Abstract
Background:
Limited evidence has suggested that circulating levels of the omega-9 fatty acid, oleic acid, may be related to greater risks of adverse cardiovascular outcomes.
Objective:
We aimed to determine whether plasma oleic acid may be independently associated with clinical and subclinical cardiovascular disease (CVD) and all-cause mortality in a large multi-ethnic cohort.
Methods:
Plasma fatty acids were measured by gas chromatography-flame ionization in 6,568 participants of the Multi-Ethnic Study of Atherosclerosis. The presence of coronary artery calcium and aortic valve calcification was determined by computed tomography, and carotid plaque was assessed by ultrasound. Incident CVD was defined as myocardial infarction, fatal coronary heart disease, resuscitated cardiac arrest, stroke, or stroke death. Heart failure (HF) was adjudicated from clinical records. Relative risk regression estimated plasma oleic acid-related rate ratios for prevalent coronary artery calcium, aortic valve calcification, and carotid plaque. Cox regression estimated hazard ratios (HRs) for CVD, HF, and all-cause mortality over a median 13-year follow-up.
Results:
Individuals in top quartiles of oleic acid showed greater rate ratios of coronary artery calcium, aortic valve calcification, and carotid plaque (all p<0.001), but associations were rendered non-significant after adjustment for other risk factors. By contrast, those in top quartiles of plasma oleic acid showed significantly greater risks of incident HF (HR: 2.03; p<0.001), CVD (HR: 1.41; p=0.008), and all-cause mortality (HR: 1.55; p<0.001) than those in referent quartiles independent of typical risk factors as well as plasma omega-3 fatty acid levels.
Conclusions:
Plasma oleic acid appears to be a risk factor for CVD events and all-cause mortality independent of typical risk factors and plasma omega-3 fatty acids. Additional studies are warranted for confirmation and to further examine whether plasma oleic acid directly contributes to, or serves as a marker of, disease pathogenesis. These findings should not be extrapolated to dietary oleic acid intake.
Keywords: fatty acids, cardiovascular disease, heart failure, all-cause death
BACKGROUND
Fatty acids (FAs) are bioactive compounds involved in numerous homeostatic processes including metabolism and regulating the immune response (1,2), but they have also been shown to influence disease pathogenesis. Indeed, cohort and intervention studies have shown that plasma levels of certain fatty acid classes are differentially related to risk of cardiovascular (CV) outcomes and, more broadly, all-cause mortality (3–12). While many of these studies have focused on saturated and polyunsaturated FAs, few have examined plasma omega-9 monounsaturated FAs and whether they may affect risk of adverse events. Oleic acid represents approximately 80% of plasma phospholipid monounsaturated FAs and served as the primary exposure variable in the present analysis.
In terms of dietary intake, oleic acid consumption has been shown to reduce blood pressure and lower risk of cardiovascular disease (CVD) development (13–15)—though some controversy remains (16,17). While it may be expected that plasma oleic acid may likewise promote CV health benefits, it has been shown that plasma and dietary oleic acid levels are only weakly correlated (18). Moreover, the few studies that have examined plasma oleic acid showed greater risk of CV events (11, 12) or null findings (8–10). It is therefore likely that other factors regulate plasma oleic acid concentrations—among these, its de novo synthesis from stearic acid by stearoyl-coenzyme CoA desaturase-1 (SCD-1) (19). Ultimately, given that dietary and circulating oleic acid levels are not well-correlated, and that limited evidence has implicated plasma oleic acid as a CV risk factor, a larger cohort study with an extended follow-up period may more comprehensively assess its potential influence on subclinical disease and adverse outcomes than previous studies to date.
Overall, this study aimed to determine whether plasma oleic acid levels are related to subclinical carotid artery atherosclerosis, coronary artery calcium (CAC), and aortic valve calcification (AVC) as well as clinical CV-related events and all-cause mortality in the MultiEthnic Study of Atherosclerosis (MESA) cohort.
MATERIALS AND METHODS
Study population
MESA study design has been previously described (20), and information regarding study protocol is available online (www.mesa-nhlbi.org). Briefly, men and women aged 45 to 84 years without clinical evidence of CV disease were recruited from six communities in the United States (Los Angeles County, CA; New York, NY; Baltimore, MD; Chicago, IL; Forsyth County, NC; and St. Paul, MN). All study participants gave informed consent, and Institutional Review Board approval was given at all MESA sites. Exam 1 was conducted in 2000 through 2002. Demographic and lifestyle information were obtained through questionnaires. Trained staff evaluated height and weight according to standard procedures, and body mass index (BMI) was calculated as weight (kg)/ height (m2).
Laboratory measurements
Blood was collected and lipids were measured using standard protocols as described previously (21). Phospholipid fatty acids were measured at baseline in EDTA plasma as done by Cao et al (22). Briefly, fatty acids were extracted from the plasma using a chloroform/methanol method. Cholesterol esters, triglycerides, phospholipids, and free fatty acids were separated by thin layer chromatography. Phospholipid fatty acids were derivatized to methyl esters and measured through gas chromatography-flame ionization detection. Measured values are presented as a percent of the total phospholipid fraction. The coefficient of variation for oleic acid measurement was 7.7%.
Dietary Intake
Dietary and nutrient intakes were evaluated in MESA at baseline as done by de Oliveira Otto et al. (23). Briefly, the average consumption frequency of specific food items were reported for the previous year by using a Block 120-item food-frequency questionnaire (24), modified to include Chinese food and beverage items (25). Dietary nutrient intakes were estimated by multiplying the frequency and serving size for each food item by the nutrient content (Nutrition Data Systems for Research; University of Minnesota). Additional information on low-fat food consumption cooking fat/oil was taken into account when calculating intakes. All nutrient values were adjusted for energy intakes by using the residuals method (26).
CV-related events and all-cause mortality
Every 9–12 months, telephone interviewers were conducted inquire about interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. The MESA mortality and morbidity review committee adjudicated HF and CVD events (20); if the participant died, the next of kin gave information on the date and cause of death, and MESA staff reviewed the National Death Index to verify participant deaths. Inpatient and outpatient medical records were reviewed in approximately 98% and 95%, respectively. Incident CVD was defined as myocardial infarction, resuscitated cardiac arrest, fatal coronary heart disease, stroke, or fatal stroke. HF included both probable and definite categories, which required HF symptoms including shortness of breath or edema. Probable HF required diagnosis of HF by a physician and HF treatment. Definite HF required one or more additional objective criteria, including pulmonary edema/congestion by chest x-ray, dilated ventricle, evidence of left ventricular diastolic dysfunction, or poor left ventricular function by either echocardiography or ventriculography.
Subclinical atherosclerosis and aortic valve disease
The presence of CAC and AVC were assessed by computed tomography imaging as described by Budoff et al. (27). The presence of carotid plaque at baseline was assessed by ultrasonography and has been described previously by Polak et al. (28). Further protocol information is available in the online supplement.
Statistical analysis
Statistical analysis was conducted using Stata (version 15.0, Stata Corp, College Station, TX). Baseline characteristics are presented as means (SD) for continuous variables and frequencies (%) for categorical variables. Missing data were excluded when calculating frequencies. Tukey-Kramer HSD was used to test differences between groups. Relative risk regression analysis estimated oleic acid-related risks of prevalent carotid plaque, CAC, and AVC at baseline using unadjusted, minimally adjusted, and fully adjusted models. Cox regression analysis estimated risks of event outcomes related to plasma oleic acid levels over a median 13- year follow up period. Covariate adjustments were made for age, education, BMI (as a continuous variable), smoking (pack-years), systolic blood pressure (as a continuous variable), blood pressure and lipid lowering medication use (as binary variables), total cholesterol, HDL-C, (log) triglycerides, alcohol use (never, former, current), diabetes status (normal fasting glucose, impaired fasting glucose, treated diabetes, untreated diabetes), combined plasma levels of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and race/ethnicity. Whether female participants had ever taken hormone replacement therapy was also included (with male individuals coded as a separate category); given the collinearity of this variable with sex, a sex- adjustment was unnecessary in models adjusted for hormone replacement therapy. Individuals with missing covariate date were excluded from analyses. Oleic acid was examined as a continuous variable (per SD), but also by quartiles in order to obtain robust associations in the case of nonlinear relationships. Interactions with race/ethnicity were tested. Unadjusted Nelson-Aalen curves were generated to show differences in cumulative hazards of adverse outcomes within quartiles of oleic acid.
RESULTS
Among 6,568 adults in the MESA cohort with plasma oleic acid measurements, there were 614 cases of incident CVD (8.2 per 1000 person years), 293 cases of incident HF (3.9 per 1000 person years), and 1,107 cases of all-cause mortality (13.5 per 1000 person years). Demographic and clinical characteristics stratified by quartiles of plasma oleic acid are presented in Table 1. Individuals in upper plasma oleic acid quartiles were more likely to be older (p<0.001), Caucasian or Hispanic (p<0.001), a current drinker (<0.001), not taking blood pressure medication (p<0.001), non-diabetic (p=0.03), have a lower BMI (p<0.001), and have higher levels of HDL-C and triglycerides (both p<0.001). Female participants in upper oleic acid quartiles were more likely to have taken hormone replacement therapy (p=0.04). Prevalence of carotid plaque, CAC, and AVC as well as all-cause mortality, CVD, and HF events were higher across successive quartiles of plasma oleic acid levels (all p<0.001).
Table 1.
Demographic and baseline clinical characteristics of MESA participants stratified by quartiles of plasma oleic acid measured at baseline.
| Factor | Quartile of plasma oleic acid, median [range] (% of total) | p-value | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| 6.4 [0.7 – 6.9] | 7.3 [6.9 – 7.6] | 8.0 [7.6 –8.5] | 9.1 [8.5 – 17.3] | ||
| N | 1644 | 1641 | 1642 | 1641 | |
| Age, years (SD) | 60.5 (9.9) | 61.9 (10.0) | 62.7 (10.4) | 63.2 (10.5) | <0.001 |
| Sex, n (% female) | 885 (53.8) | 867 (52.8) | 874 (53.2) | 846 (51.6) | NS |
| Race/ethnicity, n (%) | |||||
| Black | 710 (43.2) | 491 (29.9) | 356 (21.7) | 244 (14.9) | <0.001 |
| Chinese | 314 (19.1) | 212 (12.9) | 147 (9.0) | 120 (7.3) | |
| Caucasian | 355 (21.6) | 581 (35.4) | 751 (45.7) | 843 (51.4) | |
| Hispanic | 265 (16.1) | 357 (21.8) | 388 (23.6) | 434 (26.4) | |
| Smoking, n (%) | <0.001 | ||||
| Never | 906 (55.3) | 849 (51.9) | 810 (49.5) | 729 (44.5) | |
| Former | 563 (34.4) | 600 (36.7) | 606 (37.0) | 626 (38.2) | |
| Current | 168 (10.3) | 186 (11.4) | 222 (13.6) | 282 (17.2) | |
| Alcohol use | <0.001 | ||||
| Never | 380 (23.3) | 385 (23.6) | 301 (18.5) | 277 (17.0) | |
| Former | 440 (27.0) | 380 (23.3) | 379 (23.3) | 358 (21.9) | |
| Current | 811 (49.7) | 863 (53.0) | 947 (58.2) | 998 (61.1) | |
| BMI (kg/m2), mean (SD) | 28.7 (5.4) | 28.8 (5.6) | 28.5 (5.6) | 27.3 (5.2) | <0.001 |
| SBP (mm Hg), mean (SD) | 125.8 (20.6) | 126.6 (21.6) | 126.8 (21.6) | 126.9 (22.3) | NS |
| Blood pressure medication, n (%) | 655 (39.9) | 634 (38.6) | 598 (36.4) | 547 (33.4) | <0.001 |
| Lipid lowering medication, n (%) | 267 (16.3) | 233 (14.2) | 278 (16.9) | 275 (16.8) | NS |
| Hormone replacement therapy, n (%)* | 356 (45.8) | 401 (50.7) | 417 (52.3) | 406 (52.0) | 0.04 |
| Diabetes, n (%) | 205 (12.5) | 234 (14.3) | 189 (11.5) | 189 (11.5) | 0.03 |
| Plasma EPA+DHA, (% total), mean (SD) | 5.6 (2.4) | 4.9 (2.0) | 4.4 (1.8) | 4.1 (1.7) | <0.001 |
| Lipid levels (mg/dL) | |||||
| Total cholesterol, mean (SD) | 191.9 (34.6) | 195.5 (34.6) | 195.3 (36.6) | 193.9 (36.6) | 0.01 |
| HDL-C, mean (SD) | 49.8 (13.8) | 50.4 (14.8) | 51.2 (15.0) | 52.7 (15.6) | <0.001 |
| Triglycerides, median (IQR) | 99 (71, 141) | 108 (77, 156) | 1 17 (80, 168) | 1 23 (84, 176) | <0.001 |
| Intermediate CV endpoints, n (%)† | |||||
| Carotid plaque | 588 (36.3) | 667 (41.2) | 686 (42.5) | 736 (45.4) | <0.001 |
| Aortic valve calcification | 186 (11.3) | 182 (11.1) | 248 (15.1) | 260 (15.8) | <0.001 |
| Coronary artery calcium | 739 (45.0) | 785 (47.8) | 872 (53.1) | 878 (53.3) | <0.001 |
| Event outcomes, n (%) | |||||
| All-cause mortality | 204 (12.4) | 242 (14.7) | 304 (18.5) | 357 (21.8) | <0.001 |
| Cardiovascular disease | 165 (10.0) | 207 (12.6) | 238 (14.5) | 240 (14.6) | <0.001 |
| Heart Failure | 47 (2.9) | 69 (4.2) | 78 (4.8) | 99 (6.0) | <0.001 |
Definitions: BMI=body mass index; SBP=systolic blood pressure; diabetes=treated and untreated cases; HDL-C=high density lipoprotein-cholesterol; CV=cardiovascular; EPA=eicosapentaenoic acid; DHA=docosahexaenoic acid
Ever taken hormone replacement: males were excluded
absent or present at baseline
Relative risk regression determined cross-sectional relations between plasma oleic acid levels and carotid plaque, CAC, and AVC (Table 2). A multi-model approach was used as follows: model 1: unadjusted; model 2: adjusted for age and sex; model 3: adjusted for age, education, BMI, smoking (pack years), ever taken hormone replacement therapy, systolic blood pressure, blood pressure medication, lipid lowering medication, total cholesterol, HDL-C, (log) triglycerides, alcohol use, diabetes status, plasma levels of omega-3 fatty acids EPA and DHA, and race/ethnicity. Participants with missing covariate data were excluded. Higher levels of plasma oleic acid levels were associated with higher relative risks of prevalent carotid plaque, CAC, and AVC in unadjusted and age-sex-race adjusted models. Relations were rendered nonsignificant following adjustment for all CV-related risk factors with one exception—per SD increments in oleic acid and risk of prevalent CAC remained borderline significant (p=0.03). No overall interaction with race/ethnicity was observed.
Table 2.
Plasma oleic acid-related risk of intermediate cardiovascular endpoints in MESA participants. Regression analysis estimated relative risk ratios (95% confidence intervals) of prevalent carotid plaque, coronary artery calcium, and aortic valve calcification at baseline using a multi-model approach.
| Outcome | Oleic acid quartileG | per SD | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Carotid plaque | |||||
| Model 1 | ref | 1.14 (1.04 – 1.24) 0.004 |
1.17 (1.07 – 1.28) <0.001 |
1.25 (1.15 – 1.36) <0.001 |
1.07 (1.04 – 1.10) <0.001 |
| Model 2 | ref | 1.07 (0.99 – 1.16) | 1.08 (1.00 – 1.17) | 1.12 (1.04 – 1.21) 0.004 |
1.03 (1.00 – 1.06) 0.03 |
| Model 3 | ref | 1.04 (0.96 – 1.12) | 1.03 (0.95 – 1.11) | 1.04 (0.96 – 1.13) | 1.01 (0.98 – 1.04) |
|
Coronary artery calcium | |||||
| Model 1 | ref | 1.06 (0.99 – 1.15) | 1.18 (1.10 – 1.27) <0.001 |
1.19 (1.11 – 1.28) <0.001 |
1.07 (1.05 – 1.10) <0.001 |
| Model 2 | ref | 1.01 (0.95– 1.08) | 1.07 (1.01 – 1.13) 0.03 |
1.06 (1.00 – 1.12) | 1.03 (1.01 – 1.05) 0.002 |
| Model 3 | ref | 0.98 (0.93 – 1.04) | 1.02 (0.96 – 1.08) | 1.02 (0.96 – 1.08) | 1.02 (1.00 – 1.04) 0.03 |
|
Aortic valve calcification | |||||
| Model 1 | ref | 0.98 (0.81 – 1.19) | 1.33 (1.12 – 1.59) 0.001 |
1.40 (1.17 – 1.67) <0.001 |
1.15 (1.09 – 1.21) <0.001 |
| Model 2 | ref | 0.89 (0.74 – 1.08) | 1.03 (0.86 – 1.24) | 1.11 (0.93 – 1.32) | 1.07 (1.00 – 1.14) 0.04 |
| Model 3 | ref | 0.83 (0.68 – 1.01) | 0.99 (0.82 – 1.18) | 1.01 (0.83 – 1.23) | 1.07 (0.98 – 1.16) |
Model 1: unadjusted
Model 2: adjusted for age and sex
Model 3: adjusted for age, education, body mass index, smoking (pack-years), hormone replacement therapy, systolic blood pressure, blood pressure medication, lipid lowering medication, total cholesterol, high density lipoprotein cholesterol, (log) triglycerides, alcohol use, diabetes status, plasma EPA+DHA levels, and race/ethnicity
Carotid plaque: model 1 (N=6,479); model 2 (N=6,479); model 3 (N=6,035)
Coronary artery calcium: model 1 (N=6,568); model 2 (N=6,568); model 3 (N=6,116)
Aortic valve calcification: model 1 (N=6,571); model 2 (N=6,567); model 3 (N=6,115)
Cox regression analysis estimated oleic acid-related hazard ratios of all-cause mortality, CVD, and HF with adjustments for age, education, BMI, smoking (pack years), hormone replacement therapy, systolic blood pressure, blood pressure medication, lipid lowering medication, total cholesterol, HDL-C, (log) triglycerides, alcohol use, diabetes status, plasma EPA+DHA, and race/ethnicity (Table 3). Oleic acid was examined as a continuous (per SD) and categorical variable. Individuals with higher oleic acid levels (per SD or top quartiles) were found to have significantly greater incidence rate ratios for all-cause mortality, CVD, and HF over an approximate 13-year follow up period. No overall interaction with race/ethnicity was observed.
Table 3.
Plasma oleic acid-related risk of cardiovascular-related outcomes and all-cause mortality in MESA participants. Cox regression estimated hazard ratio (95% confidence interval) with adjustments for age, education, BMI, smoking (pack years), hormone replacement therapy, systolic blood pressure, blood pressure medication, lipid lowering medication, total cholesterol, HDL-C, (log) triglycerides, alcohol use, diabetes status, plasma EPA+DHA, and race/ethnicity.
| Event outcome | Oleic acid quartile | per SD | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| All-cause mortality | ref | 1.07 (0.88 – 1.30) | 1.27 (1.05 – 1.57) 0.01 |
1.49 (1.23 – 1.81) <0.001 |
1.19 (1.11 – 1.27) <0.001 |
| Incident CVD | ref | 1.22 (0.94 – 1.57) | 1.38 (1.08 – 1.78) 0.01 |
1.36 (1.05 – 1.77) 0.02 |
1.09 (1.00 – 1.19) 0.04 |
| Incident HF | ref | 1.23 (0.84 – 1.80) | 1.42 (0.97 – 2.08) | 1.85 (1.26 – 2.71) 0.002 |
1.16 (1.03 – 1.32) 0.02 |
Definitions: BMI=body mass index; HDL-C=high density lipoprotein cholesterol; CVD=cardiovascular disease defined as myocardial infarction, fatal coronary heart disease, resuscitated cardiac arrest, stroke, or stroke death; HF=heart failure; EPA=eicosapentaenoic acid; DHA=docosahexaenoic acid
All-cause mortality (N=6,116; 1073 incident cases)
Incident CVD (N=6,096; 591 incident cases)
Incident HF (N=6,096; 283 incident cases)
Unadjusted Nelson-Aalen cumulative hazard curves are shown in Figure 1 (panels A-C). Greater cumulative hazards for all-cause mortality (A), CVD events (B), and HF events (C) are shown in successive quartiles of plasma oleic acid.
Figure 1 (panels A-C).
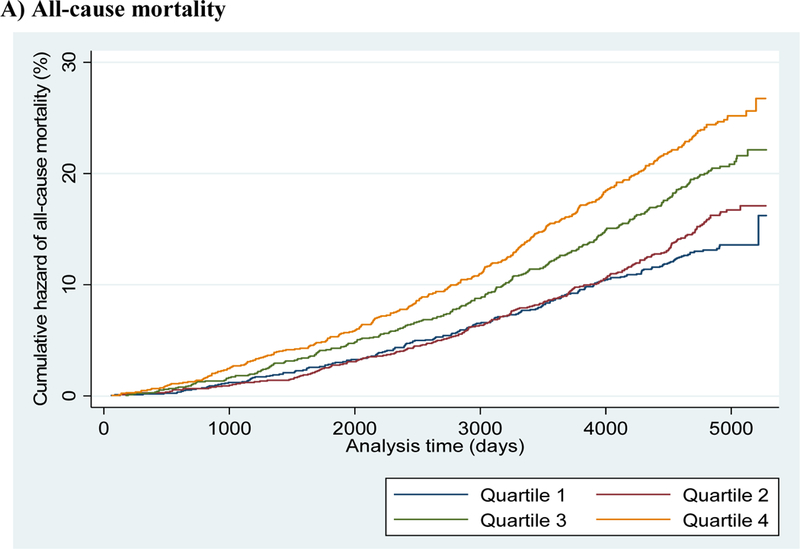
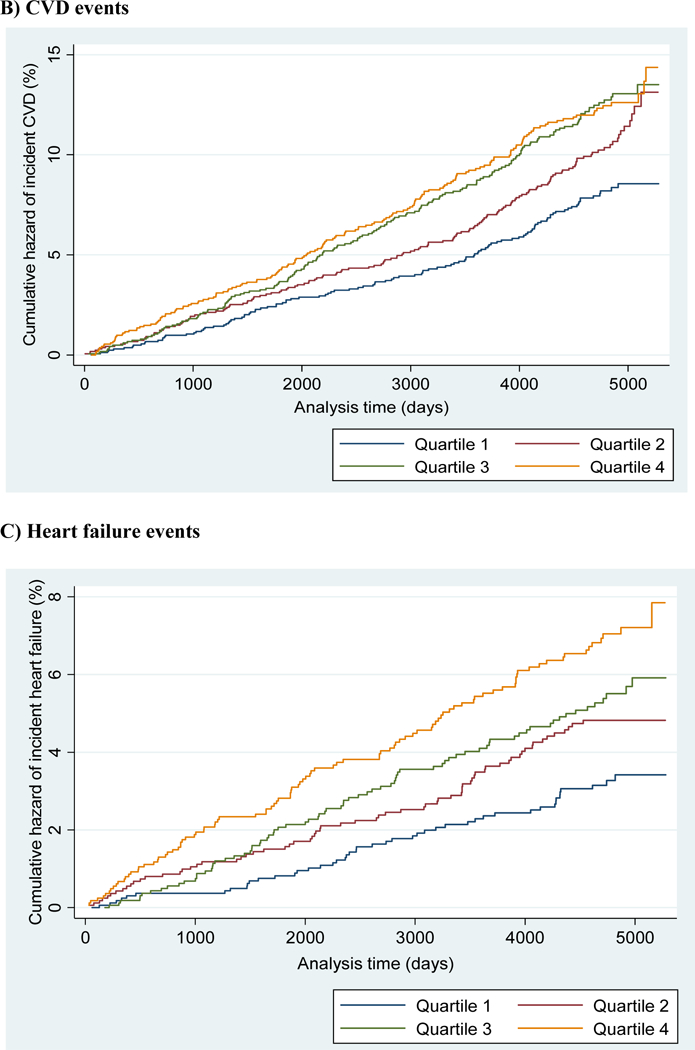
Unadjusted Nelson-Aalen cumulative hazard curves for A) all-cause mortality; B) cardiovascular disease; and C) heart failure categorized by quartiles of plasma oleic acid levels
A secondary analysis was conducted to determine whether SCD-1 activity [estimated by the plasma oleic acid to stearic acid ratio (18:1/18:0)] was associated with adverse event outcomes (Supplemental Table I). Individuals with higher SCD-1 activity were found to be at significantly greater risks of all-cause mortality, CVD, and HF. No overall interaction with race/ethnicity was observed.
Correlations between plasma oleic acid levels and demographic, clinical, lifestyle, and dietary factors are shown in Supplemental Table II. Plasma oleic acid was found to be directly, albeit weakly, correlated with numerous characteristics including smoking status, carbohydrate intake, monounsaturated fat intake, and oleic acid intake (all ρ<0.10; p<0.001). Likewise, plasma oleic acid was found to be inversely correlated with other factors—among these, BMI and fasting insulin (both ρ=−0.10; p<0.001).
DISCUSSION
Plasma phospholipid levels of oleic acid were found to be significantly related to adverse health outcomes in MESA participants. While associations between plasma oleic acid and carotid plaque, CAC, and AVC were rendered non-significant in fully adjusted models, significant associations with all-cause mortality, CVD, or HF remained after adjusting for typical CV risk factors as well as plasma omega-3 fatty acid levels of EPA and DHA. A secondary analysis of estimated SCD-1 activity showed significantly greater risks of these adverse outcomes with higher levels of SCD-1 activity. Altogether, this is the first study to show that elevated levels of plasma oleic acid are related to greater risks of multiple adverse events, including all-cause mortality, in a multi-ethnic cohort.
Previous findings
Previous case-control and prospective studies have examined plasma fatty acid profiles that included oleic acid, and results varied by both study design and the plasma fatty acid fraction that was analyzed, i.e. phospholipid, cholesterol ester, or the sum of all fractions (phospholipid, cholesterol ester, triglyceride, and free fatty acids). Case-control studies that measured plasma phospholipid oleic acid were conducted in the Women’s Health Initiative Observational Study (8), the Physician’s Health Study (9), and EPIC-Norfolk (10) cohorts. All reported no relations between oleic acid and either coronary heart disease or HF. Likewise, a prospective analysis of an ARIC subcohort (n=3,592; 197 cases) found no association between plasma phospholipid oleic acid and incident HF (11). And yet, plasma cholesterol-ester oleic acid levels were associated with greater risk of HF in ARIC (11) and were also found to be associated with cardiovascular and all-cause mortality in the Uppsala Longitudinal Study of Adult Men (29). Finally, studies that measured total plasma oleic acid levels (consisting of all fatty acid fractions) have reported mixed findings, albeit for different outcomes. A nested case control study of Singapore Chinese Health Study participants (744 cases, 744 controls) showed that higher total plasma oleic acid levels were associated with a 14% greater risk of acute myocardial infarction (p=0.03) (12), while a prospective analysis of 1,246 Three-City Study participants showed that total plasma oleic acid was related to a 73% lower risk of incident stroke over a median 5-year period (30).
While the above study findings are inconsistent, discrepancies may have resulted from differences in study designs, sample sizes, and study populations. For example, remarkable variations in mean plasma oleic acid levels were observed among cohorts. Mean oleic acid levels in the MESA sample were 7.75% of total plasma phospholipids, but approximately 9.9% in the EPIC-Norfolk study (15). In addition, oleic acid levels were more widely distributed in the current MESA sample than in the Physician’s Health (9), which may have constrained the analysis of oleic acid-related risk in the latter study. Analytical methods may have also contributed to differential results. In both the Singapore Chinese Health Study and Three-City cohorts, investigators examined concentrations of total plasma oleic acid, while most fatty acid studies typically measure the phospholipid or cholesterol ester fatty acid fraction. Additionally, the Three-City study was likely underpowered with only 27 incident stroke events in the plasma oleic acid analysis. By contrast, the present analysis includes hundreds of observations for cardiovascular events and over one thousand all-cause mortality outcomes. Taken together, null findings have predominated, but some previous evidence has suggested that elevated plasma levels of oleic acid are related to CV-related events—in stark contrast to dietary studies.
De novo lipogenesis by stearoyl-coenzyme CoA desaturase-1
It is important to recognize that dietary intake of oleic acid does not appear to strongly influence plasma levels. In a study of ARIC participants (18) and in the current MESA sample (Supplemental Table II), plasma oleic acid was only weakly correlated to dietary oleic acid consumption (both r=0.05), and including estimated dietary oleic acid intake in the present analyses only marginally reduced magnitudes of associations (data not shown). This suggests that plasma oleic acid levels (and, by extension, risk of adverse outcomes) are primarily driven by its de novo lipogenesis from fatty acid precursors. In the case of oleic acid (18:1), it is synthesized from saturated stearic acid (18:0) through activity of SCD-1 (19), a well- characterized enzyme involved in lipid handling, metabolism and metabolic dysfunction.
In addition to synthesizing oleic acid, SCD-1 has been shown to be essential in triglyceride synthesis (31) and has been implicated in development of insulin resistance (32–35), ectopic fat deposition (36, 37), and adiposity (37, 38). While it may be appealing to attribute the present findings for plasma oleic acid to SCD-1’s influence on metabolic dysfunction followed by CVD or HF development, we found little evidence that plasma oleic acid levels were related to metabolic dysfunction—indeed, plasma oleic acid levels were inversely related to prevalent diabetes and BMI (Table 1) as well as fasting levels of insulin and glucose at baseline (Supplemental Table II). As an alternative hypothesis, it has been shown in an experimental rodent model that SCD-1 mediates lipid accumulation in the myocardium resulting in myocardial dysfunction (39), but whether this phenomenon may occur in otherwise healthy human subjects is unknown.
Estimated SCD-1 activity
Numerous cohort studies have examined SCD-1 using plasma fatty acid substrate to product ratios, i.e. oleic acid to stearic acid (18:1/18:0) or palmitoleic acid to palmitic acid (16:1/16:0), as proxies of SCD-1 enzymatic activity (40, 41). Using this approach, we examined SCD-1 (18:1/18:0) in a secondary analysis and found it to be significantly associated with incident CVD, HF, and all-cause mortality (Supplemental Table I); magnitudes of associations were incrementally weaker than those observed for plasma oleic acid alone. However, these results should be interpreted with caution since the substrate-product ratios of SCD-1 activity have only been partially validated (42). This was achieved by isolating the very low density lipoprotein component followed by measurement of fatty acid substrates and products. Even with this more labor-intensive approach, investigators only showed that these SCD-1 ratios were correlated with SCD1 mRNA expression (r=0.72; p=0.004). Neither SCD-1 protein nor enzymatic activity was measured. Few subsequent studies have followed this protocol, and it remains possible that SCD-1 ratios derived from plasma phospholipid fatty acids are not reliable proxies of SCD-1 enzymatic activity in human subjects. Results related to SCD-1 activity in the current study and others should therefore be interpreted with caution without further validation.
Implications for future research
The current findings have a number of implications for future research. Foremost, the SCD-1 enzyme represents a strong target for further study given its roles in oleic acid synthesis and potential for mediating pathogenic lipid accumulation. Candidate gene and experimental studies may provide evidence as to whether SCD-1 is involved in promoting risk of adverse events. In addition, identifying dietary and lifestyle behaviors that reduce oleic acid levels may also be worthwhile. For example, we observed that carbohydrate intake was only weakly correlated with plasma oleic acid levels (r=0.07; p<0.001, Supplemental Table II); however, a recent intervention study demonstrated that step-wise increases in carbohydrate intake resulted in significantly greater levels of plasma phospholipid oleic acid (p=0.005) (43). It would be informative to further explore whether other interventions related to smoking cessation as well as dietary saturated fat and alcohol intake may also show an effect on plasma oleic acid and disease risk. Finally, it is likely that the above and other lifestyle or demographic factors modify the relations of plasma oleic acid and event outcomes; further studies of these potential interactions are therefore warranted.
Strengths and Limitations
This study represents the largest prospective analysis of plasma oleic acid and risks of clinical and subclinical disease endpoints. Statistical models were fully adjusted for conventional lipid and non-lipid CV risk factors, and the 13-year follow-up period allowed for the examination of temporality of associations. To control for the possibility that greater plasma levels of oleic acid may result in lower levels of omega-3 FAs EPA and DHA, adjustment for plasma EPA+DHA was included. In terms of limitations, approximately 450 individuals with plasma fatty acid data were excluded from analyses due to missing covariate data. It must also be acknowledged that these results may not be generalizable to other populations since MESA is a U.S.-based cohort. Finally, despite adjustments for demographic and clinical covariates in the statistical models, we cannot exclude the possibility of residual confounding.
Conclusions
These results suggest that elevated levels of plasma phospholipid oleic acid may increase risks of adverse CV-related events and all-cause mortality independent of traditional CVD risk factors. Additional studies are warranted for confirmation and to further examine whether plasma oleic acid directly contributes to disease development or is a marker of SCD-1 activity or another underlying pathogenic process. Plasma levels of oleic acid were not associated with its dietary intake, and these findings should not be extrapolated to dietary oleic acid.
Supplementary Material
HIGHLIGHTS.
Plasma oleic acid levels are related to CVD events and all-cause mortality
Associations were independent of typical CVD risk factors and plasma omega-3 levels
High plasma oleic acid may be pathogenic or a marker of SCD-1 activity
These results should not be extrapolated to dietary oleic acid intake
ACKNOWLEDGEMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
SOURCES OF FUNDING
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC- 95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01- HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS.
Footnotes
DISCLOSURES
The authors have no competing interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1).Masoodi M, Kuda O, Rossmeisl M, Flachs P, Kopecky J. Lipid signaling in adipose tissue: Connecting inflammation & metabolism. Biochim Biophys Acta. 2015;1851:503–18. [DOI] [PubMed] [Google Scholar]
- 2).Calder PC. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie. 2009;91:791–5. [DOI] [PubMed] [Google Scholar]
- 3).Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, et al. Omega-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies. 2016;176:1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Wu JH, Lemaitre RN, King IB, Song X, Psaty BM, Siscovick DS, Mozaffarian D. Circulating omega-6 polyunsaturated fatty acids and total and cause-specific mortality: the Cardiovascular Health Study. Circulation. 2014;130:1245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005;111:157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Steffen BT, Guan W, Stein JH, Tattersall MC, Kaufman JD, Sandfort V, Szklo M, Tsai MY. Plasma n-3 and n-6 Fatty Acids Are Differentially Related to Carotid Plaque and Its Progression: MESA (the Multi-Ethnic Study of Atherosclerosis). Arterioscler Thromb Vasc Biol. 2018. January 11 pii: ATVBAHA.117.310366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Matthan NR, Ooi EM, Van Horn L, Neuhouser ML, Woodman R, Lichtenstein AH. Plasma phospholipid fatty acid biomarkers of dietary fat quality and endogenous metabolism predict coronary heart disease risk: a nested case-control study within the Women’s Health Initiative observational study. J Am Heart Assoc. 2014;3:pii:e000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Morin SJ, Gaziano JM, Djousse L. Relation between plasma phospholipid oleic acid and risk of heart failure. Eur J Nutr. 2017. doi: 10.1007/s00394-017-1565-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Khaw KT, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk prospective study. PLoS Med. 2012;9:e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Yamagishi K, Nettleton JA, Folsom AR; ARIC Study Investigators. Plasma fatty acid composition and incident heart failure in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008;156:965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Sun Y, Koh HW, Choi H, Koh WP, Yuan JM, Newman JW, Su J, Fang J, Ong CN, van Dam RM. Plasma fatty acids, oxylipins, and risk of myocardial infarction: the Singapore Chinese Health Study. J Lipid Res. 2016;57:1300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Hohmann CD, Cramer H, Michalsen A, Kessler C, Steckhan N, Choi K, Dobos G. Effects of high phenolic olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Phytomedicine. 2015;22:631–40. [DOI] [PubMed] [Google Scholar]
- 14).Guasch-Ferre M, Hu FB, Martinez-Gonzalez MA, Fito M, Bullo M, Estruch R, Ros E, Corella D, Recondo J, Gomez-Gracia E, Fiol M, Lapetra J, Serra-Majem L, Munoz MA, Pinto X, Lamuela-Raventos RM, Basora J, Buil-Cosiales P, Sorli JV, Ruiz-Gutierrez V, Martinez JA, Salas-Salvado J. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014;12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Schwingshack L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Jakobsen MU, O’Reilly E, Heitmann BL, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Degirolamo C, Rudel LL. Dietary monounsaturated fatty acids appear not to provide cardioprotection. Curr Atheroscler Rep. 2010;12:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Ma J, Folsom AR, Shahar E, et al. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. Am J Clin Nutr. 1995;62:564–571. [DOI] [PubMed] [Google Scholar]
- 19).Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res. 2004;43:91–104. [DOI] [PubMed] [Google Scholar]
- 20).Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 21).Tsai MY, Johnson C, Kao WH, et al. Cholesteryl ester transfer protein genetic polymorphisms, HDL cholesterol, and subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis. Atherosclerosis. 2008;200:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006;52:2265–72.l [DOI] [PubMed] [Google Scholar]
- 23).de Oliveira Otto MC, Mozaffarian D, Kromhout D, Bertoni AG, Sibley CT, Jacobs DR Jr, Nettleton JA. Dietary intake of saturated fat by food source and incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2012;96:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69. [DOI] [PubMed] [Google Scholar]
- 25).Nettleton JA, Rock CL, Wang Y, Jenny NS, Jacobs DR. Associations between dietary macronutrient intake and plasma lipids demonstrate criterion performance of the Multi Ethnic Study of Atherosclerosis (MESA) food-frequency questionnaire. Br J Nutr. 2009;102:1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Willett WC, Stampfer M. Implications of total energy intake for epidemiologic analyses In: Willet W (ed). Nutritional epidemiology. 2nd ed. New York, NY: Oxford University Press, 1998:273–301. [Google Scholar]
- 27).Budoff MJ, Takasu J, Katz R, Mao S, Shavelle DM, O’Brien KD, Blumenthal RS, Carr JJ, Kronmal R. Reproducibility of ct measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol. 2006;13:166–172 [DOI] [PubMed] [Google Scholar]
- 28).Polak JF, Szklo M, Kronmal RA, Burke GL, Shea S, Zavodni AE, O’Leary DH. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2:e000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Warensjo E, Sundstrom J, Vessby B, Cederholm T, Riserus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population- based prospective study. Am J Clin Nutr. 2008;88:203–9. [DOI] [PubMed] [Google Scholar]
- 30).Samieri C, Feart C, Proust-Lima C, Peuchant E, Tzourio C, Stapf C, Berr C, Barberger- Gateau P. Olive oil consumption, plasma oleic acid, and stroke incidence: the Three-City Study. Neurology. 2011;77:418–25. [DOI] [PubMed] [Google Scholar]
- 31).Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, and Ntambi JM. 2000. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J. Biol. Chem. 275: 30132–30138. [DOI] [PubMed] [Google Scholar]
- 32).Rahman SM, Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Ntambi JM 2003. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down- regulates protein-tyrosine phosphatase 1B in muscle. Proc. Natl. Acad. Sci. USA. 100: 11110–11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM Stearoyl-CoA desaturase-1 deficiency reduces ceramide synthesis by downregulating serine palmitoyltransferase and increasing beta-oxidation in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005;288: E599–E607. [DOI] [PubMed] [Google Scholar]
- 34).Flowers JB, Rabaglia ME, Schueler KL, Flowers MT, Lan H, Keller MP, Ntambi JM, Attie AD. Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes. 2007;56:1228–39. [DOI] [PubMed] [Google Scholar]
- 35).Attie AD, Flowers MT, Flowers JB, Groen AK, Kuipers F, Ntambi JM. Stearoyl-CoA desaturase deficiency, hypercholesterolaemia, cholestasis and diabetes. Novartis Found Symp. 2007;286:47–53; discussion 54–7, 162–3, 196–203. [PubMed] [Google Scholar]
- 36).Miyazaki M, Dobrzyn A, Sampath H, Lee SH, Man WC, Chu K, Peters JM, Gonzalez FJ, Ntambi JM. Reduced adiposity and liver steatosis by stearoyl-CoA desaturase deficiency are independent of peroxisome proliferator-activated receptor-alpha. J Biol Chem. 2004;279:35017–24. [DOI] [PubMed] [Google Scholar]
- 37).Jiang GI, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, Ronan J, Esau C, Murphy C, Szalkowski D, Bergeron R, Doebber T, Zhang BB. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest. 2005;115:1030–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA. 2002;99: 11482–11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Dobrzyn P, Dobrzyn A, Miyazaki M, Ntambi JM. Loss of stearoyl-CoA desaturase 1 rescues cardiac function in obese leptin-deficient mice. J Lipid Res. 2010;51:2202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Lankinen MA, Stancakova A, Uusitupa M, Agren J, Pihlajamaki J, Kuusisto J, Schwab U, Laakso M. Plasma fatty acids as predictors of glycaemia and type 2 diabetes. Diabetologia. 2015;58:2533–44. [DOI] [PubMed] [Google Scholar]
- 41).Chow LS, Li S, Eberly LE, Seaquist ER, Eckfeldt JH, Hoogeveen RC, Couper DJ, Steffen LM, Pankow JS. Estimated plasma stearoyl co-A desaturase-1 activity and risk of incident diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Metabolism. 2013;62:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Peter A, Cegan A, Wagner S, Lehmann R, Stefan N, Konigsrainer A, Konigsrainer I, Haring HU, Schleicher E. Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clin Chem 2009;55:2113–2120. [DOI] [PubMed] [Google Scholar]
- 43).Volk BM, Kunces LJ, Freidenreich DJ, Kupchak BR, Saenz C, Artistizabal JC, Fernandez ML, Bruno RS, Maresh CM, Kraemer WJ, Phinney SD, Volek JS. Effects of step-wise increases in dietary carbohydrate on circulating saturated Fatty acids and palmitoleic Acid in adults with metabolic syndrome. PLoS One. 2014;9:e113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


