, 84, 169–180. doi:10.1016/j.neuroimage.2013.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Phillips JR, Lowe JR, Ohls R, Calhoun VD, & Caprihan A (2010). Resting-state functional connectivity differences in premature children. Front Syst Neurosci, 4. doi:10.3389/fnsys.2010.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doesburg SM, Ribary U, Herdman AT, Miller SP, Poskitt KJ, Moiseev A, … Grunau RE (2011). Altered long-range alpha-band synchronization during visual short-term memory retention in children born very preterm. Neuroimage, 54(3), 2330–2339. doi:10.1016/j.neuroimage.2010.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, … Edwards AD (2010). Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci U S A, 107(46), 20015–20020. doi:10.1073/pnas.1007921107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Koller JM, Earl EA, Miranda-Dominguez O, Klein RL, Van AN, … Fair DA (2017). Real-time motion analytics during brain MRI improve data quality and reduce costs. Neuroimage, 161, 80–93. doi:10.1016/j.neuroimage.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggebrecht AT, Elison JT, Feczko E, Todorov A, Wolff JJ, Kandala S, … Pruett JR Jr. (2017). Joint Attention and Brain Functional Connectivity in Infants and Toddlers. Cereb Cortex, 27(3), 1709–1720. doi:10.1093/cercor/bhw403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erberich SG, Friedlich P, Seri I, Nelson MD Jr., & Bluml S (2003). Functional MRI in neonates using neonatal head coil and MR compatible incubator. Neuroimage, 20(2), 683–692. doi:10.1016/S1053-8119(03)00370-7 [DOI] [PubMed] [Google Scholar]
- Fan Y, Shi F, Smith JK, Lin W, Gilmore JH, & Shen D (2011). Brain anatomical networks in early human brain development. Neuroimage, 54(3), 1862–1871. doi:10.1016/j.neuroimage.2010.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, … Constable RT (2015). Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci, 18(11), 1664–1671. doi:10.1038/nn.4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischi-Gomez E, Munoz-Moreno E, Vasung L, Griffa A, Borradori-Tolsa C, Monnier M, … Huppi PS (2016). Brain network characterization of high-risk preterm-born school-age children. Neuroimage Clin, 11, 195–209. doi:10.1016/j.nicl.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, & Raichle ME (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci, 8(9), 700–711. doi:10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A, 102(27), 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, & Lagercrantz H (2011). The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex, 21(1), 145–154. doi:10.1093/cercor/bhq071 [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Engstrom M, Hallberg B, Mosskin M, Aden U, … Blennow M (2009). Spontaneous brain activity in the newborn brain during natural sleep--an fMRI study in infants born at full term. Pediatr Res, 66(3), 301–305. doi:10.1203/PDR.0b013e3181b1bd84 [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, & Aden U (2007). Resting-state networks in the infant brain. Proc Natl Acad Sci U S A, 104(39), 15531–15536. doi:10.1073/pnas.0704380104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froudist-Walsh S, Karolis V, Caldinelli C, Brittain PJ, Kroll J, Rodriguez-Toscano E, … Nosarti C (2015). Very Early Brain Damage Leads to Remodeling of the Working Memory System in Adulthood: A Combined fMRI/Tractography Study. J Neurosci, 35(48), 15787–15799. doi:10.1523/JNEUROSCI.4769-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Elton A, Hernandez-Castillo CR, Smith JK, Ramirez J, & Lin W (2014). Functional Network Development During the First Year: Relative Sequence and Socioeconomic Correlations. Cereb Cortex. doi:10.1093/cercor/bhu088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Smith JK, Gilmore JH, & Lin W (2015). Development of human brain cortical network architecture during infancy. Brain Struct Funct, 220(2), 1173–1186. doi:10.1007/s00429-014-0710-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Elton A, Zhu H, Alcauter S, Smith JK, Gilmore JH, & Lin W (2014). Intersubject variability of and genetic effects on the brain’s functional connectivity during infancy. J Neurosci, 34(34), 11288–11296. doi:10.1523/JNEUROSCI.5072-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Alcauter S, & Lin W (2013). The dynamic reorganization of the default-mode network during a visual classification task. Front Syst Neurosci, 7, 34. doi:10.3389/fnsys.2013.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Giovanello KS, Smith JK, Shen D, Zhu H, & Lin W (2011). Temporal and spatial evolution of brain network topology during the first two years of life. PLoS One, 6(9), e25278. doi:10.1371/journal.pone.0025278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Shen D, Smith JK, Zhu H, & Lin W (2013). The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb Cortex, 23(3), 594–603. doi:10.1093/cercor/bhs043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, & Lin W (2009). Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci U S A, 106(16), 6790–6795. doi:10.1073/pnas.0811221106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholipour A, Kehtarnavaz N, Gopinath K, Briggs R, & Panahi I (2008). Average field map image template for Echo-Planar image analysis. Conf Proc IEEE Eng Med Biol Soc, 2008, 94–97. doi:10.1109/IEMBS.2008.4649099 [DOI] [PubMed] [Google Scholar]
- Gibson AP, Austin T, Everdell NL, Schweiger M, Arridge SR, Meek JH, … Hebden JC (2006). Three-dimensional whole-head optical tomography of passive motor evoked responses in the neonate. Neuroimage, 30(2), 521–528. doi:10.1016/j.neuroimage.2005.08.059 [DOI] [PubMed] [Google Scholar]
- Glasser MF, Smith SM, Marcus DS, Andersson JL, Auerbach EJ, Behrens TE, … Van Essen DC (2016). The Human Connectome Project’s neuroimaging approach. Nat Neurosci, 19(9), 1175–1187. doi:10.1038/nn.4361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, … Dosenbach NUF (2017). Precision Functional Mapping of Individual Human Brains. Neuron, 95(4), 791–807 e797. doi:10.1016/j.neuron.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DS, & Fair DA (2017). Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. Neuroimage, 160, 15–31. doi:10.1016/j.neuroimage.2017.01.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, & Menon V (2008). Persistent default-mode network connectivity during light sedation. Hum Brain Mapp, 29(7), 839–847. doi:10.1002/hbm.20537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffa A, Baumann PS, Thiran JP, & Hagmann P (2013). Structural connectomics in brain diseases. Neuroimage, 80, 515–526. doi:10.1016/j.neuroimage.2013.04.056 [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Gerhard S, Grant PE, Wedeen V, … Sporns O (2010). MR connectomics: Principles and challenges. J Neurosci Methods, 194(1), 34–45. doi:10.1016/j.jneumeth.2010.01.014 [DOI] [PubMed] [Google Scholar]
- Hagmann P, Sporns O, Madan N, Cammoun L, Pienaar R, Wedeen VJ, … Grant PE (2010). White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci U S A, 107(44), 19067–19072. doi:10.1073/pnas.1009073107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AR, Smith MF, Rigby AS, Wallis LI, & Whitby EH (2010). Appearances of diffuse excessive high signal intensity (DEHSI) on MR imaging following preterm birth. Pediatric Radiology, 40(8), 1390–1396. doi:10.1007/s00247-010-1633-7 [DOI] [PubMed] [Google Scholar]
- He L, & Parikh NA (2015). Aberrant Executive and Frontoparietal Functional Connectivity in Very Preterm Infants With Diffuse White Matter Abnormalities. Pediatr Neurol, 53(4), 330–337. doi:10.1016/j.pediatrneurol.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Hebden JC, Gibson A, Austin T, Yusof RM, Everdell N, Delpy DT, … Wyatt JS (2004). Imaging changes in blood volume and oxygenation in the newborn infant brain using three-dimensional optical tomography. Physics in Medicine and Biology, 49(7), 1117–1130. doi:10.1088/0031-9155/49/7/003 [DOI] [PubMed] [Google Scholar]
- Hebden JC, Gibson A, Yusof RM, Everdell N, Hillman EM, Delpy DT, … Wyatt JS (2002). Three-dimensional optical tomography of the premature infant brain. Physics in Medicine and Biology, 47(23), 4155–4166. [DOI] [PubMed] [Google Scholar]
- Hintz SR, Benaron DA, Siegel AM, Zourabian A, Stevenson DK, & Boas D (2001). Bedside functional imaging of the premature infant brain durign passive motor activation. Journal PerinatalMedicine, 29, 335–343. [DOI] [PubMed] [Google Scholar]
- Holland D, Kuperman JM, & Dale AM (2010). Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. Neuroimage, 50(1), 175–183. doi:10.1016/j.neuroimage.2009.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, & Hagmann P (2009). Predicting human resting-state functional connectiivty from structural connectivity. PNAS, 106, 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shu N, Mishra V, Jeon T, Chalak L, Wang ZJ, … He Y (2015). Development of human brain structural networks through infancy and childhood. Cereb Cortex, 25(5), 1389–1404. doi:10.1093/cercor/bht335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppi PS, Murphy BP, Maier SE, Zientara GP, Inder TE, Barnes PD, … Volpe JJ (2001). Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics, 107, 455–460. [DOI] [PubMed] [Google Scholar]
- Inder T, Neil J, Yoder B, & Rees S (2005). Patterns of cerebral injury in a primate model of preterm birth and neonatal intensive care. J Child Neurol, 20(12), 965–967. [DOI] [PubMed] [Google Scholar]
- Jakab A, Schwartz E, Kasprian G, Gruber GM, Prayer D, Schopf V, & Langs G (2014). Fetal functional imaging portrays heterogeneous development of emerging human brain networks. Front Hum Neurosci, 8, 852. doi:10.3389/fnhum.2014.00852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzard P, & Balaban RS (1995). Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med, 34, 65–73. [DOI] [PubMed] [Google Scholar]
- Kalpakidou AK, Allin MP, Walshe M, Giampietro V, McGuire PK, Rifkin L, … Nosarti C (2014). Functional neuroanatomy of executive function after neonatal brain injury in adults who were born very preterm. PLoS One, 9(12), e113975. doi:10.1371/journal.pone.0113975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolis VR, Froudist-Walsh S, Brittain PJ, Kroll J, Ball G, Edwards AD, … Nosarti, (2016). Reinforcement of the Brain’s Rich-Club Architecture Following Early Neurodevelopmental Disruption Caused by Very Preterm Birth. Cereb Cortex, 26(3), 1322–1335. doi:10.1093/cercor/bhv305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khundrakpam BS, Reid A, Brauer J, Carbonell F, Lewis J, Ameis S, … Brain Development Cooperative, G. (2013). Developmental changes in organization of structural brain networks. Cereb Cortex, 23(9), 2072–2085. doi:10.1093/cercor/bhs187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro H, Anderson PJ, Doyle LW, Neil JJ, & Inder TE (2011). High Signal Intensity on T2-Weighted MR Imaging at Term-Equivalent Age in Preterm Infants Does Not Predict 2-Year Neurodevelopmental Outcomes. American Journal of Neuroradiology, 32(11), 2005–2010. doi:10.3174/ajnr.A2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Davis EP, Sandman CA, Sporns O, O’Donnell BF, Buss C, & Hetrick WP (2014). Longer gestation is associated with more efficient brain networks in preadolescent children. Neuroimage, 100, 619–627. doi:10.1016/j.neuroimage.2014.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Pae C, Lee JD, Park ES, Cho SR, Um MH, … Park HJ (2017). Analysis of structure-function network decoupling in the brain systems of spastic diplegic cerebral palsy. Hum Brain Mapp, 38(10), 5292–5306. doi:10.1002/hbm.23738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Park HJ, Park ES, Oh MK, Park B, Rha DW, … Park CI (2011). Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain, 134(Pt 4), 1199–1210. doi:10.1093/brain/awr021 [DOI] [PubMed] [Google Scholar]
- Lee W, Donner EJ, Nossin-Manor R, Whyte HE, Sled JG, & Taylor MJ (2012). Visual functional magnetic resonance imaging of preterm infants. Dev Med Child Neurol, 54(8), 724–729. doi:10.1111/j.1469-8749.2012.04342.x [DOI] [PubMed] [Google Scholar]
- Limbrick DD Jr., Mathur A, Johnston JM, Munro R, Sagar J, Inder T, … Smyth MD (2010). Neurosurgical treatment of progressive posthemorrhagic ventricular dilation in preterm infants: a 10-year single-institution study. J Neurosurg Pediatr, 6(3), 224–230. doi:10.3171/2010.5.peds1010. [DOI] [PubMed] [Google Scholar]
- Lin W, Zhu Q, Gao W, Chen Y, Toh CH, Styner M, … Gilmore JH (2008). Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am J Neuroradiol, 29(10), 1883–1889. doi:10.3174/ajnr.A1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, & Sorenson JA (1998). Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage, 7(2), 119–132. [DOI] [PubMed] [Google Scholar]
- Martinez-Biarge M, Diez-Sebastian J, Kapellou O, Gindner D, Allsop JM, Rutherford MA, & Cowan FM (2011). Predicting motor outcome and death in term hypoxic-ischemic encephalopathy. Neurology, 76, 2055–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Biarge M, Diez-Sebastian J, Rutherford MA, & Cowan FM (2010). Outcomes after central grey matter injury in term perinatal hypoxic-ischaemic encephalopathy. Early Hum Dev, 86(11), 675–682. doi:10.1016/j.earlhumdev.2010.08.013 [DOI] [PubMed] [Google Scholar]
- Massaro AN (2015). MRI for neurodevelopmental prognostication in the high-risk term infant. Semin Perinatol, 39(2), 159–167. doi:10.1053/j.semperi.2015.01.009 [DOI] [PubMed] [Google Scholar]
- Mathur AM, Neil JJ, McKinstry RC, & Inder TE (2008). Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol, 38(3), 260–264. [DOI] [PubMed] [Google Scholar]
- McCrea HJ, & Ment LR (2008). The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol, 35(4), 777–792, vii. doi:10.1016/j.clp.2008.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevel K, & Fransson P (2016). The functional brain connectome of the child and autism spectrum disorders. Acta Paediatr, 105(9), 1024–1035. doi:10.1111/apa.13484 [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HB, Kostovic I, & van Eden CG (1992). Prenatal development of neurons in the human prefrontal cortex. II. A quantitative Golgi study. J Comp Neurol, 316(4), 485–496. doi:10.1002/cne.903160408 [DOI] [PubMed] [Google Scholar]
- Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, … Volpe JJ (2002). Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed, 87(1), F37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers EH, Hampson M, Vohr B, Lacadie C, Frost SJ, Pugh KR, … Ment LR (2010). Functional connectivity to a right hemisphere language center in prematurely born adolescents. Neuroimage, 51(4), 1445–1452. doi:10.1016/j.neuroimage.2010.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil JJ (2008). Diffusion imaging concepts for clinicians. Journal of Magnetic Resonance Imaging, 27, 1–7. [DOI] [PubMed] [Google Scholar]
- Neonatal Encephalopathy and Neurologic Outcome, Second Edition. (2014). Pediatrics. doi:10.1542/peds.2014-0724 [DOI] [PubMed] [Google Scholar]
- O’Donnell LJ, & Westin CF (2011). An introduction to diffusion tensor image analysis. Neurosurg Clin N Am, 22(2), 185–196, viii. doi:10.1016/j.nec.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidvarnia A, Metsaranta M, Lano A, & Vanhatalo S (2015). Structural damage in early preterm brain changes the electric resting state networks. Neuroimage, 120, 266–273. doi:10.1016/j.neuroimage.2015.06.091 [DOI] [PubMed] [Google Scholar]
- Pandit AS, Robinson E, Aljabar P, Ball G, Gousias IS, Wang Z, … Edwards AD (2014). Whole-brain mapping of structural connectivity in infants reveals altered connection strength associated with growth and preterm birth. Cereb Cortex, 24(9), 2324–2333. doi:10.1093/cercor/bht086 [DOI] [PubMed] [Google Scholar]
- Papile LA, Burstein J, Burstein R, & Koffler H (1978). Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infnats with beirth weights less than 1,500 gm. J Pediatr, 92(4), 529–534. [DOI] [PubMed] [Google Scholar]
- Perani D, Saccuman MC, Scifo P, Anwander A, Spada D, Baldoli C, … Friederici AD (2011). Neural language networks at birth. Proc Natl Acad Sci U S A, 108(38), 16056–16061. doi:10.1073/pnas.1102991108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Kostovic I, & Uylings HB (2008). Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb Cortex, 18(4), 915–929. doi:10.1093/cercor/bhm124 [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, & Kostovic I (2011). Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A, 108(32), 13281–13286. doi:10.1073/pnas.1105108108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Park C, & Wang Z (2014). Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol Rev, 24(1), 3–15. doi:10.1007/s11065-014-9251-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, & Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59(3), 2142–2154. doi:10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, & Petersen SE (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage, 84, 320–341. doi:10.1016/j.neuroimage.2013.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, & Petersen SE (2014). Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. doi:10.1016/j.neuroimage.2014.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, & Petersen SE (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage, 105, 536–551. doi:10.1016/j.neuroimage.2014.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Moran JM, Mavros PL, Tager-Flusberg H, Gabrieli JD, & Whitfield-Gabrieli S (2013). Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Front Hum Neurosci, 7, 573. doi:10.3389/fnhum.2013.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin MJ, Wolraich D, Als H, McAnulty G, Butler S, Conneman N, … Mulkern RV (2004). Prolonged T*2 values in newborn versus adult brain: Implications for fMRI studies of newborns. Magn Reson Med, 51(6), 1287–1291. doi:10.1002/mrm.20098 [DOI] [PubMed] [Google Scholar]
- Rubinov M, & Sporns O (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage, 52(3), 1059–1069. doi:10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Schopf V, Schlegl T, Jakab A, Kasprian G, Woitek R, Prayer D, & Langs G (2014). The relationship between eye movement and vision develops before birth. Front Hum Neurosci, 8, 775. doi:10.3389/fnhum.2014.00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalak L, & Perlman JM (2004). Hypoxic-ischemic brain injury in the term infant-current concepts. Early Hum Dev, 80(2), 125–141. doi:10.1016/j.earlhumdev.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Sidman RL, & Rakic P (1982). Development of the human central nervous system Springfield: Thomas C. C. [Google Scholar]
- Siegel JS, Mitra A, Laumann TO, Seitzman BA, Raichle M, Corbetta M, & Snyder AZ (2017). Data Quality Influences Observed Links Between Functional Connectivity and Behavior. Cereb Cortex, 27(9), 4492–4502. doi:10.1093/cercor/bhw253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Cooper RJ, Wai Lee C, Dempsey L, Edwards A, Brigadoi S, … Austin T (2014). Mapping cortical haemodynamics during neonatal seizures using diffuse optical tomography: a case study. Neuroimage Clin, 5, 256–265. doi:10.1016/j.nicl.2014.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, … Beckmann CF (2009). Correspondence of the brain’s functional architecture during activation and rest. PNAS, 106(31), 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, …Behrens TE (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 31(4), 1487–1505. doi:10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Smith-Collins AP, Luyt K, Heep A, & Kauppinen RA (2015). High frequency functional brain networks in neonates revealed by rapid acquisition resting state fMRI. Hum Brain Mapp, 36(7), 2483–2494. doi:10.1002/hbm.22786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Dosenbach NU, Smyser TA, Snyder AZ, Rogers CE, Inder TE, … Neil JJ (2016). Prediction of brain maturity in infants using machine-learning algorithms. Neuroimage, 136, 1–9. doi:10.1016/j.neuroimage.2016.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, & Neil JJ (2010). Longitudinal analysis of neural network development in preterm infants. Cereb Cortex, 20(12), 2852–2862. doi:10.1093/cercor/bhq035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, & Neil JJ (2015). Use of resting-state functional MRI to study brain development and injury in neonates. Semin Perinatol, 39(2), 130–140. doi:10.1053/j.semperi.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Shimony JS, Blazey TM, Inder TE, & Neil JJ (2013). Effects of white matter injury on resting state fMRI measures in prematurely born infants. PLoS One, 8(7), e68098. doi:10.1371/journal.pone.0068098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, & Neil JJ (2014). Resting-State Network Complexity and Magnitude Are Reduced in Prematurely Born Infants. Cereb Cortex. doi:10.1093/cercor/bhu251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, & Neil JJ (2016). Resting-State Network Complexity and Magnitude Are Reduced in Prematurely Born Infants. Cereb Cortex, 26(1), 322–333. doi:10.1093/cercor/bhu251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser TA, Smyser CD, Rogers CE, Gillespie SK, Inder TE, & Neil JJ (2016). Cortical Gray and Adjacent White Matter Demonstrate Synchronous Maturation in Very Preterm Infants. Cereb Cortex, 26(8), 3370–3378. doi:10.1093/cercor/bhv164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Mishra V, Ouyang M, Peng Q, Slinger M, Liu S, & Huang H (2017). Human Fetal Brain Connectome: Structural Network Development from Middle Fetal Stage to Birth. Frontiers in Neuroscience, 11(561). doi:10.3389/fnins.2017.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souweidane MM, Kim KH, McDowall R, Ruge MI, Lis E, Krol G, & Hirsch J (1999). Brain mapping in sedated infants and young children with passive-functional magnetic resonance imaging. Pediatr Neurosurg, 30(2), 86–92. doi:pne30086 [pii] [DOI] [PubMed] [Google Scholar]
- Sporns O, Tononi G, & Kotter R (2005). The human connectome: A structural description of the human brain. PLoS Comput Biol, 1(4), e42. doi:10.1371/journal.pcbi.0010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis EA, Adapa RM, Absalom AR, & Menon DK (2010). Changes in resting neural connectivity during propofol sedation. PLoS One, 5(12), e14224. doi:10.1371/journal.pone.0014224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, … Human Development Neonatal Research, N. (2015). Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA, 314(10), 1039–1051. doi:10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Brown JA, Dassanayake MT, Shastri R, Marusak HA, Hernandez-Andrade E, … Romero R (2014). Intrinsic functional brain architecture derived from graph theoretical analysis in the human fetus. PLoS One, 9(5), e94423. doi:10.1371/journal.pone.0094423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Grove LE, Lozon TA Jr., Vila AM, Ye Y, Nye MJ, … Romero R (2015). Age-related increases in long-range connectivity in fetal functional neural connectivity networks in utero. Dev Cogn Neurosci, 11, 96–104. doi:10.1016/j.dcn.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Scheinost D, Manning JH, Grove LE, Hect J, Marshall N, … Romero R (2017). Weak functional connectivity in the human fetal brain prior to preterm birth. Sci Rep, 7, 39286. doi:10.1038/srep39286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusor N (2014). Diffusion tensor imaging and resting state functional connectivity as advanced imaging biomarkers of outcome in infants with hypoxic-ischaemic encephalopathy treated with hypothermia. (PhD), Imperial College London. [Google Scholar]
- Tymofiyeva O, Hess CP, Ziv E, Lee PN, Glass HC, Ferriero DM, … Xu D (2013). A DTI-based template-free cortical connectome study of brain maturation. PLoS One, 8(5), e63310. doi:10.1371/journal.pone.0063310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymofiyeva O, Hess CP, Ziv E, Tian N, Bonifacio SL, McQuillen PS, … Xu D (2012). Towards the “baby connectome”: mapping the structural connectivity of the newborn brain. PLoS One, 7(2), e31029. doi:10.1371/journal.pone.0031029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K, Xu J, Auerbach EJ, Moeller S, Vu AT, Duarte-Carvajalino JM, … Consortium WU-MH (2013). Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. Neuroimage, 80, 80–104. doi:10.1016/j.neuroimage.2013.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Kersbergen KJ, de Reus MA, Keunen K, Kahn RS, Groenendaal F, … Benders MJ (2015). The Neonatal Connectome During Preterm Brain Development. Cereb Cortex, 25(9), 3000–3013. doi:10.1093/cercor/bhu095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, & Sporns O (2011). Rich-club organization of the human connectome. J Neurosci, 31(44), 15775–15786. doi:10.1523/JNEUROSCI.3539-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, & Sporns O (2013). Network hubs in the human brain. Trends Cogn Sci, 17(12), 683–696. doi:10.1016/j.tics.2013.09.012 [DOI] [PubMed] [Google Scholar]
- van Kooij BJM, de Vries LS, Ball G, van Haastert IC, Benders MJNL, Groenendaal F, & Counsell SJ (2012). Neonatal Tract-Based Spatial Statistics Findings and Outcome in Preterm Infants. American Journal of Neuroradiology, 33(1), 188–194. doi:10.3174/ajnr.A2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhatalo S, Alnajjar A, Nguyen VT, Colditz P, & Fransson P (2014). Safety of EEG-fMRI recordings in newborn infants at 3T: a study using a baby-size phantom. Clin Neurophysiol, 125(5), 941–946. doi:10.1016/j.clinph.2013.09.041 [DOI] [PubMed] [Google Scholar]
- Vassilyadi M, Tataryn Z, Shamji MF, & Ventureyra EC (2009). Functional outcomes among premature infants with intraventricular hemorrhage. Pediatr Neurosurg, 45(4), 247–255. doi:10.1159/000228982. Epub 2009 Jul 17. [DOI] [PubMed] [Google Scholar]
- Vertes PE, & Bullmore ET (2015). Annual research review: Growth connectomics--the organization and reorganization of brain networks during normal and abnormal development. J Child Psychol Psychiatry, 56(3), 299–320. doi:10.1111/jcpp.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, …Raichle ME (2007). Intrinsic functional architecture in the anaesthetized monkey brain. Nature, 447(7140), 83–86. doi:10.1038/nature05758 [DOI] [PubMed] [Google Scholar]
- Volpe JJ (2001). Neurology of the newborn (4th ed.). Philadelphia: W.B. Saunders Co. [Google Scholar]
- Volpe JJ (2009). Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. The Lancet Neurology, 8(1), 110–124. doi:10.1016/s1474-4422(08)70294-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ (2017). Confusions in Nomenclature: “Periventricular Leukomalacia” and “White Matter Injury”-Identical, Distinct, or Overlapping? Pediatr Neurol, 73, 3–6. doi:10.1016/j.pediatrneurol.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Volpe JJ, Kinney HC, Jensen FE, & Rosenberg PA (2011). Reprint of “The developing oligodendrocyte: key cellular target in brain injury in the premature infant”. Int J Dev Neurosci, 29(6), 565–582. doi:10.1016/j.ijdevneu.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Watts DJ, & Strogatz SH (1998). Collective dynamics of ‘small-world’ networks. Nature, 393, 440–442. [DOI] [PubMed] [Google Scholar]
- White BR, Liao SM, Ferradal SL, Inder TE, & Culver JP (2012). Bedside optical imaging of occipital resting-state functional connectivity in neonates. Neuroimage, 59(3), 2529–2538. doi:10.1016/j.neuroimage.2011.08.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TP, Symington I, Castellanos NP, Brittain PJ, Froudist Walsh S, Nam KW, … Nosarti C (2014). Dysconnectivity of neurocognitive networks at rest in very-preterm born adults. Neuroimage Clin, 4, 352–365. doi:10.1016/j.nicl.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert JR, Sinclair RJ, Dixit S, Damiano DL, & Burton H (2010). Somatosensory-evoked cortical activity in spastic diplegic cerebral palsy. Hum Brain Mapp, 31(11), 1772–1785. doi:10.1002/hbm.20977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Sadato N, Konishi Y, Kimura K, Tanaka M, Yonekura Y, & Ishii Y (1997). A rapid brain metabolic change in infants detected by fMRI. NeuroReport, 8(17), 3775–3778. [DOI] [PubMed] [Google Scholar]
- Yap P-T, Fan Y, Chen Y, Gilmore JH, Lin W, & Shen D (2011). Development Trends of White Matter Connectivity in the First Years of Life. PLoS One, 6(9), e24678. doi:10.1371/journal.pone.0024678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye AX, AuCoin-Power M, Taylor MJ, & Doesburg SM (2016). Disconnected neuromagnetic networks in children born very preterm: Disconnected MEG networks in preterm children. Neuroimage Clin, 11, 376–384. doi:10.1016/j.nicl.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, & Mondragon RJ (2004). The Rich-Club Phenomenon in the Internet Topology. IEEE Communications Letters, 8(3), 180–182. doi:10.1109/lcomm.2004.823426 [Google Scholar]