Abstract
Background
Previously healthy firefighters with World Trade Center (WTC) dust exposure developed airway disease. Risk factors for irritant-associated asthma/COPD overlap are poorly defined.
Methods
This study included 2,137 WTC-exposed firefighters who underwent a clinically indicated bronchodilator pulmonary function test (BD-PFT) between 9/11/2001 and 9/10/2017. A post-BD FEV1 increase of > 12% and 200 mL from baseline defined asthma, and a post-BD FEV1/FVC ratio < 0.7 identified COPD cases. Participants who met both criteria had asthma/COPD overlap. Eosinophil levels were measured on screening blood tests performed shortly after 9/11/2001 and prior to BD-PFT; a subgroup of participants also had serum IgE and 21 cytokines measured (n = 215). Marginal Cox regression models for multiple events assessed the associations of eosinophil levels or serum biomarkers with subsequent diagnosis, with age, race, smoking, WTC exposure, first post-9/11 FEV1/FVC ratio, and BMI included as covariates.
Results
BD-PFT diagnosed asthma/COPD overlap in 99 subjects (4.6%), isolated-asthma in 202 (9.5%), and isolated-COPD in 215 (10.1%). Eosinophil concentration ≥ 300 cells/μL was associated with increased risk of asthma/COPD overlap (hazard ratio [HR], 1.85; 95% CI, 1.16-2.95) but not with isolated-asthma or isolated-COPD. Serum IL-4 also predicted asthma/COPD overlap (HR, 1.51 per doubling of cytokine concentration; 95% CI, 1.17-1.95). Greater IL-21 concentration was associated with both isolated-asthma and isolated-COPD (HRs of 1.73 [95% CI, 1.27-2.35] and 2.06 [95% CI, 1.31-3.23], respectively).
Conclusions
In WTC-exposed firefighters, elevated blood eosinophil and IL-4 levels are associated with subsequent asthma/COPD overlap. Disease-specific T-helper cell type 2 biomarkers present years before diagnosis suggest patient-intrinsic predisposition to irritant-associated asthma/COPD overlap.
Key Words: asthma, airway obstruction, biomarkers, COPD, eosinophils
Abbreviations: BD, bronchodilator; FDNY, Fire Department of the City of New York; HR, hazard ratio; IFN, interferon; PFT, pulmonary function test; RV, residual volume; Th, T-helper cell type; TLC, total lung capacity; WTC, World Trade Center
FOR EDITORIAL COMMENT, SEE PAGE 1270
The collapse of the World Trade Center (WTC) on September 11, 2001 (9/11), exposed the Fire Department of the City of New York (FDNY) rescue and recovery workers to caustic dust and products of combustion.1 Subsequently, WTC-exposed rescue and recovery workers had high rates of airway injury, including excessive loss of lung function,2 obstructive ventilatory defect,3 and airway hyperreactivity.4 Firefighters with elevated postexposure blood eosinophil concentrations were at increased risk of developing COPD.5
Asthma/COPD overlap is a recently defined endotype of COPD,6, 7, 8 with patients experiencing a poorer quality of life and higher mortality compared with patients who have either isolated-COPD or isolated-asthma.9, 10, 11 Risk factors for asthma/COPD overlap are poorly defined, but among those with smoking-related COPD, elevated eosinophils in sputum and blood are biomarkers for this condition.12, 13 Longitudinal studies are needed to define risk factors for asthma/COPD overlap.14
The aim of the present study was to determine early predictors of asthma/COPD overlap among WTC-exposed firefighters with at least one post-9/11 clinically indicated pulmonary function test (PFT) with bronchodilator (BD) measurement (N = 2,137). The main predictors of interest were blood biomarkers collected during participants’ post-9/11 FDNY medical monitoring examination. We also examined these measurements in association with isolated-asthma and with isolated-COPD as a way to understand the unique predictors of asthma/COPD overlap.
Methods
Study Population
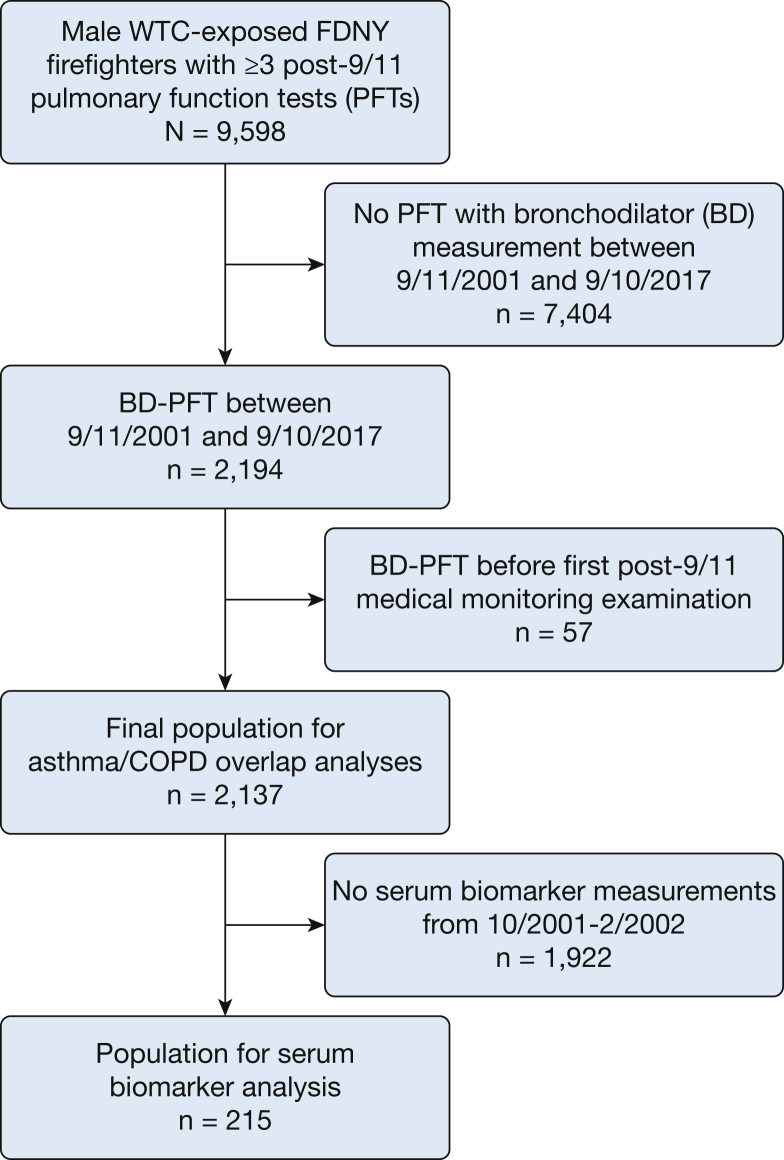
The source population consisted of 9,598 male firefighters who were actively employed by the FDNY on 9/11; first arrived at the WTC between 9/11/2001 and 9/24/2001; and had ≥ 3 post-9/11 FEV1 measurements from routine medical monitoring PFTs taken at FDNY.5 A subset of this population received at least one clinically indicated BD-PFT performed according to American Thoracic Society standards15 at a hospital-based pulmonary function laboratory between 9/11/2001 and 9/10/2017. We excluded 57 participants whose BD-PFT occurred before their first post-9/11 medical monitoring examination. The final study population included 2,137 firefighters (Fig 1).
Figure 1.
Firefighters who participated in the asthma/COPD overlap study. Shown is the source population of male firefighters who were employed by the FDNY on 9/11/2001, present at the WTC site between 9/11/2001 and 9/24/2001, and had at least three routine monitoring PFTs taken between 9/11/2001 and 9/10/2017 for FEV1 slope measurement. The final study population included those who had received a post-9/11/2001 clinically indicated PFT with BD measurement. The serum biomarker population was a subgroup who had biomarkers measured on serum drawn between 10/2001 and 2/2002. BD = bronchodilator; FDNY = Fire Department of the City of New York; PFT = pulmonary function test; WTC = World Trade Center.
Participants provided written informed consent. The Montefiore Medical Center (FWA #00002558)/Albert Einstein College of Medicine (FWA #00023382) Institutional Review Board approved this study.
Baseline Characteristics
Demographic data were retrieved from the FDNY employee database. Participants’ height, weight, self-reported smoking status (current, former, or never-smoker), and time of initial arrival at the WTC site were assessed during routine medical monitoring examinations at FDNY (both active duty firefighters and WTC-exposed retirees are scheduled to have a monitoring examination once every 12 to 18 months). Individuals were classified as having high (morning of 9/11), moderate (afternoon on 9/11 to 9/12), or low (9/13 to 9/24) WTC exposure based on their WTC site arrival time.4 Current and former smokers were grouped together as ever-smokers in these analyses. Those who consistently self-reported no cigarette smoking were classified as never-smokers.
Blood and Serum Biomarkers
Eosinophil concentration was measured from blood drawn shortly after 9/11, during the first post-9/11 monitoring examination. The median first post-9/11 blood draw date was 1/10/2002 (interquartile range: 11/26/2001 to 12/27/2002). We also had pre-9/11 blood data (eosinophil concentration) for the 1,008 participants who had blood drawn at a pre-9/11 monitoring examination. Serum biomarkers from the first post-9/11 blood draw, including IgE and cytokines, were available for a subgroup of the study cohort (n = 215). Serum was stored at –80°C; IL-4 and interferon (IFN)-γ were assayed with MilliporeSigma HSTCMAG28SPMX21 and IgE with HGAMMAG-303E.
Pulmonary Function
Participants’ most recent BD-PFT from the 9/11/2001 to 9/10/2017 period provided the pre- and post-BD FEV1 and FVC measurements used to define our main outcome. A BD response of > 12% and 200 mL increase from baseline FEV1 diagnosed asthma.16 COPD was defined according to the Global Initiative for Obstructive Lung Disease criteria, which requires a FEV1/FVC ratio < 0.7 on a post-BD PFT.7 We classified individuals who had a BD response and FEV1/FVC ≥ 0.7 as having isolated-asthma, and those who had FEV1/FVC < 0.7 and no BD response as having isolated-COPD. Individuals who met the criteria for both asthma and COPD had asthma/COPD overlap.
Total lung capacity (TLC) and residual volume (RV) measurements were also available from the BD-PFT data; these were measured prior to BD administration. We used spirometric measurements from 22,737 routine monitoring PFTs (always done without BD) taken between 9/11/2001 and 9/10/2017 to assess post-9/11 FEV1 trajectories. FEV1 values from post-9/11 monitoring PFTs that occurred prior to the BD-PFT were used to determine whether patients with asthma and/or COPD had accelerated (> 64 mL/y) or expected (≤ 64 mL/y) FEV1 decline post-9/11 but prior to our outcome determination; for individuals who had neither diagnosis, all post-9/11 FEV1 values were included in the FEV1 decline rate calculation. Pre-9/11 FEV1 and FVC measurements were available from 1,265 spirometries performed at FDNY monitoring in the year prior to 9/11 (9/11/2000 to 9/10/2001).
Statistical Analyses
Demographic and other characteristics of the study population and serum biomarker subgroup were assessed as proportions and means ± SD, with independent sample Student t tests or χ2 tests used to evaluate differences, as appropriate. Longitudinal FEV1 % predicted, FEV1/FVC ratio, and post-9/11 rate of FEV1 change were estimated in four subsets of the population defined according to outcome (isolated-asthma, isolated-COPD, asthma/COPD overlap, or neither condition) using linear mixed effects models with random intercepts. Participants’ age on 9/11, height, and race were included as fixed effects in the models with the absolute FEV1 or FEV1/FVC ratio as the outcome. Mean FEV1 % predicted and FEV1/FVC ratio values in the four groups were estimated for each 1-year period between 9/11/2000 and 9/10/2017, and mean rates of FEV1 change were determined by using the post-9/11 spirometry data.
Log-rank Mantel-Cox tests were performed to examine the univariable associations of post-9/11 FEV1 trajectory (accelerated vs expected FEV1 decline), eosinophil concentration, and smoking status with incident asthma/COPD overlap, followed by multivariable marginal Cox regression models for multiple events to evaluate shared and distinct risk factors for isolated-asthma, isolated-COPD, and asthma/COPD overlap. Censoring occurred at the time of the BD-PFT. Blood eosinophil concentration was assessed first as a binary variable (≥ 300 cells/μL vs < 300 cells/μL) and then as a continuous variable. Two sensitivity analyses were conducted: one that excluded individuals with an FEV1/FVC ratio < 0.7 on a pre-9/11 monitoring PFT (n = 69) and another that examined the relationship between pre-9/11 eosinophil concentration and the outcomes of interest (n = 1,008). Absolute change in eosinophil concentration from pre-9/11 to post-9/11 was also investigated.
A multivariable-adjusted Cox regression analysis for multiple events data was also performed in the serum biomarker subpopulation (n = 215) to determine whether log2-transformed serum IgE and 21 cytokines were associated with isolated-asthma, isolated-COPD, or asthma/COPD overlap. After Bonferroni correction, the significance cutoff for the serum biomarker analyses was set at a two-sided P value of .0024. For all other analyses, reported P values are two-sided and considered significant at the < .05 level. Multivariable models included age, race, smoking status, WTC exposure, first post-9/11 FEV1/FVC ratio, and BMI as covariates. Covariates were selected based on theory. Data analyses were performed by using SAS version 9.4 (SAS Institute, Inc). Figures were created by using Prism 7 (GraphPad Software).
Results
Baseline Characteristics
Demographic and other characteristics of the 2,137 firefighters with clinically indicated post-9/11 BD-PFT in the final study population (Fig 1) and those without BD-PFT are presented in Table 1. Compared with WTC-exposed firefighters who did not have a BD-PFT, the study population was slightly different in that it was older, had a higher BMI and post-9/11 blood eosinophil concentration, and a greater proportion of ever-smokers. These differences were more pronounced in those who would develop a post-BD FEV1/FVC ratio < 0.70. The serum biomarker subgroup was similar to the study population, with the exception of having a smaller proportion of ever-smokers.
Table 1.
Population Characteristics and Longitudinal Lung Function
| Variable | WTC-Exposed No BD-PFT (N = 7,404) | BD-PFT Study Population (N = 2,137) |
Subpopulation With Serum Biomarkers (n = 215) | P Valuea | |
|---|---|---|---|---|---|
| Post-BD FEV1/FVC ≥ 0.7 (n = 1,823) |
Post-BD FEV1/FVC < 0.7 (n = 314) |
||||
| Age on 9/11, yb | 39.9 ± 7.6 | 40.5 ± 6.7 | 44.0 ± 6.8 | 41.0 ± 6.8 | < .001 |
| BMI, kg/m2b,c | 28.7 ± 3.4 | 29.2 ± 3.5 | 28.5 ± 3.3 | 28.7 ± 3.3 | < .001 |
| Smoking status, No. (%)c | |||||
| Never | 5,031 (67.9) | 1,229 (67.4) | 142 (45.2) | 185 (86.0) | < .001 |
| Former | 2,143 (28.9) | 542 (29.7) | 148 (47.1) | 22 (10.2) | |
| Current | 230 (3.1) | 52 (2.9) | 24 (7.6) | 8 (3.7) | |
| Race, No. (%) | |||||
| White | 6,971 (94.2) | 1,719 (94.3) | 299 (95.2) | 208 (96.7) | < .001 |
| Black | 174 (2.3) | 36 (2.0) | 10 (3.2) | 4 (1.9) | |
| Hispanic | 234 (3.2) | 66 (3.6) | 5 (1.6) | 3 (1.4) | |
| Other | 25 (0.3) | 2 (0.1) | 0 | 0 | |
| WTC arrival time, No. (%) | |||||
| Morning of 9/11 | 1,129 (15.3) | 366 (20.1) | 52 (16.6) | 37 (17.2) | < .001 |
| Afternoon on 9/11–9/12 | 5,322 (71.9) | 1,295 (71.0) | 223 (71.0) | 168 (78.1) | |
| 9/13 to 9/24 | 953 (12.9) | 162 (8.9) | 39 (12.4) | 10 (4.7) | |
| Pre-9/11 spirometry | |||||
| FEV1, Lb | 4.43 ± 0.68d | 4.38 ± 0.69e | 3.94 ± 0.74f | 4.32 ± 0.69g | < .001 |
| FEV1 % predictedb | 105.9 ± 13.3d | 104.3 ± 13.7e | 95.2 ± 15.4f | 103.3 ± 14.2g | < .001 |
| FEV1/FVCb | 0.85 ± 0.05d | 0.85 ± 0.05e | 0.78 ± 0.07e,f | 0.84 ± 0.05g | < .001 |
| Post-9/11 spirometry | |||||
| FEV1, Lb,c | 4.05 ± 0.65 | 3.96 ± 0.65 | 3.46 ± 0.73 | 3.92 ± 0.70 | < .001 |
| FEV1 % predictedb,c | 97.8 ± 12.9 | 95.4 ± 13.5 | 85.2 ± 15.3 | 94.4 ± 14.4 | < .001 |
| FEV1/FVCb,c | 0.84 ± 0.05 | 0.84 ± 0.05 | 0.74 ± 0.07 | 0.83 ± 0.06 | < .001 |
| Post-9/11 FEV1 slope, mL/yb | –35.1 ± 30.8 | –37.8 ± 32.4 | –47.5 ± 36.3 | –41.1 ± 37.0 | < .001 |
| Blood eosinophil concentration | |||||
| Pre-9/11 cells/μLb | 154 ± 109h | 162 ± 117i | 186 ± 144j | 153 ± 104k | < .001 |
| Post-9/11 cells/μLb,c | 184 ± 126l | 194 ± 136m | 231 ± 175n | 198 ± 132 | < .001 |
BD = bronchodilator; PFT = pulmonary function test; WTC = World Trade Center.
ANOVA or χ2 test comparing values in first three columns.
Mean ± SD.
Value on first post-9/11 monitoring examination.
N = 6,836.
N = 1,686.
N = 285.
N = 209.
N = 3,295.
N = 857.
N = 151.
N = 109.
N = 7,388.
N = 1,815.
N = 314.
Lung Function on Monitoring and BD-PFTs
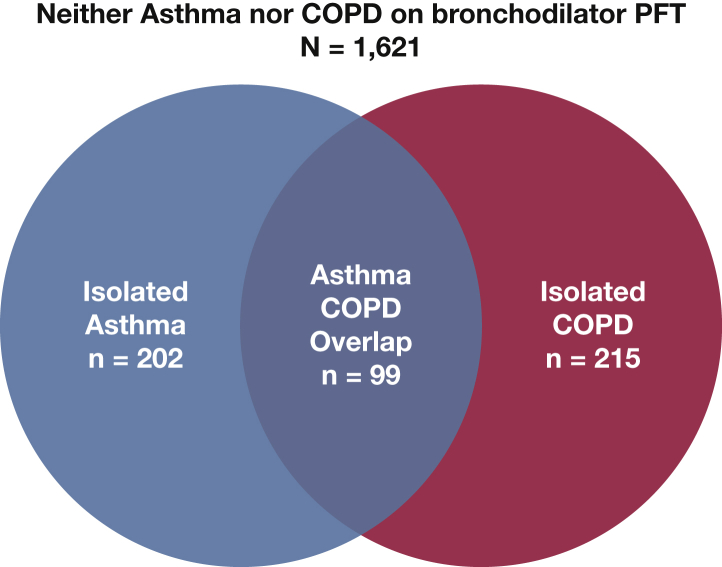
Clinically indicated BD-PFT diagnosed isolated-asthma in 202 individuals (9.5%), isolated-COPD in 215 (10.1%), and asthma/COPD overlap in 99 (4.6%) (Fig 2). At the time of BD-PFT, the asthma/COPD overlap subgroup had a lower pre-BD FEV1 % predicted, lower pre-BD FEV1/FVC ratio, and higher RV/TLC ratio than any other diagnostic category (Table 2). BD response was similar in patients with asthma/COPD overlap and isolated-asthma (22.6 ± 13.3% vs 19.9 ± 12.8% increase in FEV1, respectively; P = .09).
Figure 2.
Asthma/COPD overlap in WTC-exposed firefighters who had a BD-PFT. The Venn diagram demonstrates abnormalities on BD-PFTs obtained via the WTC treatment program. Isolated-asthma was diagnosed in 202 individuals who had FEV1 BD response of > 12% and 200 mL. Isolated-COPD was diagnosed in 215 individuals who had a post-BD FEV1/FVC ratio < 0.70. Asthma/COPD overlap was diagnosed in 99 who had both an FEV1 BD response > 12% and 200 mL, and an FEV1/FVC ratio < 0.70. The remainder of the study population (n = 1,621) did not have a BD response or airflow limitation. See Figure 1 legend for expansion of abbreviations.
Table 2.
Bronchodilator PFT Results
| Variable | Asthma/COPD Overlap | Isolated-Asthma | Isolated-COPD | Neither Diagnosis |
|---|---|---|---|---|
| Pre-BD FEV1 % predicted | 67.3 ± 14.8 | 80.9 ± 13.5a | 82.3 ± 15.2a | 96.9 ± 13.2a |
| Post-BD FEV1 % predicted | 81.3 ± 14.5 | 96.2 ± 13.7a | 85.9 ± 15.0a | 100.5 ± 13.5a |
| Pre-BD FVC % predicted | 93.3 ± 15.8 | 87.1 ± 13.9a | 101.2 ± 15.1a | 98.3 ± 12.9a |
| Post-BD FVC % predicted | 101.8 ± 14.0 | 96.0 ± 13.3a | 103.4 ± 15.1 | 98.5 ± 12.9a |
| Pre-BD FEV1/FVC | 0.56 ± 0.08 | 0.73 ± 0.07a | 0.62 ± 0.07a | 0.77 ± 0.05a |
| Post-BD FEV1/FVC | 0.62 ± 0.07 | 0.78 ± 0.05a | 0.64 ± 0.06a | 0.80 ± 0.05a |
| Pre-BD RV/TLC | 0.40 ± 0.10 | 0.33 ± 0.09a | 0.33 ± 0.08a | 0.28 ± 0.07a |
Data are expressed as mean ± SD. RV = residual volume; TLC = total lung capacity. See Table 1 legend for expansion of other abbreviations.
P < .05 vs asthma/COPD overlap subgroup.
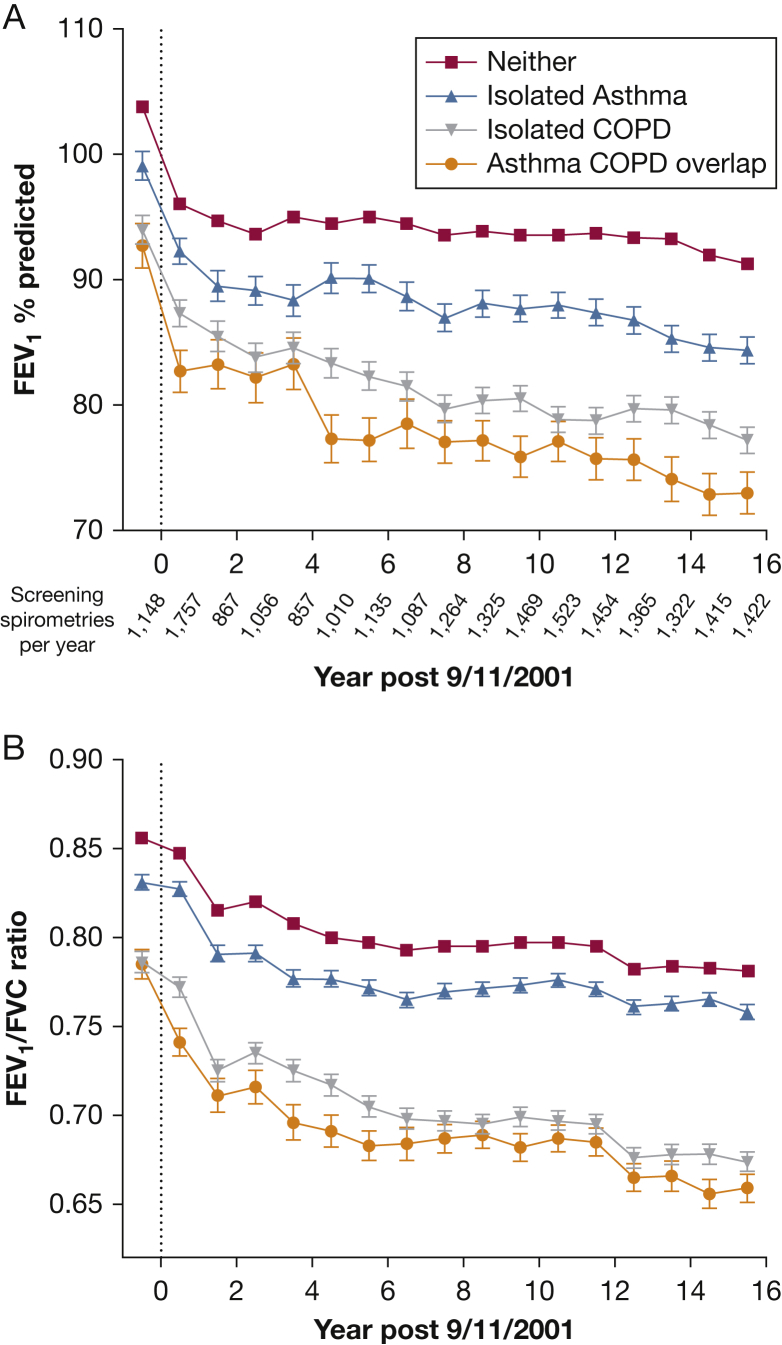
Both the pre-9/11 and first post-9/11 FEV1 % predicted in each subgroup were on average ≥ 80% on monitoring PFTs but were lowest in those who went on to develop asthma/COPD overlap (Fig 3A). The FEV1/FVC ratio on the first post-9/11 monitoring PFT was also lowest in this subgroup (Fig 3B). The annual post-9/11 FEV1 loss in individuals with asthma/COPD overlap was similar to that of the COPD subgroup (47.6 mL/y [95% CI, 43.5-51.6] and 47.2 mL/y [95% CI, 44.7-49.6], respectively), and greater than the rate of FEV1 loss in those with isolated-asthma (43.4 mL/y; 95% CI, 40.7-46.2) or neither outcome (36.8 mL/y; 95% CI, 35.9-37.6).
Figure 3.
A-B, Lung function over time. A, Mean ± SEM (SEM not shown if it is smaller than the size of the symbol) FEV1 % predicted in each year between 9/11/2000 and 9/10/2017 in the asthma/COPD overlap (orange), isolated-COPD (gray), isolated-asthma (blue), and asthma-free and COPD-free (red) groups. The vertical line at 0 represents 9/11/2001. The number of spirometries per year is shown below the x-axis. B, Mean FEV1/FVC ratio in the aforementioned groups in each year, adjusted for race, height, and age, using the same number of spirometries per year as shown in panel A.
Risk Factors for Asthma/COPD Overlap
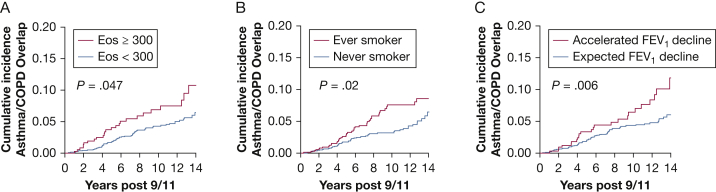
Univariable analyses showed that the incidence of asthma/COPD overlap was elevated in participants with post-9/11 eosinophil concentration ≥ 300 cells/μL (HR, 1.69; 95% CI, 1.00-2.81; P < .05) (Fig 4A), those with a history of smoking (HR, 1.67; 95% CI, 1.11-2.50; P = .02) (Fig 4B), and those experiencing post-9/11-accelerated FEV1 decline (HR, 2.05; 95% CI, 1.22-3.43; P = .006) (Fig 4C). In multivariable marginal Cox regression models for multiple events, asthma/COPD overlap was predicted by eosinophil concentration ≥ 300 cells/μL (Table 3). Eosinophil concentration was not significantly associated with isolated-asthma or isolated-COPD. When isolated-asthma and asthma/COPD overlap were compared directly, asthma/COPD overlap was still associated with elevated eosinophils (Table 4). Results were similar if analyses were restricted to those who had eosinophils measured < 15 months following 9/11 (data not shown). A unique risk factor for isolated-asthma was high-intensity WTC exposure, and for isolated-COPD, it was ever-smoking. Post-9/11-accelerated FEV1 decline was associated with all three outcomes. The observed associations did not change when eosinophil concentration was assessed as a continuous variable (data not shown).
Figure 4.
A-C, Cumulative incidence of asthma/COPD overlap in WTC-exposed firefighters who had a BD PFT. A, Cumulative incidence of asthma/COPD overlap in participants with blood EOS concentration ≥ 300 cells/μL (red) and < 300 cells/μL on first post-9/11 medical monitoring examination (blue). The level of significance shown in each panel was determined by using the log-rank test. B, Cumulative incidence in those who reported ever smoking (red) and never smoking (blue). C, Cumulative incidence in participants who had an accelerated rate of post-9/11 FEV1 decline > 64 mL/y (red) and those with expected FEV1 decline ≤ 64 mL/y (blue). EOS = eosinophil. See Figure 1 legend for expansion of other abbreviations.
Table 3.
Marginal Cox Regression Models Predicting Isolated-Asthma, Isolated-COPD, and Asthma/COPD Overlap
| Variable | Asthma/COPD Overlap vs Neither |
Isolated-Asthma vs Neither |
Isolated-COPD vs Neither |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Eosinophils ≥ 300 cells/μLa | 1.85 | 1.16-2.95 | .009 | 0.93 | 0.63-1.36 | .69 | 1.16 | 0.82-1.64 | .39 |
| Accelerated FEV1 decline | 2.17 | 1.40-3.35 | < .001 | 2.12 | 1.54-2.91 | < .001 | 2.18 | 1.59-2.99 | < .001 |
| Ever-smoker | 0.92 | 0.58-1.44 | .70 | 0.77 | 0.56-1.05 | .09 | 1.60 | 1.18-2.17 | .003 |
| WTC exposure morning of 9/11 | 1.40 | 0.84-2.32 | .19 | 1.58 | 1.14-2.20 | .006 | 0.86 | 0.59-1.26 | .44 |
The total N value was 2,124 due to missing covariates. Data were adjusted for age, race, BMI, and first post-9/11 FEV1/FVC measurement. HR = hazard ratio. See Table 1 legend for expansion of other abbreviation.
First post-9/11 measurement.
Table 4.
Marginal Cox Regression Models Predicting Isolated-Asthma, Isolated-COPD, and Asthma/COPD Overlap
| Variable | Asthma/COPD Overlap vs Isolated-Asthma |
Asthma/COPD Overlap vs Isolated-COPD |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Eosinophils ≥ 300 cells/μLa | 2.00 | 1.11-3.62 | .02 | 1.60 | 0.89-2.85 | .12 |
| Accelerated FEV1 decline | 1.02 | 0.60-1.74 | .94 | 0.99 | 0.60-1.65 | .98 |
| Ever-smoker | 1.19 | 0.69-2.05 | .52 | 0.57 | 0.34-0.98 | .04 |
| WTC exposure morning of 9/11 | 0.88 | 0.49-1.61 | .68 | 1.62 | 0.88-3.01 | .12 |
To confirm that elevated post-9/11 eosinophil concentration and accelerated FEV1 decline were associated with incident asthma/COPD overlap, a sensitivity analysis was conducted excluding patients with a pre-exposure PFT that showed a FEV1/FVC ratio < 0.7 (n = 69). First post-9/11 eosinophil concentration ≥ 300 cells/μL and accelerated FEV1 decline both remained significant predictors of asthma/COPD overlap (HR, 1.67 [95% CI, 1.03-2.71], P = .03; and HR, 2.15 [95% CI, 1.35-3.43], P = .001, respectively). To assess if pre-exposure blood eosinophil levels were indicative of a predisposition to asthma/COPD overlap, another sensitivity analysis was performed by using pre-9/11 eosinophil concentration in place of the post-9/11 measurement. The subgroup of participants who had had a pre-9/11 blood draw (n = 1,008) had baseline characteristics and lung function similar to those of the full study population (data not shown). We found that pre-9/11 eosinophil concentration ≥ 300 cells/μL was also associated with the outcome (HR, 1.42; 95% CI, 1.22-1.66; P < .001). Change in eosinophil concentration from pre-9/11 to post-9/11 was not associated with asthma/COPD overlap (data not shown).
To gain further insight into the immunologic pathways associated with isolated-asthma, isolated-COPD, and asthma/COPD overlap, we examined serum T-helper cell type 1, T-helper cell type 17 (Th17), and T-helper cell type 2 (Th2) biomarkers obtained within 6 months of 9/11. A multivariable marginal Cox regression analysis for multiple events in the serum biomarker subpopulation (n = 215) found that higher early post-9/11 IgE was associated with incident asthma/COPD overlap, but this result was not significant after adjustment for multiple comparisons (Table 5). We found that elevated IL-4 predicted asthma/COPD overlap and elevated IL-21 predicted both isolated-asthma and isolated-COPD; elevated IFN-γ was a protective factor for isolated-asthma and isolated-COPD. Early post-9/11 levels of IL-5, IL-13, IL-17, IL-23, and IL-6 were not associated with any of the three mutually exclusive outcomes (data not shown).
Table 5.
Marginal Cox Regression Models Predicting Isolated-Asthma, Isolated-COPD, and Asthma/COPD Overlap in the Subpopulation With Serum Drawn Between 9/11/2001 and 3/10/2002
| Variable | Asthma/COPD Overlap vs Neither |
Isolated-Asthma vs Neither |
Isolated-COPD vs Neither |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| IgEa | 2.31 | 1.14-4.67 | .02b | 1.21 | 0.92-1.58 | .16 | 1.14 | 0.81-1.62 | .45 |
| IFN-γa | 0.42 | 0.22-0.81 | .01b | 0.48 | 0.32-0.70 | < .001 | 0.45 | 0.28-0.70 | < .001 |
| IL-21a | 1.33 | 0.89-1.98 | .17 | 1.73 | 1.27-2.35 | < .001 | 2.06 | 1.31-3.23 | .002 |
| IL-4 substituted for IL-21 in marginal Cox regression model | |||||||||
| IL-4a | 1.51 | 1.17-1.95 | .002 | 1.68 | 1.08-2.61 | .02b | 1.35 | 0.96-1.91 | .08 |
The total N value was 215. Data were adjusted for age, race, BMI, smoking status, WTC exposure level, and first post-9/11 FEV1/FVC. IFN = interferon. See Table 1 and 3 legends for expansion of other abbreviations.
One log2 increase (doubling) of cytokine concentration.
A P value of .0024 was considered significant after Bonferroni correction.
Discussion
The WTC-exposed FDNY firefighter population is a cohort comprising previously healthy male subjects. Importantly, asthma documented during pre-employment medical evaluation precludes employment as a FDNY firefighter. Those who develop reactive airways disease during their career are removed from active duty17; therefore, the prevalence of pre-9/11 asthma in this cohort was low. The massive irritant exposure at the WTC site resulted in an acute drop in lung function, with rescue/recovery workers going on to experience air trapping, as well as fixed and reversible airflow obstruction.2, 3, 4 In the present study, we observed that elevated early post-9/11 blood eosinophil concentration predicted irritant-associated asthma/COPD overlap but not isolated-asthma or isolated-COPD. A sensitivity analysis noted that pre-911 elevated eosinophils were a risk factor for asthma/COPD overlap. This finding suggests a pre-WTC exposure predisposition to irritant-associated fixed and reversible airway injury. Although we found some overlapping biomarkers of these outcomes, the observation that there are unique biomarkers of vulnerability to asthma/COPD overlap, isolated-asthma, and isolated-COPD suggests the potential for different pathologic processes for these three diagnoses; this topic could be explored in future studies.
The FDNY WTC-exposed cohort has advantages for investigating irritant-associated airways disease. Data from a centralized post-WTC medical treatment program enabled explicit diagnostic criteria for incident isolated-asthma, isolated-COPD, and asthma/COPD overlap. Pre-9/11 lung function and blood data were available for a large subset of the cohort, enabling assessment of early indicators of susceptibility to subsequent airway injury. Our observation that pre-exposure eosinophil concentration was associated with later asthma/COPD overlap suggests patient-intrinsic vulnerability to the damaging effects of WTC dust exposure. How much the exposure itself contributed to the presentation is limited because not every assessment was performed pre-exposure.
Compared with those who developed isolated-asthma or isolated-COPD, patients with asthma/COPD overlap had a lower post-exposure FEV1 and FEV1/FVC ratio. An investigation in a cohort without WTC exposure found that low lung function in childhood was a risk factor for subsequent asthma/COPD overlap,18 and thus our observed associations between early lung function measurements and this outcome may be evidence of similar biological mechanism(s). Both the asthma/COPD overlap and isolated-COPD subgroups have progressive airway injury, with greater post-9/11 FEV1 rates of decline than individuals with isolated-asthma or neither diagnosis. The asthma/COPD overlap subgroup also experienced more air trapping, shown by higher RV/TLC at the time of BD-PFT. This finding is consistent with previous investigation of asthma/COPD overlap in never-smokers19 and could be evidence of the severity of small airways dysfunction associated with WTC exposure.20
Eosinophils and IgE are two Th2 mediators that have been extensively studied as risk factors for asthma, COPD, and asthma/COPD overlap.12, 21, 22, 23, 24, 25 In the present investigation, serum IgE was associated with asthma/COPD overlap but did not achieve significance after Bonferroni adjustment for multiple comparisons. We did observe a significant association between serum levels of the Th2 cytokine IL-4 and this outcome. IL-4 may be a biomarker on the causal pathway to irritant-associated asthma/COPD overlap because inhibiting it with dupilumab reduced asthma severity in non-WTC-exposed patients with or without high eosinophil levels.26, 27 Further investigation is required to assess the Th2 pathways that are associated with FEV1 decline, airflow limitation, and BD response following an intense irritant exposure.
The incident asthma observed in the present study is a variant of irritant-induced asthma.28 The fact that it was associated with high-intensity WTC exposure but not eosinophil concentration suggests that airway reactivity in this cohort is a form of noneosinophilic asthma.29 IFN-γ was a protective biomarker for this condition and also for isolated-COPD. High IFN-γ is associated with low IL-4 in modulation of pulmonary lymphocyte-mediated innate immunity.30 Furthermore, asthma is associated with blunted IFN-γ response.31, 32
The balance between Th2 and Th17 cytokines in airway inflammation is under active investigation.33, 34, 35 IL-21, which was found to significantly predict isolated-asthma and isolated-COPD in the present cohort, is a component of the Th17 pathway that is produced by innate lymphoid cells which regulate airway inflammation.36 Elevated levels are associated with airway inflammation in mouse models and humans.37, 38, 39 The data from the WTC-exposed FDNY cohort are consistent with a Th2 and Th17 response predicting airway remodeling and reactivity. These data support further investigation of the innate Th17 response to pulmonary irritants.
In univariable analyses, we found that in addition to high eosinophil concentration and accelerated FEV1 decline, ever-smoking was associated with asthma/COPD overlap. After adjusting for confounders, such as post-9/11 lung function, smoking was a unique risk factor for isolated-COPD but not isolated-asthma or asthma/COPD overlap. Therefore, the relationship between smoking and asthma/COPD overlap in this cohort was confounded by the other covariates. High WTC exposure level was not associated with isolated-COPD or asthma/COPD overlap, which suggests that an intense but brief irritant exposure did not increase risk of airway remodeling. In this cohort, isolated-COPD was not associated with eosinophil levels. In a population with smoking-related COPD, however, elevated blood eosinophil concentration was a biomarker of increased exacerbation.40 The variability of eosinophil effect reported in the literature may be related to the proportion of the study cohorts with an asthma component.41, 42
There are several limitations to this investigation. The FDNY firefighters are overwhelmingly white, male, and experienced a massive irritant exposure, potentially limiting generalizability of these findings; however, most findings from the FDNY cohort have been replicated in other WTC-exposed cohorts. Our definitions of isolated asthma, asthma/COPD overlap, and isolated COPD depend on results from the most recent BD-PFT. It is possible that those with isolated COPD, defined as FEV1/FVC < 0.7 and no BD response in this study, have asthma/COPD overlap because we did not proceed to methacholine challenge testing in the subgroup. Similarly, those with asthma/COPD overlap, defined as FEV1/FVC < 0.7 and a BD response, may not have persistent FEV1/FVC < 0.7 with permanent airway remodeling. A third limitation may be the use of eosinophils ≥ 300 cells/μL or < 300 cells/μL in our analyses. We modeled post-9/11 eosinophils as a continuous variable and still observed a significant association with asthma/COPD overlap. This method suggests that cut-point selection did not drive the analyses. Lastly, this study was vulnerable to selection bias. The study population with clinically indicated BD-PFT was systematically different from those without BD-PFT, with more intense WTC exposure, higher eosinophil levels, and post-WTC exposure lower lung function. This outcome precludes assessment of rates of asthma/COPD overlap in the entire cohort because undiagnosed cases are likely. Nevertheless, analyses within the BD-PFT population provide a valid assessment of risk factors for specific diagnoses within a symptomatic subgroup.
Conclusions
The data from the FDNY WTC Health Program are a valuable resource for understanding irritant-associated airways disease in a previously healthy population. High eosinophil concentrations, uniquely associated with asthma/COPD overlap in this population, may reflect biological pathways that predispose one to exaggerated inflammation and/or poor counterregulatory responses to inflammation, leading to reversible and fixed airflow obstruction. There may be potential for early interventions that involve targeting specific inflammatory pathways in an attempt to improve lung function outcomes.
Acknowledgements
Author contributions: M. D. W. had full access to all of the data in the study and agrees to be accountable for all aspects of the work so that questions related to the accuracy and integrity of the research are appropriately investigated and resolved. M. D. W. conceived of the study and designed it in conjunction with C. L., R. Z.-O., C. B. H., and D. J. P.; and M. D. W., A. S., B. P., R. Z.-O., and T. S. analyzed and interpreted the data. A. S., M. D. W., and C. L. drafted the first manuscript, with critical revisions from B. P., R. Z.-O., C. B. H., D. J. P., M. P. W., T. S., H. W. C., A. N., and K. I. B. All authors approved the final manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsors had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data; the preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
FUNDING/SUPPORT: This study was supported by National Institute for Occupational Safety and Health [Contracts 200-2011-39383, 200-2011-39378, 200-2017-93426, and 200-2017-93326]; National Institute for Occupational Safety and Health [Grants U01 OH011302 and U01 OH011300]; and National Heart, Lung, and Blood Institute [Grant R01HL119326].
References
- 1.Lioy P.J., Weisel C.P., Millette J.R. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect. 2002;110(7):703–714. doi: 10.1289/ehp.02110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldrich T.K., Gustave J., Hall C.B. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med. 2010;362(14):1263–1272. doi: 10.1056/NEJMoa0910087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiden M.D., Ferrier N., Nolan A. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest. 2010;137(3):566–574. doi: 10.1378/chest.09-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldrich T.K., Vossbrinck M., Zeig-Owens R. Lung function trajectories in World Trade Center-exposed New York City firefighters over 13 years: the roles of smoking and smoking cessation. Chest. 2016;149(6):1419–1427. doi: 10.1016/j.chest.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeig-Owens R., Singh A., Aldrich T.K. Blood leukocyte concentrations, FEV1 decline, and airflow limitation: a 15-year longitudinal study of WTC-exposed firefighters. Ann Am Thorac Soc. 2018;15(2):173–183. doi: 10.1513/AnnalsATS.201703-276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christenson S.A., Steiling K., van den Berge M. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(7):758–766. doi: 10.1164/rccm.201408-1458OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogelmeier C.F., Criner G.J., Martinez F.J. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 8.Cosentino J., Zhao H., Hardin M. Analysis of asthma-chronic obstructive pulmonary disease overlap syndrome defined on the basis of bronchodilator response and degree of emphysema. Ann Am Thorac Soc. 2016;13(9):1483–1489. doi: 10.1513/AnnalsATS.201511-761OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baarnes C.B., Andersen Z.J., Tjonneland A., Ulrik C.S. Incidence and long-term outcome of severe asthma-COPD overlap compared to asthma and COPD alone: a 35-year prospective study of 57,053 middle-aged adults. Int J Chron Obstruct Pulmon Dis. 2017;12:571–579. doi: 10.2147/COPD.S123167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauppi P., Kupiainen H., Lindqvist A. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48(3):279–285. doi: 10.3109/02770903.2011.555576. [DOI] [PubMed] [Google Scholar]
- 11.Lange P., Colak Y., Ingebrigtsen T.S., Vestbo J., Marott J.L. Long-term prognosis of asthma, chronic obstructive pulmonary disease, and asthma-chronic obstructive pulmonary disease overlap in the Copenhagen City Heart study: a prospective population-based analysis. Lancet Respir Med. 2016;4(6):454–462. doi: 10.1016/S2213-2600(16)00098-9. [DOI] [PubMed] [Google Scholar]
- 12.Gao J., Zhou W., Chen B., Lin W., Wu S., Wu F. Sputum cell count: biomarkers in the differentiation of asthma, COPD and asthma-COPD overlap. Int J Chron Obstruct Pulmon Dis. 2017;12:2703–2710. doi: 10.2147/COPD.S142466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konno S., Taniguchi N., Makita H. Distinct phenotypes of smokers with fixed airflow limitation identified by cluster analysis of severe asthma. Ann Am Thorac Soc. 2018;15(1):33–41. doi: 10.1513/AnnalsATS.201701-065OC. [DOI] [PubMed] [Google Scholar]
- 14.Woodruff P.G., van den Berge M., Boucher R.C. American Thoracic Society/National Heart, Lung, and Blood Institute Asthma-Chronic Obstructive Pulmonary Disease Overlap Workshop Report. Am J Respir Crit Care Med. 2017;196(3):375–381. doi: 10.1164/rccm.201705-0973WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2017 Update).https://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention. Accessed February 15, 2018.
- 17.Niles J.K., Webber M.P., Gustave J. The impact of the World Trade Center attack on FDNY firefighter retirement, disabilities, and pension benefits. Am J Industrial Med. 2011;54(9):672–680. doi: 10.1002/ajim.20965. [DOI] [PubMed] [Google Scholar]
- 18.Bui D.S., Burgess J.A., Lowe A.J. Childhood lung function predicts adult chronic obstructive pulmonary disease and asthma-chronic obstructive pulmonary disease overlap syndrome. Am J Respir Crit Care Med. 2017;196(1):39–46. doi: 10.1164/rccm.201606-1272OC. [DOI] [PubMed] [Google Scholar]
- 19.Gelb A.F., Yamamoto A., Verbeken E.K., Nadel J.A. Unraveling the pathophysiology of the asthma-COPD overlap syndrome: unsuspected mild centrilobular emphysema is responsible for loss of lung elastic recoil in never smokers with asthma with persistent expiratory airflow limitation. Chest. 2015;148(2):313–320. doi: 10.1378/chest.14-2483. [DOI] [PubMed] [Google Scholar]
- 20.Friedman S.M., Maslow C.B., Reibman J. Case-control study of lung function in World Trade Center Health Registry area residents and workers. Am J Respir Crit Care Med. 2011;184(5):582–589. doi: 10.1164/rccm.201011-1909OC. [DOI] [PubMed] [Google Scholar]
- 21.Hastie A.T., Martinez F.J., Curtis J.L. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967. doi: 10.1016/S2213-2600(17)30432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitaguchi Y., Komatsu Y., Fujimoto K., Hanaoka M., Kubo K. Sputum eosinophilia can predict responsiveness to inhaled corticosteroid treatment in patients with overlap syndrome of COPD and asthma. Int J Chron Obstruct Pulmon Dis. 2012;7:283–289. doi: 10.2147/COPD.S30651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosio B.G., Soriano J.B., Lopez-Campos J.L. Defining the asthma-COPD overlap syndrome in a COPD cohort. Chest. 2016;149(1):45–52. doi: 10.1378/chest.15-1055. [DOI] [PubMed] [Google Scholar]
- 24.Hirai K., Shirai T., Suzuki M. A clustering approach to identify and characterize the asthma and chronic obstructive pulmonary disease overlap phenotype. Clin Exp Allergy. 2017;47(11):1374–1382. doi: 10.1111/cea.12970. [DOI] [PubMed] [Google Scholar]
- 25.Tamada T., Sugiura H., Takahashi T. Biomarker-based detection of asthma-COPD overlap syndrome in COPD populations. Int J Chron Obstruct Pulmon Dis. 2015;10:2169–2176. doi: 10.2147/COPD.S88274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenzel S., Castro M., Corren J. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet (London, England) 2016;388(10039):31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel S., Ford L., Pearlman D. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 28.Tarlo S.M., Lemiere C. Occupational asthma. N Engl J Med. 2014;370(7):640–649. doi: 10.1056/NEJMra1301758. [DOI] [PubMed] [Google Scholar]
- 29.Carr T.F., Zeki A.A., Kraft M. Eosinophilic and noneosinophilic asthma. Am J Respir Crit Care Med. 2018;197(1):22–37. doi: 10.1164/rccm.201611-2232PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bal S.M., Bernink J.H., Nagasawa M. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nature Immunol. 2016;17(6):636–645. doi: 10.1038/ni.3444. [DOI] [PubMed] [Google Scholar]
- 31.Message S.D., Laza-Stanca V., Mallia P. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105(36):13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silver J.S., Kearley J., Copenhaver A.M. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol. 2016;17(6):626–635. doi: 10.1038/ni.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choy D.F., Hart K.M., Borthwick L.A. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7(301):301ra129. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 34.Dillon M.B., Schulten V., Oseroff C. Different Bla-g T cell antigens dominate responses in asthma versus rhinitis subjects. Clin Exp Allergy. 2015;45(12):1856–1867. doi: 10.1111/cea.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu W., Liu S., Verma M. Mechanism of TH2/TH17-predominant and neutrophilic TH2/TH17-low subtypes of asthma. J Allergy Clin Immunol. 2017;139(5):1548–1558.e1544. doi: 10.1016/j.jaci.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coquet J.M., Schuijs M.J., Smyth M.J. Interleukin-21-producing CD4(+) T cells promote type 2 immunity to house dust mites. Immunity. 2015;43(2):318–330. doi: 10.1016/j.immuni.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Lajoie S., Lewkowich I., Herman N.S. IL-21 receptor signalling partially mediates Th2-mediated allergic airway responses. Clin Exp Allergy. 2014;44(7):976–985. doi: 10.1111/cea.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qaseem A.S., Singh I., Pathan A.A. A recombinant fragment of human surfactant protein D suppresses basophil activation and T-helper type 2 and B-cell responses in grass pollen-induced allergic inflammation. Am J Respir Crit Care Med. 2017;196(12):1526–1534. doi: 10.1164/rccm.201701-0225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricciardolo F.L., Sorbello V., Folino A. Identification of IL-17F/frequent exacerbator endotype in asthma. J Allergy Clin Immunol. 2017;140(2):395–406. doi: 10.1016/j.jaci.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 40.Watz H., Tetzlaff K., Wouters E.F. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390–398. doi: 10.1016/S2213-2600(16)00100-4. [DOI] [PubMed] [Google Scholar]
- 41.Papi A., Kostikas K., Wedzicha J.A. Dual bronchodilation response by exacerbation history and eosinophilia in the FLAME study. Am J Respir Crit Care Med. 2018;197(9):1223–1226. doi: 10.1164/rccm.201709-1822LE. [DOI] [PubMed] [Google Scholar]
- 42.Song J.H., Lee C.H., Kim J.W. Clinical implications of blood eosinophil count in patients with non-asthma-COPD overlap syndrome COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2455–2464. doi: 10.2147/COPD.S129321. [DOI] [PMC free article] [PubMed] [Google Scholar]