Abstract
Purpose
To report the findings of a cross-sectional study of visual function in infants with confirmed or suspected antenatal Zika virus (ZIKV) infection seen at a single referral center in Rio de Janeiro.
Methods
Infants were examined following the ZIKV outbreak period at Fernandes Figueira Institute/FIOCRUZ. Visual function was considered abnormal if an infant could not fix and follow a standardized high-contrast target (10 cm) by 3–6 months of age. Visual function and associations with structural eye abnormalities, central nervous system (CNS) abnormalities, microcephaly, and nystagmus were assessed. Sensitivity and specificity of screening criteria for structural eye abnormalities was assessed.
Results
A total of 173 infants met inclusion criteria. Reduced visual function was found in 52 infants (30.0%) and was significantly associated with eye abnormalities (40/52; OR = 44.2; 95% CI, 16.6–117.6), CNS abnormalities (50/52; OR = 64.0; 95% CI, 14.7–277.6), microcephaly (44/52; OR = 31.5; 95% CI,12.7–77.8), and nystagmus (26/52; OR = 120.0; 95% CI, 15.6–924.5). Using microcephaly as screening criteria for the detection of eye abnormalities provided a sensitivity of 88.9% (95% CI,76.0–96.3) and specificity of 82.8% (95% CI, 75.1–88.9). Using both abnormal visual function and microcephaly increased sensitivity to 100% (95% CI, 92.1–100.0) and decreased specificity to 80.5% (95% CI, 72.5–86.9).
Conclusions
Infants with suspected antenatal ZIKV infection and reduced visual function should be referred to an ophthalmologist. Visual function assessments are helpful in screening for antenatal ZIKV exposure in resource-limited settings and can identify infants who may benefit from visual habilitation.
The Brazilian Zika virus (ZIKV) outbreak of 2015 drew attention to difficulties in ZIKV diagnostic testing and the effects of the infection on the developing fetus. Despite the fact that ZIKV infection in adults is often asymptomatic or mild, exposure to the virus during pregnancy can lead to devastating effects in infants.1,2 Congenital Zika syndrome is an extreme manifestation of in utero ZIKV infection, encompassing microcephaly and other neurologic and ocular manifestations of the disease.3 However, infants who are seemingly unaffected at birth can later manifest developmental abnormalities and findings on brain imaging, including secondary microcephaly.4 Furthermore, eye abnormalities can be present without microcephaly or other central nervous system (CNS) abnormalities.5
Historically, congenital infectious diseases such as toxoplasmosis and rubella have been important causes of reduced visual function in childhood, particularly in low- and middle-income economies.6 Congenital Zika syndrome is an additional cause of reduced visual function in childhood.7,8 Even in infants with developmental delay, simplified methods of assessing reduced visual function with fix and follow visual function assessments are practical and universally available. Early identification of reduced visual function may profoundly affect early childhood cognitive development and later school performance. The aim of the current study was to assess early visual function in infants with confirmed or suspected antenatal exposure to ZIKV infection during the Rio de Janeiro Zika outbreak of 2015–16.
Subjects and Methods
This cross-sectional study was performed at the Fernandes Figueira Institute (IFF), Oswaldo Cruz Foundation, Rio de Janeiro, Brazil, a Brazilian Ministry of Health referral center for fetal medicine, congenital anomalies, and pediatric infectious diseases. IFF and UCLA Institutional Review Board approvals were obtained, and parents or guardians provided written informed consent. This study adhered to the tents of the Declaration of Helsinki and was complied with requirements of the US Health Insurance Portability and Accountability Act of 1996. The study population was drawn in part from a cohort registered in ClinicalTrials.gov (NCT 03255369). Pregnant women or infants with suspected antenatal ZIKV infection were referred by several services, including the Acute-Febrile Illness Service of the National Institute of Infectious Diseases (INI-FIOCRUZ), a regional reference site for arboviral infections,2 Infectious Disease Departments and General Pediatric services across the city and state of Rio de Janeiro, and other governmental and private institutions in the geographic catchment area.
The study population consisted of infants referred for suspected ZIKV infection based on any of the following criteria: (1) positive RT-PCR for ZIKV during pregnancy or infancy; (2) prenatal ultrasound findings suspicious for ZIKV infection; or (3) born with clinical manifestations suggestive of congenital ZIKV infection.3 All infants who underwent an eye examination between 3–6 months of age were included. Mother-infant pairs with other serologically diagnosed perinatal infections, genetic disorders, family history of microcephaly, perinatal alcohol abuse, or illicit drug exposures were excluded.
Laboratory confirmation of ZIKV infection with RT-PCR was performed as previously described.5,9 For symptomatic pregnant women, the timing of maternal ZIKV infection was defined as the week of gestation coincident with symptom onset (first trimester, <14 weeks; second trimester, 14–25 weeks; third trimester, ≥26 weeks). Infants were evaluated from January 2016 to August 2017. Detailed demographic, medical, prenatal history information, and clinical findings were documented by pediatric infectious disease specialists. Preterm birth was defined as gestational age of <37 weeks. Microcephaly was defined as head circumference of <3 percentile for gestational age and gender. Neuroimaging was performed to evaluate CNS abnormalities and included transfontanelle ultrasounds, computerized tomography, or magnetic resonance imaging, as clinically indicated. Other CNS abnormalities associated with ZIKV included intracranial calcifications, ventriculomegaly, cerebellar anomalies, and marked cortical thinning with abnormal gyral patterns.10
All infants underwent comprehensive eye evaluation by pediatric ophthalmologists at birth or at presentation and every 3 months thereafter. Evaluation included fix-and-follow visual function, portable slit-lamp examination (KOWA Ophthalmic and Medical Equipment, Torrance, CA), ocular motility testing, cycloplegic refraction with retinoscopy (reported as spherical equivalent), and dilated fundus examination using indirect ophthalmoscopy (Keeler Ophthalmic Instruments, Malvern, PA). Visual function was considered abnormal if an infant could not fix and follow a standardized high-contrast target (10 cm) by 3–6 months of age.11 Strabismus (esotropia/exotropia) or nystagmus (presence or absence) was recorded. Optic nerve hypoplasia, optic nerve pallor, chorioretinal atrophy, and retinal pigment epithelium mottling were considered typical eye abnormalities associated with ZIKV infection.5,12–14 Posterior pole findings were documented using digital fundus imaging (RetCam, Natus Medical Incorporated, Pleasanton, CA).
Statistical Analysis
We evaluated potential associations between visual function, nystagmus and eye abnormalities, microcephaly, and other CNS abnormalities using the χ2test. All P values were two-sided and considered statistically significant if <0.05. The analysis was performed using Stata 14 (StataCorp, LP, College Station, TX). Sensitivity and specificity of using various screening criteria for the detection of structural eye abnormalities was calculated with 95% confidence intervals in Excel (Microsoft, Redwood, WA).
Results
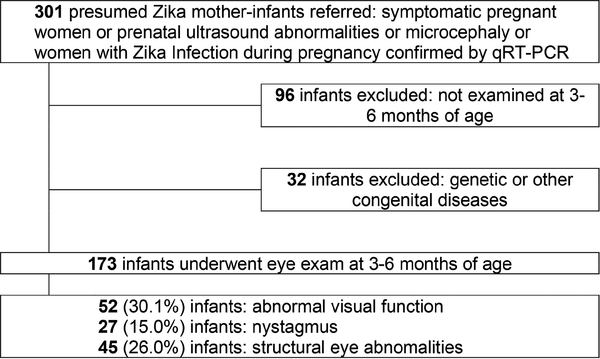
A total of 301 infants were referred to our institution for the following indications: prenatal suspicion for microcephaly, presence of other stigmata of congenital ZIKV infection, or because they were born to mothers with confirmed ZIKV infection during pregnancy as of October 1, 2015 (eFigure 1). Of these, 32 infants with genetic abnormalities or other infections and 96 who were not examined between 3 and 6 months of age were excluded. Of the remaining 173 mother-infant pairs, 117 (67.3%) had RT-PCR confirmation of infection in either mother or infant. The remaining 56 infants (32.3%) were presumed to have been exposed to ZIKV infection in utero based on maternal prenatal symptoms, prenatal ultrasound abnormalities, and/or birth defects with exclusion of genetic causes or other congenital infections. Maternal symptoms of infection during pregnancy were reported in 152 mothers (87.8%): 67 (44%) in the first trimester, 61 (40%) in the second trimester, and 24 (16%) in the third trimester.
eFIG 1.
Flowchart showing derivation of the study cohort and major visual function and eye findings.
Of the 173 infants, 91 (52%) were female. Median gestational age at birth was 39 weeks (interquartile range [IQR], 38–40 weeks): 145 infants (83.8%) were born full-term, 28 (16.2%) were born preterm. Six preterm infants could not fix and follow, and 3 had nystagmus. Median birth weight was 3045 g (IQR, 2605–3400 g).
A total of 85 of the 173 (49.1%) had abnormal CNS findings. Microcephaly was present in 62 infants (35.8%), and other CNS abnormalities were detected in 23 (13.3%) without microcephaly. There were no cases of isolated microcephaly in the absence of other CNS findings (Table 1).
Table 1.
Correlation of visual function to structural findings and nystagmus
| Structural findings | Total no. (%) [n = 173] | Fix-and-follow visual function |
|||
|---|---|---|---|---|---|
| Unable, no. (%) [n = 52] | Able, no.(%) [n = 121] | OR (95% CI) | P value | ||
| Eye abnormality | 45 (26) | 38 (73) | 7 (6) | 44.2 (16.6–117.6) | <0.0001 |
| Any CNS abnormality | 84 (49) | 50 (96) | 34 (28) | 64.0 (14.7–277.6) | <0.0001 |
| Microcephaly | 62 (36) | 44 (85) | 18 (15) | 31.5 (12.7–77.8) | <0.0001 |
| Nystagmus | 27 (16) | 26(50) | 1 (0.8) | 120.0 (15.6–924.5) | <0.0001 |
| None of the above | 87 (50) | 0 (0) | 87 (72) | 1 | - |
CI, confidence interval; CNS, central nervous system, OR, odds ratio.
Visual function (ability to fix and follow) was assessed in all infants at 3–6 months of age. Of the 173 infants, 52 (30.0%) had abnormal visual function; 4 (2.3%) had anterior segment eye abnormalities associated with ZIKV (eg, microphthalmia, iris coloboma, and microcornea); and 27 (15.6%) had nystagmus, in 26 (96%) of whom abnormal visual function was detected.
Ocular motility testing at near revealed exotropia in 2 of 173 infants (1.2%) and esotropia in 22 (12.7%). Of the 173 infants, 125 (72.5%) were hyperopic (range, 0.5–6.0 D; median, 2.00 D; IQR, 1.00–2.50); 25 (14.5%), emmetropic; and 6 (3.5%), myopic (range, −0.5 to 16.0 D; median, −3.50 D; IQR, −1.00 to −9.00 D). Poor cooperation or structural eye abnormalities hindered retinoscopy in 17 infants (10%). Of those who could be examined, 45 (26.0%) presented typical ZIKV eye abnormalities on dilated fundus examination; 12 (6.9%) had optic nerve abnormalities; 7 (4.1%) had retinal abnormalities; and 26 (15.0%) had both optic nerve and retinal findings.
Of 52 children with poor visual function, 50 (96%) had significant CNS findings. Of those, 44 (84.6%) had microcephaly. Structural eye abnormalities were present in 40 infants (76.9%) with abnormal visual function; 14 additional infants with abnormal visual function had CNS disease but no structural eye defects. Of 111 infants without microcephaly, 5 (4.5%) had structural eye abnormalities, and none of the 5 (4.5%) were able to fix and follow. Correlation of visual function with structural findings and nystagmus is shown in Table 1. Sensitivity and specificity of various screening criteria for the detection of structural eye abnormalities are provided in Table 2.
Table 2.
Sensitivity and specificity of various screening criteria for the detection of structural eye abnormalities
| Abnormality | Sensitivity | 95% CI | Specificity | 95% CI | ROC |
|---|---|---|---|---|---|
| Microcephaly | 88.9 | 76.0–96.3 | 82.8 | 75.1–88.9 | 0.859 |
| Visual function | 84.4 | 70.5–93.5 | 89.1 | 82.3–93.9 | 0.864 |
| Either | 100 | 92.1–100.0 | 80.5 | 72.5–86.9 | 0.902 |
CI, confidence interval.
Discussion
Eye manifestations in congenital ZIKV infection were first reported in January 2016 by Ventura and colleagues15 in a series of 3 infants with microcephaly. Since then, other reports have confirmed that primary eye findings include macular atrophy, retinal pigment epithelium mottling, optic nerve atrophy, and hypoplasia.5,12–14,16–22 In addition, abnormal visual function has been reported in infants infected with ZIKV and neurological abnormalities.23 In this study we were able to investigate visual function in an expanded cohort of less severely affected infants with either symptomatic pregnancies, PCR confirmation of infection, or prenatal ultrasound abnormalities.
In our previous evaluation of 112 infants with PCR-confirmed ZIKV infection, we found that structural eye abnormalities may be the only initial clinical presentation of antenatal infection and, therefore, recommended that all infants with potential antenatal ZIKV exposure undergo an eye examination. The current study included infants with suspected ZIKV infection based on prenatal ultrasound or congenital birth defects in order to assess early visual function in all infants presumably exposed to antenatal infection at our institution.
We found that 30% of infants were unable to fix and follow at 3–6 months of age, keeping in mind that approximately half of the cohort had significant CNS findings overall. Fix-and-follow as a primary outcome was readily accessible and relatively easy to test compared with other methods of assessing vision in infants, such as preferential looking tests, which require extensive training and are time consuming.24 The combined policies from the American Association of Pediatrics, the American Association for Pediatric Ophthalmology and Strabismus, the American Academy of Ophthalmology, and the American Association of Certified Orthoptists as well as the Brazilian Ministry of Health recommend fix-and-follow vision assessment as part of a healthy infant’s routine pediatric examination during the first year of age.11,25
According to the Brazilian Ministry of Health, only infants with microcephaly should be referred for an eye examination26; however, strict adherence to this guideline would have led to 5 infants with eye abnormalities and 8 infants with abnormal visual function being missed in the present cohort. Therefore, our findings support the updated Center for Disease Control and Prevention guidelines for management of antenatal ZIKV infection, which recommend that non-eye-care professionals assess visual function during routine well-child examinations.27 If any external eye abnormality or abnormal visual function is detected, referral to an ophthalmologist for a complete eye examination is warranted.
Using both abnormal visual function and microcephaly to screen for eye abnormalities had high sensitivity and specificity. Not surprisingly, infants with abnormal visual function had significantly higher rates of structural eye and CNS abnormalities. This finding also underscores the fact that visual function depends on normal development of both the eye and the brain. Therefore, a finding of poor visual function could help to identify these undiagnosed abnormalities in resource-limited settings.
According to the last Brazilian Ministry of Health report of September 2017, 11,546 pregnant women with confirmed ZIKV infection were diagnosed in Brazil between January 2016 and June 2017, and 1,023 cases of laboratory-confirmed ZIKV-related microcephaly were reported.28 There is a large number of ZIKV-affected infants that would benefit from a timely diagnosis and cost-effective early interventions, particularly while the visual system and brain have greater capacity for neuroplasticity and may develop compensatory adaptation following prior injury to these areas.29 Whenever possible, early detection of visual deficits followed by prompt corrective interventions will likely positively influence future cognitive neurodevelopment.30,31
Because our institution is a referral center for high-risk pregnancies and infants with congenital abnormalities, our study population consisted of infants born to mothers with symptomatic infection during pregnancy or infants with congenital malformations, which increases our rates of eye complications. This limits the generalizability of our findings to the overall infant population born during or immediately following the ZIKV epidemic. However, visual function testing is readily performed in low-resource settings by the general pediatric provider and could be evaluated as a primary screening tool in future studies on the general population in endemic areas. Additionally, our period of observation did not permit us to distinguish whether abnormal visual function was permanent or simply delayed. Ongoing prospective evaluations are needed to reassess visual function at a later age and explore the results of visual habilitation in ZIKV exposed children with reduced visual function.
Acknowledgments
Funding/support: Brazilian National Council for Scientific and Technological Development (441098/2016–9), National Eye Institute [R21EY028318–01 and R01AI1121207], and National Institute of Allergy and Infectious Diseases (R21AI129534–02). Dr. Tsui was supported in part by an unrestricted Research to Prevent Blindness grant given to the Stein Eye Institute. Dr. Gaw was supported in part by the Queenan Fellowship in Global Health from the Foundation for the Society of Maternal-Fetal Medicine, and the UCSF National Center of Excellence in Women’s Health. Funding sources had no involvement in design, data collection/interpretation, or manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halai UA, Nielsen-Saines K, Moreira ML, et al. Maternal Zika virus disease severity, virus load, prior dengue antibodies, and their relationship to birth outcomes. Clin Infect Dis 2017;65:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brasil P, Pereira JP Jr, Moreira ME, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 2016;375:2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 2017;171:288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Linden V, Pessoa A, Dobyns W, et al. Description of 13 infants born during October 2015-January 2016 with congenital Zika virus infection without microcephaly at birth—Brazil. MMWR Morb Mortal Wkly Rep 2016;65:1343–8. [DOI] [PubMed] [Google Scholar]
- 5.Zin AA, Tsui I, Rossetto J, et al. Screening criteria for ophthalmic manifestations of congenital Zika virus infection. JAMA Pediatr 2017;171:847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mets MB, Chhabra MS. Eye manifestations of intrauterine infections and their impact on childhood blindness. Surv Ophthalmol 2008;53:95–111. [DOI] [PubMed] [Google Scholar]
- 7.Ventura LO, Ventura CV, Lawrence L, et al. Visual impairment in children with congenital Zika syndrome. J AAPOS 2017;21:295–9.e2. [DOI] [PubMed] [Google Scholar]
- 8.Verçosa I, Carneiro P, Verçosa R, et al. The visual system in infants with microcephaly related to presumed congenital Zika syndrome. J AAPOS 2017;21:300–304.e1. [DOI] [PubMed] [Google Scholar]
- 9.Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008;14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalheiro S, Lopez A, Serra S, et al. Microcephaly and Zika virus: neonatal neuroradiological aspects. Childs Nerv Syst 2016;32:1057–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donahue SP, Baker CN; Committee on Practice and Ambulatory Medicine; American Academy of Pediatrics; Section on Ophthalmology, American Academy of Pediatrics; American Association of Certified Orthoptists; American Association for Pediatric Ophthalmology and Strabismus; American Academy of Ophthalmology. Procedures for the evaluation of the visual system by pediatricians. Pediatrics 2016;137.27543009 [Google Scholar]
- 12.Yepez JB, Murati FA, Pettito M, et al. ; Johns Hopkins Zika Center. Ophthalmic manifestations of congenital Zika syndrome in Colombia and Venezuela. JAMA Ophthalmol 2017;135:440–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ventura CV, Maia M, Ventura BV, et al. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq Bras Oftalmol 2016;79:1–3. [DOI] [PubMed] [Google Scholar]
- 14.de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016;387:228. [DOI] [PubMed] [Google Scholar]
- 16.Campos AG, Lira RP, Arantes TE. Optical coherence tomography of macular atrophy associated with microcephaly and presumed intrauterine Zika virus infection. Arq Bras Oftalmol 2016;79:400–401. [DOI] [PubMed] [Google Scholar]
- 17.de Paula Freitas B, Ventura CV, Maia M, Belfort R Jr. Zika virus and the eye. Curr Opin Ophthalmol 2017;28:595–9. [DOI] [PubMed] [Google Scholar]
- 18.Moshfeghi DM, de Miranda HA 2nd, Costa MC. Zika virus, microcephaly, and ocular findings. JAMA Ophthalmol 2016;134:945. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy M Severe eye damage in infants with microcephaly is presumed to be due to Zika virus. BMJ 2016;352:i855. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez MP, Parra Saad E, Ospina Martinez M, et al. Ocular histopathologic features of congenital Zika syndrome. JAMA Ophthalmol 2017;135:1163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ventura CV, Ventura LO. Ophthalmologic manifestations associated with Zika virus infection. Pediatrics 2018;141:S161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Oliveira Dias JR, Ventura CV, de Paula Freitas B, et al. ; Zika Virus Study Group. Zika and the eye: pieces of a puzzle. Prog Retin Eye Res 2018;66:85–106. [DOI] [PubMed] [Google Scholar]
- 23.Ventura LO, Ventura CV, Dias NC, et al. Visual impairment evaluation in 119 children with congenital Zika syndrome. J AAPOS 2018;22:218–22.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvey EM, Dobson V, Tung B, Quinn GE, Hardy RJ. Interobserver agreement for grating acuity and letter acuity assessment in 1- to 5.5-year-olds with severe retinopathy of prematurity. Invest Ophthalmol Vis Sci 1999;40:1565–76. [PubMed] [Google Scholar]
- 25.Diretrizes de Atenção à Saúde Ocular na Infância: Detecção e Intervenção Precoce paraa Prevenção de Deficiências Visuais, 2013. Available at http://bvsms.saude.gov.br/bvs/publicacoes/diretrizes_atencao_saude_ocular_infancia.pdf. [Google Scholar]
- 26.Pan American Health Organization; World Health Organization Regional Office fo the Americas. Regional Zika epidemiological update (Americas) August 25, 2017. Available at http://www.paho.org/hq/index.php?option=com_content&view=article&id=11599&Itemid=41691&lang=en.
- 27.Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection—United States, October 2017. MMWR Morb Mortal Wkly Rep 2017;66:1089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherrell H, Dunn L, Clifton V, Kumar S. Systematic review of maternal Placental Growth Factor levels in late pregnancy as a predictor of adverse intrapartum and perinatal outcomes. Eur J Obstet Gynecol Reprod Biol 2018;225:26–34. [DOI] [PubMed] [Google Scholar]
- 29.Luke B. Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: with an emphasis on US population-based studies. Am J Obstet Gynecol 2017;217:270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonnander K Early identification of children with developmental disabilities. Acta Paediatr Suppl 2000;89:17–23. [DOI] [PubMed] [Google Scholar]
- 31.Sonksen PM, Dale N. Visual impairment in infancy: impact on neurodevelopmental and neurobiological processes. Dev Med Child Neurol 2002;44:782–91. [DOI] [PubMed] [Google Scholar]