Abstract
During the 3rd trimester, large-scale neural circuits are formed in the human brain, resulting in a highly efficient and segregated connectome at birth. Despite recent findings identifying important preterm human brain network properties such as rich-club organization, how the structural network develops differentially across brain regions and among different types of connections in this period is not yet known. Here, using high resolution diffusion MRI of 77 preterm-born and full-term neonates scanned at 31.9–41.7 postmenstrual weeks (PMW), we constructed structural connectivity matrices and performed graph-theory-based analyses. Faster increases of nodal efficiency were mainly located at the brain hubs distributed in primary sensorimotor regions, superior-middle frontal, and precuneus regions during 31.9–41.7PMW. Higher rates of edge strength increases were found in the rich-club and within-module connections, compared to other connections. The edge strength of short-range connections increased faster than that of long-range connections. Nodal efficiencies of the hubs predicted individual postmenstrual ages more accurately than those of non-hubs. Collectively, these findings revealed more rapid efficiency increases of the hub and rich-club connections as well as higher developmental rates of edge strength in short-range and within-module connections. These jointly underlie network segregation and differentiated emergence of brain functions.
Keywords: baby connectome, differentiated maturation, structural connectivity, segregation, diffusion MRI, brain network
Introduction
In the last weeks before birth, large-scale brain circuit formation underlies the emergence of the human connectome at a macroscale level. Massive development of cortico-cortical axonal pathways before birth offers a structural basis to establish an adult-like brain network (e.g. Kostović and Jovanov-Milošević 2006; Kostović and Judaš 2010). The neuronal activities associated with the circuit formation undergo substantial remodeling after birth (e.g. LaMantia and Rakic 1990; Innocenti and Price 2005). As suggested by previous neuropathological studies (e.g. Huttenlocher and Dabholkar 1997), regionally differentiated developments of neuronal connections are associated with heterogeneous emergence of brain functions, with primary sensorimotor function generally emerging earlier than higher-order cognitive functions. However, little is known about the structural organization of neural networks at the macroscale level during this critical period. Knowledge of the ontogeny of the human connectome during late fetal development may provide not only insight into typical brain development, but also a reference for elucidating the complex trajectories of atypical or aberrant neurodevelopment.
Diffusion MRI (dMRI) has been extensively used to study entire brain connectivity in vivo and non-invasively. With dMRI-based tractography (e.g. Mori et al. 1999), the emergence of white matter (WM) fibers has been delineated in the fetal brain as early as the beginning of the 2nd trimester (e.g. Huang et al. 2006; Huang et al. 2009; Vasung et al. 2010; Takahashi et al. 2012; Huang and Vsung, 2014; Ouyang et al. 2015). Consistent with histological atlases (Bayer and Altman 2004), these dMRI findings have demonstrated that limbic WM fibers appear earlier while the association WM fibers constituting major cortico-cortical connectivity appear later. By the start of the 3rd trimester, except the arcuate fasciculus, all other major WM fibers can be identified with dMRI (Feng et al., 2016). Asynchronous and heterogeneous maturation of WM across regions in the 3rd trimester has also been suggested by other neuroimaging studies (e.g. Hüppi et al. 1998; Partridge et al. 2004; Bui et al. 2006; Aeby et al. 2009). In addition, it has been found that cortical microstructure of different cortical regions undergoes a differential maturation pattern (McKinstry et al. 2002; DeIpolyi et al. 2005; Huang et al. 2013; Yu et al. 2016), also assessed with dMRI.
Although literature revealed spatiotemporally heterogeneous development of both cortical regions and the WM pathways linking them, few studies have delineated differential maturation pattern of structural connectivity from the perspective of a macro-scale connectome during the 3rd trimester. The baby brain connectome (For a review, see e.g. Cao et al. 2017b) reveals the inter-regional connectivity pattern, in contrast to individual WM fiber bundles or brain regions. In a brain connectome, some regions are more interconnected with other brain regions, constituting “hubs” within the global network topography (e.g. Hagmann et al. 2008; Gong et al. 2009). Furthermore, these hub regions tend to be densely interconnected with each other forming a rich-club organization (van den Heuvel and Sporns 2011). Rich-club serves as a highly efficient backbone for integration of neuronal activity across distributed circuits and presumably forms the foundation of complex neurological functions (van den Heuvel et al. 2012). The preterm and term-born brain connectome has been investigated with dMRI tractography and subsequent graph-theory analysis (Tymofiyeva et al. 2013; Ball et al. 2014; Brown et al. 2014; van den Heuvel et al. 2015; Batalle et al. 2017; Song et al., 2017). These studies support emergence of the hub regions and rich club organization during the 3rd trimester (Ball et al. 2014; van den Heuvel et al. 2015). However, regionally differentiated maturation rates during the 3rd trimester quantified by connectomic measures of brain hubs, rich-club modules, short- and long-range connections have not yet been determined. In addition, it remains to be determined how differentiated connectional maturation contributes to the segregation process of the structural organization of the baby brain. With structural connectivity underlying functional connectivity, the present connectomic study offers a unique view for understanding the structural substrate of emerging brain functions.
In this study, we hypothesized that the structural network maturation across brain regions and among different types of connections is differentiated, underlying emergence of an efficient and segregated brain connectome at birth. Relatively high resolution (1.5 × 1.5 × 1.6 mm3) dMRI images of 77 preterm-born or term-born neonates scanned from 31.9 to 41.7 postmenstrual weeks (PMW) (Engle 2004) were acquired. The structural connectivity matrix of each neonate was constructed with dMRI tractography. With comprehensive graph theory analysis done at global, modular and regional connection levels, we examined cross-sectional age-dependent developmental rate of the preterm and term-born brain network measures across different brain regions and connections.
Materials and Methods
Preterm subjects
The study was approved by the Institutional Review Board (IRB) of the University of Texas Southwestern Medical Center. 77 normal neonates (47 males and 30 females) were recruited from Parkland Memorial Hospital at Dallas. These neonates were scanned between 31.9 to 41.7 PMW, with postmenstrual age defined in accordance with Engle’s criteria (Engle 2004). All preterm and term-born neonates underwent MR imaging as part of a study of normal prenatal and perinatal development; no neonates were scanned under clinical indications. Moreover, these neonates were recruited after rigorous screening procedures conducted by a board-certified neonatologist (LC) and an experienced pediatric radiologist (NR), based on the ultrasounds, clinical MRIs and medical records of the neonates and their mothers. Exclusion criteria included evidence of bleeding or intracranial abnormality by serial sonography; mother’s excessive drug or alcohol abuse during pregnancy; grade III-IV intraventricular hemorrhage; periventricular leukomalacia; hypoxic-ischemic encephalopathy; lung disease or brochopulmonary dysplasia; body or heart malformations; chromosomal abnormalities; necrotizing enterocolitis that requires intestinal resection or complex feeding/nutritional disorders; defects or anomalies of forebrain, brainstem or cerebellum; brain tissue dys- or hypoplasias; abnormal meninges; alterations in the pial or ventricular surface; or white matter lesions. Written and informed parental consents were obtained from the subject’s mother (or father if married). Detailed characteristics regarding this cohort are provided in Table 1.
Table 1.
Demographic information of scanned neonates (C for C-section and V for vaginal birth; B for breast-feeding and F for formula).
| Number of infants | Age range (weeks) | Age mean (weeks) | Weight range (kg) | Weight mean (kg) | Male, n (%) | White, n (%) | Mode of delivery | Feeding practice | Antibiotic Exposure during pregnancy | |
|---|---|---|---|---|---|---|---|---|---|---|
| At birth | 77 | 25.0–41.4 | 33.7 | 0.8–4.0 | 2.1 | 47 (61) | 59 (77) | C:29; V:48 | B: 77; F: 0 | Yes |
| At scan | 77 | 31.9–41. 7 | 37.2 | 1.4–4.1 | 2.6 | 47 (61) | 59 (77) | C:29; V:48 | B: 77; F: 0 | Yes |
MRI Acquisition
All neonates were scanned with a Philips 3.0 T Achieva MR scanner at the Children’s Medical Center, Dallas. They were well-fed before scanning. During scan, all neonates were asleep naturally without sedation. Earplugs, earphones and extra foam padding were applied to reduce the sound of the scanner while the neonates were asleep. A single-shot EPI sequence (SENSE factor = 2.5) was used for dMRI acquisition, with the following parameters: TE=78ms, TR=6850ms, in-plane field of view = 168 × 168mm2, in-plane imaging matrix = 112 × 112, in-plane imaging resolution =1.5 × 1.5mm2, slice thickness =1.6mm without gap, slice number=60, 30 independent diffusion encoding directions with b value = 1000 s/mm2. The images were reconstructed to 256 × 256 in-plane matrix. Two repetitions were conducted for dMRI acquisition, resulting in a scan time of 11 minutes. As described in our previous publication (Huang et al. 2015), with 30 diffusion weighted image (DWI) volumes and 2 repetitions, we accepted those dMRI datasets with less than 5 DWI volumes affected by severe motion. The affected volumes were replaced by the good volumes of another dMRI repetition during post processing.
Data preprocessing
Small motion and eddy current of dMRI of each neonate were corrected by registering all the DWIs to the b0 image using a 12-parameter (affine) automated image registration (AIR) algorithm (Woods et al. 1998). After AIR, six independent elements of the 3×3 diffusion tensor were determined by multivariate least-square fitting of DWIs (Basser et al. 1994). The tensor was diagonalized to obtain three eigenvalues (λ1−3 ) and eigenvectors (V1−3). The diffusion metrics, such as fractional anisotropy (FA) and apparent diffusion coefficient (ADC) images were then calculated. All above-mentioned procedures were conducted offline using DTIStudio (Jiang et al. 2006).
Network construction
Nodes and edges, the two fundamental elements of a network, were defined using the following procedures to construct the individual structural network.
Network node definition.
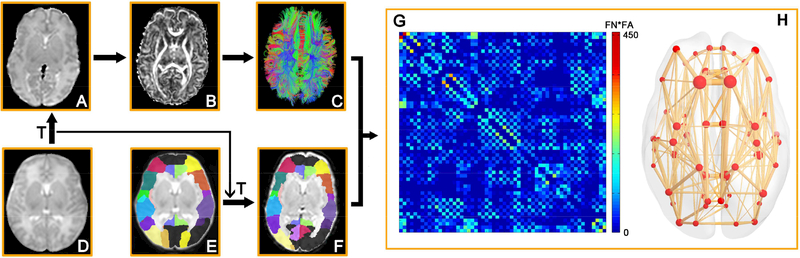
The nodes of each subject in the native dMRI space were obtained by transferring the parcellated cortical regions in the Johns Hopkins University (JHU) neonate atlas (Oishi et al. 2011). The contrasts of the single-subject b0 (ss-b0) image in the JHU atlas space (Fig. 1D) and individual neonate subject’s b0 image in the native space (Fig. 1A) were used to drive the registration. Briefly, a 12-parameter affine registration (incorporating rigid transformation) was used to transform the ss-b0 image in the JHU atlas space (Fig. 1D) to the b0 image in the native dMRI space of each neonate (Fig. 1A), followed by a non-linear transformation. The transformation T(•), combining the 12-parameter affine registration and non-linear transformation, was used to map JHU atlas labels (Fig. 1E) to the native space of individual neonate (Fig. 1F). Trilinear and nearest neighbor interpolation were used for transforming the non-atlas images and atlas labels, respectively. Discrete atlas label values were preserved with the nearest-neighbor interpolation. The structural network (Fig. 1G) of each neonate was constructed with 58 cortical regions (Fig. 1F) representing 58 nodes of the brain network. The registration procedures were conducted using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/). Of note, the cortical regions were dilated by 9 voxels in order to allow traced WM fibers (see Network edge definition below) to reach the cortical nodes. To avoid overlap of atlas labels, every dilated voxel was assigned to the label of cortical voxel which has the shortest Euclidean distance from the dilated voxel. The voxels in the dilated cortical regions with ADC greater than 1.9 × 10−3mm2/s were likely to be those of cerebrospinal fluid (CSF). These voxels were removed to minimize spurious structural connections between the nodes.
Figure 1.
Flowchart of brain network construction. The b0 image of the single-subject template in the JHU atlas space (D) was registered to the b0 image of each neonate subject in its native space (A) with the transformation T(•). (B) and (C) show FA map and dMRI tractography results in the native space, respectively. The JHU atlas labels (E) were transferred to the native space (F) with the transformation T(•). With delineation of network edges (C) and nodes (F) in the native space, connectivity matrix (G) and network graph (H) were established. The flowchart was drawn demonstrating analysis of a representative neonate dataset. Reconstructed whole-brain fiber streamlines (C) and 3D representation of the structural network (H) were generated using TrackVis (http://trackvis.org/) and BrainNet Viewer software (Xia et al. 2013), respectively.
Network edge definition.
Network edges were defined with reconstructed WM fibers by dMRI tractography. Brute-force deterministic fiber tractography (Mori et al. 1999; Huang et al. 2004) in the whole brain was performed with Diffusion Toolkit (http://trackvis.org/). Due to low FA in preterm brains, the FA threshold was set to 0.1 and angle threshold was 35o for tractography. Only reconstructed fibers with two end points located in the dilated cortical ribbon were kept, as shown in Fig. 1C. Two regions were considered structurally connected if there exists at least one streamline fiber with two end-points located in these two regions. FA and the streamline number both have been proved as good markers for characterization of tissue microstructure and WM changes during development (Wimberger et al. 1995; Drobyshevsky et al. 2005; Huang et al. 2006; Huang et al. 2009; Takahashi et al. 2012). Therefore, we defined the number of fibers multiplied by the mean FA (FN × FA) of all connected fibers between two regions as the edge weight. As a result, we constructed a weighted structural network (Fig. 1H) for each neonate, represented by a symmetric 58 × 58 connectivity matrix (Fig. 1G).
Network analysis
To describe the topological organization of the neonatal structural connectome, the following graph metrics were estimated, with detailed definitions of these network metrics provided in the Supplemental Materials.
Global network organization.
For global network metrics, we quantified the network sparsity, network strength (Sp), global efficiency (Eglob), local efficiency (Eloc), shortest path length (Lp), clustering coefficient (Cp) and small-world parameters (λ, γ and σ) (Rubinov and Sporns 2010).
Regional network metrics.
To estimate the regional topological properties of the brain networks, we calculated the nodal efficiency (Enodal) (Achard and Bullmore 2007), betweenness centrality, weighted degree centrality and eigenvector centrality (van den Heuvel and Sporns 2013). Linear fitting was conducted to measured nodal efficiency. The fitted nodal efficiency at each age was mapped to the cortical surface of a neonate template brain to generate the fitted nodal efficiency at each PMW.
Hub distribution.
The brain hubs were identified based on group-averaged backbone network. The method in the literature (Gong et al., 2009) was followed to identify the backbone network with minimal inclusions of false positive connections. Specifically, a nonparametric one-tailed sign test (p < 0.05, FDR corrected) with the null hypothesis that the number of fibers was zero was conducted to exclude possible false positive connections. The connections that reached the statistical significance were averaged across neonate subjects to generate the backbone network. The nodes of the backbone network were identified as hubs if any of three graph-based regional centrality metric (betweenness centrality, weighted degree centrality or eigenvector centrality) measurements was greater than “mean+std”. Next, the rich-club coefficient (ᶲ) and normalized rich-club (RC) coefficient (ᶲnorm) were calculated based on the backbone network. Based on categories of the nodes that network connections (i.e. edges) were associated with, network edges were categorized into rich-club, feeder and local edges (van den Heuvel and Sporns 2011).
Short- and long- range connections/edges.
The length of each traced streamline fiber was the physical distance of this fiber. The length of individual network edge was defined as the average length of all streamline fibers connecting two nodes associated with this edge. Edge length was calculated after brain normalization process to remove the effects of brain sizes. Edge length in the backbone network was the average of corresponding edge length across individual subjects. Edge length threshold used to differentiate short- and long-range connections was obtained from another average of all edge lengths in the backbone network. Connections with edge length smaller/greater than this edge length threshold were defined as short-/long- range connections, respectively.
Modular parcellation.
Module detection was performed with an optimized simulated annealing approach (Guimera et al. 2004) to parcellate the brain network into different modules (Newman and Girvan 2004). Briefly, the aim of this module identification process is to find a specific partition (p) which yields the largest network modularity, Q(p). Q(p) quantifies the difference between the number of intra-module links of the actual network and that of the random network in which connections are linked at random. The modular analysis was performed on individual network of each neonate brain to calculate the modularity and module number of individual network. Individual network was not used for modular parcellation. Instead, backbone network was used to obtain a consistent module parcellation across neonates and to localize age-related changes within and between specific modules. Based on the modular parcellation of the backbone network, the participation coefficient (PC) of each node was calculated (Guimera et al. 2004; Sporns et al. 2007; Rubinov and Sporns 2010) to assess the contribution of each node to modular segregation or integration. The hub regions were then categorized as connector hubs (with PC > 0.5) which occupied high inter-module connections and provincial hubs (with PC < 0.5) which occupied high intra-module connections. All network analyses were performed using GRETNA software (http://www.nitrc.org/projects/gretna/) (Wang et al. 2015) and the results were visualized using BrainNet Viewer software (https://www.nitrc.org/projects/bnv/) (Xia et al. 2013).
Statistical analysis
Age effects on network properties.
To examine the age effects on the network topological properties, a general linear model (GLM) analysis was implemented between each global network metric and postmenstrual age across all subjects, with gender and total brain volume (TBV) as covariates:
The slope of each metric against age β1was used to represent the developmental rate. The same procedure was repeated for nodal efficiency. Bonferroni correction was conducted on the age-effect analysis of nodal efficiency measurements. The corrected p value was set as 0.05/number of comparisons with number of comparisons 58.
Network-based statistic (NBS).
To identify structural connections showing significant age effects from the whole connectome, we used the NBS approach (Zalesky et al. 2010). First, the same GLM analysis with gender and TBV as covariates was applied for the entire connectome. A threshold of p < 0.005 was used to yield t statistic matrix of suprathreshold connections. The nonparametric NBS approach was used for controlling family wise error (FWE). After 10000 permutations, the statistical significance of the observed component sizes in the un-corrected connection matrix was evaluated. The interconnected sub-network components with a corrected p < 0.005 were considered statistically significant.
Clustering analysis
To group the brain regions with similar developmental trajectories, we used a data-driven k-means clustering method (Seber 2009). The brain regions with significant age-dependent nodal efficiency increase were used as the input, and developmental curve of each region’s efficiency was used as the feature of clustering. The k-means algorithm was initialized with randomized estimates for the trajectory centers and iterated multiple times to convergence. Ten repetitions with different random initial cluster centroids were used to minimize the effect of the start condition. The whole process was repeated for five different clustering models with cluster number 2, 3, 4, 5 and 6, respectively. The two-cluster model was chosen because of its highest silhouette value (Rousseeuw 1987), shown in Supplemental Fig 1. Of the note, highest silhouette value of a model indicates that this model fits best for clustering the nodes based on age-dependent nodal efficiency increase pattern.
Prediction of the neonate age using support vector regression
A support vector regression (SVR) with a linear kernel function was used to test the prediction power of nodal efficiency on individual neonate postmenstrual age. Default settings with C = 1 and epsilon = 0.001 in the LIBSVM Toolbox (http://www.csie.ntu.edu.tw/~cjlin/libsvm/) were used to evaluate the SVR model (Dosenbach et al. 2010; Iuculano et al. 2014). A Leave-one-out cross-validation (LOOCV) was used to evaluate the prediction accuracy of the model. Each neonate was designated as the test data in turn while remaining ones were used to train the SVR predictor which aimed at predicting the test neonate’s age. Pearson correlation coefficient between the actual and predicted ages was calculated to assess the prediction accuracy. Nodal efficiencies of all regions, hubs and non-hub regions of training samples were used as features for the SVR predictor, respectively. In each iteration, the hub distribution obtained from the training sample was similar to that obtained from the whole cohort.
Evaluation of the effects of different parcellation schemes
To evaluate potential effects of the parcellation schemes (e.g. Zalesky et al., 2010) on the results, the neonate cortex was further randomly subdivided into 256 nodes of equal size to examine the age-dependent network property changes with a high-resolution parcellation. For each neonate, a high-resolution structural connectivity matrix in 256 by 256 was constructed. Same network analysis procedures and statistical analyses used in the low-resolution networks (58 nodes) were repeated.
Results
Differentiated nodal efficiency increases with faster nodal efficiency increases at the brain hubs
The global structural network of neonate brain became stronger and more efficient from 31.9 to 41.7 PMW, as shown in the scatter plots of network strength, global efficiency, local efficiency, number of edges (network sparsity) and small-worldness σ in Supplemental Fig. 2.
Differential nodal efficiency increases across brain regions:
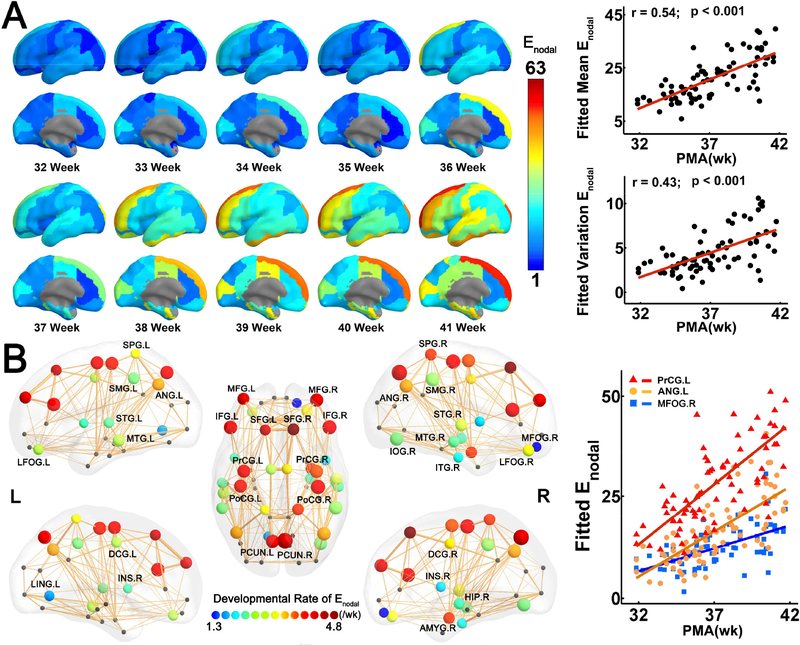
The heterogeneous distribution of nodal efficiency across brain regions is shown by the fitted nodal efficiency maps of all weeks from 31.9 to 41.7PMW (Fig. 2A, left panel). Higher efficiency in prefrontal cortex, precentral and postcentral gyrus and lower efficiency in occipital cortex can be observed (Fig. 2A, left panel). Both the mean and standard deviation (SD) of nodal efficiency increased significantly with age (Mean: r = 0.54, p = 6.5 × 10−7; SD: r = 0.43, p = 1.2× 10−4) (Fig. 2A, right panel), indicating that both the average and variability of nodal efficiency across brain regions increased with age. As shown in Fig 2B, 34 cortical regions widely distributed in bilateral frontal, parietal, temporal and limbic regions exhibited significant age-related linear increases (p < 0.05, Bonferroni corrected). Importantly, the developmental rates of nodal efficiency varied across regions. More rapid increases were found in the precentral and postcentral gyrus, superior, middle and inferior frontal gyrus and precuneus relative to other brain regions (Fig. 2B). The scatter plots of three representative regions with distinguished nodal efficiency increase rates (left precentral gyrus: PrCG.L, left angular gyrus: ANG.L, and right medial fronto-orbital gyrus: MFOG.R) are shown in the right panel of Fig. 2B.
Figure 2.
Heterogeneous development of nodal efficiency (Enodal) across brain regions. (A) On the left panel, fitted nodal efficiency maps at each week from 32 to 41 PMW demonstrate heterogeneous nodal efficiency distribution across the cortical surface. On the right panel, both the mean and standard deviation of nodal efficiency increased significantly with age. (B) On the left panel, 34 brain regions with significant and heterogeneous age-related increases of nodal efficiency are displayed as small spheres with colors (from blue to red) encoding different increase rates of nodal efficiency and sizes encoding the R values of the correlation between the nodal efficiency and age. Scatter plots on the right panel show significant age-related increases in nodal efficiency of three representative regions, namely, PrCG.L, ANG.L and MOFG.R, from highest to lowest efficiency increase rate. Colors of the dots and the fitted lines for each representative region in the scatter plots are consistent with those encoding nodal efficiency increase rates shown on the left panel. Abbreviations: AMYG: amygdala; ANG: angular gyrus; DCG: dorsal cingulate gyrus; FFG: fusiform gyrus; HIP: hippocampus; INS: insular cortex; IOG: inferior occipital gyrus; L/R: left/right; LFOG: lateral fronto-orbital gyrus; MFOG: medial fronto-orbaital gyrus; PCG: posterior cingulate gyrus; PCUN: precuneus; PrCG/PoCG: precentral/postcentral gyrus; SFG/MFG/IFG: superior/middle/inferior frontal gyrus; SMG: supramarginal gyrus; SPG: superior parietal gyrus; STG/MTG/ITG: superior/middle/inferior temporal gyrus.
Hub distribution, rich-club organization and faster nodal efficiency increases at the brain hubs:
Hub regions (red balls) are mainly distributed in the bilateral superior and middle frontal cortex, precentral and postcentral gyrus, superior parietal cortex and cingulate cortex (Figure 3A). Consistent hub distribution across different PMW age groups can be observed (Supplemental Fig. 3). Moreover, a characteristic rich-club organization with the normalized RC exceeding 1 (ϕ norm =1.3) was identified for the backbone network. With a data-driven two-cluster model for categorizing nodal efficiency, among 34 brain regions (shown in Fig. 3B) with significant age-dependent nodal efficiency increases, all 12 cluster-1 brain regions (blue circles in Fig. 3A and listed in Table 2) were part of the 18 hub regions (red balls in Fig. 3A) of the neonate connectome, indicating distinctively higher rate of efficiency increases during 31.9–41.7PMW at hub regions. Note that not all hub regions were characterized by statistically significant efficiency increases; however, those with significant efficiency increases were all cluster-1 brain regions. Fig. 3B shows significantly steeper efficiency increase trend line at the hub regions compared to non-hub regions (t = 7.55, interaction p < 10−3). Furthermore, by correlating the nodal efficiency increase rates and nodal efficiency measurements at 34 brain regions with significant age-dependent changes, we found significantly positive correlation between efficiency increase rate and efficiency measurements themselves (r = 0.70, p < 10−3) (Fig. 3C).
Figure 3.
Hub distribution of the neonate connectome and higher developmental rates in hub regions compared to non-hubs. (A) A 3D representation of the hub distribution of the neonate structural connectome with the hub nodes in red and non-hub nodes in gray overlaid on the group-averaged backbone network. Cluster-1 nodes identified by a data-driven clustering analysis were marked with blue circle. Of note, all cluster-1 nodes were hub regions of the connectome. (B) Scatter plot showing more rapid age-related increases of nodal efficiency (Enodal) in hub regions than non-hub regions. Partial correlation between age and regional efficiency were fitted separately in all hub regions (red dots) and non-hub regions (gray dots). Interaction effect between age and hub category was significant (p <10−3). (C) Scatter plot showing significant linear correlation between nodal efficiency and developmental rate (of nodal efficiency) across the brain regions. In (C), each dot represents one of the 34 brain regions with significant nodal efficiency increases, with the hub/non-hub regions represented by red/gray dots. See the legend of Figure 2 for abbreviations of brain regions.
Table 2.
Brain hub regions with the statistically significant age-related increases in nodal efficiency. The hub regions were sorted by descending developmental rate. See the legend of Figure 2 for abbreviation of brain regions.
| Regions | Mean Enodal (std) | R value | P value | Developmental rate |
|---|---|---|---|---|
| SFG.R | 46.3 (19.09) | 0.51 | 3.28 × 10−6 | 4.85 |
| SFG.L | 46.8 (19.66) | 0.47 | 1.92 × 10−5 | 4.57 |
| MFG.L | 40.0 (17.05) | 0.51 | 2.91 × 10−6 | 4.15 |
| MFG.R | 39.5 (16.20) | 0.55 | 3.05 × 10−7 | 4.07 |
| PrCG.L | 40.3 (13.52) | 0.54 | 4.81 × 10−7 | 3.27 |
| PoCG.R | 38.2 (12.55) | 0.59 | 3.07 × 10−8 | 3.22 |
| PoCG.L | 39.0 (12.88) | 0.55 | 4.13 × 10−7 | 3.11 |
| SPG.R | 38.5 (13.81) | 0.50 | 5.36 × 10−6 | 3.00 |
| PrCG.R | 37.6 (12.11) | 0.55 | 3.44 × 10−7 | 2.85 |
| DCG.R | 44.2 (13.30) | 0.45 | 4.35 × 10−5 | 2.51 |
| SPG.L | 37.7 (12.88) | 0.44 | 9.33 × 10−5 | 2.43 |
| DCG.L | 42.1 (11.57) | 0.46 | 2.97 × 10−5 | 2.24 |
Faster edge strength increases in rich-club, in short-range connections and in intra-module connections
Faster edge strength increases in rich-club:
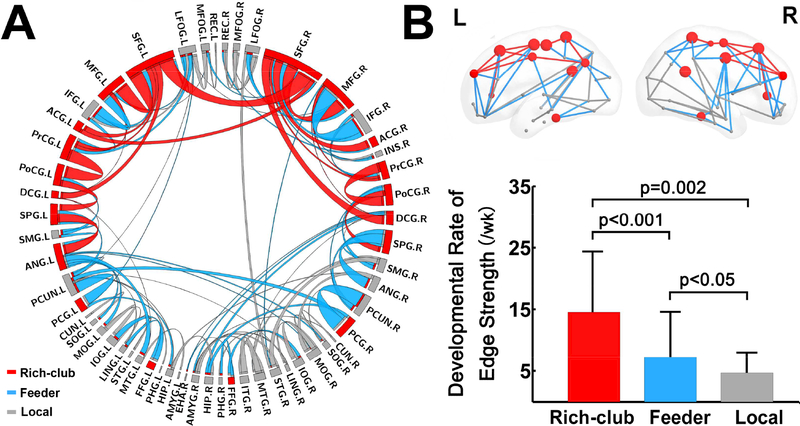
NBS analysis revealed 107 significantly increasing edges (12% of all edges) connecting 55 nodes, which were widely distributed in bilateral frontal, parietal, temporal and limbic areas (Fig. 4A). The developmental rate varied across the edges, reflected by differentially encoded edge width (Fig. 4A). Higher increase rates of edge strength were found in a few symmetric and short-range connections, including bilateral connections between superior and middle frontal gyrus, between precentral and postcentral gyrus, and between precuneus and posterior cingulate gyrus (Fig. 4A). With the connections classified into rich-club, feeder, and local connections ( Figs 4A and 4B), highest rate of age-related increase was found in rich-club connections, followed by feeder and local connections (Fig. 4B).
Figure 4.
Components with significant age-related alterations revealed by NBS analysis and differential development rates of edge strengths in rich-club organization. (A) NBS components are shown in a circle view with the color of edges encoded by the category of rich-club (red) feeder (blue) and local (gray) edges and size of edges encoded by the developmental rate. (B) The bar plot showing significant differences in edge strength developmental rate among the rich-club, feeder and local connections. See the legend of Figure 2 for abbreviations of brain regions.
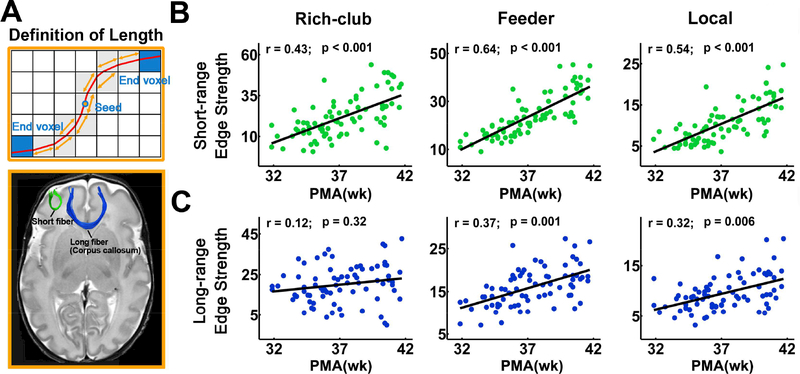
Faster edge strength increases in short-range connections:
Green and blue fibers representing typical short- and long-range connections are demonstrated in Fig. 5A. Amongst short-range connections, rich-club (r = 0.43, p = 1.1 × 10−4), feeder (r = 0.64, p = 5.9 × 10−10) and local (r = 0.54, p = 7.2× 10−7) connections (Fig. 5B) all increased significantly with age. By contrast, amongst long-range connections, only feeder (r = 0.37, p = 1.0 × 10−3) and local (r = 0.32, p = 5.8 × 10−3) connections increased significantly with age while no significant age-dependent changes were found for rich-club connections (r = 0.12, p = 0.32) (Fig. 5C).
Figure 5.
Development pattern of short- and long-range connections with age. (A) Definition of physical fiber length is shown in the upper panel. The physical length of a streamline reconstructed from deterministic tractography by following the main diffusion direction within each voxel was the length of the red curve. An illustration of short- and long-range fibers is presented in the lower panel. (B) Scatter plots showing significantly and relatively sharp age-related edge strength increases in different categories of short-range connections. (C) Scatter plots showing non-significant age-related changes of edge strength of rich-club long-range connections as well as significant but relatively mild age-related edge strength increases of feeder and local long-range connections. PMA: postmenstrual age.
Neonate brain modular organization and faster edge strength increases in intra-module connections:
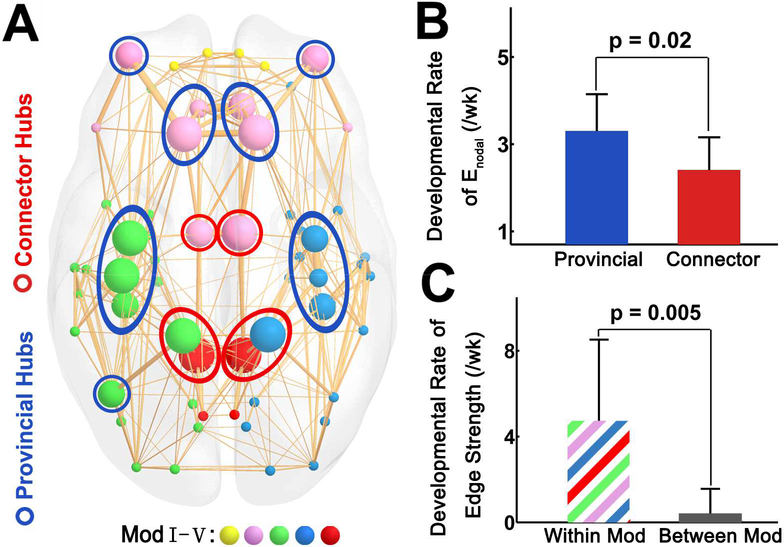
Significant modular organization was found in structural networks of all neonates (Q > 0.39 for all subjects). No significant age-dependent alterations in the modularity and module number were found (all p > 0.05), suggesting that the major modular organization remained stable during 31.9–41.7 PMW. A significant modular architecture of the backbone network was identified (Q = 0.42), separating the brain into five different modules (Fig. 6A, left panel). Hub regions were evenly distributed in different modules with provincial hubs located in the center of modules and connector hubs (the bilateral dorsal and posterior cingulate gyrus, bilateral superior parietal gyrus) located in the boundaries (Fig. 6A). By comparing the developmental rates of these two types of hubs, we found the rates of provincial hubs are significantly higher than those of connector hubs (t = 2.6, p = 0.02) (Fig. 6B). Moreover, we found that developmental rates of within-module connections were higher than those of between-module connections (t = 3.4, p = 0.005) (Fig. 6C). R values, p values and age-dependent edge strength increase rates from the correlation of edge strength and age for within-module and between-module connections are listed in Supplemental Table S1.
Figure 6.
Higher developmental rates of edge strength of provincial hubs compared with connector hubs, and higher developmental rates of within-module connections compared with between-module connections. (A) A 3D representation of the modular structure of the group-averaged backbone network with nodes in colors corresponding to different modules and size encoding average regional efficiency. Module Ⅰ was composed of bilateral orbital-frontal regions (yellow). Module Ⅱ was composed of bilateral prefrontal regions and dorsal cingulate gyrus (purple). Module Ⅲ mainly consisted of left pre/postcentral gyrus, temporal and superior parietal regions (green). Module Ⅳ mainly consisted of right pre/postcentral gyrus, temporal and parietal regions (blue). Module Ⅴ consisted of bilateral posterior parietal regions (red). The provincial hubs and connector hubs were marked with blue and red circles, respectively. (B) The bar plot showing higher developmental rate of provincial hubs than that of connector hubs. (C) The bar plot showing higher developmental rate of within-module (Within Mod) connections compared with that of between-module (Between Mod) connections.
Age prediction and reproducible findings with a high-resolution parcellation scheme
Age prediction:
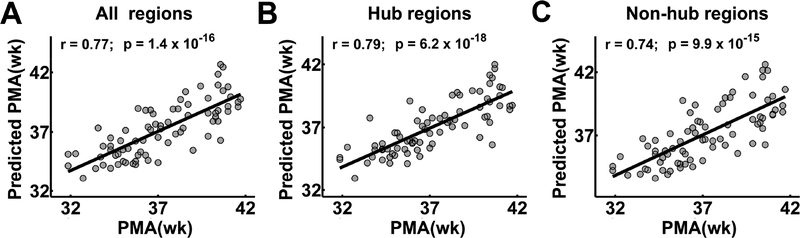
Fig. 7A shows that the postmenstrual ages of the neonates can be predicted by the nodal efficiency of brain structural connectome, with a correlation r = 0.77 between the actual and the predicted postmenstrual age. It is noteworthy that hub regions showed higher prediction accuracy (r = 0.79) (Fig. 7B) than non-hub regions (r = 0.74) (Fig. 7C), suggesting a stronger association between hubs and postmenstrual age as compared to non-hubs.
Figure 7.
Prediction of individual ages based on nodal efficiency of all brain regions (A), hub regions (B) and non-hub regions (C). The scatter plots depict actual versus predicted age. Pearson correlation coefficient between the actual and predicted ages are shown to assess the prediction accuracy. PMA: postmenstrual age.
Reproducible findings with a high-resolution parcellation scheme:
Similar age-related development trend lines of global and regional network properties were observed when the analyses were repeated utilizing the higher resolution parcellation. This included significant age-dependent increases in global and local network efficiency (Eglob: r = 0.61, p = 5.9 × 10−8; Eloc: r = 0.63, p = 1.2 × 10−9) (Fig. 8B). Brain regions with the most rapid age-dependent increases of nodal efficiency were mainly distributed in the precentral and postcentral gyrus, precuneus and posterior parietal cortex (Fig. 8C), consistent with the findings from low-resolution parcellation. Similar hub distributions were also found, mainly located in the bilateral orbito-frontal cortex, bilateral precentral and postcentral cortex, bilateral superior parietal cortex and temporal cortex (Fig. 8D). Finally, significantly higher developmental rate (t = 2.93, p = 0.004) (Fig. 8E) was also found in hub regions than non-hub regions with high-resolution network analysis. These results jointly indicated that the maturation patterns of the neonate connectome are largely independent of cortical parcellation schemes.
Figure 8.
Reproducible age-dependent alterations with a high-resolution parcellation scheme. (A) Parcellation with the JHU-58-region neonate atlas (Oishi et al. 2011) and high resolution cortical parcellation with 256 ROIs. (B) Scatter plots showing significantly linear increases of global and local efficiency with age in low- and high-resolution networks. PMA: postmenstrual age. (C) Similar distributions of brain regions with significant age-dependent changes of nodal efficiency for low- and high-resolution network. These regions are displayed as small spheres with colors encoding the developmental rates of nodal efficiency and sizes encoding the R values. (D) Similar hub distributions of low- and high-resolution networks with the hub nodes in red and non-hub nodes in gray. Nodes in (C) and (D) are overlaid on group-averaged network backbones. (E) Bar plots showing significant higher developmental rate of nodal efficiency (Enodal) in hub regions compared with non-hub regions in both low- and high-resolution networks.
Discussion
Using connectomic analyses of dMRI images with relatively high resolution (1.5 × 1.5 × 1.6 mm3) from 77 neonates scanned at 31.9 to 41.7 PMW, we found rapid and regionally differentiated maturation of structural brain networks. Faster connectivity increases take place mainly at the brain hubs and rich-club regions compared to other regions, resulting in a more segregated structural connectome near term-equivalency. The brain hubs with faster age-dependent nodal efficiency increases are distributed in primary sensorimotor regions, superior-middle frontal and precuneus regions, while the hub distribution remains almost unchanged during 31.9–41.7PMW. Faster connectional maturation at these hub regions was supported by a data-driven cluster analysis. Compared to long-range or between-module connections, short-range and within-module connections appeared to develop more rapidly during 31.9–41.7PMW, laying the foundation for efficient network communication. Efficiency measures of all brain regions, especially those of hub regions, accurately predicted neonatal age in PMW. These findings shed light on spatiotemporal principles of brain connectome development during this critical period, offering references for aberrant brain organization that may be associated with many neurodevelopmental disorders. The highly accurate prediction of age at identified hubs suggests that these core regions may serve as biomarkers indicating the ontogeny of early brain development. Collectively, rapid increases of hub and rich-club connections may result in structural segregation and underlie emergence of certain primary functions of the brain during 31.9–41.7PMW.
Segregation of neonate brain structural connectome
Dramatic global and local efficiency increases were found with global graphic analysis (Fig. S1), suggesting that overall white matter maturation contributes to a more efficient and compact network during 31.9–41.7PMW. These findings are consistent to the literature (van den Heuvel et al. 2015). Despite overall increases, the pattern of age-dependent nodal efficiency increases is not uniform across the brain regions (Fig. 2), with some regions demonstrating rapid increases in nodal efficiency while other regions remain almost unchanged. This differentiated increase pattern contributes to the segregation of baby brain connectome during 31.9–41.7 PMW. It is noteworthy that the regions with higher nodal efficiency exhibited higher developmental rate too (Fig. 2). Figure 3 further demonstrated that brain regions with fastest efficiency increases coincided with the brain hubs. More accurate prediction of individual age was found at the hub regions than other regions (Fig. 7). These results suggest that the selective strengthening of hubs is prominent during the final weeks before birth. On the other hand, the hub distribution across the ages during 31.9–41.7PMW is almost unchanged (Fig. S2). This supports the idea that the increased segregation of the developing connectome is achieved by increasing the connectivity to and from key hubs, established early in gestation, rather than by altering the hub distribution. Brain hubs occupy a dominant position in information transfer (Xu et al. 2010) and have higher levels of metabolic energy consumption and higher rates of cerebral blood flow than peripheral nodes (Liang et al. 2013; Tomasi et al. 2014). The rich club of hub regions observed here in the bilateral precentral and postcentral gyrus, posterior cingulate gyrus, superior and middle frontal cortex, are consistent with prior observation in neonates (Ball et al. 2014; Pandit et al. 2014; van den Heuvel et al. 2015). Moreover, these hubs show a strong correspondence with those of the adult structural connectome (Hagmann et al. 2008; Gong et al. 2009; van den Heuvel and Sporns 2011). Our findings and previous studies jointly suggest that these hubs are not only critical for the neonate brain to get ready for postnatal neural growth, but also play a key role in organizing the connectome throughout brain development. In addition, functional network segregation was observed in a subset of same preterm cohort by analyzing resting-state fMRI dataset (Cao et al. 2017a), which infer that the structural segregation in the present study is likely to underlie the functional segregation.
Higher increase rates of edge strength in rich-club edges were found in short-range connections, compared to those in long-range connections (Fig. 5). Moreover, higher developmental rates were found in provincial hubs than in connector hubs (Fig. 6B) and in within-module connections than between-module connections (Fig. 6C). The module distribution of these neonate brains at 31.9–41.7PMW (Fig. 6A) is similar to that of adult brains (Hagmann et al. 2008). Edges within modules and provincial hubs mainly contribute to connections within particular systems, as compared to global integration. Faster increases of edge strength and nodal efficiency in particular systems make the network more specialized and segregated during maturation. These results are consistent to the general understanding of a typical developmental course of a structural network characterized by the progressive maturation from local and proximity-based connections, supporting primary functions, to a more distributed and integrative topology, supporting higher cognitive functions (Hagmann et al. 2010; Yap et al. 2011; Bullmore and Sporns 2012; van den Heuvel et al. 2012; Tymofiyeva et al. 2013; Collin et al. 2014; Vertes and Bullmore 2015).
Differentiated maturation of brain regions with higher rates of efficiency increases at the brain hubs
Differentiated maturation of brain regions is reflected by not only heterogeneously distributed nodal efficiency, but also varying developmental rates of the nodal efficiency, as demonstrated from Figure 2. Among all brain nodes with significant efficiency increases, higher increase rates were found at certain brain hubs (Fig. 3). From Table 2, hub regions with significant nodal efficiency changes are the left and right precentral and postcentral gyrus, left and right dorsal cingulate gyrus, left and right precuneus and as well as frontal gyri. As elaborated below, these regions were consistently found to play an important role in early brain development. The left and right precentral and postcentral gyrus are essential for primary sensorimotor functions. Previous functional connectivity studies (Doria et al. 2010; Smyser et al. 2010; Fransson et al. 2011) found these regions among the earliest appearing functional networks identified from resting-state fMRI. Moreover, the identified hubs of the neonate functional connectome are largely confined to the primary sensorimotor regions (Fransson et al. 2011; Gao et al. 2011; Cao et al. 2017a), which distinguishes the neonate brain from the adult brain. This differential pattern is observed by PET studies (Chugani et al. 1987; Chugani 1998). These observed early maturation of sensory areas may not only be helpful for basic survival functions at birth (Buckner and Krienen 2013) but also support the later maturation of higher order and multimodal integrative areas (Guillery 2005). The left and right precuneus, as a functional core of the default-mode-network (Fransson and Marrelec, 2008), are also regions with high rates of nodal efficiency increases (Figure 3). Higher rates of efficiency increases of these gyri may underlie the emergence of primitive default-mode network in infancy (Gao, et al., 2009), a process that is critical for the development of sense of self (e.g. Uddin et al. 2007). Other hubs with significant nodal efficiency increases include frontal gyri (Fig. 3 and Table 2). Higher rates of efficiency increases in the frontal lobe during preterm development have been observed in recent structural connectivity studies (Brown, et al., 2014; Pandit et al., 2014). Active frontal cortical maturation has also been reflected by cortical FA changes. Sharp decrease of frontal cortical FA, a measure quantifying the dendritic arborization in the cerebral cortex, has been consistently found in several cortical development studies of the preterm brains (DeIpolyi, et al., 2005; Ball et al., 2013; Yu et al., 2016). Observation of structural connectivity hubs at superior and middle frontal cortex could be related to active cortical microstructural activities in these regions.
Emergence of rich-club organization in early developing brain
Figure 4 shows rich-club organization (van den Heuvel and Sporns 2011) consisting of the hubs exhibited in Figure 3A. Despite early emergence of rich-club organization has been revealed in recent studies of neonate structural connectome (Ball et al. 2014; van den Heuvel et al. 2015), the present study revealed developmental rate of rich-club regions and other network properties by employing a neonate dataset with a relatively large sample and evenly distributed ages from 31.9–41.7 PMWs, offering new insight into the asynchronous development across brain regions. Faster increases at brain hubs may drive emergence of rich-club organization in the period of 31.9–41.7 PMW. Rich-club organization was also contributed by more rapidly strengthened edges in rich-club connections than those in other connections, as demonstrated by fastest edge strength increases in rich-club connections, followed by the feeder connections and the local connections (Fig. 4B). The “rich-get-richer” principle of network evolution (Barabasi and Albert 1999) means that new connections are preferentially associated to the nodes with many connections. This principle is reflected by the findings of more rapid growth of hubs and rich-club connections in the present study. Accelerated growth of hub regions possibly underlies important functional roles of hub regions after birth and functional segregation of brain regions. Accelerated growth of hub regions also leads to a wider degree distribution, including more highly-connected hubs as well as more non-hub nodes with few local connections (Kaiser 2017),
Short-range and long-range connections
As can be observed from Figure 4, most of edges with significant strength increases were short-range connections. The present study revealed that faster increasing edge strength of short-range connectivity compared to that of long-range connectivity may facilitate the structural connectome segregation process centered at rich-club organization (Figs. 4 and 5) during 31.9–41.7PMW. Developmental rates of short-range cortical-cortical connections in rich-club edges were higher than those of long-range connections (Fig. 5). Relatively higher long-range edge strength at earlier PMW (e.g. 32PMW) (Fig. 5) suggests that these long-range connections lay the foundation for the following network reconfiguration process after 32PMW. Particular growth of short rich-club edges may also enhance local neuronal operations and segregation of modules, supported by modular analysis as shown in Figure 6. Delineation of the brain connectivity pathways using dMRI has revealed white matter morphological dynamics from the early 2nd trimester to birth (Huang et al. 2006; Huang et al. 2009; Takahashi et al. 2012; Ouyang et al. 2015). Among all white matter tract groups, long-range association tracts connecting cortical regions are those emerging relatively late. For example, the arcuate fasciculus, key for the language function development, is not well developed until 2 years of age (e.g. Zhang et al, 2007). Other connectomic studies have found that increasing edges in prenatal and preterm developmental stages consisted of many short local connections and limited long-range connections (Takahashi et al. 2012; Brown et al. 2014). Faster increasing short-range connections during 31.9–41.7PMW may constitute pivotal edges of the rich-club backbone and mediate specialized functional process in local integration (Park and Friston 2013; Ouyang et al., 2017).
Limitations, technical considerations and future directions
We tested the effects of different cortical parcellation schemes and found that the maturation patterns of the neonate connectome were largely independent of the cortical parcellation scheme used, as demonstrated by Figure 8. Besides the effects of different parcellation schemes on connectomic analysis results, several issues need to be further considered for future studies. First, the dataset used in this study was obtained using a cross-sectional design. Future studies with longitudinal design may be considered to eliminate the effects of individual differences, even though the age-related structural connectomic changes are dominant in this early developmental period. Second, deterministic tractography was used for the reconstruction of WM tracts, which may have resulted in the loss of existing fibers due to the “fiber-crossing” problem (Mori and van Zijl 2002). Tractography techniques more robust to fiber-crossing, such as probabilistic tractography (Behrens et al. 2007), can be considered to better define the network edges in future studies. Third, it has been found that preterm birth was associated with altered microstructural development (e.g. Boardman et al. 2010; Rathbone et al. 2011) and adverse neurodevelopmental outcomes (Woodward et al. 2006). Despite preterm birth effects, MRI examinations of preterm infants have been predominantly used to understand brain development during the 3rd trimester. Several other studies using dMRI also indicated dramatic reconfiguration during the last 10 weeks prior to normal time of birth (e.g. Ball et al. 2013; Brown et al. 2014; van den Heuvel et al. 2015; Song et al., 2017). Exposure to the extrauterine environment could constitute part of the observed network reorganization (Karolis et al. 2016; Batalle et al. 2017), but these effects would be relatively subtle compared with more profound effects of development during the 3rd trimester (Bourgeois et al. 1989; Kostović 1990). Nevertheless, it is likely that disruption of the network could become apparent in years subsequent to premature birth. Recent advances of in-utero MRI (e.g. Kasprian et al. 2008; Thomason et al. 2013; Mitter et al. 2015; van den Heuvel and Thomason 2016) could alleviate these preterm effects. Fifth, segregation has also been found in the functional connectome development during this period, in our previous study (Cao et al. 2017a). It is noteworthy that the cohort of that functional connectome study (Cao et al. 2017a) was a subset of the cohort used in the present one. Finally, with same segregation processes found in the structural connectome, the mechanistic relationship on how structural connections underlie functional ones has yet to be delineated. Recently developed approaches, such as those used for understanding network level structure-function relationships (Mišić et al. 2016), could offer insights into mechanistic structure-function relationship in this specific developmental period.
Supplementary Material
Acknowledgments:
This study was sponsored by NIH (Grant Nos. MH092535 and MH092535-S1, HH), the 973 program (Grant No. 2013CB837300, NS), the National Natural Science Foundation of China (Grant Nos. 81471732, 81671761, NS; 81628009, HH), the Fundamental Research Funds for the Central Universities (Grant No. 2017XTCX04, NS), and the Interdisciplinary Research Funds of Beijing Normal University (NS). The authors thank Michelle Slinger at Children’s Hospital of Philadelphia for her contribution to writing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achard S, Bullmore E, 2007. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol 3, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeby A, Liu Y, De Tiège X, Denolin V, David P, Balériaux D, Kavec M, Metens T, Van Bogaert P, 2009. Maturation of thalamic radiations between 34 and 41 weeks’ gestation: A combined voxel-based study and probabilistic tractography with diffusion tensor imaging. American Journal of Neuroradiology 30, 1780–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Aljabar P, Zebari S, Tusor N, Arichi T, Merchant N, Robinson EC, Ogundipe E, Rueckert D, Edwards AD, Counsell SJ, 2014. Rich-club organization of the newborn human brain. Proc. Natl. Acad. Sci. U. S. A 111, 7456–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Srinivasan L, Aljabar P, Counsell SJ, Durighel G, Hajnal JV, Rutherford MA, Edwards AD, 2013. Development of cortical microstructure in the preterm human brain. Proc. Natl. Acad. Sci. U. S. A 110, 9541–9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabasi AL, Albert R, 1999. Emergence of scaling in random networks. Science 286, 509–512. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D, 1994. MR diffusion tensor spectroscopy and imaging. Biophysical journal 66, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalle D, Hughes EJ, Zhang H, Tournier J-D, Tusor N, Aljabar P, Wali L, Alexander DC, Hajnal JV, Nosarti C, 2017. Early development of structural networks and the impact of prematurity on brain connectivity. NeuroImage 149, 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J, 2004. Atlas of human central nervous system development; Volume 2: The Human Brain During the Third Trimester: CRC. [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW, 2007. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JP, Craven C, Valappil S, Counsell SJ, Dyet L, Rueckert D, Aljabar P, Rutherford MA, Chew A, Allsop JM, 2010. A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. Neuroimage 52, 409–414. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Jastreboff PJ, Rakic P, 1989. Synaptogenesis in visual cortex of normal and preterm monkeys: evidence for intrinsic regulation of synaptic overproduction. Proc. Natl. Acad. Sci. U. S. A 86, 4297–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Miller SP, Booth BG, Andrews S, Chau V, Poskitt KJ, Hamarneh G, 2014. Structural network analysis of brain development in young preterm neonates. Neuroimage 101, 667–680. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, 2013. The evolution of distributed association networks in the human brain. Trends. Cogn. Sci 17, 648–665. [DOI] [PubMed] [Google Scholar]
- Bui T, Daire JL, Chalard F, Zaccaria I, Alberti C, Elmaleh M, Garel C, Luton D, Blanc N, Sebag G, 2006. Microstructural development of human brain assessed in utero by diffusion tensor imaging. Pediatric Radiology 36, 1133–1140. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O, 2012. The economy of brain network organization. Nat. Rev. Neurosci 13, 336–349. [DOI] [PubMed] [Google Scholar]
- Cao M, He Y, Dai Z, Liao X, Jeon T, Ouyang M, Chalak L, Bi Y, Rollins N, Dong Q, 2017a. Early development of functional network segregation revealed by connectomic analysis of the preterm human brain. Cereb. Cortex 27, 1949–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Huang H, He Y, 2017b. Developmental connectomics from infancy through early childhood. Trends Neurosci 40, 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani H,T, 1998. A critical period of brain development: Studies of cerebral glucose utilization with PET. Preventive Medicine 27, 184–188. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC, 1987. Positron emission tomography study of human brain functional development. Ann. Neurol 22, 487–497. [DOI] [PubMed] [Google Scholar]
- Collin G, Sporns O, Mandl RC, van den Heuvel MP, 2014. Structural and functional aspects relating to cost and benefit of rich club organization in the human cerebral cortex. Cereb. Cortex 24, 2258–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeIpolyi AR, Mukherjee P, Gill K, Henry RG, Partridge SC, Veeraraghavan S, Jin H, Lu Y, Miller SP, Ferriero DM, Vigneron DB, Barkovich AJ, 2005. Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: diffusion tensor imaging versus cortical gyration. NeuroImage 27, 579–586. [DOI] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, Counsell SJ, Murgasova M, Aljabar P, Nunes RG, 2010. Emergence of resting state networks in the preterm human brain. Proc. Natl. Acad. Sci. U. S. A 107, 20015–20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR, Barch DM, Petersen SE, Schlaggar BL, 2010. Prediction of individual brain maturity using fMRI. Science 329, 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, Li L, Ji X, Trommer B, Beardsley DJ, Luo NL, Back SA, Tan S, 2005. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J. Neurosci 25, 5988–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle WA, 2004. Age terminology during the perinatal period. Pediatrics 114, 1362–1364. [DOI] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, Lagercrantz H, 2011. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb. Cortex 21, 145–154. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G, 2008. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage 42, 1178–1184. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Aden U, 2007. Resting-state networks in the infant brain. Proc. Natl. Acad. Sci. U. S. A 104, 15531–15536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Li H, Oishi K, Mishra V, Ouyang M, Jeon T, Peng Y, Liu S, Huang H, 2016. Age-specific gray and white matter DTI atlas for human brain at 33, 36 and 39 postmenstrual weeks. OHBM Conference, Geneva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Giovanello KS, Smith JK, Shen DG, Zhu HT, Lin WL, 2011. Temporal and spatial evolution of brain network topology during the first two years of life. PLoS One 6, e25278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, Lin W, 2009. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl. Acad. Sci. U. S. A 106, 6790–6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C, 2009. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb. Cortex 19, 524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW, 2005. Is postnatal neocortical maturation hierarchical? Trends Neurosci. 28, 512–517. [DOI] [PubMed] [Google Scholar]
- Guimera R, Sales-Pardo M, Amaral LA, 2004. Modularity from fluctuations in random graphs and complex networks. Phys. Rev. E 70, 025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ, 1998. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatric research 44, 584–590. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O, 2008. Mapping the structural core of human cerebral cortex. PLoS Biol. 6, e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Sporns O, Madan N, Cammoun L, Pienaar R, Wedeen VJ, Meuli R, Thiran JP, Grant PE, 2010. White matter maturation reshapes structural connectivity in the late developing human brain. Proc. Natl. Acad. Sci. U. S. A 107, 19067–19072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Jeon T, Sedmak G, Pletikos M, Vasung L, Xu X, Yarowsky P, Richards LJ, Kostović I, Šestan N, 2013. Coupling diffusion imaging with histological and gene expression analysis to examine the dynamics of cortical areas across the fetal period of human brain development. Cereb. Cortex 23, 2620–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shu N, Mishra V, Jeon T, Chalak L, Wang ZJ, Rollins N, Gong G, Cheng H, Peng Y, Dong Q, He Y, 2015. Development of human brain structural networks through infancy and childhood. Cereb. Cortex 25, 1389–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, Miller MI, Mori S, 2009. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J. Neurosci 29, 4263–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, van Zijl P, Mori S, 2004. Analysis of noise effects on DTI‐based tractography using the brute‐force and multi‐ROI approach. Magn Reson Med 52, 559–565. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards LJ, Yarowsky P, Donohue P, Graham E, van Zijl PCM, Mori S, 2006. White and gray matter development in human fetal, newborn and pediatric brains. NeuroImage 33, 27–38. [DOI] [PubMed] [Google Scholar]
- Huang H, Vasung L, 2014. Gaining insights of fetal brain development with diffusion MRI and histology. Int J Dev Neurosci 32, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS, 1997. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology 387, 167–178. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Price DJ, 2005. Exuberance in the development of cortical networks. Nat. Rev. Neurosci 6, 955–965. [DOI] [PubMed] [Google Scholar]
- Iuculano T, Rosenberg-Lee M, Supekar K, Lynch CJ, Khouzam A, Phillips J, Uddin LQ, Menon V, 2014. Brain organization underlying superior mathematical abilities in children with autism. Biological Psychiatry 75, 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S, 2006. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Computer methods and programs in biomedicine 81, 106–116. [DOI] [PubMed] [Google Scholar]
- Kaiser M, 2017. Mechanisms of connectome development. Trends Cogn. Sci 21, 703–717. [DOI] [PubMed] [Google Scholar]
- Karolis VR, Froudist-Walsh S, Brittain PJ, Kroll J, Ball G, Edwards AD, Dell’Acqua F, Williams SC, Murray RM, Nosarti C, 2016. Reinforcement of the brain’s rich-club architecture following early neurodevelopmental disruption caused by very preterm birth. Cereb. Cortex 26, 1322–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprian G, Brugger PC, Weber M, Krssák M, Krampl E, Herold C, Prayer D, 2008. In utero tractography of fetal white matter development. Neuroimage 43, 213–224. [DOI] [PubMed] [Google Scholar]
- Kostović I, 1990. Structural and histochemical reorganization of the human prefrontal cortex during perinatal and postnatal life. Prog. Brain Res 85, 223–239; discussion 239–240. [DOI] [PubMed] [Google Scholar]
- Kostović I, Jovanov-Milošević N, 2006. The development of cerebral connections during the first 20–45 weeks’ gestation. Seminars in Fetal and Neonatal Medicine Elsevier, pp. 415–422. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M, 2010. The development of the subplate and thalamocortical connections in the human foetal brain. Acta paediatrica 99, 1119–1127. [DOI] [PubMed] [Google Scholar]
- LaMantia A, Rakic P, 1990. Axon overproduction and elimination in the corpus callosum of the developing rehsus monkey. J. Neurosci 10, 2156–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zou QH, He Y, Yang YH, 2013. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc. Natl. Acad. Sci. U. S. A 110, 1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry RC, Mathur A, Miller JH, Ozcan A, Snyder AZ, Schefft GL, Almli CR, Shiran SI, Conturo TE, Neil JJ, 2002. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb. Cortex 12, 1237–1243. [DOI] [PubMed] [Google Scholar]
- Mitter C, Prayer D, Brugger PC, Weber M, Kasprian G, 2015. In vivo tractography of fetal association fibers. PloS one 10, e0119536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mišić B, Betzel RF, De Reus MA, Van Den Heuvel MP, Berman MG, McIntosh AR, Sporns O, 2016. Network-level structure-function relationships in human neocortex. Cereb. Cortex 26, 3285–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PCM, 1999. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol 45, 265–269. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC, 2002. Fiber tracking: principles and strategies - a technical review. N. M. R. Biomed 15, 468–480. [DOI] [PubMed] [Google Scholar]
- Newman ME, Girvan M, 2004. Finding and evaluating community structure in networks. Phys. Rev. E 69, 026113. [DOI] [PubMed] [Google Scholar]
- Oishi K, Mori S, Donohue PK, Ernst T, Anderson L, Buchthal S, Faria A, Jiang H, Li X, Miller MI, van Zijl PCM, Chang L, 2011. Multi-contrast human neonatal brain atlas: Application to normal neonate development analysis. NeuroImage 56, 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang A, Jeon T, Sunkin SM, Pletikos M, Sedmak G, Sestan N, Lein ES, Huang H, 2015. Spatial mapping of structural and connectional imaging data for the developing human brain with diffusion tensor imaging. Methods (San Diego, Calif) 73, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Kang H, Detre J, Roberts T, Huang H, 2017. Short-range connections in the developmental connectome during typical and atypical brain maturation. Neurosci Biobehav Rev 83:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit AS, Robinson E, Aljabar P, Ball G, Gousias IS, Wang Z, Hajnal JV, Rueckert D, Counsell SJ, Montana G, Edwards AD, 2014. Whole-brain mapping of structural connectivity in infants reveals altered connection strength associated with growth and preterm birth. Cereb. Cortex 24, 2324–2333. [DOI] [PubMed] [Google Scholar]
- Park H-J, Friston K, 2013. Structural and functional brain networks: from connections to cognition Science 342, 1238411. [DOI] [PubMed] [Google Scholar]
- Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, Lu Y, Glenn OA, Ferriero DM, Barkovich AJ, Vigneron DB, 2004. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. NeuroImage 22, 1302–1314. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A, 2001. Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain Research Bulletin 54, 255–266. [DOI] [PubMed] [Google Scholar]
- Rathbone R, Counsell S, Kapellou O, Dyet L, Kennea N, Hajnal J, Allsop J, Cowan F, Edwards A, 2011. Perinatal cortical growth and childhood neurocognitive abilities. Neurology 77, 1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseeuw PJ, 1987. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. Journal of computational and applied mathematics 20, 53–65. [Google Scholar]
- Rubinov M, Sporns O, 2010. Complex network measures of brain connectivity: uses and interpretations. NeuroImage 52, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Seber GA, 2009. Multivariate observations[M]. John Wiley & Sons; 252. [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ, 2010. Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex 20, 2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Mishra V, Ouyang M, Peng Q, Slinger M, Liu S, Huang H, 2017. Human fetal brain connectome: structural network development from middle fetal stage to birth. Front. Neurosci 11:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kotter R, 2007. Identification and classification of hubs in brain networks. PLoS One 2, e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Folkerth RD, Galaburda AM, Grant PE, 2012. Emerging cerebral connectivity in the human fetal brain: an MR tractography study. Cereb. Cortex 22, 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Dassanayake MT, Shen S, Katkuri Y, Alexis M, Anderson AL, Yeo L, Mody S, Hernandez-Andrade E, Hassan SS, Studholme C, Jeong JW, Romero R, 2013. Cross-hemispheric functional connectivity in the human fetal brain. Sci. Transl. Med 5, 173–ra24.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang R, Wang G-J, Volkow ND, 2014. Functional connectivity and brain activation: a synergistic approach. Cereb. Cortex 24, 2619–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymofiyeva O, Hess CP, Ziv E, Lee PN, Glass HC, Ferriero DM, Barkovich AJ, Xu D, 2013. A DTI-based template-free cortical connectome study of brain maturation. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP, 2007. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn. Sci 11, 153–157. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MI, Thomason ME, 2016. Functional connectivity of the human brain in utero. Trends Cogn. Sci 20, 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Kahn RS, Goni J, Sporns O, 2012. High-cost, high-capacity backbone for global brain communication. Proc. Natl. Acad. Sci. U. S. A 109, 11372–11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Kersbergen KJ, de Reus MA, Keunen K, Kahn RS, Groenendaal F, de Vries LS, Benders MJNL, 2015. The neonatal connectome during preterm brain development. Cereb. Cortex 25, 3000–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O, 2011. Rich-club organization of the human connectome. J. Neurosci 31, 15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O, 2013. Network hubs in the human brain. Trends Cogn Sci 17, 683–696. [DOI] [PubMed] [Google Scholar]
- Vasung L, Huang H, Jovanov‐Milošević N, Pletikos M, Mori S, Kostović I, 2010. Development of axonal pathways in the human fronto-limbic brain: histochemical characterization and diffusion tensor imaging. J. Anat 217, 400–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes PE, Bullmore ET, 2015. Annual Research Review: Growth connectomics - the organization and reorganization of brain networks during normal and abnormal development. Journal of Child Psychology and Psychiatry 56, 299–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang X, Xia M, Liao X, Evans A, He Y, 2015. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci 9, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberger DM, Roberts TP, Barkovich AJ, Prayer LM, Moseley ME, Kucharczyk J, 1995. Identification of “premyelination” bydiffusion-weighted mri. Journal of Computer Assisted Tomography 19, 28–33. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC, 1998. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography 22, 139–152. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE, 2006. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. New England Journal of Medicine 355, 685–694. [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y, 2013. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One 8, e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X-K, Zhang J, Small M, 2010. Rich-club connectivity dominates assortativity and transitivity of complex networks. Phys. Rev. E 82, 046117. [DOI] [PubMed] [Google Scholar]
- Yap PT, Fan Y, Chen Y, Gilmore JH, Lin W, Shen D, 2011. Development trends of white matter connectivity in the first years of life. PLoS One 6, e24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Ouyang A, Chalak L, Jeon T, Chia J, Mishra V, Sivarajan M, Jackson G, Rollins N, Liu S, Huang H, 2016. Structural development of human fetal and preterm brain cortical plate based on population-averaged templates. Cereb. Cortex 26, 4381–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET, 2010. Network-based statistic: identifying differences in brain networks. NeuroImage 53, 1197–1207. [DOI] [PubMed] [Google Scholar]
- Zhang J, Evans A, Hermoye L, Lee SK, Wakana S, Zhang W, Donohue P, Miller MI, Huang H, Wang X, van Zijl PCM, Mori S, 2007. Evidence of slow maturation of the superior longitudinal fasciculus in early childhood by diffusion tensor imaging. Neuroimage 38, 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.