Thiopurines are key to the treatment of acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL) in children and adults, and 6-mercaptopurine (6-MP) is commonly used in consolidation and maintenance therapy1–3. Leukopenia is a dose-limiting toxicity of 6-MP partly explained by hypomorphic variants of TPMT2–5 and clinical importance of TMPT genotyping is well established. Recently, NUDT15 was identified as a novel thiopurine regulator conferring 6-MP sensitivity most prominently in Asians and Hispanics4–9. Patients with bi-allelic NUDT15 variants are extremely sensitive to 6-MP, and only 5–10% of the standard dose is sufficient to maintain the target leukocyte count4,8.
Thus far, a total of seven variants in NUDT15 with low diphosphatase activity resulting in excess myelosuppression by 6-MP have been identified4,10,11 and haplotypes with different combinations of variants are known to exist (Figure 1A). Given the clinical importance of NUDT15 genotyping for individualized dosing of 6-MP6,12, the diplotype should be precisely determined, especially for those with heterozygous genotype at multiple variants. For example, a case with heterozygous c.36_37insGGAGTC and c.415C>T, which is the most frequent combination4, should be determined as compound heterozygosity (*3/*6) or mono-allelic variants (*1/*2), because the two diplotypes have significantly different impacts on total NUDT15 activity4.
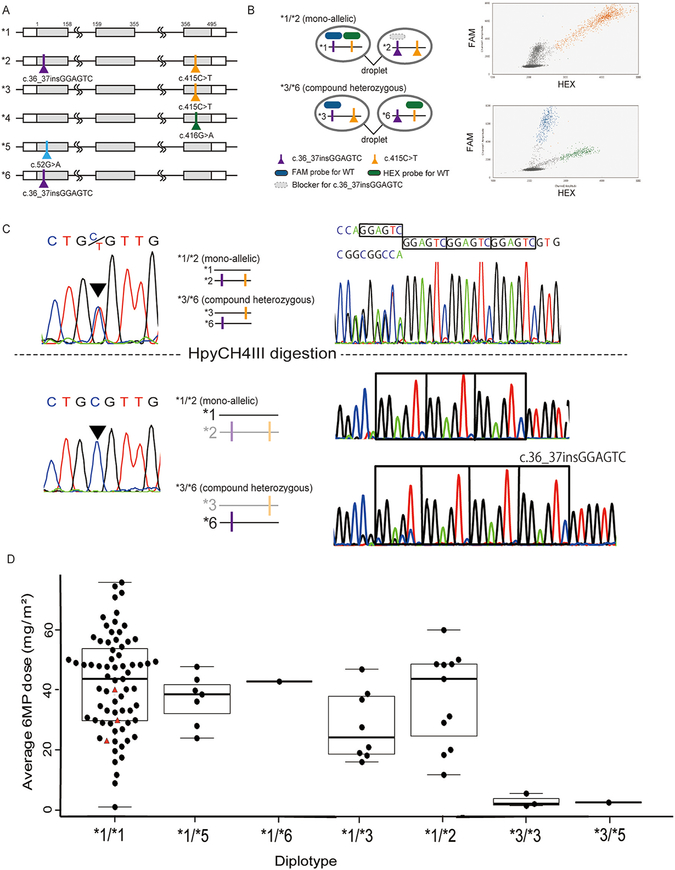
Figure 1. Diplotyping of NUDT15 variants and tolerated 6-MP dose.
(A) Major haplotypes of NUDT15. (B) Diplotype analysis by droplet digital PCR. Variant-specific probes (FAM for c.36_37insGGAGTC and HEX for c.415C>T) were able to distinguish compound heterozygous variants (*3/*6) and mono-allelic variants (*1/*2). (C) Diplotype analysis by restriction enzyme-PCR. They were cleaved with HpyCH4III and PCR was performed to confirm the results of ddPCR. (D) Average 6MP doses according to diplotype. Three cases without a NUDT15 variant (*1/*1) had a TPMT heterozygous variant (c.719A>G), shown in red triangles.
However, a convenient method to determine the diplotype of NUDT15 has not been established, and as shown in the previous study, the diplotype is currently inferred using a public catalogue of human variants4. Herein we demonstrated a method for identifying the diplotype using droplet digital PCR (ddPCR), and confirmed the results by wild-type specific PCR by a restriction enzyme.
In total 138 Japanese children with ALL or LBL were enrolled (Supplementary Table 1). Patients were treated at the National Center for Child Health and Development, the University of Tokyo Hospital, the Hirosaki University Hospital or the Saitama Children’s Medical Center. For maintenance therapy, the 6-MP and MTX dosages were adjusted to maintain a leukocyte count of 1,500–3,000/μl by at least monthly blood tests. In this study, a tolerable 6-MP and MTX dose was defined as the average of the doses per day (per week for MTX) administered during the first 6 months of maintenance therapy. When maintenance therapy was interrupted during the 6 months and 6-MP/MTX was held, the period was calculated as 0 mg/m2.
This study was approved by the institutional ethics board of the National Center for Child Health and Development (#1035), and informed consent was obtained from the patients or guardians.
Germline DNA and RNA were extracted from peripheral blood during remission. We genotyped the seven known variants of NUDT15 loci (c.36_37insGGAGTC, c.37_42delGGAGTC, c.52G>A, c.101G>C, c.103A>G, c.415C>T, and c.416G>A) by Sanger sequencing, as reported previously4,11. For all cases, TMPT variants were also genotyped, for c.280G>C (rs1800462), c.460G>A (rs1800460), and c.719A>G (rs1142345). The primer sequences are listed in Supplementary Table 2.
The diplotype of each case was assessed by ddPCR utilizing characteristics of droplets that could separate template DNA molecules13,14. PCR reaction using fluorescent probes specific to the variants was performed for each droplet to determine the allelic configuration of NUDT15 (Figure 1B).
The results of diplotyping were validated by restriction enzyme-PCR (RE-PCR). The synthesized cDNA was digested by HpyCH4~, which specifically digests the c.415C>T allele. PCR was performed using the digested cDNA as a template, followed by analysis with capillary sequencing to provide a wild-type allele-specific nucleotide sequence for cases with heterozygous c.415C>T (Figure 1C).
The genomic NUDT15 was subcloned into pUC19 cloning vector, and the diplotyping results were also confirmed by Sanger sequencing of the vectors.
The details of NUDT15 diplotyping and the primer sequences are shown in the Supplemental Method section.
The genotyping results are shown in Supplementary Table 1. Thirty-eight (27.5%) cases had one or more variants in the NUDT15 gene, and three cases (2%) had a homozygous c.415C>T variant. Twenty cases (14.4%) had one heterozygous variant of c.36_37insGGAGTC (n = 1), c.52G>A (n = 7) or c.415C>T (n = 12). Fifteen cases were heterozygous at two variants, 14 patients had c.36_37insGGAGTC and c.415C>T, and one case had c.52G>A and c.415C>T. Three cases had TPMT heterozygous variants of c.719A>G, whose NUDT15 was wild-type.
Because c.415C>T and c.36_37insGGAGTC can be located either on the same allele (i.e., *1/*2) or on different alleles (*3/*6), we sought to define diplotype of the 14 patients carrying the two variants (i.e., c.415C>T and c.36_37insGGAGTC). As confirmed that both ddPCR and RE-PCR were capable of detecting all the combinations with or without c.36_37insGGAGTC or c.415C>T (*1, *2, *3, and *6) using an artificial mixture of subcloned vectors (Figure 1B and 1C), these two methods consistently demonstrated that c.36_37insGGAGTC exists on the same allele of c.415C>T in all of the 14 cases, plausibly determining their diplotypes as *1/*2. Considering the allele frequencies of *2, *3 and *6 in our cohort (5.1%, 6.9% and 0.4%, respectively), there is still a possibility to encounter a patient showing compound heterozygosity (i.e., *3/*6) who is at higher risk for excessive 6-MP toxicity compared to patients with *1/*2. Thus, our methods can be informative to distinguish *1/*2 from *3/*6, and eventually mitigate 6-MP toxicity.
We were unable to validate the diplotyping of the case with the two heterozygous variants of c.52G>A and c.415C>T by either ddPCR or RE-PCR, although a reason for this failure is still unclear. We then cloned the whole genomic region of NUDT15 to the TA-cloning pUC19 vector confirming that the two variants were located on different alleles with compound heterozygotes for *3/*5 (Supplementary Figure 1A and 1B).
Based on these results, the allele frequency for one or more hypomorphic variants of NUDT15 in our cohort was estimated at 15.2% (Table 1). We next evaluated the average dose of 6-MP required to maintain the target leukocyte count in 103 patients receiving maintenance therapy for more than 6 months. The median of the tolerated dose in all cases was 39.4 mg/m2 (1.1–75.5 mg/m2). The tolerated 6-MP doses were extremely low among the three patients with homozygous c.415C>T variants (1.4, 2.0, and 5.6 mg/m2), and the patient with compound heterozygous variants required significant reduction of 6-MP (2.5 mg/m2). On the other hand, even though 14 cases had two heterozygous variants (c.36_37insGGAGTC and c.415C>T), they were able to tolerate a significantly higher dose of 6-MP than those with bi-allelic variants because the two variants were located on the same allele (Figure 1D).
Table 1.
Distribution of diplotypes of NUDT15
| NUDT15 diplotype | Number of patients |
|---|---|
| No variant | |
| *1/*1 | 100 (72.5) |
| Mono-allelic variants | |
| *1/*2 | 14 (10.1) |
| *1/*3 | 12 (8.7) |
| *1/*5 | 7 (5.1) |
| *1/*6 | 1 (0.7) |
| Bi-allelic variants | |
| *3/*3 | 3 (2.2) |
| *3/*5 | 1 (0.7) |
Diplotypes of the 138 cases determined in our study are shown.
Using a combination of methods (e.g., ddPCR, TA cloning), we were able to resolve NUDT15 haplotype in 100% of the cases in this cohort. As all four variants detected in our cohort were previously reported to have lower enzymatic activity of NUDT15 at the same degree, we classified the children into three groups based on diplotype: the normal activity (normal/normal), intermediate activity (normal/low), and low activity (low/low) groups. Sensitivity of the low activity group (n = 4) was significantly higher than that of the intermediate activity cases (n = 27) and the normal activity cases (n = 72), and the median tolerable dosage was 2.3 mg/m2, 36.7 mg/m2, and 43.5 mg/m2, respectively (Supplementary Figure 2A).
Even though we reproduced high sensitivity to 6-MP is conferred by the NUDT15 variants, the tolerable dose for Asian cases without the NUDT15 variants were significantly low, and it was concordant to the previous reports4,7,8. Especially, 25% of cases had sensitive to 6-MP (~30 mg/m2) and some cases showed extremely high sensitivity (<5 mg/m2) even without having known variants of either TPMT or NUDT15. Extremely sensitive cases are also found in non-Asian population. More comprehensive approaches including whole genome sequencing should be done to identify potential novel variants responsible for high sensitivity to 6-MP.
Patients with low NUDT15 activity are able to tolerate the standard dose of MTX (Supplementary Figure 2B), and Asian population can tolerate approximately 20 mg/m2, which is almost identical to that seen in North American patients. Therefore, preemptive genotyping of NUDT15 can help to avoid not only excess myelosuppression and serious complications such as infection, but also any unnecessary reduction in concomitant MTX during maintenance therapy.
Our study has some limitations. First, the number of cases is still small to identify optimal doses for each allelic genotype including rare variants and diplotypes, and to find statistical difference between intermediate activity cases and normal activity cases. Second, in this study, we only included newly diagnosed cases, and it should be noted that relapsed patients would likely not tolerate comparable doses of 6-MP. Further studies with larger cohort are required to solve these issues.
In conclusion, we established a novel method of NUDT15 diplotype analysis by ddPCR which was validated by allele-specific sequencing. Thiopurines are used not only hematologic malignancies, but also auto-immune diseases such as inflammatory bowel disease, thus clinical importance of NUDT15 genotyping is widely recognized15. The diplotype information for NUDT15 is essential for predicting 6-MP sensitivity, and its possible clinical applications should be considered.
Supplementary Material
Acknowledgements
The authors would like to thank Ms. Natsuko Ariizumi, Ms. Shinobu Kobayashi, and Ms. Yoshie Fukumasa for their technical assistance. We also thank Mr. Yukinori Yatsuda (Bio-rad) for his assistance with the ddPCR analysis. The authors thank Mr. James R. Valera of the Department of Education for Clinical Research of the National Center for Child Health and Development for his assistance in proofreading and editing this manuscript.
This study was supported in part by the Japan Society for the Promotion of Science (JSPS) through a Grant-in-Aid for Scientific Research (KAKENHI, grant number 17H04234), by a grant from the National Center for Child Health and Development (grant numbers 28–5), by grant 17km0405107h0005, 17ck0106253h0001, and 17ck0106219h0002 from the Japan Agency for Medical Research and development, (AMED), and by foundation for National Institutes of Health (GM118578). T.M is supported by the Garwood Fellowship at St. Jude Children’s Research Hospital.
References
- 1.Kato M, Ishimaru S, Seki M, Yoshida K, Shiraishi Y, Chiba K et al. Long-term outcome of 6-month maintenance chemotherapy for acute lymphoblastic leukemia in children. Leukemia 2017; 31: 580–584. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen SN, Grell K, Nersting J, Abrahamsson J, Lund B, Kanerva J et al. DNA-thioguanine nucleotide concentration and relapse-free survival during maintenance therapy of childhood acute lymphoblastic leukaemia (NOPHO ALL2008): a prospective substudy of a phase 3 trial. Lancet Oncol 2017; 18: 515–524. [DOI] [PubMed] [Google Scholar]
- 3.Kato M & Manabe A Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatrics international : official journal of the Japan Pediatric Society 2018; 60: 4–12. [DOI] [PubMed] [Google Scholar]
- 4.Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet 2016; 48: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zgheib NK, Akika R, Mahfouz R, Aridi CA, Ghanem KM, Saab R et al. NUDT15 and TPMT genetic polymorphisms are related to 6-mercaptopurine intolerance in children treated for acute lymphoblastic leukemia at the Children’s Cancer Center of Lebanon. Pediatr Blood Cancer 2017; 64: 146–150. [DOI] [PubMed] [Google Scholar]
- 6.Soler AM, Olano N, Mendez Y, Lopes A, Silveira A, Dabezies A et al. TPMT and NUDT15 genes are both related to mercaptopurine intolerance in acute lymphoblastic leukaemia patients from Uruguay. Br J Haematol 2017. [DOI] [PubMed] [Google Scholar]
- 7.Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol 2015; 33: 1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka Y, Kato M, Hasegawa D, Urayama KY, Nakadate H, Kondoh K et al. Susceptibility to 6-MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br J Haematol 2015; 171: 109–115. [DOI] [PubMed] [Google Scholar]
- 9.Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet 2014; 46: 1017–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moriyama T, Nishii R, Lin TN, Kihira K, Toyoda H, Jacob N et al. The effects of inherited NUDT15 polymorphisms on thiopurine active metabolites in Japanese children with acute lymphoblastic leukemia. Pharmacogenet Genomics 2017; 27: 236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moriyama T, Yang YL, Nishii R, Ariffin H, Liu C, Lin TN et al. Novel variants in NUDT15 and thiopurine intolerance in children with acute lymphoblastic leukemia from diverse ancestry. Blood 2017; 130: 1209–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang DC, Yang CP, Liu HC, Jaing TH, Chen SH, Hung IJ et al. NUDT15 gene polymorphism related to mercaptopurine intolerance in Taiwan Chinese children with acute lymphoblastic leukemia. Pharmacogenomics J 2016; 16: 536–539. [DOI] [PubMed] [Google Scholar]
- 13.Regan JF, Kamitaki N, Legler T, Cooper S, Klitgord N, Karlin-Neumann G et al. A rapid molecular approach for chromosomal phasing. PloS one 2015; 10: e0118270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hidaka N, Iwama E, Kubo N, Harada T, Miyawaki K, Tanaka K et al. Most T790M mutations are present on the same EGFR allele as activating mutations in patients with non-small cell lung cancer. Lung Cancer 2017; 108: 75–82. [DOI] [PubMed] [Google Scholar]
- 15.Chao K, Wang X, Cao Q, Qian J, Wu K, Zhu X et al. Combined Detection of NUDT15 Variants Could Highly Predict Thiopurine-induced Leukopenia in Chinese Patients with Inflammatory Bowel Disease: A Multicenter Analysis. Inflamm Bowel Dis 2017; 23: 1592–1599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.