Abstract
Background:
In the Multicenter AIDS Cohort Study, we examined whether fibroblast growth factor-23 (FGF-23), a bone-derived phosphaturic hormone involved in bone metabolism, is associated with incident frailty. Further, we examined whether this association differs by HIV serostatus and race.
Methods:
Of 715 men assessed for frailty and selected for FGF-23 measurements using stored blood samples (2007–2011), 512 men were non-frail at/prior to the baseline visit. Frailty was defined by the presence of ≥3 of the following on two consecutive 6-month visits within 1 year: unintentional weight loss ≥10 pounds, weakness, slowness, low energy and low physical activity. We determined the association of FGF-23 levels with incident frailty using proportional hazards models adjusting for sociodemographics, co-morbidities and kidney function.
Results:
Sixty-five percent were HIV-infected; 29% were black. Median baseline FGF-23 levels were lower in HIV-infected versus HIV-uninfected men (33.7 vs. 39.9 rU/mL, p=0.006) but similar by race. During a median follow-up of 6.6 years, 32 men developed frailty; they had higher baseline FGF-23 levels versus men who remained non-frail (45 vs. 36 rU/mL, p=0.02). FGF-23 (per doubling) was associated with a 1.63-fold risk of frailty (95%CI:1.19, 2.23); results did not differ by HIV serostatus. Conversely, FGF-23 was associated with a 2.72-fold risk of frailty among blacks (95%CI:1.51, 4.91) but had minimal association among non-blacks (HR=1.26, 95%CI:0.77, 2.05; p-interaction=0.024).
Conclusion:
Among men with or at-risk for HIV infection, higher FGF-23 was associated with greater risk of frailty, particularly in blacks. The mechanisms by which FGF-23 may contribute to frailty warrant further study.
Introduction
In an era in which combination antiretroviral therapy has resulted in durable virologic suppression, the life expectancy of treated HIV-infected individuals nearly approximates those of HIV-uninfected individuals.1,2 However, this progress has been tempered by the early onset of multiple chronic age-related diseases, the prevalence of which in one report was similar in the HIV-infected population to HIV-uninfected persons who were 15 years older.3 Moreover, individuals aged 50 or older now comprise nearly half of persons living with HIV and nearly 20% of those newly diagnosed with HIV in the U.S.4
With the aging of the HIV-infected population, management focus has pivoted towards chronic age-related, non-infectious conditions, such as frailty. Treated HIV-infected individuals appear to be more likely to develop frailty than HIV-uninfected persons.5,6 Regardless of HIV serostatus, frailty, a condition characterized by accelerated physiological decline and maladaptive responses to stressors,7 is independently associated with substantial risks for adverse health outcomes, including chronic disease and infection-related hospitalizations and death.8,9
The mechanisms by which frailty develops in the context of HIV infection remain to be fully elucidated. The cumulative effects of multi-morbidity, persistent immune activation, and heightened systemic inflammation likely play a major role in frailty pathogenesis.7,10 One potential contributor to frailty pathogenesis that has not been well-studied among HIV-infected persons is disturbances to the bone-endocrine axis that arise either through co-morbid conditions such as chronic kidney disease (CKD) or through systemic inflammation. A growing body of evidence links CKD to frailty.11,12 Furthermore, CKD, like HIV infection, is accompanied by persistent systemic inflammation, which in turn, may further impact hormones important for maintaining bone health.
One such hormone is fibroblast growth factor-23 (FGF-23) which is involved in the regulation of bone mineral metabolism. Primarily secreted by osteocytes, FGF-23 lowers serum phosphate levels by augmenting phosphate excretion within the renal proximal tubules13 and by inhibiting parathyroid hormone secretion.14 It also lowers levels of active vitamin D13 and enhances calcium reabsorption in the renal distal tubules.15 A growing body of work suggests a bi-directional association between systemic inflammation and FGF-23 expression.16,17 In addition, experimental and epidemiological studies indicate that FGF-23 may affect cardiac smooth muscle cells and promote the development of left ventricular hypertrophy.18 Among patients with CKD, higher serum FGF-23 levels have been associated with significant risk for progressive kidney disease, cardiovascular morbidity, and mortality.18–22 FGF-23’s effects, however, may occur before overt CKD develops23 and may extend beyond the kidney and heart, since FGF-23 is expressed in other organs.24
Despite the importance of FGF-23 in bone mineral metabolism, its potential relationship with inflammation, and its observed association with age-related co-morbidities, only one study among elderly HIV-uninfected adults examined FGF-23’s association with frailty.25 In this prior study, higher serum FGF-23 levels were associated with significantly higher odds of frailty. To our knowledge, no study has investigated this association in HIV-infected populations. To explore the potential role of FGF-23 in frailty development among individuals with or at-risk for HIV infection, we examined whether concentrations of FGF-23 were associated with incident frailty in the Multicenter AIDS Cohort Study (MACS). Since HIV infection involves persistent inflammation and abnormalities in bone mineral metabolism and because racial/ethnic differences exist in markers of bone mineral metabolism and in susceptibility to frailty,26 we also evaluated whether FGF-23’s association with frailty differed by HIV serostatus and race.
Methods
Study Design and Population
The MACS is an ongoing, prospective cohort study of the natural and treated history of HIV infection among men who have sex with men. As of July 1, 2017, it included a total of 3912 HIV-infected and 3443 HIV-uninfected participants recruited in four waves (4954 in 1984–85; 866 in 1987; 1350 in 2001–2003; and 383 in 2010+) from four sites located in the United States (Baltimore, MD/Washington, DC; Chicago, IL; Los Angeles, CA; Pittsburgh, PA/Columbus, OH). Details on study design and methods have been described elsewhere.27–29 Briefly, MACS collects data regarding participants’ demographics, medical history, HIV treatment and behaviors at semi-annual study visits using standardized questionnaires. Blood and urine specimens are collected during regular study visits. Unused specimens are stored in a central repository for future testing. All participants have provided written informed consent to study protocols. Study protocols were approved by local Institutional Review Boards. The current investigation was also approved by the University of California, San Francisco and San Francisco VA Medical Center Institutional Review Boards.
From October 2007 onwards, frailty assessments have been conducted at all MACS sites semi-annually. To assess the association between FGF-23 levels and frailty, we selected 715 HIV-infected and -uninfected men enrolled in MACS cardiovascular ancillary study30 who had fasting plasma or serum specimens available between 2007 and 2011 (with oversampling of HIV-infected men by design). We measured FGF-23 levels at the earliest MACS study visit on or after October 2007. The visit at which specimens were chosen was designated as the baseline visit. During the sampling process, we excluded HIV-infected men who were naïve to highly-active antiretroviral therapy (HAART) at baseline. From those with FGF-23 measurements, we excluded men who were missing frailty data at the baseline visit (n=47) or were frail at or prior to the baseline visit (n=147). The final study population comprised 521 participants.
Measurement of Plasma FGF-23 Concentrations
Fasting (≥8 hours) plasma or serum specimens were stored at −70ºC in a central repository and just thawed before the testing. A two-site enzyme-linked immunosorbent assay (ELISA) (Kainos Laboratories, Inc., Tokyo, Japan) kit was used to measure the intact form of FGF-23 at Dr. Richard Semba’s laboratory at the Johns Hopkins School of Medicine. The intra-assay and inter-assay coefficients of variation at this laboratory were both <5%.
Assessment of Frailty
The Fried Criteria for frailty were operationalized in 2007 to identify MACS participants with frailty phenotype.31 The frailty phenotype was defined by the presence of 3 of 5 conditions: 1) unintentional weight loss (self-reported unintentional weight loss of ≥10 pounds during the previous 6 months); 2) weakness (scored lower than the 20th percentile of HIV-uninfected men in grip strength test measured with a Jamar hydraulic handheld dynamometer); 3) low energy (self-reported difficulty in performing activities in past 4 weeks due to health); 4) slowness (walked slower than the 20th percentile of HIV-uninfected men over a course of 4 meters); 5) low physical activity level (self-reported limitation in vigorous activities due to health). Measures of these components were standardized across study sites and have been assessed consistently at semi-annual visits beginning October 1, 2007. For the current analysis, the presence of frailty was confirmed at consecutive visits within 1 year. An individual was considered non-frail if he never fulfilled the frailty phenotype definition during the study follow-up, or only met the frailty phenotype definition at a single study visit which was not confirmed on a consecutive visit within 1 year.
Covariates
Demographic characteristics were collected at the MACS enrollment visit. Variables that were assessed at the baseline visit (or at the closest visit within 2 years prior to the baseline visit) included body mass index (BMI), blood pressure, serum creatinine, fasting blood glucose and fasting lipid levels, spot urine protein-to-creatinine ratios and self-reported cigarette smoking, alcohol use and injection drug use. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to estimate glomerular filtration rate (eGFR, mL/min/1.73m2) from serum creatinine.32 Proteinuria was defined as a spot urine protein-to-creatinine ratio (UPCR) ≥200 mg/g.33 Hepatitis C virus (HCV) serostatus was determined by the presence of HCV RNA or antibody in serum. Diabetes mellitus was defined as a fasting blood glucose ≥126 mg/dL or a self-reported diagnosis with the use of glucose-lowering medications. Hypertension was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg, or a self-reported diagnosis with use of anti-hypertensive medications. Dyslipidemia was defined as a total cholesterol ≥200 mg/dL, low-density lipoprotein ≥130 mg/dL, high-density lipoprotein <40 mg/dL, triglyceride ≥150 mg/dL or a self-reported diagnosis with the use of lipid-lowering medications. HIV serostatus was assessed using an ELISA test and confirmed with a Western blot. CD4+ T-cell counts were determined using standardized flow cytometry; plasma HIV-1 RNA levels were measured using Amplicor HIV-1 monitor ultrasensitive test (Roche Diagnostics, 50 copies/mL lower limit of detection) or TaqMan HIV-1 test (Roche Diagnostics, 20 copies/mL lower limit of detection). We considered other HIV-specific factors including history of clinically defined AIDS diagnosis, current and cumulative exposure to HAART, nucleoside reverse transcriptase inhibitors (NRTI), non-nucleoside reverse transcriptase inhibitors (NNRTI), protease inhibitors (PI) and tenofovir disoproxil fumarate (TDF). HAART was defined according to the Department of Health and Human Services/ Henry J. Kaiser Family Foundation Panel guidelines.34
Statistical Analysis
FGF-23 levels were categorized into tertiles for the comparison of demographic, behavioral and clinical characteristics at the baseline visit. Continuous variables were compared using one-way ANOVA or the Kruskal-Wallis test, as appropriate. Fisher’s exact test was used for categorical variables.
To assess the association of FGF-23 levels with incident frailty, we first constructed Kaplan-Meier plots to graphically explore the proportion of men who were frailty-free by FGF-23 tertiles. We then used multivariable Cox proportional hazards models to evaluate the independent association between FGF-23 levels (per doubling on linear scale) and the risk of developing frailty. Three separate models were constructed with staged adjustment for potential confounders. The first model adjusted for age and race. The second model additionally adjusted for HIV serostatus and markers of kidney function and injury – eGFR and proteinuria. The fully adjusted model included additional adjustment for the enrollment cohort (ie. pre-2001 and 2001+) and other frailty-associated factors including BMI, HCV serostatus, hypertension, diabetes mellitus, dyslipidemia and smoking. All covariates were assessed prior to or at the baseline visit. The proportional hazards assumption was graphically explored using log-log plots and evaluated using Schoenfeld residuals. Potential effect modification of the associations between FGF-23 levels and frailty by HIV serostatus and by race were assessed in stratified analyses and with the inclusion of interaction terms. The likelihood ratio test was used to test the statistical significance of potential effect modification. We also explored whether FGF-23 levels were associated with presentation of individual components of frailty (including weakness, slowness, unintentional weight loss, low energy and low physical activity). We repeated the multivariable Cox regression analyses evaluating each individual frailty component at the time of incident frailty as the outcomes. As a sensitivity analysis, we compared men with incident frailty to those who never met the definition of the frailty phenotype.
Among 521 participants included in the present study, 39 (7.5%) were missing data on one or more covariates at the baseline visit. To address the potential bias due to missing covariates, we conducted multiple imputation using the Markov Chain Monte Carlo methods as a sensitivity analysis. In all analyses, p-values less than 0.05 were considered statistically significant.
Results
Baseline participant characteristics
In total, 521 non-frail men were included in the study with a mean age of 49.8 years (standard deviation [SD]: 7.1 years); 147 (28%) were black, and 335 (64%) were HIV-infected. The median eGFR and UPCR levels were 92.8 mL/min/1.73m2 (interquartile range [IQR]: 81.0, 104.1) and 80 mg/g (IQR: 59, 114). The overall median baseline FGF-23 level was 36.2 rU/mL (IQR: 23.7, 53.8). When compared by HIV serostatus, the median FGF-23 levels were lower among HIV-infected men (33.7 vs 39.9 rU/mL, p=0.006). Black men also had slightly lower FGF-23 levels compared non-black men (median 34.2 vs 37.1 rU/mL, p=0.037).
Table 1 displays participant characteristics at the baseline visit across FGF-23 tertiles. Compared to individuals in the lowest FGF-23 tertile, those with higher levels were more likely to be diabetic and have lower UPCR levels, and less likely to be HIV-infected. Among HIV-infected men, 88% were on HAART, and 79% were virologically suppressed; the median CD4+ count was 575 (IQR: 430, 755) cells/mm3.
Table 1.
Baseline characteristics of HIV-uninfected and HIV-infected men by FGF-23 tertiles
| Characteristics | FGF-23 Tertiles |
P-values | ||
|---|---|---|---|---|
| <28.4 rU/mL | 28.4–45.4 rU/mL | >45.4 rU/mL | ||
| Median Follow-up, y (IQR) | 6.6 (5.6–7.0) | 6.7 (6.2–7.0) | 6.5 (5.6–6.9) | 0.106 |
| Pre-2001 cohort, n (%) | 82 (47) | 93 (54) | 102 (59) | 0.099 |
| Mean age, y (SD) | 50.1 (7) | 50.6 (7) | 51.3 (7) | 0.236 |
| Race, n (%) | ||||
| Black | 56 (32) | 49 (28) | 42 (24) | 0.362 |
| Non-black | 118 (68) | 124 (57) (7)1 | 132 (76) | |
| Cigarette smoking status, n (%) | ||||
| Current | 51 (30) | 37 (21) | 48 (28) | 0.311 |
| Past | 79 (46) | 79 (46) | 81 (47) | |
| Never | 43 (25) | 57 (33) | 45 (26) | |
| Injection drug use, n (%) | 3 (2) | 3 (2) | 3 (2) | 1.000 |
| Diabetes mellitus, n (%)* | 6 (4) | 17 (10) | 20 (12) | 0.010 |
| Hypertension, n (%) | 57 (34) | 61 (36) | 65 (38) | 0.679 |
| Dyslipidemia, n (%) | 136 (78.2) | 140 (80.9) | 141 (81.0) | 0.766 |
| HCV seropositive, n (%) | 10 (6) | 6 (4) | 6 (3) | 0.503 |
| Mean LDL, mg/dL (SD) | 111.8 (32.1) | 117.7 (34.5) | 112.4 (34.7) | 0.211 |
| Mean HDL, mg/dL (SD) | 49.1 (14.6) | 50.1 (16.5) | 48.3 (13.7) | 0.518 |
| Mean triglyceride, mg/dL (SD) | 162.9 (130.8) | 173.0 (226.3) | 174.5 (120.6) | 0.777 |
| Mean body mass index, kg/m2 (SD) | 26.1 (4.2) | 26.6 (4.5) | 26.5 (4.5) | 0.420 |
| Median eGFR, ml/min/1.73m2 (IQR) | 93.9 (81.1–105.2) | 92.1 (82.7–104.0) | 93.0 (79.5–102.7) | 0.704 |
| Median UPCR, mg/g (IQR) | 88.0 (63.0–130.0) | 77.0 (60.0–110.0) | 71.5 (55.0–105.0) | 0.005 |
| HIV-infected, n (%) | 128 (74) | 104 (60) | 103 (59) | 0.007 |
| History of AIDS, n (%) | 12 (9) | 9 (9) | 11 (11) | 0.894 |
| Median current CD4 count, cells/mm3 (IQR) | 560 (436–705) | 586 (422–837) | 599 (426–738) | 0.546 |
| Median nadir CD4 count, cells/mm3 (IQR) | 284 (184–390) | 311 (209–437) | 281 (177–399) | 0.435 |
| HIV-1 RNA level <50 copies/mL, n (%) | 105 (82) | 80 (77) | 80 (78) | 0.576 |
| Current HAART use, n (%) | 116 (91) | 91 (88) | 87 (85) | 0.364 |
| Median cumulative HAART use, y (IQR) | 7.1 (4.7–9.8) | 7.3 (4.4–9.8) | 7.3 (4.4–10.7) | 0.616 |
| Current NRTI use, n (%) | 118 (92) | 92 (89) | 89 (86) | 0.349 |
| Median cumulative NRTI use, y (IQR) | 9.3 (6.1–11.7) | 9.5 (6.4–11.9) | 9.8 (6.5–12.8) | 0.529 |
| Current NNRTI Use, n (%) | 73 (57.) | 45 (43) | 47 (46) | 0.078 |
| Median cumulative NNRTI use, y (IQR) | 3.5 (0.9–6.9) | 2.2 (0.5–5.7) | 2.6 (0.3–5.3) | 0.236 |
| Current PI use, n (%) | 49 (38) | 55 (53) | 48 (47) | 0.082 |
| Median cumulative PI use, y (IQR) | 4.0 (0.0–7.6) | 4.9 (0.6–8.6) | 5.2 (0.1–9.9) | 0.115 |
| Current TDF use, n (%) | 94 (73) | 66 (64) | 52 (51) | 0.002 |
| Median cumulative TDF use, y (IQR) | 2.6 (0.6–4.6) | 1.9 (0.0–4.5) | 1.8 (0.0–3.9) | 0.054 |
Abbreviations: SD, standard deviation; IQR, interquartile range; HCV, hepatitis C virus; LDL low density lipoprotein; HDL, high density lipoprotein; eGFR, estimated glomerular filtration rate; UPCR, urine protein-to-creatinine ration; HAART, highly active antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TDF, tenofovir disoproxil fumarate.
Of note, 63% of HIV-infected men were receiving TDF-containing HAART, for which cumulative use up to the time of the baseline visit was inversely associated with FGF-23 levels (0.94% lower per year; 95% confidence interval [CI]: 0.91, 0.95%).
Incidence of frailty and distribution of frailty components
The median follow-up was 6.6 years (IQR: 5.8, 7.0). Over a course of 3,148 person-years (PY), 32 men developed frailty, of whom 19 were HIV-infected. The overall incidence rate of frailty was 1.02 per 100-PY and was similar among HIV-infected and -uninfected men (0.95 vs. 1.13 per 100 PYs, respectively, p=0.63). When compared by race, the incidence rate of frailty was higher among blacks compared to non-blacks (1.64 vs. 0.78 per 100 PYs, respectively, p=0.03). Median FGF-23 levels at the baseline visit were higher in men who later developed frailty compared with those who did not (45.1 vs 35.7 rU/mL, p=0.02).
Among the 32 individuals who developed frailty, slowness (93%) and low physical activity (88%) were the two most common conditions at the incident frailty visit. Additionally, low energy, weakness and unintentional weight loss were present in 75%, 59% and 4%, respectively, at the incident frailty visit.
Association of FGF-23 levels with incident frailty
Figure 1 shows the Kaplan-Meier curves of frailty by FGF-23 tertiles in the overall study population. Compared to individuals in the lowest FGF-23 tertile, the proportion of individuals who survived frailty-free by the end of study follow-up was lower in those with higher FGF-23 levels. Results from the multivariable Cox model (Table 2) showed that each two-fold higher concentration of FGF-23 at baseline was associated with 1.63-fold (95% CI: 1.19, 2.23) risk of frailty in the minimally adjusted model. This association remained statistically significant after further adjustment for HIV serostatus, kidney function, enrollment cohort, and frailty-related comorbidities and in the fully adjusted model with multiple imputation for missing covariates.
Figure 1.
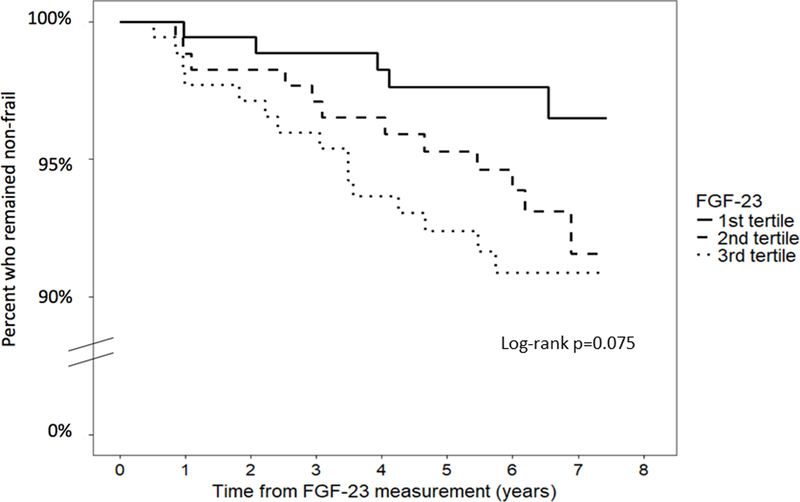
Time to frailty by FGF-23 tertiles among HIV-uninfected and HIV-infected men
Table 2.
Association of FGF-23 levels (per doubling) with risk of frailty overall and by HIV serostatus and race
| Overall (n=521) | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Age-, race-adjusted | 1.63 (1.19–2.23) | 0.002 |
| + HIV and kidney function* | 1.60 (1.16–2.21) | 0.004 |
| + other frailty-associated factors† | 1.61 (1.15–2.24) | 0.005 |
| Multiple imputation‡ | 1.66 (1.19–2.31) | 0.003 |
|
Fully adjusted models by HIV serostatus | ||
| HIV-infected (n=335) | 1.70 (1.18–2.45) | 0.005 |
| + HIV-related factors€ | 1.67 (1.15–2.41) | 0.007 |
| HIV-uninfected (n=186) | 1.21 (0.56–2.63) | 0.623 |
|
Fully adjusted models by race¶ | ||
| Blacks (n=147) | 2.72 (1.51–4.91) | 0.001 |
| Non-blacks (n=374) | 1.26 (0.77–2.05) | 0.354 |
Note: P-interaction for FGF-23 and HIV serostatus = 0.33; p-interaction for FGF-23 and race = 0.024
Kidney function assessments included eGFR and UPCR>200 mg/g
Additionally adjusted for pre-2001 cohort, BMI, HCV serostatus, hypertension, diabetes mellitus, dyslipidemia, and smoking. HCV serostatus was not adjusted for in the stratified analysis that included only HIV-uninfected men because of low number of persons with HCV seropositivity in this group.
Fully adjusted model with missing covariates addressed with multiple imputation
Additionally adjusted for cumulative TDF use, cumulative HAART use and prior AIDS
Adjusted for HIV serostatus, age, eGFR, UPCR>200 mg/g, BMI, HCV serostatus, hypertension, diabetes mellitus, and smoking. Cohort and dyslipidemia were not adjusted because they strongly correlated with race.
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; UPCR, urine protein-to-creatinine ratio; BMI, body mass index; HCV, hepatitis C virus; TDF, tenofovir disoproxil fumarate; HAART, highly active antiretroviral therapy
While the magnitude of association between FGF-23 and frailty appeared greater in HIV-infected than HIV-uninfected men, this difference was not statistically significant (p-interaction=0.33) (Table 2). In contrast, race significantly modified the association between FGF-23 and frailty (p-interaction=0.024). FGF-23 levels (per doubling) were independently associated with 2.72-fold (95% CI: 1.51, 4.91) increased risk of frailty among blacks but not among non-blacks (HR=1.26, 95% CI: 0.77, 2.05).
Sensitivity analyses yielded overall consistent results when FGF-23 values were modeled as tertiles (Supplemental Table 1). We also observed similar results when men with incident frailty were compared to those who never fulfilled the definition of the frailty phenotype (adjusted HR=1.67 [95% CI:1.21–2.30] per doubling of FGF-23).
Association of FGF-23 levels with individual clinical presentations of frailty
When fully adjusted analyses were repeated using individual frailty components as the outcomes, FGF-23 levels were independently associated with slowness (HR=1.88, 95% CI: 1.32, 2.67), low physical activity (HR=1.49, 95% CI: 1.05, 2.10), low energy (HR=1.61, 95% CI: 1.10, 2.36) and weakness (HR=1.67, 95% CI: 1.00, 2.78), but not with weight loss (HR=1.32, 95% CI: 0.51, 3.39).
Discussion
Among middle-aged men with or at-risk for HIV infection, we found that higher FGF-23 levels were associated with increased risk of frailty. The magnitude of this association was only minimally attenuated with adjustment for sociodemographic factors, HIV serostatus, kidney function, and aging-related co-morbid conditions and was comparable to the risk observed in association with 10 additional years of age in studies focused on populations of advanced age.35,36 In addition, FGF-23’s association with frailty was more pronounced among blacks compared to non-blacks, but did not differ significantly by HIV serostatus. Examination of the associations of FGF-23 with individual clinical presentations of frailty revealed that higher FGF-23 levels were significantly associated with measured slowness and marginally with weakness as well as self-reported low physical activity and energy. In contrast, the FGF-23 levels were not significantly associated with weight loss in the preceding 6 months although the number of men who experienced weight loss was small (n=4). In total, our findings suggest that FGF-23 may either play a direct role or reflect pathways involved in the pathogenesis of frailty.
Our results are consistent with those of the only prior epidemiological study evaluating the link between FGF-23 and frailty.25 In that community-based study of older adults (aged ~77 years), each doubling of FGF-23 levels was associated with 1.38-fold higher odds of frailty.25 With regards to the associations of FGF-23 with individual clinical presentations of frailty observed in our study, some, but not all, are consistent with those reported in the study by Beben and colleagues.25 These differential observations across the two studies may arise from heterogeneity in study population composition, including age and co-morbidities.
While the exact mechanisms underlying the association between FGF-23 and frailty remain unclear, we speculate that they likely encompass a complex interplay of vitamin D, klotho and inflammation. First, experimental models have shown that FGF-23 inhibits 1α-hydroxylase and stimulates 24-hydroxylase activity, resulting in lower levels of 1,25-OH2 vitamin D.13,37 In turn, lower levels of active vitamin D may contribute to frailty by adversely impacting skeletal muscle integrity38 and function39,40 as well as by modulating inflammation.41–43 While the association of vitamin D deficiency with frailty has not been well-studied in the HIV-infected population specifically, a recent meta-analysis of HIV-uninfected study populations demonstrated that 25-OH vitamin D deficiency is associated with 1.25 to 1.55 increased odds of frailty.44 Furthermore, among HIV-uninfected men aged 70 and older in the Concord Health and Ageing in Men Project (CHAMP), the lowest quartile of 1,25-OH2 vitamin D (<62 pmol/L) was cross-sectionally associated with higher odds of frailty, independent of sociodemographic factors, comorbid conditions as well as 25-OH vitamin D and parathyroid hormone levels.45
Second, the transmembrane form of klotho, a hormone that has been associated with longevity,46 physical function,47–49 and cognitive health,50 is a co-factor necessary for FGF-23’s interaction with its receptor to regulate renal tubular phosphate excretion.51 A small study comparing HIV-infected with healthy HIV-uninfected individuals demonstrated that the adipocyte expression of transmembrane klotho is reduced among treated and untreated HIV-infected individuals;52 however, the clinical significance of this reduction needs further study. The circulating form of klotho has been associated inversely with mortality risk46 and positively with muscular strength47,48 in older HIV-uninfected populations. Whether these associations are independent of FGF-23 remains unclear as these prior studies did not assess circulating klotho concurrently with FGF-23 levels. Future studies are needed to elucidate the relative impact of klotho and FGF-23 on frailty and to investigate the potential influence of HIV infection and race on these hormones and their associations with age-related outcomes.
Finally, several studies support the potential role of chronic inflammation in the development of frailty in older and HIV-infected populations.53,54 Experimental studies implicate a positive feedback loop between inflammation and FGF-23 that appears independent of klotho. Specifically, cell studies have demonstrated that inflammation stimulates FGF-23 production by osteocytes;16 in turn, FGF-23 induces production of inflammatory cytokines by hepatocytes.17 Cross-sectional findings from the Chronic Renal Insufficiency Cohort (CRIC) Study, comprised of HIV-uninfected individuals with CKD, corroborate a link between FGF-23 and inflammation and indicated that higher concentrations of FGF-23 were associated with inflammation.55 Prospectively, FGF-23 and inflammation are additively associated with increased risk of mortality in persons with CKD.56 Whether similar combined associations exist for the outcome of frailty, particularly among HIV-infected individuals needs further study. However, we observed that HIV-infected men had lower baseline levels of FGF-23 compared to HIV-uninfected men, despite the HIV-infected population being characterized by persistent systemic inflammation. The potential impact of inflammation on FGF-23 levels may be counterbalanced by TDF-mediated renal tubular phosphate wasting thus leading to lower FGF-23 concentrations.
In our study, we also noted that the association of higher FGF-23 levels with incident frailty was more pronounced among blacks than non-blacks. While disparities in co-morbid conditions, such as kidney disease, may contribute to these observed racial differences, our findings remained significant after accounting for age, smoking, HIV serostatus, comorbidities, kidney function, proteinuria, and BMI. Studies of HIV-uninfected older adults and individuals with CKD have shown that blacks have lower FGF-2357,58 and 25-OH vitamin D levels but higher concentrations of 1,25-OH2 vitamin D and intact parathyroid hormone (PTH).59 We observed similar differences in FGF-23 by race in the present study. These racial differences extend to differences in bone integrity, with blacks having greater bone density60 but paradoxically higher prevalence of frailty compared to whites.61 Apart from the present study, rigorous investigation of the clinical consequences of these racial differences in FGF-23 is lacking.
Our study has several strengths and limitations to consider. The study utilized prospectively collected longitudinal data from a well-characterized, diverse population of HIV-infected and -uninfected men. This allowed for adjustment for important potential confounders. In addition, incident frailty was confirmed on two sequential visits. However, since our study population was comprised only of men, our findings may not be generalizable to women. We were unable to more comprehensively examine the bone-endocrine axis and its association with frailty due to the lack of data on other biomarkers of bone mineral health.
In conclusion, we found that higher FGF-23 concentrations are independently associated with incident frailty among HIV-infected and -uninfected men, particularly among blacks. This association is largely consistent across objective and self-reported components of the frailty phenotype. These results add to the growing evidence of a potential role played by the complex bone-endocrine axis in frailty pathogenesis. These findings also highlight the need to expand our understanding of FGF-23 and its impact on aging processes, beyond the context of CKD.
Supplementary Material
Acknowledgements:
This study was supported by NIH/NIDDK grant R03DK096975. Jordan E. Lake was supported by NIH/NIAID grant K23 AI110532. Richard D. Semba was supported by NIH/NIA grant R01AG027012. Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Jay Bream, Todd Brown, Barbara Crain, Adrian Dobs, Michelle Estrella, W. David Hardy, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Dana Gabuzda, Frank J. Palella, Jr., Sudhir Penugonda, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (Co-P I), Aaron Aronow, Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D’Souza (Co-PI), Alison, Abraham, Keri Althoff, Jennifer Deal, Priya Duggal, Sabina Haberlen, Eithne Keelagan, Alvaro Muñoz , Derek Ng, Eric C. Seaberg, Sol Su, Pamela Surkan. Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://aidscohortstudy.org/.
Source of Funding: This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R03DK096975 to MME. JEL was supported by the National Institute of Allergy and Infectious Diseases (NIAID) grant K23 AI110532, and RDS was supported by the National Institute of Aging (NIA) grant R01AG027012. Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Jay Bream, Todd Brown, Adrian Dobs, Michelle Estrella, W. David Hardy, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), Sheila Badri, Dana Gabuzda, Frank J. Palella, Jr., Sudhir Penugonda, John P. Phair, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (PI), Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Lawrence A. Kingsley, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D’Souza (PI), Alison Abraham, Keri Althoff, Michael Collaco, Priya Duggal, Sabina Haberlen, Eithne Keelaghan, Heather McKay, Alvaro Muñoz , Derek Ng, Anne Rostich, Eric C. Seaberg, Sol Su, Pamela Surkan, Nicholas Wada. Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://aidscohortstudy.org/.
Footnotes
Conflicts of Interest: MS and MME report receiving honoraria from Gilead Sciences. FJP consults for Gilead Sciences, Janssen Pharmaceuticals, Merck and Co., and ViiV. JEL reports consulting for Gilead Sciences and Merck and research funding from Gilead. The remaining authors had no conflicts to declare.
References
- 1.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Annals of internal medicine 2007;146(2):87–95. [DOI] [PubMed] [Google Scholar]
- 3.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2011;53(11):1120–1126. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. HIV Surveillance Report, 2014. Vol. 26 Published November 2015. Accessed June 28, 2017. [Google Scholar]
- 5.Kooij KW, Wit FW, Schouten J, et al. HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls. Aids 2016;30(2):241–250. [DOI] [PubMed] [Google Scholar]
- 6.Althoff KN, Jacobson LP, Cranston RD, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014;69(2):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piggott DA, Varadhan R, Mehta SH, et al. Frailty, Inflammation, and Mortality Among Persons Aging With HIV Infection and Injection Drug Use. J Gerontol A Biol Sci Med Sci 2015;70(12):1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piggott DA, Muzaale AD, Mehta SH, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One 2013;8(1):e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piggott DA, Muzaale AD, Varadhan R, et al. Frailty and Cause-Specific Hospitalization Among Persons Aging With HIV Infection and Injection Drug Use. J Gerontol A Biol Sci Med Sci 2017;72(3):389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong C, Gange SJ, Buchacz K, et al. First Occurrence of Diabetes, Chronic Kidney Disease, and Hypertension Among North American HIV-Infected Adults, 2000–2013. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2017;64(4):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. American journal of kidney diseases : the official journal of the National Kidney Foundation 2004;43(5):861–867. [DOI] [PubMed] [Google Scholar]
- 12.Wilhelm-Leen ER, Hall YN, M KT, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med 2009;122(7):664–671 e662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 2004;113(4):561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest 2007;117(12):4003–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrukhova O, Smorodchenko A, Egerbacher M, et al. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J 2014;33(3):229–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.David V, Martin A, Isakova T, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney international 2016;89(1):135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh S, Grabner A, Yanucil C, et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney international 2016;90(5):985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011;121(11):4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semba RD, Fink JC, Sun K, et al. Serum Fibroblast Growth Factor-23 and Risk of Incident Chronic Kidney Disease in Older Community-Dwelling Women. Clin J Am Soc Nephrol 2011. [DOI] [PMC free article] [PubMed]
- 20.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. Jama 2011;305(23):2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta R, Cai X, Lee J, et al. Association of Fibroblast Growth Factor 23 With Atrial Fibrillation in Chronic Kidney Disease, From the Chronic Renal Insufficiency Cohort Study. JAMA Cardiol 2016;1(5):548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scialla JJ, Xie H, Rahman M, et al. Fibroblast growth factor-23 and cardiovascular events in CKD. Journal of the American Society of Nephrology : JASN 2014;25(2):349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney international 2010;78(10):975–980. [DOI] [PubMed] [Google Scholar]
- 24.Erben RG. Update on FGF23 and Klotho signaling. Mol Cell Endocrinol 2016;432:56–65. [DOI] [PubMed] [Google Scholar]
- 25.Beben T, Ix JH, Shlipak MG, et al. Fibroblast Growth Factor-23 and Frailty in Elderly Community-Dwelling Individuals: The Cardiovascular Health Study. J Am Geriatr Soc 2016;64(2):270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorgetti V, dos Reis LM, Ott SM. Ethnic differences in bone and mineral metabolism in healthy people and patients with CKD. Kidney international 2014;85(6):1283–1289. [DOI] [PubMed] [Google Scholar]
- 27.Detels R, Jacobson L, Margolick J, et al. The multicenter AIDS Cohort Study, 1983 to. Public Health 2012;126(3):196–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudley J, Jin S, Hoover D, Metz S, Thackeray R, Chmiel J. The Multicenter AIDS Cohort Study: retention after 9 1/2 years. Am J Epidemiol 1995;142(3):323–330. [DOI] [PubMed] [Google Scholar]
- 29.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987;126(2):310–318. [DOI] [PubMed] [Google Scholar]
- 30.Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Annals of internal medicine 2014;160(7):458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012. Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter., Suppl. 2013; 3: 1–150. Kidney international 2013;Suppl. 2013(3):1–150. http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf. Accessed March 1, 2015. [Google Scholar]
- 34.Infection. DHJKFFPoCPftToH. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. November 2014. revision. In: Services DoHaH, ed. https://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultAndAdolescentGL.pdf2014.
- 35.Myers V, Drory Y, Goldbourt U, Gerber Y. Multilevel socioeconomic status and incidence of frailty post myocardial infarction. Int J Cardiol 2014;170(3):338–343. [DOI] [PubMed] [Google Scholar]
- 36.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc 2005;53(8):1321–1330. [DOI] [PubMed] [Google Scholar]
- 37.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 2004;19(3):429–435. [DOI] [PubMed] [Google Scholar]
- 38.Ceglia L, Niramitmahapanya S, da Silva Morais M, et al. A randomized study on the effect of vitamin D(3) supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab 2013;98(12):E1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2011;22(3):859–871. [DOI] [PubMed] [Google Scholar]
- 40.Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr 2002;75(4):611–615. [DOI] [PubMed] [Google Scholar]
- 41.Kruit A, Zanen P. The association between vitamin D and C-reactive protein levels in patients with inflammatory and non-inflammatory diseases. Clin Biochem 2016;49(7–8):534–537. [DOI] [PubMed] [Google Scholar]
- 42.Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 2008;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Vita F, Lauretani F, Bauer J, et al. Relationship between vitamin D and inflammatory markers in older individuals. Age (Dordr) 2014;36(4):9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou J, Huang P, Liu P, et al. Association of vitamin D deficiency and frailty: A systematic review and meta-analysis. Maturitas 2016;94:70–76. [DOI] [PubMed] [Google Scholar]
- 45.Hirani V, Naganathan V, Cumming RG, et al. Associations between frailty and serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentrations in older Australian men: the Concord Health and Ageing in Men Project. J Gerontol A Biol Sci Med Sci 2013;68(9):1112–1121. [DOI] [PubMed] [Google Scholar]
- 46.Semba RD, Cappola AR, Sun K, et al. Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci 2011;66(7):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Semba RD, Cappola AR, Sun K, et al. Relationship of low plasma klotho with poor grip strength in older community-dwelling adults: the InCHIANTI study. Eur J Appl Physiol 2012;112(4):1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Semba RD, Ferrucci L, Sun K, et al. Low Plasma Klotho Concentrations and Decline of Knee Strength in Older Adults. J Gerontol A Biol Sci Med Sci 2016;71(1):103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shardell M, Semba RD, Kalyani RR, Hicks GE, Bandinelli S, Ferrucci L. Serum 25-Hydroxyvitamin D, Plasma Klotho, and Lower-Extremity Physical Performance Among Older Adults: Findings From the InCHIANTI Study. J Gerontol A Biol Sci Med Sci 2015;70(9):1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shardell M, Semba RD, Rosano C, et al. Plasma Klotho and Cognitive Decline in Older Adults: Findings From the InCHIANTI Study. J Gerontol A Biol Sci Med Sci 2016;71(5):677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006;444(7120):770–774. [DOI] [PubMed] [Google Scholar]
- 52.Gallego-Escuredo JM, Domingo P, Gutierrez Mdel M, et al. Reduced levels of serum FGF19 and impaired expression of receptors for endocrine FGFs in adipose tissue from HIV-infected patients. Journal of acquired immune deficiency syndromes 2012;61(5):527–534. [DOI] [PubMed] [Google Scholar]
- 53.Margolick JB, Bream JH, Martinez-Maza O, et al. Frailty and Circulating Markers of Inflammation in HIV+ and HIV- Men in the Multicenter AIDS Cohort Study. Journal of acquired immune deficiency syndromes 2017;74(4):407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 2002;162(20):2333–2341. [DOI] [PubMed] [Google Scholar]
- 55.Munoz Mendoza J, Isakova T, Ricardo AC, et al. Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol 2012;7(7):1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munoz Mendoza J, Isakova T, Cai X, et al. Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney international 2017;91(3):711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ix JH, Katz R, Kestenbaum BR, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). Journal of the American College of Cardiology 2012;60(3):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. Journal of the American Society of Nephrology : JASN 2011;22(10):1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gutierrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2011;22(6):1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner DR, Heyward VH. Measures of body composition in blacks and whites: a comparative review. Am J Clin Nutr 2000;71(6):1392–1402. [DOI] [PubMed] [Google Scholar]
- 61.Hirsch C, Anderson ML, Newman A, et al. The association of race with frailty: the cardiovascular health study. Ann Epidemiol 2006;16(7):545–553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


