Abstract
Women who have experienced significant adversities during childhood and adulthood are at risk for excessive inflammation during pregnancy, but the mechanisms are unclear. Using structural equation modeling, we examined pathways from childhood abuse history and current socioeconomic status (SES) to inflammatory markers through indicators of health risk, recent stressors, and psychological distress in 214 women assessed at mid-pregnancy (5–31 weeks gestation). Self-reported data on socioeconomic indicators, childhood trauma history, pre-pregnancy body mass index (BMI), smoking, sleep quality, interpersonal conflict, recent life events, perceived stress, and depressive symptoms were collected, and serum levels of C-reactive protein (CRP) and interleukin (IL)-6 were determined. In separate models, pre-pregnancy BMI, sleep quality, and interpersonal conflict statistically explained the relationship between adversity and inflammation. These three intermediate variables were then entered into a multiple mediation analysis to examine unique effects. Childhood abuse history and current SES both demonstrated significant indirect effects on CRP through pre-pregnancy BMI, and current SES showed a significant indirect effect on IL-6 through all intermediate variables. When examining each indirect pathway individually, pre-pregnancy BMI and interpersonal conflict emerged as parallel pathways by which low current SES leads to elevated IL-6; the indirect pathway through sleep quality was no longer significant. Pre-pregnancy BMI and interpersonal conflict are two independent mechanisms by which adversity is associated with increased inflammation during pregnancy. Women who have been exposed to significant adversity may be at particular risk for obesity, sleep disruption, and interpersonal conflict, with implications for immune dysregulation during pregnancy.
Keywords: childhood trauma, socioeconomic status (SES), C-reactive protein (CRP), interleukin-6 (IL-6), body mass index (BMI), obesity, interpersonal conflict, sleep disruption, stress, pregnant women
1. Introduction
In comparison to non-pregnancy, healthy pregnancy is characterized by increased inflammatory responses to biological stimuli as well as mild elevations in serum inflammatory markers, such as C-reactive protein (CRP) and pro-inflammatory cytokines including interleukin-6 (IL-6) and IL-8 (Christian and Porter, 2014; Curry et al., 2008; Gillespie et al., 2016; Sharma et al., 2007). Although it is normal and healthy for a woman to experience an enhanced inflammatory state during pregnancy in comparison to non-pregnancy, excessive systemic inflammation during pregnancy is linked to poor maternal health and adverse birth outcomes for both the mother and her offspring (for review see Christian, 2015). For example, excessive inflammation during pregnancy is associated with increased risk for gestational hypertension, preeclampsia, spontaneous abortion, preterm birth, and perinatal depression (Blair et al., 2015; Christian et al., 2009; Coussons-Read et al., 2012; Romero et al., 2007). In addition to being associated with pregnancy complications, excessive inflammation in pregnant women also increases the offspring’s risk for developing chronic illnesses and psychopathology during childhood (Gaillard et al., 2016; Graham et al., 2018; Lee et al., 2015). Given the adverse impact of inflammation on both the health of the mother and her offspring, it is important to identify the factors and processes that contribute to systemic inflammation during pregnancy for treatment and prevention efforts.
One risk factor that has been linked to excessive inflammation during pregnancy is exposure to chronic adversity. Women who have experienced significant adversities during childhood (e.g., abuse) and adulthood (e.g., poverty or low socioeconomic conditions) are at risk for excessive inflammation, which has implications for pregnancy (Miller et al., 2017; Mitchell et al., 2018; Walsh et al., 2016). A history of childhood exposure to abuse and low socioeconomic status (SES) have been independently associated with a chronic inflammatory state characterized by elevations in inflammatory markers across the lifespan (Azad et al., 2012; Baumeister et al., 2016; Danese et al., 2007; Pollitt et al., 2008), in addition to adverse pregnancy outcomes characterized by excessive inflammation (Bushnik et al., 2017; Gavin et al., 2012; Silva et al., 2008; Smith et al., 2016). However, it is still unclear how chronic adversities experienced during childhood and adulthood affect inflammatory states in pregnant women.
It is likely that a history of childhood abuse and low SES during adulthood lead to heightened inflammation during pregnancy through multiple independent and interrelated pathways. Potential explanatory pathways linking adversities across the lifespan to inflammation during pregnancy include indicators of health risk, recent stressors, and psychological distress, each of which have been linked with upregulated inflammatory activity during pregnancy (for review see Christian, 2012, 2015; Dunkel Schetter, 2011). Health risk indicators that are associated with a history of childhood abuse and low SES include obesity, cigarette smoking, and sleep disturbance (for review see Adler et al., 1994; Kendall-Tackett, 2002; Pampel et al., 2010). Childhood abuse and low SES are linked to exposure to frequent stressors in adulthood, such as stressful life events and interpersonal conflict (Johnson et al., 2002; Kim et al., 2009; Matthews et al., 2000; Messman-Moore and Coates, 2007; Widom et al., 2008). Psychological distress, such as perceived stress and depressive symptoms, during adulthood is also associated with exposure to childhood abuse and low SES (Bifulco et al., 2002; Hager and Runtz, 2012; Lynch et al., 1997; Weiss et al., 1999).
Despite advancements in statistical procedures and psychoneuroimmunology models of pregnancy, few empirical studies have used a lifespan approach with a multiple determinants model to study perinatal health. The unique effects of childhood and adulthood adversities on inflammatory markers like CRP and IL-6 during pregnancy, in addition to potential explanatory pathways, can be tested using structural equation modeling (SEM). SEM can estimate both the strength of a direct pathway between two variables (e.g., adversity → inflammation) and also indirect pathways involving candidate mediators (e.g., adversity → obesity → inflammation). By examining both the independent and interrelated pathways that link risk factors during and prior to pregnancy to elevated inflammatory markers in pregnant women, it may be possible to identify clearer targets for intervention in high-risk individuals.
Addressing gaps in the literature, the present study used SEM to examine pathways from self-reported childhood abuse history and current SES to inflammation during pregnancy through health risk indicators, recent stressors, and psychological distress in a racially diverse sample of pregnant women from varied socioeconomic backgrounds. It was hypothesized that (a) greater childhood abuse history and lower current SES would both be uniquely associated with elevated maternal serum CRP and IL-6 during pregnancy independent of race, days gestation at sampling, maternal age, and pregnancy complications (i.e., hypertension, preeclampsia, and gestational diabetes), and (b) health risk indicators (i.e., obesity, cigarette smoking, and sleep disturbance), recent stressors (i.e., interpersonal conflict, recent life events), and psychological distress (i.e., perceived stress, depressive symptoms) would explain the relationship between adversity and inflammation during pregnancy.
2. Methods and materials
2.1. Study Design
Participants included 214 pregnant adult (ages 18 and older) women who were recruited largely from faculty, staff, and students at The Ohio State University (OSU) and OSU Wexner Medical Center (OSUWMC). Participants were also recruited from the OSUWMC Prenatal Clinic and surrounding community of Columbus, Ohio. The broader study examined the effects of prior vaccination on maternal and cord blood antibody levels in a sample of pregnant women (Christian et al., 2017). Data collection for the current study occurred between October 2013 and September 2016 and consisted of two visits (baseline and 30 days later). The current secondary analyses focused on serum inflammatory markers and psychosocial data collected at the first study visit, with the exception of the Test of Negative Social Exchange (TENSE) and the Childhood Trauma Questionnaire (CTQ), which were administered at the 30-day follow-up visit.
2.2. Participants
Exclusion criteria included major immune conditions (e.g., MRSA, systemic lupus erythematosus, no active cancer diagnosis) or consumption of more than two alcoholic beverages per week per self-report or medical record review at time of enrollment. Participants were also ineligible for the study if they were not at least 18 years old, beyond 31 weeks gestation, or did not intend to deliver at OSUWMC. Women reporting acute illness, such as cold or flu symptoms, or antibiotic use within 10 days of a study visit were rescheduled. Written informed consent for research participation was obtained from all participants. In addition, HIPAA authorizations were obtained from all participants, and each received modest compensation. The study was approved by The Ohio State University Biomedical Institutional Review Board.
2.3. Measures
2.3.1. Demographics and pregnancy information
Age, race, and ethnicity were determined via self-report at the first study visit. Education level, annual household income, and perceived social class were collected by self-report as indicators of socioeconomic status. Due date and pregnancy complications (i.e., hypertension, preeclampsia, and gestational diabetes) were collected via medical record review. The presence of pregnancy complications was coded 1 = yes, 0 = no.
2.3.2. Childhood abuse
The 28-item Childhood Trauma Questionnaire (CTQ-Short Form) was used to retrospectively measure childhood maltreatment and includes the subscales of emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect (Bernstein et al., 2003). Participants rated each item on a Likert scale from 1 (“never true”) to 5 (“very often true”). The CTQ has shown predictive validity for perinatal health outcomes (Mohler et al., 2008; Shea et al., 2007). The three subscales of emotional abuse, physical abuse, and sexual abuse were used in analyses.
2.3.3. Pre-pregnancy body mass index (BMI)
Pre-pregnancy BMI (kg/m2) was determined using self-reported weight in pounds prior to pregnancy and height as measured at the first study visit.
2.3.4. Cigarette smoking
Cigarette smoking was assessed via self-report at the first study visit and was defined as current (coded 1) or not current (coded 0).
2.3.5. Sleep quality
The 19-item Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep duration, perceived sleep quality, and disturbances over the past month (Buysse et al., 1989). The PSQI possesses good internal consistency reliability and construct validity across various populations (Carpenter and Andrykowski, 1998), including pregnant women (Skouteris et al., 2009).
2.3.6. Interpersonal conflict
A modified version of the 21-item Test of Negative Social Exchange (TENSE) was administered to measure negative social experiences and interpersonal conflict (Finch et al., 1999). Participants indicated on a 5-point scale (1 = “not at all,” 5 = “about every day”) how often they had experienced various negative social interactions with important people in their life during the past month. Items on the TENSE are designed to measure anger (e.g., “Lost his or her temper with me”), insensitivity (e.g., “Took my feelings lightly”), manipulation (e.g., “Tried to get me to do things I didn’t want to”), impatience (e.g., “Tried to rush me along”), and rejection (e.g., “Didn’t want to be with me”). The TENSE possesses good psychometric properties (Finch et al., 1999; Ruehlman and Karoly, 1991).
2.3.7. Recent life events
The Prenatal Life Events Scale (PLES) was used to assess stress from life events occurring during the past year (Lobel et al., 2008). Participants reported which of 29 events they had experienced and rated the extent to which each event was undesirable or negative. The total number of life events that participants had experienced during the last year was used in analyses.
2.3.8. Perceived stress
The 10-item Perceived Stress Scale (PSS) was used to measure the subjective experiences of stress and coping with stress during the last month (Cohen et al., 1983). The PSS assesses a construct independent of depressive symptomatology (Cohen et al., 1983) and has been associated with birth outcomes in perinatal populations (Zambrana et al., 1999).
2.3.9. Depressive symptoms
The Center for Epidemiological Studies - Depression Scale (CES-D) was administered to assess depressive symptoms during the last week (Radloff, 1977). This well-validated 20-item scale assesses cognitive, emotional, interpersonal, and somatic symptoms of depression (Shafer, 2006), and has been used in a number of empirical studies assessing depressive symptoms in pregnant women (Nast et al., 2013).
2.4. Serum Parameters
Whole blood was collected into vacutainer tubes while participants were in seated positions. Samples were immediately centrifuged, aliquoted, and placed in −80°C freezer storage until analysis. Samples were assayed for CRP and IL-6 in duplicate.
High-sensitivity CRP was measured using a solid-phase chemiluminescence immunometric assay with the IMMULITE 1000 Immunoassay System (Siemens Healthcare Diagnostics, Inc., 1717 Deerfield Rd., Deerfield, IL.). Analytical sensitivity for this assay was 0.1 mg/L, and functional sensitivity was 0.3 mg/L. Inter- and intra-assay coefficients of variation were 7.3% and 3.1%, respectively.
Serum levels of IL-6 were measured by electrochemiluminescence in multiplex V-PLEX kits using a MESO QuickPlex SQ 120 instrument (Meso Scale Discovery, 1601 Research Blvd, Rockville, MD). Inter- and intra-assay coefficients of variation were 6.4% and 4.0%, respectively. Sample concentrations were extrapolated from a standard curve, and the mean calculated concentration of two sample replicates was used in analyses. The lower limit of detection for IL-6 was 0.06 pg/mL. Seven participants had IL-6 concentrations that were below fit curve range for both replicates, and two participants had IL-6 concentrations that were below fit curve range for one replicate; IL-6 values were treated as missing data for these nine participants.
2.5. Data Screening
Variables were examined for outliers and deviations from normality before analyses. Using a cut-off of 3 standard deviations from the mean, one outlier was identified for CRP (125.0 mg/L), and one outlier was identified for IL-6 (13.04 pg/mL). These two values were removed and treated as missing data. Variables were transformed if skewness was above 2.0 or if kurtosis was above 7.0 (West et al., 1995); logarithmic transformations were applied to CRP, the CTQ subscales for physical abuse and sexual abuse, and the TENSE due to extreme deviations from normality (all had a right skew before logarithmic transformations). Logarithmic transformations improved the distributions of the four transformed variables, but the CTQ subscales for physical abuse and sexual abuse, and the TENSE were still not normally distributed.
2.6. Missing Data
All variables were assessed for missing data. Across study variables, < 7% of data points were missing. Missing data were imputed using regression imputation with maximum likelihood estimates (Arbuckle, 2017). None of the imputed values were out of range. Descriptive statistics were generated with raw, non-imputed data, and the remaining analyses were conducted with imputed data.
2.7. Statistical Analyses
Descriptive statistics and bivariate correlations were computed using IBM SPSS 24.0. SEM using maximum likelihood estimations were used to evaluate the hypothesized models using AMOS 25 (Arbuckle, 2017). Maximum likelihood estimation was used because it is robust to moderate violations of the normality assumption (Anderson and Gerbing, 1984). We examined whether latent variables reflecting childhood abuse history and current SES were related to CRP and IL-6 during pregnancy, and whether these associations were explained by health risk indicators, recent stressors, and depressive symptoms.
Following the recommendations of Anderson and Gerbing (1988), we first evaluated the measurement model using an analysis equivalent to confirmatory factor analysis before assessing the structural relationships. The measurement model consisted of two exogenous latent variables using the observed indicators for childhood abuse history and current SES. The history of childhood abuse latent variable was constructed using the CTQ subscales of emotional, physical, and sexual abuse. The current SES latent variable was constructed using education, annual household income, and perceived social class. To identify the latent variables, one observed indicator of each latent variable was set to 1.0. The covariance between the latent factors was estimated in all models.
Second, we then evaluated the structural model to simultaneously estimate the independent effects of the latent variables of childhood abuse history and current SES in predicting CRP and IL-6 (Model 0). The covariance between the error terms of CRP and IL-6 was estimated in all structural models. Third, candidate mediators were entered into the structural model in single (separate) mediation analyses to estimate the viability of indirect pathways by which childhood abuse history and current SES can lead to inflammation during pregnancy (Models 1–7). Fourth, the candidate mediators found to be significant were then entered into a final multiple mediation analysis simultaneously (Model 8). To assess the strength and significance of specific indirect effects, focused estimand-based analyses were conducted (Arbuckle, 2017).
The nominal α-level for the significance tests for paths in all models was set to α = .05. A residual analysis was performed for each model to ensure that all of the standardized residuals were less than two in absolute value. Structural and mediation analyses were adjusted for race, days gestation at sampling, maternal age, and pregnancy complications by including paths from these covariates to each endogenous variable and by covarying all of the covariates and the exogenous latent variables. Overall model fit was examined using the χ2 test statistic, the BollenStine (B-S) bootstrapping procedure, the comparative fit index (CFI), and the root mean square error of approximation (RMSEA) with a 90% confidence interval (CI). Conventional criteria for acceptable model fit include a nonsignificant χ2 test statistic, a nonsignificant B-S bootstrap (Bollen and Stine, 1992), a CFI ≥ .95, a RMSEA ≤ .06, and a RMSEA maximum upper bound of 90% CI ≤ 0.10 (Hu and Bentler, 1999). The significance of indirect effects was evaluated using Monte Carlo parametric bootstrap resampling procedures with 5,000 samples and 95% biascorrected (BC) CI.
3. Results
3.1. Descriptive Statistics
Characteristics of study participants are presented in Table 1. A total of 214 women participated in the study. The average maternal age was 29.38 years (SD = 4.93; range = 18–42), 65.9% (n = 141) of the sample was White, and 52.3% (n = 112) reported having a college degree or higher. The sample was fairly diverse in terms of annual household income, with 22.4% (n = 48) reporting less than $15,000 and 21.0% (n = 45) reporting more than $100,000. The mean gestational age at the first study visit was 17.70 weeks (SD = 7.00, range: 5–31 weeks). In this sample, 19.6% (n = 42) had pregnancy complications (i.e., hypertension, preeclampsia, and gestational diabetes) per self-report or chart review, and 13.1% (n = 28) reported that they currently smoke cigarettes. Observed pre-pregnancy BMI values ranged from 18.18 to 56.88.
Table 1.
Characteristics of study participants (N = 214).
| Variable | Mean (SD) or n (%) |
|---|---|
| Maternal age [mean (SD)] | 29.3 (5.0) |
| Race [n (%)] | |
| White | 141 (65.9) |
| Black/African American | 60 (28.0) |
| Asian American or Multiracial | 13 (6.1) |
| Ethnicity [n (%)] | |
| Non-Hispanic | 207 (96.7) |
| Hispanic | 7 (3.3) |
| Education [n (%)] | |
| Less than high school | 17 (7.9) |
| High school | 25 (11.7) |
| Some college or 2-year degree | 60 (28.0) |
| College degree | 44 (20.6) |
| Graduate school | 68 (31.8) |
| Income [n (%)] | |
| <$15,000 | 48 (22.4) |
| $15,000-$29,999 | 33 (15.4) |
| $30,000-$49,999 | 26 (12.1) |
| $50,000-$74,999 | 27 (12.6) |
| $75,000-$99,999 | 35 (16.4) |
| >$100,000 | 45 (21.0) |
| Perceived social class [n (%)] | |
| Loer | 29 (13.6) |
| orking | 51 (23.8) |
| Loer top | 85 (39.7) |
| Upper top or higher | 49 (22.9) |
| Days gestation [mean (SD)] | 123.9 (49.0) |
| Pregnancy complications [n (%)] | 42 (19.6) |
| Current smoker [n (%)] | 28 (13.1) |
| Pre-pregnancy BMI [n (%)] | |
| Undereight | 1 (0.5) |
| Normal eight | 103 (48.1) |
| Overeight | 48 (22.4) |
| Obese | 62 (29.0) |
3.2. Measurement Model
The results of the factor analysis used to evaluate the measurement model, which included the exogenous latent variables of childhood abuse history and current SES, indicated acceptable model fit, χ2 (8) = 12.864, p = .117; B-S bootstrap p = .166, CFI = .993, RMSEA = .053 [.000, .105]. Standardized parameter estimates and squared multiple correlation values for the factor analysis are provided in Figure 1. All indicators loaded onto their intended latent variables, and the standardized factor loadings were all significant (p’s < .001).
Figure 1.
Measurement model.
Note. Squared multiple correlations are shown above the observed indicator boxes. Error terms are not shown for visual clarity. SES = socioeconomic status. **p < .001.
Table 2 displays the bivariate correlations between the inflammatory markers, latent variables, candidate mediators, and covariates. Consistent with hypotheses, childhood abuse history demonstrated positive associations with CRP and IL-6, while inverse associations were observed for current SES with CRP and IL-6 (p’s < 0.01). CRP and IL-6 were positively associated (r = .54, p < .01), while the childhood abuse history and current SES latent variables were negatively associated (r = −.48, p < .01).
Table 2.
Bivariate correlations between study variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| 1. CRP± | — | ||||||||||||
| 2. IL-6 | .54** | — | |||||||||||
| 3. Childhood abuse history | .29** | .30** | — | ||||||||||
| 4. Current SES | −.22** | −.26** | −.48** | — | |||||||||
| 5. BMI | .58** | .59** | .31** | −.26** | — | ||||||||
| 6. Smoking | .18** | .11 | .35** | −.49** | .09 | — | |||||||
| 7. PSQI | .12 | .25** | .55** | −.30** | .20** | .13 | — | ||||||
| 8. TENSE± | .09 | .24** | .48** | −.32** | .07 | .17* | .29** | — | |||||
| 9. PLES | .18** | .23** | .66** | −.52** | .24** | .38** | .44** | .51** | — | ||||
| 10. PSS | .08 | .18* | .52** | −.25** | .05 | .06 | .37** | .45** | .41** | — | |||
| 11. CES-D | .08 | .23** | .61** | −.41** | .13 | .13 | .44** | .40** | .38** | .74** | — | ||
| 12. Maternal age | −.09 | −.05 | −.18** | .67** | −.05 | −.18** | −.11 | −.13 | −.29** | −.07 | −.20** | — | |
| 13. Days gestation | .12 | .14* | .00 | −.06 | −.03 | .01 | .08 | .07 | .02 | .03 | .05 | −.10 | — |
Note. Smoking was defined as current (coded 1) or not current (coded 0) at the first study visit. CRP = C-reactive protein. IL-6 = interleukin-6. SES = socioeconomic status. BMI = (pre-pregnancy) body mass index. PSQI = Pittsburgh Sleep Quality Index. TENSE = Test of Negative Social Exchange. PLES = Prenatal Life Events Scale. PSS = Perceived Stress Scale. CES-D = Center for Epidemiological Studies – Depression. ±Variable has been log transformed.
p < .05.
p < .01.
In relation to the candidate mediators, pre-pregnancy BMI, smoking, and recent life events (PLES) were each positively associated with CRP (Table 2). In addition, pre-pregnancy BMI, sleep quality (PSQI), interpersonal conflict (TENSE), recent life events (PLES), perceived stress (PSS), and depressive symptoms (CES-D) were each positively associated with IL-6. As predicted, all seven candidate mediators were positively associated with childhood abuse history and negatively associated with current SES, supporting further planned analyses.
3.3. Structural Model
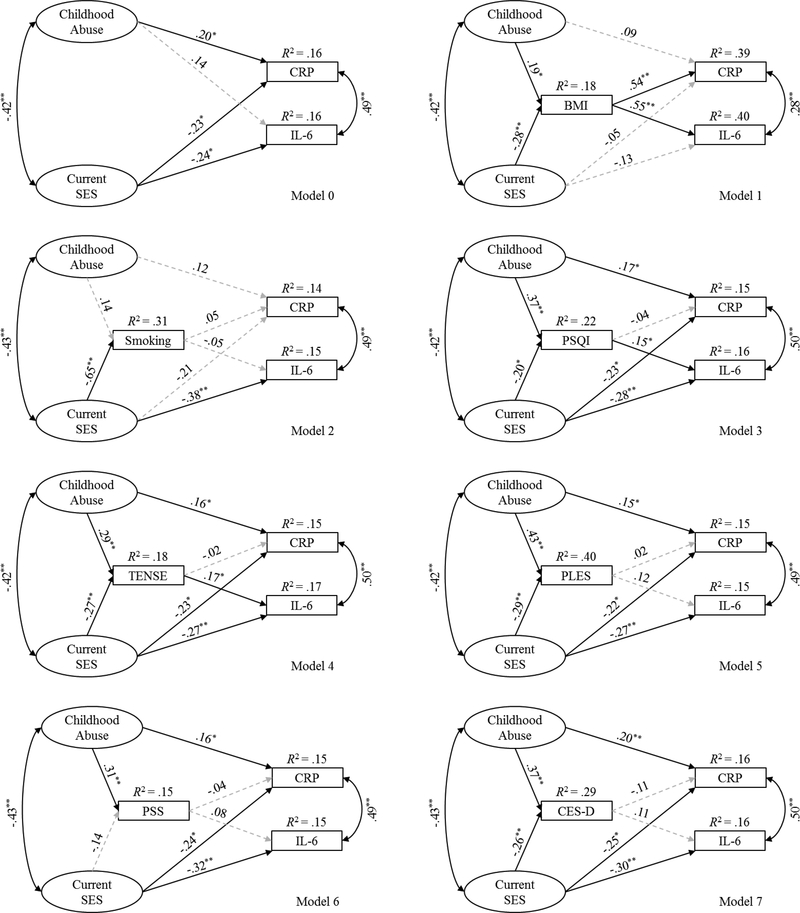
The latent variables of childhood abuse history and current SES were then tested in a structural model to examine the independent effects of childhood abuse history and current SES in predicting CRP and IL-6. Analyses adjusted for race, days gestation at sampling, maternal age, and pregnancy complications. The structural model (Model 0; Fig. 2) demonstrated good fit, χ2 (32) = 35.773, p = .269; B-S bootstrap p = .329, CFI = .996, RMSEA = .024 [.000, .058]. See Figure 2 for standardized parameter estimates and the proportion of variance accounted for (R2) in the endogenous variables. Childhood abuse history predicted CRP but not IL-6, while current SES predicted both CRP and IL-6. To clarify the model, the nonsignificant path between childhood abuse history and IL-6 was dropped. The trimmed model also indicated a good fit to the data, χ2 (33) = 36.409, p = .313; B-S bootstrap p = .349, CFI = .996, RMSEA = .022 [.000, .056]. Childhood abuse history predicted CRP (β = .155, p = .026), and current SES predicted CRP (β = −.228, p = .035) and IL-6 (β = −.346, p < .001). The trimmed model explained 14.8% of the variance in CRP and 14.5% of the variance in IL-6.
Figure 2.
Standardized regression coefficients for Models 0 through 7.
Note. Significant paths are shown in black solid lines, and nonsignificant paths are shown in gray dashed lines. The observed indicators for the two latent variables, error terms, and covariates (i.e., race, days gestation at sampling, maternal age, and pregnancy complications) are not shown for visual clarity. CRP = C-reactive protein. IL-6 = interleukin-6. SES = socioeconomic status. BMI = (pre-pregnancy) body mass index. PSQI = Pittsburgh Sleep Quality Index. TENSE = Test of Negative Social Exchange. PLES = Prenatal Life Events Scale. PSS = Perceived Stress Scale.
CES-D = Center for Epidemiological Studies – Depression. *p < .05. **p < .01.
3.4. Candidate Mediators in Single Mediation Analyses
In order to test indirect pathways that may explain the associations of childhood abuse history and current SES with inflammatory markers, seven single mediation analyses were conducted using the trimmed structural model (Models 1–7; Fig. 2). Each of the single mediation models demonstrated good fit (see Table 3 for model fit indices). Figure 2 provides the standardized parameter estimates and the proportion of variance accounted for in the endogenous variables for Models 1–7, and Table 4 shows the unstandardized indirect effects of each candidate mediator after controlling for the direct paths from childhood abuse history and current SES to the inflammatory markers. Pre-pregnancy BMI statistically explained the relationship between childhood abuse history and CRP. Pre-pregnancy BMI, PSQI, and TENSE statistically explained the relationship between current SES and IL-6 in separate models. Pre-pregnancy BMI also statistically explained the relationship between current SES and CRP. None of the other indirect effects were statistically significant (p’s > .05).
Table 3.
Model fit indices for the single mediation models.
| Model | χ2 (df), p-value | B-S bootstrap p-value | CFI | RMSEA [CI] |
|---|---|---|---|---|
| 1 | 37.842 (37), p = .431 | .492 | .999 | .010 [.000, .050] |
| 2 | 46.236 (37), p = .142 | .181 | .990 | .034 [.000, .062] |
| 3 | 45.643 (37), p = .156 | .197 | .991 | .033 [.000, .062] |
| 4 | 49.518 (37), p = .082 | .112 | .987 | .040 [.000, .067] |
| 5 | 46.793 (37), p = .130 | .168 | .990 | .035 [.000, .063] |
| 6 | 41.441 (37), p = .283 | .333 | .995 | .024 [.000, .056] |
| 7 | 43.618 (37), p = .211 | .256 | .993 | .029 [.000, .059] |
Note. B-S = Bollen-Stine. CFI = comparative fit index. RMSEA = root mean square error of approximation with a 90% confidence interval (CI).
Table 4.
Unstandardized indirect effects of childhood abuse history and current SES on CRP and IL-6 via candidate mediators for the single mediation analyses.
| Model | Indirect Paths | Unstandardized Coefficient (SE) | 95% BC CI | p-value |
|---|---|---|---|---|
| 1 | Abuse → BMI → CRP | .011 (.005) | .002, .021 | .019 |
| SES → BMI → CRP | −.041 (.017) | −.077, −.010 | .007 | |
| SES → BMI → IL-6 | −.030 (.012) | −.056, −.008 | .007 | |
| 2 | Abuse → Smoking → CRP | .001 (.001) | −.001, .005 | .322 |
| SES → Smoking → CRP | −.009 (.015) | −.040, .018 | .476 | |
| SES → Smoking → IL-6 | .006 (.011) | −.015, .029 | .588 | |
| 3 | Abuse → PSQI → CRP | −.001 (.003) | −.008, .004 | .617 |
| SES → PSQI → CRP | .002 (.005) | −.005, .015 | .472 | |
| SES → PSQI → IL-6 | −.009 (.004) | −.019, −.001 | .041 | |
| 4 | Abuse → TENSE → CRP | −.001 (.002) | −.006, .004 | .700 |
| SES → TENSE → CRP | .002 (.006) | −.008, .015 | .634 | |
| SES → TENSE → IL-6 | −.008 (.005) | −.023, −.001 | .014 | |
| 5 | Abuse → PLES → CRP | .001 (.004) | −.007, .009 | .845 |
| SES → PLES → CRP | −.001 (.007) | −.016, .011 | .798 | |
| SES → PLES → IL-6 | −.007 (.005) | −.020, .001 | .071 | |
| 6 | Abuse → PSS → CRP | −.001 (.002) | −.007, .003 | .559 |
| SES → PSS → CRP | .001 (.004) | −.003, .014 | .410 | |
| SES → PSS → IL-6 | −.002 (.003) | −.013, .001 | .171 | |
| 7 | Abuse → CES-D → CRP | −.004 (.003) | −.012, .001 | .264 |
| SES → CES-D → CRP | .008 (.007) | −.001, .027 | .094 | |
| SES → CES-D → IL-6 | −.006 (.005) | −.026, .003 | .069 |
Note. Significant p−values are bolded. BC CI = bias-corrected confidence interval. Abuse = childhood abuse history latent variable. BMI = (pre-pregnancy) body mass index. CRP = Creactive protein. SES = current socioeconomic status latent variable. IL-6 = interleukin-6. PSQI = Pittsburgh Sleep Quality Index. TENSE = Test of Negative Social Exchange. PLES = Prenatal Life Events Scale. PSS = Perceived Stress Scale. CES-D = Center for Epidemiological Studies – Depression.
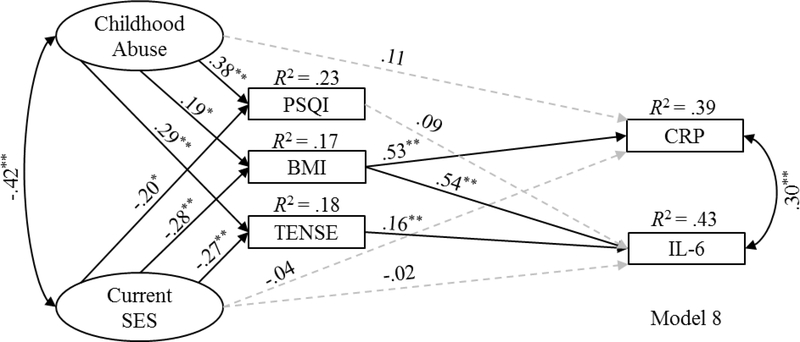
3.5. Candidate Mediators in a Multiple Mediation Analysis
The three intermediate variables that exhibited significant indirect effects (i.e., pre-pregnancy BMI, PSQI, and TENSE) were then entered into the final multiple mediation analysis (Model 8). The nonsignificant paths evidenced in the single mediation analyses between both PSQI and TENSE with CRP were not included in the final multiple mediation analysis. Model 8 indicated a good fit to the data, χ1 (50) = 66.498, p = .059; B-S bootstrap p = .082, CFI = .985, RMSEA = .039 [.000, .063].2 Figure 3 provides the standardized parameter estimates and the proportion of variance accounted for in the endogenous variables for Model 8. Childhood abuse history showed a significant indirect effect on CRP (standardized coefficient = .100, SE = .043, 95% BC CI = .017, .188, p = .019). Current SES showed a significant indirect effect on CRP (standardized coefficient = −.146, SE = .060, 95% BC CI = −.274, −.038, p = .009) and IL-6 (standardized coefficient = −.210, SE = .064, 95% BC CI = −.343, −.088, p = .002).3
Figure 3.
Standardized regression coefficients for Model 8 (multiple mediation analysis).
Note. Solid and dashed lines reflect significant and nonsignificant paths, respectively. The observed indicators for the two latent variables, error terms, and covariates (i.e., race, days gestation at sampling, maternal age, and pregnancy complications) are not shown for visual clarity. CRP = C-reactive protein. IL-6 = interleukin-6. SES = socioeconomic status. PSQI = Pittsburgh Sleep Quality Index. BMI = (pre-pregnancy) body mass index. TENSE = Test of Negative Social Exchange. *p < .05. **p < .01.
Focused estimand-based analyses were conducted to assess the strength and significance of each specific indirect path in the omnibus indirect effect estimate for current SES on IL-6. This analysis demonstrated that the current SES → pre-pregnancy BMI → IL-6 indirect path was significant (standardized coefficient = −.149, SE = .060, 95% BC CI = −.274, −.037, p = .010), as was the current SES → TENSE → IL-6 indirect path (standardized coefficient = −.043, SE = .024, 95% BC CI = −.105, −.009, p = .009); however, the current SES → PSQI → IL-6 indirect path was no longer significant (standardized coefficient = −.018, SE = .016, 95% BC CI = −.066, 001, p = .078). A comparison of the difference between the significant indirect paths through pre-pregnancy BMI and TENSE was nonsignificant (difference = −.107, SE = .066, 95% BC CI = −.241, .022, p = .106), suggesting that the two parallel indirect paths did not differ in strength.
4. Discussion
Consistent with the larger literature (Miller et al., 2017; Mitchell et al., 2018; Walsh et al., 2016), the current study provides further evidence that adversities experienced during childhood and adulthood are associated with elevated inflammatory markers during pregnancy. Specifically, bivariate correlations revealed that a history of childhood abuse was positively associated with both CRP and IL-6 in a sample of pregnant women, while current SES was negatively associated with both CRP and IL-6. When the unique effects of childhood abuse history and low current SES on inflammatory markers were examined after adjusting for race, days gestation at sampling, maternal age, and pregnancy complications (i.e., hypertension, preeclampsia, and gestational diabetes) using SEM, low current SES continued to be associated with both CRP and IL-6, whereas childhood abuse history was associated with CRP but not IL-6.
The current findings in a sample of pregnant women replicate and extend the results of studies in non-pregnant samples that have shown lower current SES to be associated with elevations in CRP and IL-6 (Elliot and Chapman, 2016; Gimeno et al., 2007; Hemingway et al., 2003; Koster et al., 2006; Osler et al., 2015). These results also extend upon studies that have demonstrated a history of childhood abuse to be associated with elevated CRP in non-pregnant individuals after adjusting for SES (Baumeister et al., 2016; Coelho et al., 2014; Danese et al., 2007). Although empirical studies in pregnant women are limited, these data support prior research from our group linking childhood abuse history to elevated CRP, but not IL-6, after adjusting for current SES within a different and smaller cohort of 77 women (Mitchell et al., 2018). In contrast to previous research (Baumeister et al., 2016), the association between childhood abuse history and IL-6 was not replicated in our sample, further adding to mixed findings (for review see Coelho et al., 2014). As described, although childhood abuse history and IL-6 were positively correlated (r = .30, p < .01), childhood abuse history was no longer associated with IL-6 when entered into the structural model with current SES to simultaneously estimate their unique effects. This finding suggests that, in some cohorts, associations between childhood abuse history and IL-6 may be better accounted for by other related risk factors. In order to understand the processes by which adversity may lead to elevations in inflammatory markers, indicators of health risk (i.e., pre-pregnancy BMI, cigarette smoking, and sleep quality), recent stressors (i.e., interpersonal conflict, recent life events), and psychological distress (i.e., perceived stress, depressive symptoms) were examined as potential pathways through which childhood abuse history and current SES leads to elevations in CRP and IL-6, adjusting for race, days gestation at sampling, maternal age, and pregnancy complications. Both greater childhood abuse history and lower current SES were linked to elevated CRP via pre-pregnancy BMI. In addition, three significant indirect pathways emerged in single mediation analyses between current SES and IL-6, demonstrating that lower current SES was linked to elevated IL-6 via pre-pregnancy BMI, sleep quality, and interpersonal conflict. When simultaneously estimating the unique effects of these three explanatory pathways, pre-pregnancy BMI and interpersonal conflict emerged as parallel pathways by which low current SES leads to elevations in IL-6 during pregnancy; the indirect path through sleep quality was no longer significant after controlling for the effects of both pre-pregnancy BMI and interpersonal conflict. These data demonstrate that obesity and interpersonal conflict are two independent mechanisms by which low current SES is associated with elevated IL-6 during pregnancy.
Of note, pre-pregnancy BMI accounted for a substantial proportion of variance in both CRP and IL-6, more than any of the other candidate mediators or adversity variables combined. For example, when interpersonal conflict was entered as a candidate mediator in a single mediation analysis, the model explained 17.0% of the variance in IL-6. When pre-pregnancy BMI was entered as a candidate mediator in a single mediation analysis, the model explained 39.9% of the variance in IL-6. When pre-pregnancy BMI, sleep quality, and interpersonal conflict were entered together as candidate mediators, the model explained 43.3% of the variance in IL-6, only 3.4% more than pre-pregnancy BMI alone. This result demonstrates that obesity status appears to be the biggest driver of inflammatory markers during pregnancy and suggests that clinical interventions that target maternal obesity prior to pregnancy may mitigate the effects of adversity on perinatal health. As described, pre-pregnancy BMI explained the effect of childhood abuse history on CRP, and pre-pregnancy BMI also explained the effect of current SES on both CRP and IL-6. These data in a sample of pregnant women replicate and extend the results of studies in non-pregnant samples that have shown early childhood adversity to affect CRP indirectly through BMI (Matthews et al., 2014; Raposa et al., 2014). Regarding data during pregnancy, a recent study demonstrated that pre-pregnancy BMI explained the relationship between childhood physical abuse and both CRP and IL-6 after adjusting for indicators of SES in a diverse sample of 77 pregnant women (Mitchell et al., 2018). Although the finding that prepregnancy BMI explained the relationship between childhood abuse history and IL-6 was not replicated, the finding that pre-pregnancy BMI explained the relationship between childhood abuse history and CRP was replicated in the current study.
In addition to pre-pregnancy BMI, interpersonal conflict was the other independent pathway by which low current SES was associated with elevated IL-6 in the current study. One explanation that may account for this finding is that individuals of lower SES are more likely to interpret ambiguous social situations as more negative than individuals of higher SES (Chen et al., 2010; Chen and Matthews, 2001; Flory et al., 1998). In addition, individuals of low SES are exposed to more frequent stressors, such as more interpersonal conflict, than individuals of high SES (Evans et al., 2013; Gallo and Matthews, 2003; Matthews et al., 2000). It is possible that this cumulative exposure to stress taxes neuroendocrine systems, further contributing to excessive inflammation in individuals of low SES.
Although sleep quality explained the relationship between current SES and IL-6 in a single mediation analysis, sleep quality no longer accounted for this relationship when entered into a multiple mediation analysis with pre-pregnancy BMI and interpersonal conflict. Sleep disruption has been shown to confer risk for obesity (for review see Broussard and Van Cauter, 2016) and interpersonal conflict (Gordon and Chen, 2014). Of note, poor sleep quality as operationalized as higher scores on the PSQI was positively correlated with both pre-pregnancy BMI (r = .20, p < .01) and interpersonal conflict (r = .29, p < .01), whereas pre-pregnancy BMI and interpersonal conflict were not correlated (r = .07, p = .39). These findings highlight the importance of accounting for the shared variance among correlated risk factors in order to identify clearer targets for treatment and prevention efforts.
Inconsistent with our hypotheses, cigarette smoking, recent life events, perceived stress, and depressive symptoms did not explain the relationship between adversity and inflammation in the current study. Cigarette smoking likely did not account for the relationship between adversity and inflammation due to low statistical power: only 13.1% of women endorsed cigarette use at the baseline study visit. Regardless, these nonsignificant results are particularly noteworthy because when entered into the structural model in order to examine independent and interrelated pathways, cigarette smoking was no longer associated with childhood abuse history or CRP, recent life events was no longer associated with CRP or IL-6, perceived stress was no longer associated with current SES or IL-6, and depressive symptoms was no longer associated with IL6. Similar to the findings for sleep quality, these findings further highlight the importance of examining the unique variance among correlated risk factors for intervention efforts.
Several limitations must be considered when interpreting the results of the current study. We utilized retrospective self-reports of childhood abuse and self-reported weight prior to pregnancy. Self-reported weight prior to pregnancy is strongly associated with measured weight (Shin et al., 2014), and self-reported childhood abuse history as measured by the CTQ-Short Form demonstrates good criterion-related validity with therapist ratings of childhood abuse (Bernstein et al., 2003). Nevertheless, it is possible that our findings may have been affected by response biases. In addition, we did not include a non-pregnant comparison group in our study. As noted earlier, a healthy pregnancy is characterized by an enhanced inflammatory state in comparison to non-pregnancy. The inclusion of a non-pregnant comparison group would help clarify whether our results are specific to pregnant women or generalizable to women of reproductive age. We also did not obtain data from a large enough sample size to permit us to conduct subgroup analyses by trimester. While there is variability in sampling timing, our sample was predominately assessed in mid-pregnancy (57.5% of our participants were assessed during their second trimester). Although we controlled for days gestation at sampling in our study, it is possible that our results could vary by trimester. In addition, given that 51.4% of participants in our sample were either overweight (n = 48) or obese (n = 62), the observed effects may only be applicable to cultures with high rates of obesity. It is also important to note that causal inferences cannot be made from our results due to the cross-sectional, correlational study design.
Finally, the current analyses focused on risk pathways to inflammation and did not examine protective pathways (e.g., social support) that may buffer against the development of excessive inflammation in pregnant women who have experienced adversity. Although the multiple mediation model explained 39.1% of the variance in CRP and 43.3% of the variance in IL-6, the residual variances suggest that other explanatory pathways need to be considered in the relationship between adversity and inflammation during pregnancy. Perinatal health research is thus needed that incorporates multiple risk and protective pathways across the lifespan in order to better understand the factors and processes that contribute to and protect against systemic inflammation during pregnancy (Chen and Miller, 2013).
Despite these limitations, the current study contributes to the existing literature by elucidating pathways that link risk factors during and prior to pregnancy to inflammation during pregnancy. The strengths of the present study include the use of a lifespan approach to examine the unique effects of childhood and adulthood adversities on two markers of inflammation, in addition to multiple explanatory pathways, in a large, racially diverse sample of pregnant women from varied socioeconomic backgrounds. Our results extend previous studies by identifying both independent and interrelated pathways that help explain how adversities experienced during childhood and adulthood lead to elevations in inflammatory markers during pregnancy. Based on these findings, additional research is needed to determine whether clinical interventions targeting obesity and interpersonal conflict in pregnant women reduce the adverse impact of inflammatory dysregulation on both the health of the mother and her offspring.
5. Conclusions
The results of this multiple pathway, lifespan analysis demonstrate plausible explanations for how adversities experienced during childhood and adulthood lead to inflammation during pregnancy. Our results suggest that women who have been exposed to significant adversity may be at particular risk for obesity, sleep disruption, and interpersonal conflict, in addition to related immune dysregulation during pregnancy. In addition, pre-pregnancy BMI and interpersonal conflict appear to be two independent pathways by which adversity is associated with increased inflammation during pregnancy. Our research suggests that maternal obesity and interpersonal conflict may be targets of intervention for pregnant women who have experienced adversity; thus, intervention research is warranted.
Highlights.
Childhood abuse predicted higher serum C-reactive protein (CRP) in pregnant women.
Lower socioeconomic status (SES) predicted higher CRP and interleukin (IL)-6.
Both childhood abuse and lower SES were linked to higher CRP via body mass.
Lower SES was linked to higher IL-6 via body mass, sleep, and social conflict.
Body mass and interpersonal conflict are independent pathways between SES and IL-6.
Acknowledgements
This study was supported by NINR (R01NR013661, LMC). The project described was also supported by Award Number Grant UL1TR001070 from the National Center For Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Covarying the error terms of the three candidate mediators in the multiple mediation analysis as recommended by Preacher and Hayes (2008) did not substantially change the parameter estimates or the model fit indices, χ2 (47) = 62.152, p = .068; B-S bootstrap p = .095, CFI = .987, RMSEA = .039 [.000, .063].
The multiple mediation analysis was also conducted without the covariates. With the exception of a significant χ2, the fit indices were acceptable for this model, χ2 (34) = 52.006, p = .025; B-S bootstrap p = .139, CFI = .982, RMSEA = .050 [.018, .076]. There remained a significant indirect effect of childhood abuse history on CRP (standardized coefficient = .127, SE = .059, 95% BC CI = .014, .243, p = .029). There also remained a significant indirect effect of current SES on IL-6 (standardized coefficient = −.134, SE = .056, 95% BC CI = −.248, −.027, p = .013); however, the indirect effect of current SES on CRP was no longer significant without inclusion of the covariates (standardized coefficient = −.090, SE = .049, 95% BC CI = −.189, .008, p = .071)
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL, 1994. Socioeconomic status and health: The challenge of the gradient. American Psychologist 49, 15–24. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Gerbing DW, 1984. The effect of sampling error on convergence, improper solutions, and goodness-of-fit indexes for maximum-likelihood confirmatory factor-analysis. Psychometrika 49, 155–173. [Google Scholar]
- Anderson JC, Gerbing DW, 1988. Structural equation modeling in practice - a review and recommended 2-step approach. Psychol Bull 103, 411–423. [Google Scholar]
- Arbuckle JL, 2017. IBM SPSS Amos 25 user’s guide. Amos Development Corporation, Crawfordville, FL. [Google Scholar]
- Azad MB, Lissitsyn Y, Miller GE, Becker AB, HayGlass KT, Kozyrskyj AL, 2012. Influence of socioeconomic status trajectories on innate immune responsiveness in children. PLoS One 7, e38669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V, 2016. Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatr 21, 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W, 2003. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Neglect 27, 169–190. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Moran PM, Baines R, Bunn A, Stanford K, 2002. Exploring psychological abuse in childhood: II. Association with other abuse and adult clinical depression. Bulletin of the Menninger Clinic 66, 241–258. [DOI] [PubMed] [Google Scholar]
- Blair LM, Porter K, Leblebicioglu B, Christian LM, 2015. Poor sleep quality and associated inflammation predict preterm birth: Heightened risk among African Americans. Sleep 38, 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen KA, Stine RA, 1992. Bootstrapping goodness-of-fit measures in structural equation models. Sociol Method Res 21, 205–229. [Google Scholar]
- Broussard JL, Van Cauter E, 2016. Disturbances of sleep and circadian rhythms: Novel risk factors for obesity. Current Opinion in Endocrinology, Diabetes, and Obesity 23, 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnik T, Yang S, Kaufman JS, Kramer MS, Wilkins R, 2017. Socioeconomic disparities in small-for-gestational-age birth and preterm birth. Health Rep 28, 3–10. [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, 1989. The Pittsburgh Sleep Quality Index - a new instrument for psychiatric practice and research. Psychiat Res 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA, 1998. Psychometric evaluation of the Pittsburgh Sleep Quality Index. Journal of Psychosomatic Research 45, 5–13. [DOI] [PubMed] [Google Scholar]
- Chen E, Cohen S, Miller GE, 2010. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychological Science 21, 31–37. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews KA, 2001. Cognitive appraisal biases: An approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Annals of Behavioral Medicine 23, 101–111. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, 2013. Socioeconomic status and health: Mediating and moderating factors. Annual Review of Clinical Psychology 9, 723–749. [DOI] [PubMed] [Google Scholar]
- Christian LM, 2012. Psychoneuroimmunology in pregnancy: Immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neurosci Biobehav Rev 36, 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, 2015. Stress and immune function during pregnancy: An emerging focus in mind-body medicine. Curr Dir Psychol Sci 24, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Beverly C, Mitchell AM, Karlsson E, Porter K, Schultz-Cherry S, Ramilo O, 2017. Effects of prior influenza virus vaccination on maternal antibody responses: Implications for achieving protection in the newborns. Vaccine 35, 52835290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Franco A, Glaser R, Iams JD, 2009. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun 23, 750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Porter KP, 2014. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine 70, 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho R, Viola TW, Walss-Bass C, Brietzke E, Grassi-Oliveira R, 2014. Childhood maltreatment and inflammatory markers: A systematic review. Acta Psychiat Scand 129, 180–192. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J Health Soc Behav 24, 385–396. [PubMed] [Google Scholar]
- Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D’Anna K, Argys L, Ross RG, Brandt C, Cole S, 2012. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav Immun 26, 650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry AE, Vogel I, Skogstrand K, Drews C, Schendel DE, Flanders WD, Hougaard DM, Thorsen P, 2008. Maternal plasma cytokines in early-and mid-gestation of normal human pregnancy and their association with maternal factors. Journal of Reproductive Immunology 77, 152–160. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R, 2007. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences 104, 1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel Schetter C, 2011. Psychological science on pregnancy: Stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol 62, 531–558. [DOI] [PubMed] [Google Scholar]
- Elliot AJ, Chapman BP, 2016. Socioeconomic status, psychological resources, and inflammatory markers: Results from the MIDUS study. Health Psychology 35, 12051213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Li D, Whipple SS, 2013. Cumulative risk and child development. Psychol Bull 139, 1342. [DOI] [PubMed] [Google Scholar]
- Finch JF, Okun MA, Pool GJ, Ruehlman LS, 1999. A comparison of the influence of conflictual and supportive social interactions on psychological distress. J Pers 67, 581–621. [DOI] [PubMed] [Google Scholar]
- Flory JD, Matthews KA, Owens JF, 1998. A social information processing approach to dispositional hostility: Relationships with negative mood and blood pressure elevations at work. Journal of Social and Clinical Psychology 17, 491–504. [Google Scholar]
- Gaillard R, Rifas-Shiman SL, Perng W, Oken E, Gillman MW, 2016. Maternal inflammation during pregnancy and childhood adiposity. Obesity 24, 1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LC, Matthews KA, 2003. Understanding the association between socioeconomic status and physical health: Do negative emotions play a role? Psychol Bull 129, 10–51. [DOI] [PubMed] [Google Scholar]
- Gavin AR, Nurius P, Logan-Greene P, 2012. Mediators of adverse birth outcomes among socially disadvantaged women. J Womens Health 21, 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie S, Porter K, Christian LM, 2016. Adaptation of the inflammatory immune response across pregnancy and postpartum in Black and White women. Journal of Reproductive Immunology 114, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Brunner EJ, Lowe GD, Rumley A, Marmot MG, Ferrie JE, 2007. Adult socioeconomic position, C-reactive protein and interleukin-6 in the Whitehall II prospective study. European Journal of Epidemiology 22, 675–683. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Chen S, 2014. The role of sleep in interpersonal conflict: Do sleepless nights mean worse fights? Social Psychological and Personality Science 5, 168–175. [Google Scholar]
- Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, Potkin SG, Entringer S, Wadhwa PD, Fair DA, Buss C, 2018. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol Psychiatry 83, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager AD, Runtz MG, 2012. Physical and psychological maltreatment in childhood and later health problems in women: An exploratory investigation of the roles of perceived stress and coping strategies. Child Abuse Neglect 36, 393–403. [DOI] [PubMed] [Google Scholar]
- Hemingway H, Shipley M, Mullen MJ, Kumari M, Brunner E, Taylor M, Donald AE, Deanfield JE, Marmot M, 2003. Social and psychosocial influences on inflammatory markers and vascular function in civil servants (the Whitehall II study). American Journal of Cardiology 92, 984–987. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM, 1999. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling 6, 1–55. [Google Scholar]
- Johnson JG, Cohen P, Gould MS, Kasen S, Brown J, Brook JS, 2002. Childhood adversities, interpersonal difficulties, and risk for suicide attempts during late adolescence and early adulthood. Archives of General Psychiatry 59, 741–749. [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett K, 2002. The health effects of childhood abuse: Four pathways by which abuse can influence health. Child Abuse Neglect 26, 715–729. [DOI] [PubMed] [Google Scholar]
- Kim J, Talbot NL, Cicchetti D, 2009. Childhood abuse and current interpersonal conflict: The role of shame. Child Abuse Neglect 33, 362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster A, Bosma H, Penninx BW, Newman AB, Harris TB, Van Eijk JTM, Kempen GI, Simonsick EM, Johnson KC, Rooks RN, 2006. Association of inflammatory markers with socioeconomic status. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 61, 284–290. [DOI] [PubMed] [Google Scholar]
- Lee BK, Magnusson C, Gardner RM, Blomstrom A, Newschaffer CJ, Burstyn I, Karlsson H, Dalman C, 2015. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun 44, 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA, 2008. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol 27, 604–615. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Kaplan GA, Shema SJ, 1997. Cumulative impact of sustained economic hardship on physical, cognitive, psychological, and social functioning. New England Journal of Medicine 337, 1889–1895. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Chang YF, Thurston RC, Bromberger JT, 2014. Child abuse is related to inflammation in mid-life women: Role of obesity. Brain Behav Immun 36, 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Räikkönen K, Everson SA, Flory JD, Marco CA, Owens JF, Lloyd CE, 2000. Do the daily experiences of healthy men and women vary according to occupational prestige and work strain? Psychosomatic Medicine 62, 346–353. [DOI] [PubMed] [Google Scholar]
- Messman-Moore TL, Coates AA, 2007. The impact of childhood psychological abuse on adult interpersonal conflict: The role of early maladaptive schemas and patterns of interpersonal behavior. Journal of Emotional Abuse 7, 75–92. [Google Scholar]
- Miller GE, Culhane J, Grobman W, Simhan H, Williamson DE, Adam EK, Buss C, Entringer S, Kim KY, Felipe Garcia-Espana J, Keenan-Devlin L, McDade TW, Wadhwa PD, Borders A, 2017. Mothers’ childhood hardship forecasts adverse pregnancy outcomes: Role of inflammatory, lifestyle, and psychosocial pathways. Brain Behav Immun 65, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AM, Porter K, Christian LM, 2018. Examination of the role of obesity in the association between childhood trauma and inflammation during pregnancy. Health Psychol 37, 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler E, Matheis V, Marysko M, Finke P, Kaufmann C, Cierpka M, Reck C, Resch F, 2008. Complications during pregnancy, peri- and postnatal period in a sample of women with a history of child abuse. J Psychosom Obst Gyn 29, 193–198. [DOI] [PubMed] [Google Scholar]
- Nast I, Bolten M, Meinlschmidt G, Hellhammer DH, 2013. How to measure prenatal stress? A systematic review of psychometric instruments to assess psychosocial stress during pregnancy. Paediatr Perinat Ep 27, 313–322. [DOI] [PubMed] [Google Scholar]
- Osler M, Bruunsgaard H, Lykke Mortensen E, 2015. Lifetime socio-economic position and depression: An analysis of the influence of cognitive function, behaviour and inflammatory markers. The European Journal of Public Health 25, 1065–1069. [DOI] [PubMed] [Google Scholar]
- Pampel FC, Krueger PM, Denney JT, 2010. Socioeconomic disparities in health behaviors. Annual Review of Sociology 36, 349–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G, 2008. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. J Epidemiol Community Health 62, 484–491. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF, 2008. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 40, 879–891. [DOI] [PubMed] [Google Scholar]
- Radloff LS, 1977. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurements 1, 385–401. [Google Scholar]
- Raposa EB, Bower JE, Hammen CL, Najman JM, Brennan PA, 2014. A developmental pathway from early life stress to inflammation: The role of negative health behaviors. Psychological Science 25, 1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Gotsch F, Pineles B, Kusanovic JP, 2007. Inflammation in pregnancy: Its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev 65, S194–S202. [DOI] [PubMed] [Google Scholar]
- Ruehlman LS, Karoly P, 1991. With a little flak from my friends: Development and preliminary validation of the Test of Negative Social Exchange (TENSE). Psychological Assessment: A Journal of Consulting and Clinical Psychology 3, 97–104. [Google Scholar]
- Shafer AB, 2006. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol 62, 123–146. [DOI] [PubMed] [Google Scholar]
- Sharma A, Satyam A, Sharma JB, 2007. Leptin, IL–10 and inflammatory markers (TNF∝, IL–6 and IL–8) in pre–eclamptic, normotensive pregnant and healthy non–pregnant women. American Journal of Reproductive Immunology 58, 21–30. [DOI] [PubMed] [Google Scholar]
- Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M, 2007. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: Preliminary results. Psychoneuroendocrinology 32, 1013–1020. [DOI] [PubMed] [Google Scholar]
- Shin D, Chung H, Weatherspoon L, Song WO, 2014. Validity of prepregnancy weight status estimated from self-reported height and weight. Matern Child Hlth J 18, 1667–1674. [DOI] [PubMed] [Google Scholar]
- Silva LM, Coolman M, Steegers EAP, Jaddoe VWV, Moll HA, Hofman A, Mackenbach JP, Raat H, 2008. Low socioeconomic status is a risk factor for preeclampsia: The Generation R Study. J Hypertens 26, 1200–1208. [DOI] [PubMed] [Google Scholar]
- Skouteris H, Wertheim EH, Germano C, Paxton SJ, Milgrom J, 2009. Assessing sleep during pregnancy: A study across two time points examining the Pittsburgh Sleep Quality Index and associations with depressive symptoms. Women’s Health Issues 19, 45–51. [DOI] [PubMed] [Google Scholar]
- Smith MV, Gotman N, Yonkers KA, 2016. Early childhood adversity and pregnancy outcomes. Matern Child Hlth J 20, 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K, Basu A, Werner E, Lee S, Feng T, Osborne LM, Rainford A, Gilchrist M, Monk C, 2016. Associations among child abuse, depression, and interleukin-6 in pregnant adolescents: Paradoxical findings. Psychosom Med 78, 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EL, Longhurst JG, Mazure CM, 1999. Childhood sexual abuse as a risk factor for depression in women: Psychosocial and neurobiological correlates. Am J Psychiat 156, 816–828. [DOI] [PubMed] [Google Scholar]
- West SG, Finch JF, Curran PJ, 1995. Structural equation models with nonnormal variables: Problems and remedies In: Hoyle RH (Ed.), Structural Equation Modeling: Concepts, Issues, and Applications. Sage Publications, Inc, Thousand Oaks, CA, US, pp. 56–75. [Google Scholar]
- Widom CS, Czaja SJ, Dutton MA, 2008. Childhood victimization and lifetime revictimization. Child Abuse Neglect 32, 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrana RE, Dunkel-Schetter C, Collins NL, Scrimshaw SC, 1999. Mediators of ethnic associated differences in infant birth weight. J Urban Health 76, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]