Abstract
The development of a Pd(II)-catalyzed enantioselective fluorination of C(sp3)–H bonds would offer a new approach to making chiral organofluorines. However, such a strategy is particularly challenging because of the difficulty in differentiating prochiral C(sp3)–H bonds through Pd(II)-insertion, as well as the sluggish reductive elimination involving Pd–F bonds. Here, we report the development of a Pd(II)-catalyzed enantioselective C(sp3)–H fluorination using a chiral transient directing group strategy. In this work, a bulky, amino amide transient directing group was developed to control the stereochemistry of C–H insertion step and selectively promote C(sp3)–F reductive elimination pathway from Pd(IV)–F intermediate. Stereochemical analysis revealed that while the desired C(sp3)–F formation proceeds via an inner-sphere pathway with retention of configuration, the undesired C(sp3)–O formation occurs through an SN2-type mechanism. The elucidation of the dual mechanism allows us to rationalize the profound ligand effect on controlling reductive elimination selectivity from high-valent Pd species.
Graphical Abstract

The presence of a C–F bond can uniquely influence the physical and biological properties of a molecule, such as conformation, lipophilicity, and metabolic stability1,2. Therefore, fluorine incorporation has been widely used as a means to tailor the properties of a molecule. The success of this strategy is evidenced by the fact that over 25% of agrochemicals3 and 20% of drug molecules4 in the market contain at least one C–F bond in the structure. Accordingly, significant progress has been made in the field of fluorination of organic molecules5–7. Asymmetric methods that directly construct a C(sp3)–F stereocenter have also been achieved through multiple approaches8 including α-fluorination of enolate or enolate-equivalents9–11, fluoro-difunctionalization of olefins12–14, and asymmetric addition of nucleophilic fluorides to carbon electrophiles15–17. As an alternative approach, the development of a direct, enantioselective fluorination of a C(sp3)–H bond via metal-insertion would be highly appealing. Although a number of C(sp3)–H fluorination methods using palladium insertion18–21 have been reported, an asymmetric version remains to be developed. Radical-mediated processes22–26 have been shown to be an effective strategy for direct C(sp3)–H fluorination, however, only a single example of enantioselective benzylic C–H fluorination with low enantioselectivity (25% enantiomeric excess) has been reported27. Such limitations of asymmetric C(sp3)–H fluorination encouraged us to devise a new, highly enantioselective C(sp3)–H fluorination using Pd(II)-insertion chemistry.
We have recently developed a Pd(II)-catalyzed enantioselective C(sp3)–H arylation using a chiral amino acid transient directing group28. This finding led us to investigate the feasibility of developing a new chiral transient directing group to enable an enantioselective C(sp3)–H fluorination. The major pitfall of C(sp3)–H fluorination using Pd(II/IV) catalysis is the relatively sluggish C–F reductive elimination from Pd(IV) species, which renders [F+] a broadly useful bystanding oxidant that favors the reductive elimination of other C(sp3)–C(sp3) or C(sp3)–heteroatom bonds (Figure 1a)29–31. In addition, C(sp3)–[Pd(IV)(Ln)]–F species have been proposed to undergo SN2-type reactions with various oxygen- and nitrogen-nucleophiles, forming C(sp3)–O and C(sp3)–N bonds instead of C(sp3)–F bond32–34. These observations call into the question whether such reductive elimination selectivity could be biased towards fluorination by modifying the ligand environment of a Pd(IV)–F intermediate. Herein, we report the first Pd(II)-catalyzed enantioselective C(sp3)–H fluorination via metal-insertion. We achieved this goal using a transient directing group strategy (Figure 1b), in which the transient directing group serves as a chiral ligand that controls the stereochemistry in the C–H insertion step and promotes C(sp3)–F formation over the undesired C(sp3)–O formation during the reductive elimination step. Stereochemical analysis of the products suggests the reductive elimination proceeds via two distinct reaction pathways to provide the C(sp3)–F and C(sp3)–O products respectively. The identification of the dual pathways of Pd(IV) reductive elimination provides a rationale for the profound ligand effect observed on the reductive elimination selectivity (Figure 1c).
Figure 1. Enantioselective C(sp3)–H Fluorination.
a, The possibility of multiple reductive elimination pathways from Pd(IV) species generated with [F+] as oxidant presents a challenging selectivity issue. b, This work: Pd(II)-catalyzed enantioselective C(sp3)–H fluorination using chiral transient directing group strategy. c, Profound ligand effect was observed on reductive elimination selectivity (C(sp3)–O vs. C(sp3)–F). Stereochemical analysis of products suggests that such ligand effect origins from the dual mechanism of Pd(IV) reductive elimination step.
Results and discussion
Our initial efforts were focused on evaluating oxidants that can avoid the undesired oxidation of aldehyde (1a) and yet are capable of oxidizing Pd(II) to Pd(IV) species. After extensive screening (Supplementary Table 1), we were able to observe a mixture of fluorinated and acetoxylated aldehyde products when N-Fluoro-2,4,6-trimethylpyridinium salt ([F+]) was used in AcOH solvent (Table 1). With simple glycine (TDG1) as the transient directing group (entry 1), [F+] mainly served as a bystanding oxidant, providing the acetoxylation product 2a as the major product over the fluorination product 3a with a ratio of 9.7:1. As sterics on the amino acid transient directing groups increases, we began to observe the formation of more 3a (16%) and less 2a (47%) (entries 2, 3). It is noteworthy that chiral TDG3 (entry 3) gave 2a with 88% enantiomeric excess (e.e.) and 3a with 90% e.e., which implies that C–H insertion is highly stereoselective. Considering the previously observed beneficial effect of tertiary amides such as DMF and NMP in Pd(II)-catalyzed C–H fluorination reactions35,36, we prepared the amino acid-derived diethyl amide TDG4 for our reaction. Importantly, TDG4 switched the preference of the reaction pathways to afford 3a as the major product for the first time with high enantioselectivity, albeit still low chemoselectivity (entry 4). To further enhance the chemoselectivity in favor of fluorination over acetoxylation, we evaluated various solvents that could replace the solvent quantity of AcOH. We found the use of benzene as solvent with 10% AcOH improved the selectivity to 1:6.7 in favor of fluorination (entry 5). The lower ee value of the acetoxylation product 2a in this case is likely to originate from the competing inner-sphere C–O reductive elimination. Indeed, systematically lowering the concentration of AcOH slows down the bimolecular outer-sphere pathway and further decreases the ee values (Supplementary Fig. 3). Through further screening of acid additives (Supplementary Table 4), we identified C6F5CO2H as the most effective carboxylic acid which allows the fluorination to proceed with moderate efficiency, good chemoselectivity, and high enantioselectivity (entry 6). Notably, the control of reductive elimination selectivity from Pd(IV) intermediates during catalysis has only been demonstrated with C(sp2)–O vs. C(sp2)–N bond formation37. The loss of both reactivity and selectivity with amino acid directing group TDG3 (entry 7) and the less hindered amide directing groups TDG5-7 (entry 8–10) indicates the importance of the amide moiety and the bulky quaternary substituent of the transient directing group (Supplementary Table 5).
Table 1.
Reaction conditions: substrate 1a (0.10 mmol, 1.0 equiv.), [F+]BF4− (0.15 mmol, 1.5 equiv.), NBu4PF6 (0.05 mmol, 0.5 equiv.), Pd(OAc)2 (10 mol%), Transient DG (20 mol%), Solvent (entry 1–4: 0.2 M, entry 5–10: 0.25 M), 70 °C, 24 h.
The yield was determined by 1H NMR analysis of the crude product using CH2Br2 as the internal standard.
5 equiv. of C6F5CO2H was used.
R = C6F5.
The high enantioselectivities observed in both C(sp3)–O and C(sp3)–F forming processes allow us to probe the mechanism of the reductive elimination pathway through the lens of stereochemistry. Thus, the absolute stereochemistry of both 2a and 3a were identified by X-ray crystallography (Figure 2a). Interestingly, the opposite absolute configuration of these two products was observed, indicating the involvement of two distinct reaction pathways. The configuration of 3a is consistent with our previous arylation reaction28, suggesting that fluorination proceeds through a classic inner-sphere reductive elimination process with retention of stereochemistry, while 2a is formed through an SN2-type mechanism to achieve inversion of stereochemistry. To further support this mechanistic hypothesis, we conducted a series of experiments to rule out the possibility of 2a and 3a forming through an identical mechanism from two different diastereomeric palladacycles. First, we showed that C(sp3)–H insertion process is irreversible via deuterium incorporation experiments, because no deuterium incorporation was observed in substrate and products in either absence or presence of [F+] with both TDG3 and TDG4 (Figure 2b). Second, stoichiometric reaction between the pre-formed imine 7 and Pd(OAc)2 with AcOD-d4 at 70 °C allowed us to isolate the palladacycle intermediate 8, which bears the same stereochemistry with 3a as expected (Figure 2c). When intermediate 8 was treated with [F+] in AcOH, 2a and 3a with opposite configuration were formed, consistent with the catalytic reaction (Table 1, entry 4), although the C(sp3)–O/C(sp3)–F ratio and the enantioselectivity slightly vary. These combined experimental data renders the alternative hypothesis that two diastereomeric palladacycles undergo different bond-formations unlikely. Lastly, we verified the linear relationship between the e.e. of 3a and TDG4 in order to exclude the possibility of an external Pd-bound fluoride undergoing SN2 pathway for C(sp3)–F formation (Supplementary Fig. 2). It has been shown by Doyle and coworkers that Pd-bound fluoride species can act as nucleophile in Pd-catalyzed allylic fluorination38. Indeed, our mechanistic hypothesis of the competitive nature of C(sp3)–Pd(IV) reductive elimination mechanism (SN2 vs. inner-sphere) is supported by a number of previous kinetic and computational studies32,33,39–41. It is intriguing that under our system the ratio of C(sp3)–O vs. C(sp3)–F can serve as a measurement for the competition between the inner-sphere reductive elimination and SN2 pathway from Pd(IV) intermediate during catalysis. It is noteworthy that a related study of controlling the stereochemical course of reductive elimination has been conducted using stoichiometric Pt(IV) complexes42.
Figure 2. Experimental evidence for the dual mechanism of Pd(IV) reductive elimination.
a, Stereochemical analysis of acetoxylation & fluorination reveals opposite configuration. b, Deuterium incorporation experiments show that C(sp3)–H insertion process is irreversible under our catalytic conditions. c, Bicyclic palladacycle 8 was synthesized and characterized via X-ray crystallography. Identical stereochemical outcome was observed with the catalytic conditions when 8 was reacted with [F+].
Based on these mechanistic investigations, we propose a rationale for the switch of chemoselectivity of the C(sp3)–F and C(sp3)–O bond forming processes as depicted in Figure 3. A number of literature precedents propose that the formation of a cationic, five-coordinate Pd(IV) species must precede both C(sp2)–F and C(sp3)–F reductive elimination43–45. However, using an amino acid-type transient directing group (TDG1-3) will lead to a neutral, five-coordinate Pd(IV) intermediate (Figure 3, I–III) and render the desired C(sp3)–F reductive elimination slower than competing pathways, which is the SN2 C(sp3)–O formation in our case. Among the amino acid-type transient directing groups, we clearly observed a steric effect (Figure 3, I–III) which complies with the study of Gagné and coworkers on C(sp3)–F reductive elimination from high-valent Pt and Pd species46. When the carboxylic acid moiety of the transient directing group is replaced with a neutral diethyl amide group, a cationic, five-coordinate Pd(IV) intermediate suitable for C(sp3)–F reductive elimination can be formed (Figure 3, IV), which shifts the selectivity towards C(sp3)–F formation without affecting the overall yield. While it was previously proposed that tertiary amide additives such as NMP promote fluorination via accelerating the Pd(II)/(IV) oxidation step35,36, our result suggests that the role of tertiary amide motif is more likely to promote the C(sp3)–F reductive elimination step. Indeed, computational analysis showed that the switch from TDG3 to TDG4 reduces the barrier for inner-sphere C(sp3)–F and SN2-type C(sp3)–O reductive elimination by 4.5 kcal/mol and 2.8 kcal/mol, respectively, which is qualitatively in accordance with the observed higher fluorination selectivity with TDG4. Our hypothesis is that the switch from neutral to cationic Pd(IV) not only reduces the energy level of σ*(Pd–C), which would accelerate both reductive elimination pathways, but also affect the property of Pd–F bonding, which would have a larger influence on the inner-sphere C(sp3)–F reductive elimination. To support this hypothesis, we performed frontier molecular orbital (FMO) and natural bond orbital (NBO) analysis to show that the switch from TDG3 to TDG4 leads to 1) a decrease in the energy level of LUMO (σ*(Pd–C)), and 2) increases in the resonance stabilization energy in both σ(Pd–C)→σ*(Pd–F) and σ(Pd–F)→σ*(Pd–C) interactions (See Supplementary Information). Finally, the selectivity and efficiency of fluorination is further promoted when AcOH is replaced with C6F5CO2H (Figure 3, V). Extensive screening of benzoic acids shows that 2,3,5,6-tetrafluoro-substitution is essential for obtaining satisfactory yield of the fluorination product (Supplementary Table 4). The use of electron-deficient benzoic acid not only reduces the C(sp3)–O bond formation due to poor nucleophilicity, but also promotes C(sp3)–F reductive elimination as would be expected from an electron-withdrawing anionic ligand bound to Pd(IV).
Figure 3. Controlling reductive elimination pathways from putative Pd(IV) intermediates.
Trends of reductive elimination selectivity observed with anionic and neutral transient directing groups.
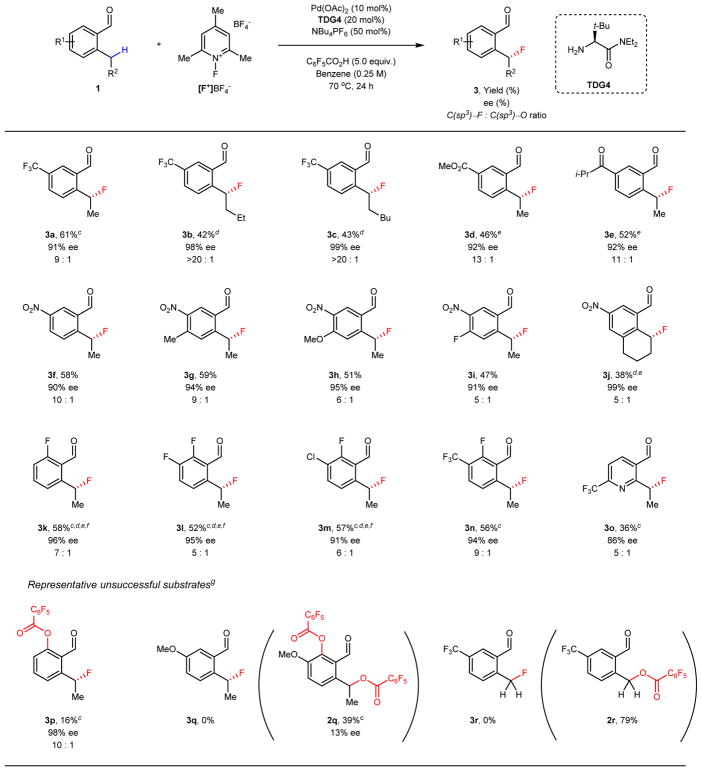
With the optimized reaction conditions in hand, we examined the substrate scope of our enantioselective fluorination (Table 2). Substrates with longer alkyl chains on the 2-position of benzaldehyde were tolerated with reduced efficiency and higher enantioselectivity (3b–c). With these bulkier substrates, only trace amount of C(sp3)–O products were observed, which is in accordance with our mechanistic hypothesis. Next, substrates bearing electron-withdrawing groups were tested. Carbonyl substituents, such as ester (3d) and ketone (3e), gave the fluorinated products in synthetically useful yields and excellent enantioselectivities. Nitro-substituted benzaldehyde (3f) also provided the fluorinated product in moderate yield. Unfortunately, substrates solely bearing electron-donating functional groups did not afford fluorination products under our current reaction conditions. Thus, we tested substrates that contain both a –NO2 group and an additional functional group. To our delight, these proved to be suitable substrates for our enantioselective fluorination (3g–i). Interestingly, a tetralin scaffold could also be fluorinated with excellent enantioselectivity albeit low yield (3j). Benzaldehydes containing ortho-F also underwent C(sp3)–H fluorination with moderate yields and excellent enantioselectivity (3l–m). Lastly, a heterocyclic substrate was also fluorinated with high enantioselectivity as well (3o). When a non-substituted 2-ethylbenzaldehyde (1p) was subjected to the reaction conditions, the reaction suffered not only from low efficiency, but also from the undesired C(sp2)–H activation (3p). With a substrate bearing an electron-donating –OMe group (1q), both C(sp2)–O and C(sp3)–O formations were favored over C–F formation, affording 2q as the major product with very low e.e. values. It is possible that 2q is formed through a distinct mechanism, most likely through a quinone methide-type intermediate which undergoes a nucleophilic addition of C6F5CO2H. Such drastic effect of a para–OMe group on reductive elimination of benzylic site has been reported by Sanford and coworkers47. We also observed that substrate 1r with primary C(sp3)–H bond gives 2r as the sole product which is expected from the SN2 reaction at a less hindered primary carbon center.
Table 2.
Scope of 2-alkylbenzaldehyde substrates for Pd(II)-catalyzed enantioselective C(sp3)–H fluorination.a,b
Reaction conditions: substrate 1 (0.10 mmol, 1.0 equiv.), [F+]BF4− (0.15 mmol, 1.5 equiv.), NBu4PF6 (50 mol%), C6F5CO2H (0.50 mmol, 5.0 equiv.), Pd(OAc)2 (10 mol%), TDG4 (20 mol%), Benzene (0.4 mL), 70 °C, 24 h.
Isolated yield as a mixture with substrate 1 unless otherwise noted. C(sp3)–F : C(sp3)–O ratio was determined by 1H NMR analysis.
Due to the high volatility of the product, yield was determined by 1H NMR analysis.
[F+]PF6− (0.15 mol, 1.5 equiv.) was used instead of [F+]BF4−/NBu4PF6.
DCM (0.4 mL) was used as solvent.
TDG4 (25 mol%) was used.
Full table of unsuccessful substrates are shown in the Supplementary Information.
The incompatibility of our reaction with substrates bearing electron-donating groups prompted us to employ the well-known SNAr reactions of the fluorinated products bearing an ortho-F group (3k–3n) to install a wide range of functional groups. To test this approach, we subjected our ortho-F-benzaldehyde product 3k into various SNAr reaction conditions (Figure 4). To our delight, the –F group can be smoothly substituted to form C–N (4a, 4b), C–O (4c), and C–S (4d) bonds without eroding the enantiopurity of the compound. Furthermore, we demonstrated that 3k can serve as a precursor for heterocyclic scaffolds that bear a stereogenic 1-fluoroethyl moiety. We were able to obtain benzothiophene (4e), anthranil (4f), and quinazoline48 (4g) compounds without eroding the enantiopurity. While a 1,2,4-triazoloquinoxaline scaffold49 (4h) can also be accessed, partial racemization was observed.
Figure 4. Access to diverse chiral organofluorines.
a, NaN3 (4.0 equiv.), HMPA (0.2 M), r.t., 36 h. b, piperidine (3.0 equiv.), K2CO3 (3.0 equiv.), DMF (0.2 M), 100 °C, 12 h. c, PhOH (3 equiv.), K2CO3 (3.0 equiv.), DMF (0.2 M), 100 °C, 2 h. d, NaSMe (3.0 equiv.), DMF (0.2 M), 60 °C, 4 h. e, Ethyl thioglycolate (3.0 equiv.), K2CO3 (3.0 equiv.), DMF (0.2 M), 60 °C, 3 h. f, NaN3 (3.0 equiv.), DMF (0.2 M), 100 °C, 12 h. g, guanidine carbonate (2.5 equiv.), DMA (0.2 M), 150 °C, 1 h. h, 3-amino-1,2,4-triazole (2.0 equiv.), Cs2CO3 (3.0 equiv.), DMF (0.2 M), 100 °C, 2 h.
In summary, we have developed a Pd(II)-catalyzed enantioselective C(sp3)–H fluorination method using a chiral transient directing group. The choice of anionic or neutral transient directing groups to favor the formation of neutral or cationic Pd(IV) intermediates offers an effective method for controlling the dual reductive elimination pathways. The use of a bulky amino amide transient DG was critical in achieving high enantioselectivity and promoting C–F reductive elimination. We are currently applying this design principle to achieve Pd-catalyzed enantioselective fluorination of other alkyl C–H bonds.
Methods
General procedure for the enantioselective fluorination
A sealed tube with magnetic stir bar was charged with Pd(OAc)2 (10 mol%, 2.2 mg), NBu4PF6 (0.05 mol, 19.4 mg), [F+]BF4− (0.15 mmol, 34.0 mg), C6F5CO2H (0.5 mmol, 106.0 mg), substrate (0.1 mmol), and TDG4 (20 mol%, 3.7 mg) in air. Then, benzene (0.4 mL) or DCM (0.4 mL) was added as solvent. The reaction mixture was stirred at room temperature for 10 minutes, then at 70 °C for 24 hours. Upon completion, the reaction mixture was cooled to room temperature, diluted with DCM. The organic layer was washed with sat. NaHCO3 (aq) solution for 2 times. The organic layer was dried with Na2SO4, filtered through a silica plug with ethyl acetate, and concentrated in vacuo. The crude reaction mixture was purified on silica gel using hexanes/ethyl acetate as eluent to afford the desired product as a mixture with the corresponding substrate. Isolated yield were calculated based on the isolated mass and the ratio of substrate and product determined by analysis of 1H NMR spectrum. Pure analytical samples for characterization were isolated by multiple preparative TLC. Full experimental details and characterization of compounds can be found in the Supplementary Information.
Data Availability
All characterization data, computational data, and experimental protocols are provided in the Supplementary Information or are available from the authors upon request. Metrical parameters for the structure of compound 5a, 6, and 8 are available free of charge from the Cambridge Crystallographic Data Centre under reference numbers CCDC 1556389, CCDC 1556390, and CCDC 1577327 respectively.
Supplementary Material
Acknowledgments
We gratefully acknowledge The Scripps Research Institute, the NIH (NIGMS, 2R01GM084019), and Shanghai RAAS Blood Products Co., Ltd. for their financial support. H.P. thanks the Korea Foundation for Advanced Studies and Eli Lilly for graduate fellowship.
Footnotes
Author Contributions
H.P. developed the enantioselective fluorination reaction. H.P. and K.H. expanded the substrate scope. P.V. conducted the computational studies. J.-Q.Y. conceived and supervised the project.
Readers are welcome to comment on the online version of this article.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.O’Hagan D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem Soc Rev. 2008;37:308–319. doi: 10.1039/b711844a. [DOI] [PubMed] [Google Scholar]
- 2.Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem Soc Rev. 2008;37:320–330. doi: 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara T, O’Hagan D. Successful fluorine-containing herbicide agrochemicals. J Fluorine Chem. 2014;167:16–29. [Google Scholar]
- 4.Wang J, et al. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011) Chem Rev. 2014;114:2432–2506. doi: 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- 5.Furuya T, Kamlet AS, Ritter T. Catalysis for fluorination and trifluoromethylation. Nature. 2011;473:470–477. doi: 10.1038/nature10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollingworth C, Gouverneur V. Transition metal catalysis and nucleophilic fluorination. Chem Commun. 2012;48:2929–2942. doi: 10.1039/c2cc16158c. [DOI] [PubMed] [Google Scholar]
- 7.Sather AC, Buchwald SL. The Evolution of Pd0/PdII-Catalyzed Aromatic Fluorination. Acc Chem Res. 2016;49:2146–2157. doi: 10.1021/acs.accounts.6b00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Wu T, Phipps RJ, Toste FD. Advances in Catalytic Enantioselective Fluorination, Mono-, Di-, and Trifluoromethylation, and Trifluoromethylthiolation Reactions. Chem Rev. 2015;115:826–870. doi: 10.1021/cr500277b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hintermann L, Togni A. Catalytic Enantioselective Fluorination of β-Ketoesters. Angew Chem Int Ed. 2000;39:4359–4362. doi: 10.1002/1521-3773(20001201)39:23<4359::AID-ANIE4359>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Marigo M, Fielenbach D, Braunton A, Kjærsgaard A, Jørgensen KA. Enantioselective Formation of Stereogenic Carbon–Fluorine Centers by a Simple Catalytic Method. Angew Chem Int Ed. 2005;44:3703–3706. doi: 10.1002/anie.200500395. [DOI] [PubMed] [Google Scholar]
- 11.Beeson TD, MacMillan DWC. Enantioselective Organocatalytic α-Fluorination of Aldehydes. J Am Chem Soc. 2005;127:8826–8828. doi: 10.1021/ja051805f. [DOI] [PubMed] [Google Scholar]
- 12.Rauniyar V, Lackner AD, Hamilton GL, Toste FD. Asymmetric Electrophilic Fluorination Using an Anionic Chiral Phase-Transfer Catalyst. Science. 2011;334:1681–1684. doi: 10.1126/science.1213918. [DOI] [PubMed] [Google Scholar]
- 13.Talbot EPA, Fernandes TdA, McKenna JM, Toste FD. Asymmetric Palladium-Catalyzed Directed Intermolecular Fluoroarylation of Styrenes. J Am Chem Soc. 2014;136:4101–4104. doi: 10.1021/ja412881j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochrane NA, Nguyen H, Gagné MR. Catalytic Enantioselective Cyclization and C3-Fluorination of Polyenes. J Am Chem Soc. 2013;135:628–631. doi: 10.1021/ja3116795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalow JA, Doyle AG. Enantioselective Ring Opening of Epoxides by Fluoride Anion Promoted by a Cooperative Dual-Catalyst System. J Am Chem Soc. 2010;132:3268–3269. doi: 10.1021/ja100161d. [DOI] [PubMed] [Google Scholar]
- 16.Katcher MH, Doyle AG. Palladium-Catalyzed Asymmetric Synthesis of Allylic Fluorides. J Am Chem Soc. 2010;132:17402–17404. doi: 10.1021/ja109120n. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Stockdale DP, Mixdorf JC, Topczewski JJ, Nguyen HM. Iridium-Catalyzed Enantioselective Fluorination of Racemic, Secondary Allylic Trichloroacetimidates. J Am Chem Soc. 2015;137:11912–11915. doi: 10.1021/jacs.5b07492. [DOI] [PubMed] [Google Scholar]
- 18.Hull KL, Anani WQ, Sanford MS. Palladium-Catalyzed Fluorination of Carbon–Hydrogen Bonds. J Am Chem Soc. 2006;128:7134–7135. doi: 10.1021/ja061943k. [DOI] [PubMed] [Google Scholar]
- 19.Braun MG, Doyle AG. Palladium-Catalyzed Allylic C–H Fluorination. J Am Chem Soc. 2013;135:12990–12993. doi: 10.1021/ja407223g. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Yin XS, Chen K, Zhang SQ, Shi BF. Stereoselective Synthesis of Chiral β-Fluoro α-Amino Acids via Pd(II)-Catalyzed Fluorination of Unactivated Methylene C(sp3)–H Bonds: Scope and Mechanistic Studies. J Am Chem Soc. 2015;137:8219–8226. doi: 10.1021/jacs.5b03989. [DOI] [PubMed] [Google Scholar]
- 21.Zhu RY, et al. Ligand-Enabled Stereoselective β-C(sp3)–H Fluorination: Synthesis of Unnatural Enantiopure anti-β-Fluoro-α-amino Acids. J Am Chem Soc. 2015;137:7067–7070. doi: 10.1021/jacs.5b04088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rueda-Becerril M, et al. Fluorine Transfer to Alkyl Radicals. J Am Chem Soc. 2012;134:4026–4029. doi: 10.1021/ja211679v. [DOI] [PubMed] [Google Scholar]
- 23.Bloom S, et al. A Polycomponent Metal-Catalyzed Aliphatic, Allylic, and Benzylic Fluorination. Angew Chem Int Ed. 2012;51:10580–10583. doi: 10.1002/anie.201203642. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, et al. Oxidative Aliphatic C–H Fluorination with Fluoride Ion Catalyzed by a Manganese Porphyrin. Science. 2012;337:1322–1325. doi: 10.1126/science.1222327. [DOI] [PubMed] [Google Scholar]
- 25.Xia JB, Zhu C, Chen C. Visible Light-Promoted Metal-Free C–H Activation: Diarylketone-Catalyzed Selective Benzylic Mono- and Difluorination. J Am Chem Soc. 2013;135:17494–17500. doi: 10.1021/ja410815u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatalova-Sazepin C, Hemelaere R, Paquin JF, Sammis GM. Recent Advances in Radical Fluorination. Synthesis. 2015;47:2554–2569. [Google Scholar]
- 27.Huang X, et al. Late Stage Benzylic C–H Fluorination with [18F]Fluoride for PET Imaging. J Am Chem Soc. 2014;136:6842–6845. doi: 10.1021/ja5039819. [DOI] [PubMed] [Google Scholar]
- 28.Zhang FL, Hong K, Li TJ, Park H, Yu JQ. Functionalization of C(sp3)–H bonds using a Transient Directing Group. Science. 2016;351:252–256. doi: 10.1126/science.aad7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engle KM, Mei TS, Wang X, Yu JQ. Bystanding F+ Oxidants Enable Selective Reductive Elimination from High-Valent Metal Centers in Catalysis. Angew Chem Int Ed. 2011;50:1478–1491. doi: 10.1002/anie.201005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yahav A, Goldberg I, Vigalok A. Synthesis of the Elusive (R3P)2MF2 (M = Pd, Pt) Complexes. J Am Chem Soc. 2003;125:13634–13635. doi: 10.1021/ja0377753. [DOI] [PubMed] [Google Scholar]
- 31.Rosewall CF, Sibbald PA, Liskin DV, Michael FE. Palladium-Catalyzed Carboamination of Alkenes Promoted by N-Fluorobenzenesulfonimide via C–H Activation of Arenes. J Am Chem Soc. 2009;131:9488–9489. doi: 10.1021/ja9031659. [DOI] [PubMed] [Google Scholar]
- 32.Pérez-Temprano MH, Racowski JM, Kampf JW, Sanford MS. Competition between sp3-C–N vs. sp3-C–F Reductive Elimination from PdIV Complexes. J Am Chem Soc. 2014;136:4097–4100. doi: 10.1021/ja411433f. [DOI] [PubMed] [Google Scholar]
- 33.Camasso NM, Pérez-Temprano MH, Sanford MS. C(sp3)–O Bond-Forming Reductive Elimination from PdIV with Diverse Oxygen Nucleophiles. J Am Chem Soc. 2014;136:12771–12775. doi: 10.1021/ja507056u. [DOI] [PubMed] [Google Scholar]
- 34.Sibbald PA, Rosewall CF, Swartz RD, Michael FE. Mechanism of N-Fluorobenzenesulfonimide Promoted Diamination and Carboamination Reactions: Divergent Reactivity of a Pd(IV) Species. J Am Chem Soc. 2009;131:15945–15951. doi: 10.1021/ja906915w. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Mei TS, Yu JQ. Versatile Pd(OTf)2·2H2O-Catalyzed ortho-Fluorination Using NMP as a Promoter. J Am Chem Soc. 2009;131:7520–7521. doi: 10.1021/ja901352k. [DOI] [PubMed] [Google Scholar]
- 36.Chan KSL, Wasa M, Wang X, Yu JQ. Palladium(II)-Catalyzed Selective Monofluorination of Benzoic Acids Using a Practical Auxiliary: A Weak-Coordination Approach. Angew Chem Int Ed. 2011;50:9081–9084. doi: 10.1002/anie.201102985. [DOI] [PubMed] [Google Scholar]
- 37.He G, Lu G, Guo Z, Liu P, Chen G. Benzazetidine synthesis via palladium-catalysed intramolecular C–H amination. Nat Chem. 2016;8:1131–1136. [Google Scholar]
- 38.Katcher MH, Norrby PO, Doyle AG. Mechanistic Investigations of Palladium-Catalyzed Allylic Fluorination. Organometallics. 2014;33:2121–2133. [Google Scholar]
- 39.Racowski JM, Sanford MS. Carbon–Heteroatom Bond-Forming Reductive Elimination from Palladium(IV) Complexes. Top Organomet Chem. 35:61–84. doi: 10.1002/anie.201107816. [DOI] [PubMed] [Google Scholar]
- 40.Chen K, Zhang SQ, Jiang HZ, Xu JW, Shi BF. Practical Synthesis of anti-β-Hydroxy-α-Amino Acids by PdII-Catalyzed Sequential C(sp3)–H Functionalization. Chem Eur J. 2015;21:3264–3270. doi: 10.1002/chem.201405942. [DOI] [PubMed] [Google Scholar]
- 41.Canty AJ, et al. Computational study of C(sp3)–O bond formation at a PdIV centre. Dalton Trans. 2017;46:3742–3748. doi: 10.1039/c7dt00096k. [DOI] [PubMed] [Google Scholar]
- 42.Geier MJ, Dadkhah Aseman M, Gagné MR. Anion-Dependent Switch in C–X Reductive Elimination Diastereoselectivity. Organometallics. 2014;33:4353–4356. doi: 10.1021/om5006929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furuya T, et al. Mechanism of C–F Reductive Elimination from Palladium(IV) Fluorides. J Am Chem Soc. 2010;132:3793–3807. doi: 10.1021/ja909371t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Racowski JM, Gary JB, Sanford MS. Carbon(sp3)–Fluorine Bond-Forming Reductive Elimination from Palladium(IV) Complexes. Angew Chem Int Ed. 2012;51:3414–3417. doi: 10.1002/anie.201107816. [DOI] [PubMed] [Google Scholar]
- 45.Kaspi AW, Yahav-Levi A, Goldberg I, Vigalok A. Xenon Difluoride Induced Aryl Iodide Reductive Elimination: a Simple Access to Difluoropalladium(II) Complexes. Inorg Chem. 2008;47:5–7. doi: 10.1021/ic701722f. [DOI] [PubMed] [Google Scholar]
- 46.Zhao SB, Becker JJ, Gagné MR. Steric Crowding Makes Challenging Csp3–F Reductive Eliminations Feasible. Organometallics. 2011;30:3926–3929. doi: 10.1021/om200515f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMurtrey KB, Racowski JM, Sanford MS. Pd-Catalyzed C–H Fluorination with Nucleophilic Fluoride. Org Lett. 2012;14:4094–4097. doi: 10.1021/ol301739f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hynes JB, Campbell JP. Synthesis of 2-aminoquinazolines from ortho-fluorobenzaldehydes. J Heterocyclic Chem. 1997;34:385–387. [Google Scholar]
- 49.Niu X, et al. An efficient one-pot synthesis of 1,2,4-triazoloquinoxalines. Tetrahedron. 2014;70:4657–4660. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All characterization data, computational data, and experimental protocols are provided in the Supplementary Information or are available from the authors upon request. Metrical parameters for the structure of compound 5a, 6, and 8 are available free of charge from the Cambridge Crystallographic Data Centre under reference numbers CCDC 1556389, CCDC 1556390, and CCDC 1577327 respectively.