Abstract
Objectives
The purpose of this study was to test the efficacy of MOUTh (Managing Oral Hygiene Using Threat Reduction), a non-pharmacologic, relationship-based intervention versus control on 2 primary outcomes for nursing home (NH) residents with dementia who resisted mouth care: 1) reduction in the occurrence and intensity of care-resistant behaviors (CRBs), and 2) improvement in oral health. Two secondary outcomes were also examined, 1) the duration of mouth care and 2) the completion of oral hygiene activities.
Background
Persons with dementia who exhibit care-resistant behaviors (CRBs) are at risk for inadequate mouth care and subsequent systemic illnesses.
Materials and methods
The study used a randomized repeated measures design. Recruitment occurred in 9 nursing homes that varied in size, ownership, reimbursement patterns, and location. 101 nursing home residents with dementia were randomized at the individual level to experimental (n=55) or control groups (n=46). 100 participants provided data for the analyses.
Results
Compared to the control group, persons in the experimental group had twice the odds of allowing mouth care and completing oral hygiene activities; they also allowed longer duration of mouth care (d = 0.56), but showed only small reductions in the intensity of CRBs (d = 0.16) and small differential improvements in oral health (d = 0.18).
Conclusion
The data suggest that this intervention facilitates mouth care among persons with dementia. The management of refusal behavior may be a clinically more realistic approach than reducing or eradicating refusals.
Keywords: Dementia, nursing home, oral health, care-resistant behavior, resistiveness to care, care-rejection
1. INTRODUCTION
Mouth care is oral infection control.1 Inadequate mouth care results in bacterial plaque biofilm accumulation on both teeth and dentures. An increase in plaque biofilm quantity and maturation includes more anaerobic, pathogenic bacterial species as plaque is allowed to remain on intraoral surfaces.2 Furthermore, mechanical debridement is the most effective method of plaque removal as chemical and antimicrobial means lack adequate penetration into the liquid solid-surface biofilms.2 In addition to serving as the primary etiologic factor for dental caries (tooth decay) and periodontal diseases,3,4 increased plaque retention is associated with increased rates of nosocomial pneumonia and the pathogens identified in those cases are overwhelmingly genetically identical to those flora in the oral cavity.5,6 Periodontal diseases have further been associated with systemic problems such as atherosclerotic vascular disease, rheumatoid arthritis, cancer, obesity, and diabetes/impaired glycemic control.7–11
Care-rejection or care-resistant behavior (CRB), refers to actions taken by an older adult to avoid receiving any type of assistance or care activity. 12 For nursing home (NH) residents with dementia, overall prevalence of CRB is 63.4% and increases with dementia severity. 13,14 Nursing home staff report CRBs as a significant barrier to the provision of oral hygiene in persons with dementia,15 and report insufficient education and guidance in preventing and managing these behaviors. 16 Attempts to provide mouth care often trigger CRB; 95% of the NH nurses who encounter CRBs in the context of oral hygiene simply omit mouth care.16 Therefore, NH residents with dementia who demonstrate CRB have more caries and worse oral hygiene than those who do not; in fact, CRB increases the older adult’s risk for poor oral health threefold. 16,17 We anticipated that preventing or reducing CRB would result in the ability to “get into the mouth” and complete mouth care, which in turn, would result in better oral health for NH residents with dementia. Thus, the purpose of this study was to test the efficacy of MOUTh (Managing Oral Hygiene Using Threat Reduction), a non-pharmacologic, relationship-based intervention versus control (usual care) on 2 primary outcomes for NH residents with dementia who resisted mouth care: reduction in the occurrence and intensity of CRBs, and improvement in oral health. We also examined 2 secondary outcomes, duration of oral care and completion of oral hygiene activities.
2. MATERIALS AND METHODS
The study used a randomized repeated measures design, was registered as a clinical trial (NCT01363258, www.clinicaltrials.gov), and complied with the CONSORT recommendations. The protocol has been published.18 Participants were randomized to either control or experimental group at the individual level using a computer-generated random assignment list prior to the intervention phase. Implementation of the study procedures occurred sequentially.
2.1 Setting and sample
Recruitment occurred sequentially in 9 United States NHs that were selected using convenience sampling. Four were located in northeastern United States and served predominantly rural communities. Five were located in southeastern United States and served predominantly urban communities. Sizes ranged from 60 to 240 beds. Two were owned by local governments (county), one by a religious community, and the rest by secular companies. Three of the 4 Pennsylvania facilities were non-profit; four of the 5 Alabama facilities were for-profit. The nursing homes selected were from a convenience sample.
NH staff at the facility were asked to contact the legally authorized representatives for each potential participant who met the inclusion criteria. Once consent was obtained from the legally authorized representatives, participants were evaluated by authors VW and CJT to confirm eligibility. Initial inclusion criteria for the NH residents were: age 55 or older; dentate with least 2 adjacent teeth to allow for assessment of interdental cleaning, or edentulous but using a complete denture in at least one arch (maxillary or mandibular); diagnosed with any type of dementia based on health records review; able to grasp a toothbrush; and identified by staff as being resistant to mouth care or identified in the most recently completed Minimum Data Set document as exhibiting care-rejection behaviors. Based on data from an earlier pilot study,19 this study design required recruiting at least 80 individuals (40 per group) which would provide 80% power to detect an effect size (Cohen’s d) of 0.29 at a 0.05 significance level test, under the assumptions of 42 repeated measurements per individual and an intra-subject correlation of 0.2.
2.2 Procedures
The following descriptive data were collected once, at the beginning of the study, from both medical records and interviews with participants: functional status (Katz Activities of Daily Living20), degree of dementia (Global Deterioration Scale21), degree of cognitive impairment (Mini-Mental State Examination22), comorbid conditions (Charlson Co-morbidity Index23), and demographic data (age, gender, duration in facility). Oral health assessments were conducted prior to the beginning of the study and then weekly thereafter, by a member of the research team blinded to group assignment.
Participants were randomized at the individual level in each NH using a random numbers generator program and assigned to either the control or experimental group by 2 of the authors (VW, CJT), see Figure 1. All outcomes were assessed by trained research assistants blinded to treatment assignment. Eighty-nine research assistants provided mouth care; 47 served as experimental mouth-care providers and 42 as control mouth-care providers. The research assistants included 3rd and 4th year nursing students, undergraduate public health students, undergraduate kinesiology students, and certified nursing assistants. Blinding was accomplished by training members of the control team separately from members of the experimental team. Team members were yoked, meaning that the CRB raters were permanently assigned to either the control mouth-care providers or the experimental mouth-care providers for the duration of the study in individual NHs. Yoking was done to prevent the accidental “sharing” of the experimental threat-reduction strategies by well-meaning CRB raters. Ten percent of all control mouth-care provider interactions were observed by members of the research team (RJ, VW, CJT) for potential diffusion or for the accidental use of threat-reduction strategies intuitively learned by a control mouth-care provider. No cross-contamination or accidental use of threat-reduction strategies were observed during mouth care delivered by control group mouth-care providers.
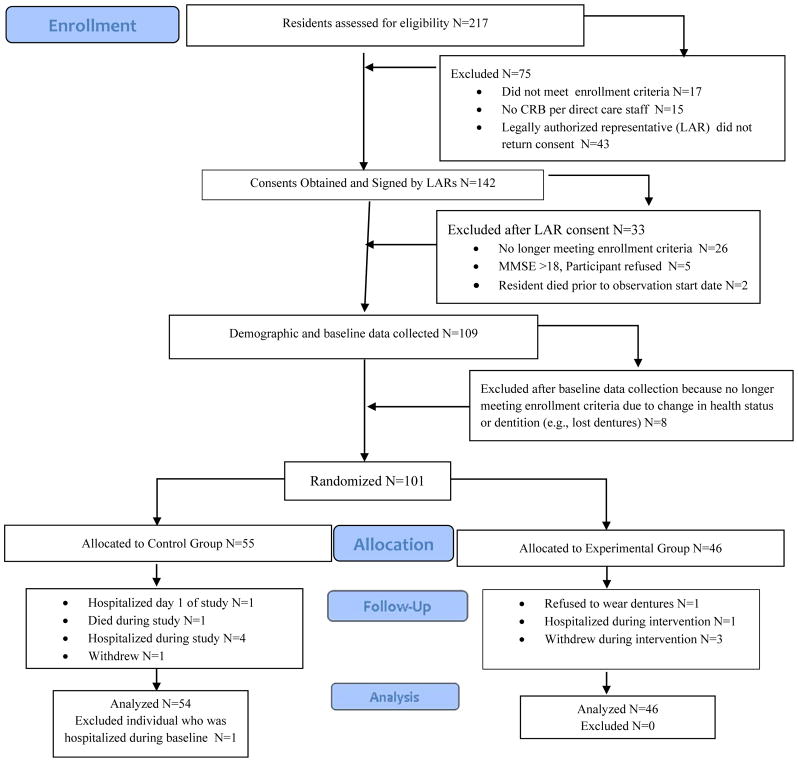
Figure 1.
Flow Diagram of Recruitment, Enrollment, Group assignment, and Number of Participants Who Contributed Data to the Analyses.
All participants were observed receiving twice daily mouth care from NH staff for 7 days in order to assess delivery of usual mouth care procedures without threat-reduction strategies and to generate baseline levels of CRB. Starting on day 8, participants received mouth care twice daily for three weeks from the trained research assistants. Both the control and experimental mouth care providers followed an evidence-based best mouth care protocol designed for teeth and dentures that has been described previously.19 Briefly, all tooth and tongue dorsum surfaces were brushed using a soft toothbrush and fluoride toothpaste. Interdental cleaning was accomplished using interdental brushes. After interdental cleaning, participants rinsed and spit using non-alcoholic antimicrobial (0.07% cetylpyridium chloride) mouth rinse. Participants assigned to the experimental group, however, received mouth care from mouth-care providers who had received additional training in both the recognition of CRB and strategies to reduce CRB (MOUTh intervention). These strategies are described in the “Procedures” section, below.
Treatment fidelity was assessed for mouth care providers delivering the experimental protocol by observing the 10% of all mouth care interactions between the experimental mouth care providers and participants assigned to the experimental group. RJ, VW, and CJT used checklists to assess which combination of threat-reduction strategies were being employed by the experimental mouth-care providers. Inter-rater reliability between the CRB raters and an expert CRB rater was also assessed throughout the intervention phase; none fell below 90%.
Although consent was obtained from the legally authorized representatives for each NH resident, assent was also obtained from each participant for each mouth care episode. Assent was considered “granted” if the person with dementia verbally or nonverbally agreed to receive mouth care after being approached by a mouth care provider. Mouth care providers were trained to approach participants no more than 3 times to obtain assent. If assent could not be obtained, the mouth care interaction was classified as “not done, unable to obtain assent.”
2.3 Intervention description
The Managing Oral Hygiene Using THreat Reduction (MOUTh) intervention contained 3 components: an evidence-based mouth care protocol for older adults with natural dentition and dentures,24–28 recognition of CRBs,29 and strategies to reduce threat perception during the provision of mouth care. 30 Over-the-counter, readily available mouth care products were supplied by the research team. Persons who were dentate received soft, manual toothbrushes; plastic interdental brushes; fluoride-containing toothpaste; alcohol-free, antibacterial mouthwash; and lip balm. Persons without dentition and who exclusively wore removable dental prostheses received soft, manual toothbrushes; denture cleaning paste; denture brushes; and lip balm. Partially dentulous participants who wore removable dental prostheses received both sets of supplies. Experimental mouth care providers were trained to recognize milder CRB and to implement strategies to prevent any escalation of CRBs. These strategies to reduce threat perception were grounded in the neurobiology of threat reduction and pilot tested with favorable results.30,31
The MOUTh intervention was originally conceptualized as a “toolbox” of strategies to prevent and reduce CRBs, which were initially derived from two sources: existing practices culled from extant nursing and dental literature and new techniques developed within pilot work. 19,30,31 These strategies included: establishing rapport by approaching the resident at or below eye level with a pleasant and calm demeanor; providing mouth care in front of a sink and in front of a mirror (to access procedural or implicit memories); avoiding elderspeak, a type of sing-song “baby talk”; chaining, which involved starting the mouth care and having the older adult finish the task; cueing by using gestures, pantomimes, and short, 1-step commands; distraction; bridging, where the older adult was asked to hold a toothbrush during mouth care; rescue, where a second experimental mouth care provider replaced the first experimental mouth care provider if care-resistant behaviors were escalating; and hand-over-hand, which involved either the older adult placing his or hand over that of the experimental mouth care provider, or the experimental mouth care provider gently guiding the older adult’s hands.32 The experimental mouth care providers were expected to select strategies on a trial and error basis.
2.4 Measurement of primary outcomes
Occurrence and intensity of CRB were operationalized as a binary indicator and a numeric score, respectively, obtained from the revised Resistiveness to Care Scale (RTC-r).33 The RTC-r is a modification of the original Resistiveness to Care Scale for Dementia of the Alzheimer’s Type.34,35 The RTC-r is a 13-item instrument used to identify specific CRBs and intensities of CRBs. CRB occurrence was simply a binary indicator of whether resistive behaviors were present or not. The intensity score was obtained by weighting the behaviors in the following manner: behaviors classified as mild were summed and multiplied by 1, behaviors classified as moderate were summed and multiplied by 2, and behaviors classified as extreme were summed and multiplied by 3. All scores (mild + moderate + severe) were then summed, resulting in the intensity score. In this study, inter-rater reliability was estimated at 0.87 (intraclass correlation) (p<0.001) across 2,328 mouth care observations.
The oral health of the participants was assessed using the Oral Health Assessment Tool (OHAT), a tool developed specifically for NH residents. 36,37 The OHAT is comprised of 8 items each assigned a score ranging from 0 (healthy) to 2 (unhealthy). Overall scores can range from 0 to 16. Six of the 8 items were nurse-sensitive measures of oral health: presence of pain; appearances of the lips, tongue, gums and tissues, and saliva; and overall oral cleanliness. Two other categories informed the score but were less responsive to nursing interventions: state of natural teeth (decayed, broken, or worn down) and condition of dentures (loose fit, broken teeth or areas). Participants’ oral cavities were assessed using the OHAT prior to the study, after 1 week of baseline mouth care, and at weekly intervals throughout the remainder of the study, for a total of five OHAT data collection points. A mouth care assessor blinded to group membership conducted the examinations. The mouth care assessor was a registered nurse who was trained by the principal investigator. The intraclass correlation coefficient was 0.744 for intra-rater reliabilty.
2.5 Measurement of secondary outcomes
The duration of mouth care was measured using a stopwatch and recorded on the RTC-r forms. Completion of mouth care was operationalized as a dichotomous response to the item, “Mouth care completed,” added to the RTC-r. For dentate participants, mouth care was considered completed if all teeth were brushed and interdental surfaces cleaned (using the interdental brushes). If mouthwash was omitted for safety reasons (for example, some participants drank it), mouth care was still considered complete. For edentulous participants, mouth care was considered completed if the dentures were removed from the mouth and cleaned. Dentures were only returned back into the mouth during the morning mouth care sessions; dentures were left in a denture cup with clean, fresh water after the evening mouth care sessions. For persons with partial edentulism and a removable partial denture prosthesis, mouth care was considered complete if the aforementioned conditions were met for both dentition and removable dental appliances.
2.6 Statistical analyses
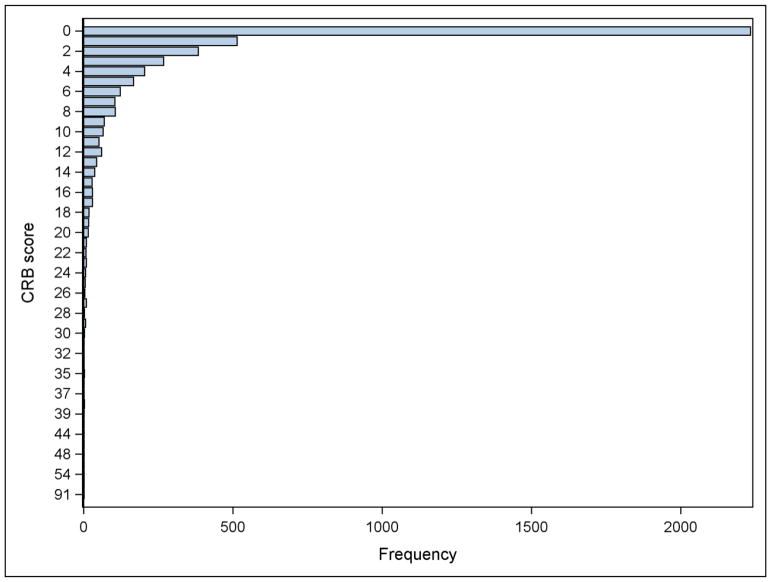
The NH residents were randomized at the individual level, which lessened the effect of NH site as a confounding variable. Participant characteristics were tabulated by study group in order to examine balance in covariates at baseline. An imbalanced characteristic (months in nursing home) was used as adjusting covariate in all subsequent between-group comparisons. The initial datasets included 4706 attempted instances of mouth care (1155 in the observation week and 3551 in the intervention weeks). During the intervention weeks, assent for mouth care was obtained in 2886 (82.3%) instances, and completion of mouth care activities was recorded in 2869 (80.1%) instances. There were 622 (13% of total) instances where residents refused care and therefore care was not completed. In 87 (2% of total) instances, mouth care was initiated but not completed yet CRBs were recorded. Therefore, the final number of instances of mouth care used for analysis of CRBs included 3998 instances of mouth care (1091 in the observation week and 2907 in the intervention weeks). Between-group differences in proportions of assent to mouth care and completion of mouth care during the intervention weeks (3551 instances of attempted mouth care) were examined using repeated measures models fitted using generalized linear mixed models fitted with a dichotomous outcome binomial distributions (logit link)..38,39 The intention-to-treat principle was also used for the remaining analyses, and thus the analytical datasets included data from the 3998 instances of mouth care where CRBs were recorded, regardless of whether mouth care was completed or not. Intervention effects were examined in terms of duration of mouth care, occurrence of CRBs (binary indicator), intensity CRBs when they occur (weighted scores), and OHAT scores. For duration of mouth care and OHAT scores, the analyses were conducted using linear mixed models that allowed covariance structures for repeated measures on the same participants and nesting of participants within nursing homes. Intervention effect sizes (Cohen’s d) were computed using the standard deviation estimated from models that included data from the baseline observation week only. The distribution of the CRB scores was examined graphically and found to have a mass of observations at zero and to be right skewed for those scores greater than zero (see Figure 2). Thus, the analysis of intervention effect in terms of resisting behaviors had two components. The first component was the probability of CRB occurrence (e.g., whether or not the score was zero, adjusting for the duration of mouth care, since the longer the duration the higher the likelihood of observing resisting behaviors). The second analysis included only those instances of mouth care in which CRBs occurred, using the weighted scores to capture the intensity of these behaviors, and adjusting for the duration of mouth care. Generalized linear mixed models fitted with binomial (logit link) and gamma (log link) distributions, with additional dispersion parameters, were used to estimate and test intervention effects in terms of occurrence and intensity of CRBs, respectively. Gamma models can be used to model outcomes with positive right skewed distributions,40 which occurred in this study. Intervention effect size (Cohen’s d) for the binomial model was computed using the method by Chinn,41 and for the gamma model, using reverse-link estimates from the repeated measures model and a standard deviation estimated from a model that included data from the baseline observation week only. Inference was conducted in the link scale, but inverse-link estimates in the original scales (proportions and CRB scores, for the binomial and gamma models, respectively) were computed to facilitate interpretation. Intervention effect sizes were examined in terms of their relevance (i.e., their magnitude), benefit (whether the sign or direction of the effect suggested benefit to the intervention group), and significance from null hypothesis tests. Effect sizes (Cohen’s d) of magnitude 0.3 were considered large enough to be clinically relevant, corresponding to the mid-point between a small (d=0.2) and a medium (d=0.5) effect size, as categorized by Cohen.42 Multiple significance testing on study outcomes was addressed using a False Discovery Rate (FDR) approach.43 Significance was held at the 10% FDR level.
Figure 2.
Graphical representation of care-resistant behavior (CRB) frequency (N=4656 instances of mouth care).
2.7 Experimental ethics
This study received approval from both The Pennsylvania State University’s Institutional Review Board (US) and the University of Alabama at Birmingham’s Institutional Review Board (US).
3 RESULTS
The CONSORT diagram of subject flow throughout the study is depicted in Figure 1. One-hundred nine NH residents were enrolled; 101 were randomized, 100 contributed data for analyses, and 91 completed the 3-week intervention period. Table 1 lists the demographics of the sample. The majority (77.0%) was female and white (85.0%). They had moderate to severe dementia and were dependent on others for care. Cohen’s d equivalent effect sizes for the categorical characteristics were estimated using the method by Rosenthal and Rubin.44 Between-group imbalance at baseline was observed for months in nursing home, with participants in the experimental group, on average, having resided 11 months longer at their nursing homes, compared to their control counterparts (d = 0.47, p = 0.022). Therefore, all models assessing between-group differences were adjusted for months in nursing home. All models also included a random effect for NH; however, the model results showed no effect on the outcome variables.
Table 1.
Comparison between control and experimental group demographic characteristics at baseline (brackets contain standard deviations [SD])
| Characteristic | All, n=100 M (SD) |
Control, n=46 M (SD) |
Experimental, n=54 M (SD) |
P valuea | Effect sizeb |
|---|---|---|---|---|---|
| Age in years | 81.55 (9.91) | 80.48 (11.54) | 82.80 (8.92) | 0.260 | 0.23 |
| Gender | 76.0% Female 24.0% Male |
74.1% Female 25.9% Male |
78.3.% Female 21.7% Male |
0.625 | 0.1 |
| Race | 84.0% White 16.0% Black |
85.2% White 14.8% Black |
82.6% White 17.4% Black |
0.726 | 0.07 |
| Months in Nursing Home | 22.66 (23.63) | 17.39 (15.99) | 28.74 (29.16) | 0.022 | 0.47 |
| GDSc (1–7, higher numbers indicate worsening dementia) | 5.71 (.89) | 5.69 (1.03) | 5.73 (.71) | 0.821 | 0.05 |
| MMSEd (1–30, lower numbers indicate worsening cognitive function) | 6.98 (6.89) | 6.54 (7.31) | 7.47 (6.41) | 0.505 | 0.13 |
| Katz Index of ADLse (1–18, higher numbers indicate greater dependency) | 9.53 (3.16) | 9.33 (2.67) | 9.76 (3.63) | 0.514 | 0.13 |
| Charlson Co-Morbidity Index | 2.50 (2.06) | 2.40 (1.53) | 2.60 (2.56) | 0.629 | 0.1 |
| OHATf (0–16, higher numbers indicate worse oral health) | 5.35 (2.77) | 5.67 (2.85) | 5.04 (2.63) | 0.282 | 0.22 |
P values from t-tests or Chi-squared tests as appropriate
Effect sizes are Cohen’s d or d-equivalent (Rosenthal and Rubin, 2003).
Global Deterioration Scale
Activities of Daily Living
Mini Mental State Exam
Oral Health Assessment Tool
3.1 Assent and completion of mouth care
Not all participants received mouth care at every potential session. Reasons for not receiving mouth care included failure to gain assent, unavailability, and somnolence. During the intervention period, in order to complete mouth care, mouth care providers were required to gain assent. During that period, when available for mouth care, NH residents in the experimental group had twice the odds of assenting to mouth care compared to residents in the control group (Table 2). Participants in the experimental group had also twice the odds of completing mouth care. These differences were relevant (d > 0.3) and significant at the 10% FDR level.
Table 2.
Estimates of the expected proportions of assent to mouth care and mouth care completed by group during the intervention weeks, estimated with repeated measures models (N=100, 3551 instances of mouth care)(brackets contain standard error)
| Outcome | Experimental | Control | Between-group difference | |||
|---|---|---|---|---|---|---|
| Proportion (SE) | Proportion (SE) | Odds Ratio (SE) | Effect sizeb | P | FDRc | |
| Assent to mouth carea | 0.87 (0.03) | 0.76 (0.05) | 2.1 (1.42) | 0.41 | 0.0357 | 0.0714 |
| Completion of mouth carea | 0.85 (0.03) | 0.74 (0.05) | 2.04 (1.39) | 0.39 | 0.0305 | 0.0714 |
Estimates from repeated measures models adjusted for months in nursing home.
Cohen’s d in the log odds scale (Chinn, 2000).
False Discovery Rate-adjusted P values
3.2 Duration of mouth care
Descriptive statistics for the additional outcomes of interest by study group and week are shown in Table 3 (raw data) and intervention effects estimated with longitudinal models are shown in Table 4. At baseline, the average time spent on mouth care was roughly 2.6 minutes. At the intervention weeks, the time spent on mouth care increased in both study groups to over four minutes, but the magnitude of the increase, on average, was higher in the experimental group by 0.72 minutes. This difference was relevant (d = 0.56) and statistically significant.
Table 3.
Descriptive statistics for outcomes of interest by study group and week (N=100 participants, 3998 instances of mouth care, brackets contain standard deviation [SD])
| Week | Group | Duration of mouth care in minutes | Occurrence of Care-Resistant Behaviors (CRB) | Intensity of Care-Resistant Behaviors | Oral Health Assessment Tool Scores (OHAT) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of mouth care sessions | Mean (SD) | Number of mouth care sessions | Proportion | Number of mouth care sessions | Mean (SD) | Number of examinations | Mean (SD) | ||
| 0a | Experimental | 534 | 2.5 (1.21) | 551 | 0.46 | 251 | 6.48 (6.41) | 89c | 4.72 (2.37) |
| Control | 524 | 2.58 (1.34) | 540 | 0.51 | 276 | 5.25 (6.4) | 88c | 4.66 (2.71) | |
| 1b | Experimental | 545 | 5.23 (2.47) | 553 | 0.58 | 318 | 7.6 (7.05) | 46 | 3.8 (2.41) |
| Control | 484 | 4.59 (2.33) | 485 | 0.56 | 272 | 5.31 (5.09) | 43 | 3.88 (2.57) | |
| 2 | Experimental | 500 | 4.9 (2.27) | 503 | 0.56 | 283 | 7.08 (7.18) | 44 | 3.25 (2.22) |
| Control | 449 | 4.23 (1.89) | 454 | 0.54 | 245 | 4.96 (5.23) | 40 | 3.73 (2.24) | |
| 3 | Experimental | 471 | 4.96 (2.47) | 473 | 0.55 | 262 | 7.05 (8.57) | 45 | 2.82 (1.9) |
| Control | 435 | 4.04 (1.85) | 439 | 0.51 | 223 | 4.87 (5.13) | 39 | 2.87 (2.03) | |
Week 0 was the baseline week
Weeks 1 through 3 were the intervention weeks
This number represents two oral examinations for each participant, one prior to the Baseline Week and one after the Baseline Week but prior to Week 1.
Table 4.
Examination of intervention effects with longitudinal models (N=100 participants, 3998 instances of mouth care, brackets contain standard error [SE])
| Outcome | Group | Observation Week | Follow-up Weeks | Change from Observation Week to Follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Between group differencesa | FDRd | |||||||
|
|
||||||||
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Effect sizeb | Pc | |||
| Duration of mouth care in minutes | Experimental | 2.57 (0.3) | 4.96 (0.28) | 2.39 (0.13) | 0.72 (0.11) | 0.56 | <.0001 | 0.0006 |
| Control | 2.58 (0.3) | 4.26 (0.29) | 1.67 (0.14) | |||||
| Occurrence of care-resistant behaviorse | ||||||||
| Log oddsf | Experimental | −0.12 (0.22) | 0.06 (0.20) | 0.18 (0.1) | 0.02 (0.11) | 0.01 | 0.8837 | 0.8837 |
| Control | 0.11 (0.22) | 0.28 (0.21) | 0.16 (0.1) | |||||
| Proportiong | Experimental | 0.47 (0.05) | 0.52 (0.05) | |||||
| Control | 0.53 (0.05) | 0.57 (0.05) | ||||||
| Intensity of care-resistant behaviorse | ||||||||
| Log meanf | Experimental | 1.84 (0.13) | 1.59 (0.11) | −0.25 (0.1) | −0.16 (0.11) | −0.16 | 0.1433 | 0.215 |
| Control | 1.58 (0.14) | 1.49 (0.11) | −0.09 (0.09) | |||||
| Meang | Experimental | 6.3 (0.86) | 4.91 (0.53) | |||||
| Control | 4.84 (0.66) | 4.42 (0.48) | ||||||
| Oral health assessment score | Experimental | 4.86 (0.68) | 3.42 (0.61) | −1.43 (0.65) | −0.33 (0.32) | −0.18 | 0.2961 | 0.3553 |
| Control | 4.93 (0.68) | 3.83 (0.62) | −1.1 (0.66) | |||||
All between-group comparisons adjusted for months in nursing home
Cohen’s d, using baseline standard deviations of 1.28 for duration of moth care, 6.17 for intensity of care-resistant behaviors,1.83 for oral health assessment, and 1.81 for the log odds (Chinn, 2000).
Time by group interaction test
False Discovery Rate-adjusted P values
Controlling for duration of mouth care
Modeling and testing was conducted in the link scale
Inverse-link original scale estimates
3.3 Care-resistant behavior
During the first week of the study (baseline period), after adjusting for duration of mouth care and months residing in nursing home, CRB occurred in about 47% of the experimental group mouth care sessions and 53% of the control group mouth care sessions (Table 4). During the intervention weeks, occurrence of CRB increased in both groups at similar levels (d = 0.01, p = 0.8837). In terms of the intensity of CRB, at baseline the experimental group exhibited slightly higher intensity compared to the control group. During the intervention weeks, the intensity of CRB decreased in both groups, but the decrease was slightly higher in the experimental group (Table 4). Although the effect of the intervention on CRB intensity was in a direction that indicated benefit, the magnitude of the effect was small (d=-0.16) and not statistically significant.
3.4 Oral health
Between baseline and week 3, mean OHAT scores improved for both groups (decreasing scores indicate improvement in oral health; see Table 3). When comparing baseline to the intervention weeks (Table 3), the experimental group had a slightly larger improvement (i.e., decrease) in OHAT scores compared to the control group. However, the magnitude of the intervention effect was small (d = −0.18) and not statistically significant.
4 DISCUSSION
The purpose of this study was to test the efficacy of MOUTh (Managing Oral Hygiene Using Threat Reduction), a non-pharmacologic, relationship-based intervention versus control on 2 primary outcomes for NH residents with dementia who resist mouth care: reduction in the occurrence and intensity of CRBs, and improvement in oral health. We found that the frequency of CRBs increased in both groups while the intensity of the behaviors trended downward in the experimental group. We also examined 2 secondary outcomes, duration of oral care and completion of oral hygiene activities. Participants in the experimental group experienced longer durations of mouth care and were twice as likely to receive completed mouth care compared to participants in the control group. Oral health, operationalized as OHAT scores, showed important clinical improvements for both groups. Although the experimental group presented with a larger change in mean scores than the control group during the 3-week follow-up period, the change was small in magnitude and not statistically significant. This finding may reflect the reality that mouth care may be a low priority in nursing home facilities and that use of alternative mouth care techniques, including oral chlorhexidine swabs (e.g. “toothettes”) or other mechanisms for mouth care may be non-ideal.
A strength of this study was the ecological validity of the MOUTh intervention. The behavioral techniques and protocols were easily taught to the mouth care providers, and only readily available, over-the-counter mouth care products were used. Some researchers have incorporated chlorhexidine and enhanced fluoride products as part of the mouth care protocol.45 While these products demonstrate efficacy, their use in NHs may be problematic because nursing assistants are not allowed to deliver prescribed substances to NH residents. Furthermore, the chronic use of oral care products containing chlorhexidine may contribute to multi-drug resistant dental plaque bacteria.46
The use of 9 facilities that differed in size, geography, ownership, and reimbursement patterns contributed to the generalizability of the findings. However, the use of research staff to perform the intervention, while supporting internal validity, compromised external validity. We did not measure dental or denture plaque in this study; changes in dental and denture plaque may have provided more quantifiable and reliable measurements of the effectiveness of the mouth care. This study also excluded persons with dysphagia.
Another limitation was the use of the OHAT to quantify oral health. While the OHAT is a well-recognized clinical instrument that was originally developed for use in long-term care settings as a screening tool for dental problems, it may not be sufficiently sensitive to identify the changes related solely to plaque accumulation and gingival inflammation.36,37 Two of the items are not responsive to the delivery of regular mouth care: the condition of natural teeth (the presence of decayed, broken, or missing teeth) and the condition of denture(s) (broken areas, missing teeth, poorly fitting). Because of its emphasis on capturing unhealthy changes, and its designation of “0” for scores related to healthy findings, the instrument may possess a floor effect in capturing47 healthy changes. Other studies have used plaque and gingival indices to measure levels of oral hygiene control and gingivitis.47,48 Utilization of a more sensitive measure may allow for a more precise assessment of changes to oral hygiene using such experimental measures.
To our knowledge, and based on the results of a recently published systematic review,49 this study represents the first randomized clinical trial of a non-pharmacological intervention designed to reduce CRBs during mouth care and improve oral health outcomes. The inclusion of participants with both dementia and CRBs was novel; this is a population that has been excluded in the past, 30 and continues to be excluded now,50 by researchers testing interventions for improving mouth care in NHs. Additionally, this study addressed the “big research gap”49 for strategies that oral care providers can use to accomplish mouth care in persons with dementia residing in NHs. Mouth care providers who had been trained in the behavioral strategies of MOUTh were twice as likely to clear the first hurdle of care, gaining assent and cooperation. Both the control and experimental mouth-care providers had been trained to gain assent by saying, “Please come with me to brush your teeth/clean your dentures.” If the participant responded, “No,” or “I just brushed my teeth,” the control mouth-care providers simply repeated the assent process up to two more times. The experimental mouth care providers, on the other hand, were observed employing the same behavioral strategies used to minimize CRB to gain assent and cooperation outside of the mouth care activities. For example, if the participant responded negatively during the first assent attempt, the experimental mouth-care providers immediately countered using specific MOUTh strategies, such as establishing rapport (e.g., “That blue sweater is so pretty on you”), using gestures and pantomime, or entering the person’s reality (e.g., when interacting with a retired attorney, “I know you just brushed your teeth, but the judge is waiting in chambers.”). Strategies implemented by oral care providers were instrumental with gaining assent and cooperation during the initial contact with the resident.
The experimental mouth care providers were trained to recognize milder CRB and to implement strategies to prevent escalation, which may have resulted in milder presentations of CRB compared to the control group participants. Our findings are supported by Volicer and colleagues, who observed that clinicians who pressed onwards with care because they were not cognizant of the milder presentations of CRB often unwittingly created situations where CRB escalated to combative behavior.13 These findings strongly suggest that management of CRBs, not their prevention or reduction, results in the successful implementation of oral hygiene activities in persons with dementia.
An important clinical implication of this study is that the quality of mouth care may be more important than the frequency of mouth care.51 No individual received twice daily mouth care throughout the full length of the study due to failure to achieve assent and/or inability of the oral care provider to deliver mouth care to the participant. Yet, oral health improved for both groups. In spite of practice guidelines that recommend a minimum of twice daily mouth care,37 we were unable to find empirical data supporting the optimum dosage of mouth care for dentate older adults. In an experimental gingivitis model, data suggest that complete plaque removal every 48 hours is sufficient to prevent development of gingival inflammation,51 but that plaque may develop more quickly at sites with inflammation, likely due to subgingival bacterial reservoirs.52 It is possible that frequency of mouth care delivery may increase CRBs as patients may remember the experience with a negative connotation.
For future studies, it may be beneficial to employ another measurement of oral health and hygiene that encompasses a fuller continuum of healthy to unhealthy changes and provides more quantitative assessment of oral hygiene measures. Next logical steps include testing mouth care protocols tailored to persons with dysphagia, who comprise 40% of NH residents;53,54 and determining the optimal dosage of mouth care that reduces plaque, while at the same time, minimizes refusals and escalations of CRB.
The MOUTh intervention needs to be evaluated in real-world settings where direct care staff are trained to use MOUTh in daily mouth care activities. Our team recently received funding from the Centers of Medicare and Medicaid services to train NH staff to use the MOUTh strategies; we will be evaluating the MOUTh protocol in 7 NHs in central Alabama. Two of the outcome measures for this project are dental and denture plaque changes, which will be obtained using an intraoral camera.
In addition to implementing the MOUTh protocol in NHs, we will also explore opportunities to test its efficacy in assisted living facilities. Assisted living facilities are rapidly replacing NHs as the preferred setting for residential long-term care for persons with dementia. Nationally, 1 million older adults reside in assisted living facilities55 89% of whom have dementia.56,57 Comparatively, 61% of the 1.7 million older adults who reside in NHs have dementia.58 More exploration is needed to determine which components of the MOUTh intervention would optimally affect the oral health of persons with dementia residing in assisted living facilities.
5 Conclusions
We were able to provide evidence-based mouth care to NH residents with dementia who exhibited CRB. The MOUTh intervention did not reduce the frequency of CRB occurrences. The intervention reduced the intensity of CRB exhibited by the experimental group compared to the control group; however, the magnitude of the effect was small and not statistically significant. There experimental group demonstrated a larger improvement in oral health than the control group, but the results were not statistically significant. NH residents in the experimental group were twice as likely to both assent to mouth care and receive completed mouth care, compared to the control group; these findings were statistically significant. NH residents in the experimental group received longer durations of mouth care compared to residents in the control group. We concluded that the MOUTh intervention showed efficacy for managing CRB during mouth care, not eradicating it, resulting in higher rates of completion of mouth care activities for NH residents randomized to experimental conditions.
Acknowledgments
Funding Sources: The project described was supported by Award Number R01NR012737 from the National Institute of Nursing Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
Contributor Information
Rita A. Jablonski, Professor, School of Nursing, Nurse Practitioner, Memory Disorders Clinic, University of Alabama at Birmingham, 1720 2nd Avenue South, Birmingham, AL 35294-1210.
Ann M. Kolanowski, Professor, College of Nursing, The Pennsylvania State University.
Andres Azuero, Associate Professor, School of Nursing, University of Alabama at Birmingham.
Vicki Winstead, Scientist I, School of Nursing, University of Alabama at Birmingham.
Corteza Jones-Townsend, Clinical Research Associate, Novartis Pharmaceuticals.
Maria L. Geisinger, Associate Professor, School of Dentistry, University of Alabama at Birmingham.
References
- 1.Fulmer T, Jablonski RA, Mertz E, George M, Russell S. Oral health. Nursing research and practice. 2012;2012:809465. doi: 10.1155/2012/809465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- 3.van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73(3):672–681. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 4.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 5.El-Solh AA. Association between pneumonia and oral care in nursing home residents. Lung. 2011;189(3):173–180. doi: 10.1007/s00408-011-9297-0. [DOI] [PubMed] [Google Scholar]
- 6.van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Oral health care and aspiration pneumonia in frail older people: a systematic literature review. Gerodontology. 2013;30(1):3–9. doi: 10.1111/j.1741-2358.2012.00637.x. [DOI] [PubMed] [Google Scholar]
- 7.Orlandi M, Suvan J, Petrie A, et al. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: a systematic review and meta-analysis. Atherosclerosis. 2014;236(1):39–46. doi: 10.1016/j.atherosclerosis.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Payne JB, Golub LM, Thiele GM, Mikuls TR. The Link Between Periodontitis and Rheumatoid Arthritis: A Periodontist’s Perspective. Curr Oral Health Rep. 2015;2:20–29. doi: 10.1007/s40496-014-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mai X, Genco RJ, LaMonte MJ, et al. Periodontal Pathogens and Risk of Incident Cancer in Postmenopausal Females: The Buffalo OsteoPerio Study. Journal of periodontology. 2016;87(3):257–267. doi: 10.1902/jop.2015.150433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson TC, Weldon JC, Worthington HV, et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2015;(11):CD004714. doi: 10.1002/14651858.CD004714.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Putten GJ, De Visschere L, van der Maarel-Wierink C, Vanobbergen J, Schols J. The importance of oral health in (frail) elderly people – a review. European Geriatric Medicine. 2013;4(5):339–344. [Google Scholar]
- 12.Ishii S, Streim JE, Saliba D. A conceptual framework for rejection of care behaviors: review of literature and analysis of role of dementia severity. Journal of the American Medical Directors Association. 2012;13(1):11–23. e11–12. doi: 10.1016/j.jamda.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Volicer L, Van der Steen JT, Frijters DH. Modifiable factors related to abusive behaviors in nursing home residents with dementia. Journal of the American Medical Directors Association. 2009;10(9):617–622. doi: 10.1016/j.jamda.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Ishii S, Streim JE, Saliba D. Potentially reversible resident factors associated with rejection of care behaviors. Journal of the American Geriatrics Society. 2010;58(9):1693–1700. doi: 10.1111/j.1532-5415.2010.03020.x. [DOI] [PubMed] [Google Scholar]
- 15.Jablonski RA, Munro CL, Grap MJ, Schubert CM, Ligon M, Spigelmyer P. Mouth care in nursing homes: knowledge, beliefs, and practices of nursing assistants. Geriatric nursing (New York, NY) 2009;30(2):99–107. doi: 10.1016/j.gerinurse.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willumsen T, Karlsen L, Naess R, Bjorntvedt S. Are the barriers to good oral hygiene in nursing homes within the nurses or the patients? Gerodontology. 2012;29(2):e748–755. doi: 10.1111/j.1741-2358.2011.00554.x. [DOI] [PubMed] [Google Scholar]
- 17.Zuluaga DJ, Ferreira J, Montoya JA, Willumsen T. Oral health in institutionalised elderly people in Oslo, Norway and its relationship with dependence and cognitive impairment. Gerodontology. 2012;29(2):e420–426. doi: 10.1111/j.1741-2358.2011.00490.x. [DOI] [PubMed] [Google Scholar]
- 18.Booker S, Murff S, Kitko L, Jablonski R. Mouth care to reduce ventilator-associated pneumonia. Am J Nurs. 2013;113(10):24–30. doi: 10.1097/01.NAJ.0000435343.38287.3a. quiz 31. [DOI] [PubMed] [Google Scholar]
- 19.Jablonski RA, Kolanowski A, Therrien B, Mahoney EK, Kassab C, Leslie DL. Reducing care-resistant behaviors during oral hygiene in persons with dementia. BMC Oral Health. 2011;11:30. doi: 10.1186/1472-6831-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the Index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:94–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 21.Reisberg B, Ferris S, De Leon M, Crook T. The global deterioration scale for assessment of primary degenerative dementia. American Journal of Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State:” A practical method for grading the congitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Chalmers J, Johnson V, Tang JH, Titler MG. Evidence-based protocol: oral hygiene care for functionally dependent and cognitively impaired older adults. J Gerontol Nurs. 2004;30(11):5–12. doi: 10.3928/0098-9134-20041101-06. [DOI] [PubMed] [Google Scholar]
- 25.Gil-Montoya JA, de Mello AL, Cardenas CB, Lopez IG. Oral health protocol for the dependent institutionalized elderly. Geriatric Nursing. 2006;27(2):95–101. doi: 10.1016/j.gerinurse.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Felton D, Cooper L, Duqum I, et al. Evidence-based guidelines for the care and maintenance of complete dentures: a publication of the American College of Prosthodontists. Journal of prosthodontics : official journal of the American College of Prosthodontists. 2011;20(Suppl 1):S1–S12. doi: 10.1111/j.1532-849X.2010.00683.x. [DOI] [PubMed] [Google Scholar]
- 27.American Dental Association. [Accessed 8/24/2010];Oral health topics: dentures. 2010 http://www.ada.org/2648.aspx?currentTab=2.
- 28.American Dental Association. [Accessed 8/24/2010];Oral Health Topics:Cleaning Your Teeth & Gums. 2010 http://www.ada.org/2624.aspx?currentTab=2.
- 29.Mahoney EK, Hurley AC, Volicer L. Instruction Manual for the Resistiveness to Care Scale (RTC-DAT) Boston, MA: Boston College School of Nursing; 1999. [Google Scholar]
- 30.Jablonski RA, Therrien B, Mahoney EK, Kolanowski A, Gabello M, Brock A. An intervention to reduce care-resistant behavior in persons with dementia during oral hygiene: a pilot study. Special care in dentistry : official publication of the American Association of Hospital Dentists, the Academy of Dentistry for the Handicapped, and the American Society for Geriatric Dentistry. 2011;31(3):77–87. doi: 10.1111/j.1754-4505.2011.00190.x. [DOI] [PubMed] [Google Scholar]
- 31.Jablonski RA, Therrien B, Kolanowski A. No more fighting and biting during mouth care: applying the theoretical constructs of threat perception to clinical practice. Research and theory for nursing practice. 2011;25(3):163–175. doi: 10.1891/1541-6577.25.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jablonski-Jaudon RA, Kolanowski AM, Winstead V, Jones-Townsend C, Azuero A. Maturation of the MOUTh Intervention: From Reducing Threat to Relationship-Centered Care. J Gerontol Nurs. 2016;42(3):15–23. doi: 10.3928/00989134-20160212-05. quiz 24–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jablonski-Jaudon R, Winstead V, Jones-Townsend C, Azuero A, Kolanowski A, Mahoney EK. Revising the Resistiveness to Care Scale. Journal of Nursing Measurement. 2015 doi: 10.1891/1061-3749.24.2.E72. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volicer L, Hurley AC, Mahoney EK. Using the Resistiveness to Care-Dementia of the Alzheimer Type (RTC-DAT) Scale. In: Volicer L, Hurley AC, editors. Assessment scales for advanced dementia. Baltimore, MD: Health Professions Press; 2015. pp. 185–196. [Google Scholar]
- 35.Mahoney EK, Hurley AC, Volicer L, et al. Development and testing of the Resistiveness to Care Scale. Research in nursing & health. 1999;22(1):27–38. doi: 10.1002/(sici)1098-240x(199902)22:1<27::aid-nur4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 36.Chalmers J, King PL, Spencer AJ, Wright FA, Carter KD. The oral health assessment tool--validity and reliability. Australian Dental Journal. 2005;50(3):191–199. doi: 10.1111/j.1834-7819.2005.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 37.Johnson V. Evidence-based practice guideline: oral hygiene care for functionally dependent and cognitively impaired older adults. Journal of gerontological nursing. 2012;38(11):11. doi: 10.3928/00989134-20121003-02. [DOI] [PubMed] [Google Scholar]
- 38.Burton P, Gurrin L, Sly P. Extending the simple linear regression model to account for correlated responses: an introduction to generalized estimating equations and multi-level mixed modelling. Stat Med. 1998;17(11):1261–1291. doi: 10.1002/(sici)1097-0258(19980615)17:11<1261::aid-sim846>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 40.Azuero A, Pisu M, McNees P, Burkhardt J, Benz R, Meneses K. An application of longitudinal analysis with skewed outcomes. Nurs Res. 2010;59(4):301–307. doi: 10.1097/NNR.0b013e3181e507f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19(22):3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 42.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- 43.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;(57):289–300. Series B. [Google Scholar]
- 44.Rosenthal R, Rubin D. R-equivalent: A simple effect size indicator. Psychological Methods. 2003;8(4):492–496. doi: 10.1037/1082-989X.8.4.492. [DOI] [PubMed] [Google Scholar]
- 45.Sloane PD, Zimmerman S, Chen X, et al. Effect of a person-centered mouth care intervention on care processes and outcomes in three nursing homes. Journal of the American Geriatrics Society. 2013;61(7):1158–1163. doi: 10.1111/jgs.12317. [DOI] [PubMed] [Google Scholar]
- 46.Saleem HG, Seers CA, Sabri AN, Reynolds EC. Dental plaque bacteria with reduced susceptibility to chlorhexidine are multidrug resistant. BMC Microbiol. 2016;16:214. doi: 10.1186/s12866-016-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischman SL. Clinical index systems used to assess the efficacy of mouthrinses on plaque and gingivitis. J Clin Periodontol. 1988;15(8):506–510. doi: 10.1111/j.1600-051x.1988.tb01022.x. [DOI] [PubMed] [Google Scholar]
- 48.Loe H. The Gingival Index, the Plaque Index and the Retention Index Systems. Journal of periodontology. 1967;38(6):Suppl:610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 49.Hoben M, Kent A, Kobagi N, Huynh KT, Clarke A, Yoon MN. Effective strategies to motivate nursing home residents in oral care and to prevent or reduce responsive behaviors to oral care: A systematic review. PLoS One. 2017;12(6):e0178913. doi: 10.1371/journal.pone.0178913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu JH, Liu MF. Oral health of older adults in lont-term care facilities. J Oral Health Dent Care. 2017;1(2):008. [Google Scholar]
- 51.Lang NP, Cumming BR, Loe H. Toothbrushing frequency as it relates to plaque development and gingival health. Journal of periodontology. 1973;44(7):396–405. doi: 10.1902/jop.1973.44.7.396. [DOI] [PubMed] [Google Scholar]
- 52.Ramberg P, Lindhe J, Dahlen G, Volpe AR. The influence of gingival inflammation on de novo plaque formation. J Clin Periodontol. 1994;21(1):51–56. doi: 10.1111/j.1600-051x.1994.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 53.Ortega O, Parra C, Zarcero S, Nart J, Sakwinska O, Clave P. Oral health in older patients with oropharyngeal dysphagia. Age Ageing. 2014;43(1):132–137. doi: 10.1093/ageing/aft164. [DOI] [PubMed] [Google Scholar]
- 54.Easterling CS, Robbins E. Dementia and dysphagia. Geriatr Nurs. 2008;29(4):275–285. doi: 10.1016/j.gerinurse.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 55.Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-Term Care Providers and services users in the United States: data from the National Study of Long-Term Care Providers, 2013–2014. Vital Health Stat 3. 2016;(38):x–xii. 1–105. [PubMed] [Google Scholar]
- 56.Samus QM, Onyike CU, Johnston D, et al. 12-month incidence, prevalence, persistence, and treatment of mental disorders among individuals recently admitted to assisted living facilities in Maryland. Int Psychogeriatr. 2013;25(5):721–731. doi: 10.1017/S1041610212002244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zimmerman S, Sloane PD, Reed D. Dementia prevalence and care in assisted living. Health Aff (Millwood) 2014;33(4):658–666. doi: 10.1377/hlthaff.2013.1255. [DOI] [PubMed] [Google Scholar]
- 58.Centers for Medicare and Medicaid Services. CMS Nursing Home Data Compendium. 2015 [Google Scholar]