Abstract
Viruses use diverse molecular mechanisms to exploit and evade the immune response. Herpesviruses, in particular, encode functional chemokine and chemokine receptor homologs pirated from the host, as well as secreted chemokine-binding proteins with unique structures. Multiple functions have been described for herpesvirus chemokine components, including attraction of target cells, blockade of leukocyte migration, and modulation of gene expression and cell entry by the virus. Here we review current concepts about how human herpesvirus chemokines, chemokine receptors, and chemokine-binding proteins may be used to shape a proviral state in the host.
Keywords: Chemokines, Chemokine receptors, Viral infection
Introduction
Herpesviruses are large DNA viruses divided into α, β, and γ subfamilies that cause acute, latent, and chronic infections in mammalian hosts [1]. The high prevalence of herpesviruses in human populations worldwide may be related to their ability to establish life-long latency and to their exceptional capacity to modulate the host immune response. At the molecular level, herpesvirus survival strategies are mediated in part by viral genes pirated from the host. The host chemokine system, which, among other functions, coordinates leukocyte trafficking, is a particularly rich source of such genes. In particular, human herpesviruses (HHV) encode both viral chemokines (vCK) and viral chemokine receptors (vCKR) [2, 3, 4], which are structurally homologous to human chemokines and human G protein-coupled chemokine receptors (GPCR), respectively. They also encode vCK-binding proteins (vCKBP), which lack homology to any other known protein yet bind promiscuously to many chemokines and interfere with their cellular activities. These herpesvirus-encoded proteins may modulate host chemokine functions to ultimately subvert the antiviral immune response. In addition, novel functions unrelated to conventional chemokine activities and leukocyte trafficking have been identified. For example, most HHV deploy vCK in the viral envelope as key components of cell entry.
vCK, vCKR, and vCKBP are differentially represented among the HHV subfamilies. Only α and β HHV are known to encode vCKBP (Fig. 1), whereas only the β and γ HHV subfamilies have been shown to encode vCKR (Fig. 2). On the other hand, possibly due to their implication in common cell entry mechanisms, all known HHV encode at least one vCK (Fig. 3). Here we review the current state of knowledge of the HHV chemokine system.
Fig. 1.
vCKBP encoded by HHV. Graphic representation of the vCKBP identified in alphaherpesvirus (HSV1, HSV2, and VZV) and betaherpesvirus (HCMV). The chemokines bound by each vCKBP are indicated. Since it has been shown that HCMV UL21.5 mRNA is packaged into virions, the expression of UL21.5 protein from this encapsidated mRNA as well as from the viral DNA genome is represented. vCKBP are depicted in the color of the corresponding HHV. sgG2, soluble gG2.
Fig. 2.
vCKR encoded by HHV. Graphic representation of the vCKR identified in betaherpesvirus (HCMV, HHV6, and HHV7) and gammaherpesvirus (EBV and KSHV). Chemokines bound by each vCKR are indicated. Unknown ligands for orphan vCKR are shown in gray. The lightning bolt symbol indicates vCKR with reported constitutive activity.
Fig. 3.
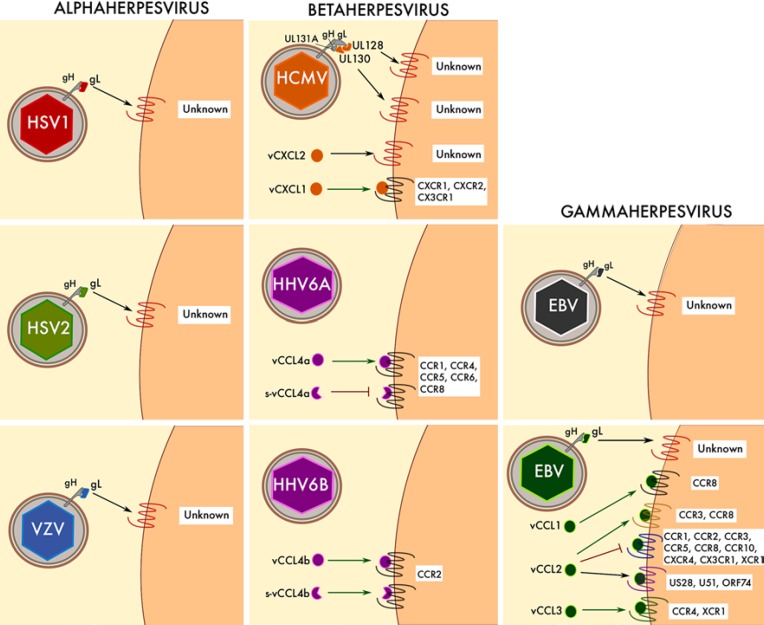
vCK encoded by HHV. Graphic representation of the vCK identified in alphaherpesvirus (HSV1, HSV2, and VZV), betaherpesvirus (HCMV, HHV6A, and HHV6B) and gammaherpesvirus (EBV and KSHV). vCK are shown in the same color as the corresponding virus. Other non-chemokine viral proteins (gH and HCMV gL and UL131A) are shown in gray. Cellular receptors for each vCK are indicated. Agonistic and antagonistic activities are represented with green and red arrows, respectively. Unknown receptors for vCK are shown in red. s-vCCL4a/b, spliced variants of vCCL4a/b.
Alphaherpesviruses
The Alphaherpesvirinae subfamily includes the neurotropic herpesviruses herpes simplex virus 1 (HSV-1), HSV-2, and varicella-zoster virus (VZV). After the initial infection of epithelial cells in the oropharynx or genital tract, these viruses establish latency in ganglia of the peripheral nervous system [5]. Upon reactivation, they typically cause painful but self-limiting infections, including cold sores (HSV-1), genital ulcers (HSV-2), and shingles (VZV) [5]. However, they can also cause more serious conditions, including stromal keratitis, acute encephalitis, and postherpetic neuralgia. Unlike in β and γ HHV, which are mostly lymphotropic, no vCKR have been identified in α HHV. Instead, all 3 α HHV express vCKBP in their viral envelope: glycoprotein G (gG) in HSV-1 and HSV-2 and glycoprotein C (gC) in VZV. Interestingly, these are the only known vCKBP that do not inhibit but instead potentiate the activity of their chemokine ligands. The conserved envelope protein gL of this HHV subfamily has been shown to adopt the canonical chemokine fold.
vCKBP Encoded by Alphaherpesviruses
HSV-1 and HSV-2 gG
The US4 ORF of HSV-1 and HSV-2 encodes a viral envelope vCKBP termed gG. Interestingly, the sequence of gG is more divergent (< 14% amino acid identity) than any other envelope glycoprotein common to HSV-1 and HSV-2. Furthermore, HSV-2 gG (gG2) but not HSV-1 gG (gG1) can be cleaved and released from the virion as a soluble vCKBP (SgG2) [6, 7]. However, despite these structural and topological differences, gG1 and gG2 are functionally very similar. They both bind the same small set of human chemokines with CCL22, a gG2-specific ligand, as the only exception (Fig. 1) [8]. Surprisingly, unlike gG of nonhuman alphaherpesviruses, gG1 expressed in the viral envelope or the plasma membrane of infected cells and SgG2 enhance the chemotactic activity of their ligands via a mechanism that has been proposed to mimic that of glycosaminoglycan (GAG)-chemokine interactions [8, 9]. Consistent with this, gG itself is a GAG-binding protein whose interaction with chemokines does not block ligand binding to signaling receptors [10]. Therefore, it has been suggested that gG attached to the cell surface via GAG or expressed in the plasma membrane of the infected cell may bind chemokines to present them to their cognate cellular receptors [10]. In addition, gG has been reported to delay receptor internalization upon chemokine binding [10]. How HSV would benefit from chemokine potentiation by gG is currently unknown. Existing hypotheses include the recruitment to the site of infection of more cells susceptible to being infected and the induction of an activated proviral state in cells neighboring the infected cell.
Varicella-Zoster Virus gC
An orthologue of HSV gG is missing in VZV. Instead, VZV expresses an envelope glycoprotein named gC that has been recently characterized as a vCKBP able to bind 27 different chemokines with nanomolar affinity (Fig. 1) [11]. Like HSV gG, gC boosts the chemotactic activity of its ligands; however, its role in VZV pathogenesis is not yet defined. It has been reported that a gC deletion mutant of VZV has a reduced capacity to reach the dermis from the epidermis [12]. Since the activity of some GPCR is known to loosen epithelial tight junctions [13], it was proposed that gC may facilitate viral dissemination across the epidermis [11].
vCK Encoded by Alphaherpesviruses
Glycoprotein L
Herpesvirus cell entry is a complicated process that requires the action of multiple viral glycoproteins; however, gB and the heterodimer gH/gL play a conserved central role in all HHV [14]. It is assumed that, upon binding to specific cellular receptors, gH/gL dimers activate gB to initiate fusion with the target cell plasma membrane [15]. In certain HHV, gH/gL can interact with additional viral proteins to form diverse complexes that may ultimately determine the cellular tropism of the virus. In fact, in β HHV, gH/gL dimers need these supplementary interacting partners to be efficiently exposed in the viral envelope [16]. On the contrary, uncomplexed gH/gL dimers are known to mediate the cell entry of α HHV by their interaction with cellular integrins [17]. Importantly, it has been shown that gL of α HHV adopts a classical chemokine fold. In the gH/gL dimer of HSV-2, gL folds as a C chemokine with a single disulfide bond [18], validating computational predictions of chemokine homology to α HHV gL [19]. This opens up the possibility that gH/gL dimers may use cellular GPCR in addition to integrins to induce α HHV entry in particular cell types.
Betaherpesviruses
Human cytomegalovirus (HCMV) and the 3 roseolovirus subtypes HHV-6A, HHV-6B, and HHV7 comprise the HHV Betaherpesvirinae subfamily. Though typically asymptomatic in healthy adults, β HHV constitute an important health concern in neonates and infants [20, 21]. In addition, HCMV infections are a significant risk factor for graft rejection in transplants. A link between HHV-6 and multiple sclerosis and other neuroinflammatory conditions has also been proposed [22, 23]. The 230-kb genome of HCMV is the largest for the herpesviruses and encodes more than 160 ORF [24]. Four of these ORF encode GPCR homologues US27, US28, UL33, and UL78. US28 is a promiscuous vCKR binding 10 human chemokines and 1 vCK, whereas the remaining 3 homologues are classified as orphan receptors, with no chemokine ligands identified to date (Fig. 2). Similarly, vCXCL1 is the only one of the 4 different HCMV-encoded chemo kines - vCXCL1, vCXCL2, UL128, and UL130 - whose cellular receptors have been identified (Fig. 3). Furthermore, HCMV UL21.5 ORF encodes the only herpesvirus vCKBP outside of the α HHV subfamily (Fig. 1). On the other hand, 2 vCKR named U12 and U51 are conserved in all roseoloviruses and they are most homologous to CXCR4 (HHV6-U12, 19% identity), CCR3 (HHV6-U51, 14% identity), CCR7 (HHV7-U12, 20% identity), and CCR4 (HHV7- U51, 18% identity). As might be expected from these quite divergent sequences, U12 and U51 bind to distinct repertoires of chemokines (Fig. 2). In contrast, ORF U83 encodes a vCK unique to HHV-6 named vCCL4. Interestingly, HHV-6A vCCL4 (vCCL4a) and HHV-6B vCCL4 (vCCL4b) display remarkably different receptor specificities (Fig. 3).
vCKBP Encoded by Betaherpesviruses
HCMV UL21.5
ORF UL21.5 of HCMV encodes a secreted vCKBP shown so far to bind only CCL5 (Fig. 1) [25]. However, it is important to note that a comprehensive chemokine screening of this vCKBP has not yet been reported. The UL21.5 interaction with CCL5 is known to block binding of the chemokine to its cellular receptors [25], although inhibition of CCL5-mediated chemotaxis has not yet been assessed. Interestingly, UL21.5-encoding mRNA is packaged into virions [26], which might allow HCMV to interfere with cellular migration even before the initiation of viral transcription.
vCK Encoded by Betaherpesviruses
HCMV vCXCL1
ORF UL146 of HCMV encodes vCXCL1, a vCK that shares 31% amino acid identity with human CXCL8. Consistent with this, vCXCL1 is known to act as an agonist at the cellular CXCL8 receptors CXCR1 and CXCR2 [27], which are primarily expressed in neutrophils. Since neutrophils are known to contribute to the vascular dissemination of HCMV, it has been proposed that vCXCL1 may promote virus spreading by recruiting neutrophils to the site of infection [27, 28]. It has also been reported that vCXCL1 signals through CX3CR1 to attract NK cells [29]. However, the affinity of the vCXCL1 interaction with CX3CR1 is significantly lower than that with CXCR2, which raises concerns about the relevance of the interaction in vivo.
Located in a hypervariable region of the HCMV genome termed UL/b', vCXCL1 is one of the most structurally diverse genes across HCMV strains (up to 60% amino acid variability). However, the N-terminal ELR motif that characterizes all ligands of CXCR1 and CXCR2 is unaltered in most vCXCL1 clinical variants [30]. Although some functional differences have been found among these different vCXCL1 genotypes [31], the correlation between vCXCL1 variant and clinical outcome remains controversial. Nevertheless, it is important to note that lab strains lacking the UL/b' genomic region are attenuated in vivo [32], which suggests that ORF located in this area, including vCXCL1, may be important HCMV virulence factors.
HCMV vCXCL2
UL147, another ORF in the UL/b' genomic region of HCMV, encodes a second viral CXC chemokine named vCXCL2 [33]. vCXCL2 lacks an N-terminal ELR motif and is most highly related to CXCL9 (27.5% amino acid identity). However, its putative cellular receptor and biological activities remain undefined.
HCMV UL128 and UL130
The cell entry protein gL of β HHV lacks the chemokine homology observed for its counterparts in α HHV. However, gH/gL dimers of HCMV can interact with UL128 and UL130, CC, and C chemokine homologs encoded by the virus, respectively, as well as with the accessory protein UL131A to form the pentameric complex gH/gL/UL128-UL130-UL131A [34]. This complex is required for HCMV infection in epithelial, endothelial, and monocytic cells but it is dispensable in fibroblasts, where a different gH/gL/gO complex plays a major role [35, 36, 37]. Importantly, the ablation of any one of the 5 components of the complex suffices to disrupt HCMV infectivity in these cell types. For instance, HCMV lab strains carrying deletions of the UL/b' genomic region, where UL131A is located, are unable to infect nonfibroblast cells [38]. Although the mechanism by which the pentamer promotes HCMV cell entry remains unknown, vaccine strategies targeting this envelope complex are effective in blocking HCMV entry in key cell types [39, 40], including cytotrophoblasts [41], which are thought to be the main placental target for congenital HCMV infections.
A recent crystal structure of the pentamer confirmed the protein-protein interactions within the complex as well as a chemokine fold for UL128 and UL130 [42]. In addition, this study claimed that the great flexibility of the complex could allow UL128 and UL130 to interact with cellular chemokine receptors to promote viral cell entry. However, a bona fide cellular receptor for the pentamer remains to be identified. Furthermore, whether these 2 proteins can act as soluble vCK, independently of their complex with gH/gL and UL131A, is not clear. Chemokine-like activities have not been ascribed to UL130 yet and those reported for UL128 appear to contradict one another. Initially, it was shown that recombinant UL128 impaired monocyte chemotaxis induced by CCL5 and CCL2 [43]. However, a later study showed that UL128 protein induced chemotaxis of human peripheral blood mononuclear cells [44]. Further investigation will be required to resolve these controversies and to clarify whether the cellular effects of complexed and free UL128 and UL130 can be mediated by classical chemokine receptors.
HHV-6 vCCL4
The conserved ORF U83 of HHV-6A and HHV-6B encodes 2 related but functionally distinct chemokines, i.e., vCCL4a and vCCL4b, respectively. While vCCL4a is known to induce chemotaxis by signaling through CCR1, CCR4, CCR5, CCR6, and CCR8, vCCL4b is a specific CCR2 agonist (Fig. 3) [45, 46]. Both vCK could presumably attract cells to the site of infection to facilitate viral spread and latency, but the broader specificity of vCCL4a could allow HHV-6A to attract a higher diversity of cells and expand the virus cell tropism. Interestingly, 2 splicing variants of these vCK are expressed during the virus cell cycle. Full-length vCCL4 is expressed mostly at late stages associated with active virus replication, whereas truncated forms of the chemokine N-terminus are produced early after virus cell entry and during latency [45, 47]. Importantly, truncated vCCL4a and vCCL4b maintain the receptor specificity of the full-length vCK. However, while the splicing truncation of vCCL4b does not affect its agonist activity at CCR2, the short form of vCCL4a is known to antagonize those receptors activated by full-length vCCL4a [45, 48]. This could potentially provide HHV-6A with the interesting ability to fine-tune its chemokine modulatory effects to benefit specific phases of viral infection.
vCKR Encoded by Betaherpesviruses
HCMV US27
HCMV US27 is a late-phase protein predominantly expressed in intracellular vesicles suggestive of a role in endocytosis [49]. Its primary sequence is most highly homologous to CX3CR1 (22% amino acid identity) and contains the familiar DRY box motif at the end of transmembrane helix III as well as a C-terminal domain rich in serine and threonine residues. This latter domain is thought to target the receptor to intracellular vesicles since a chimeric US27 construct containing the C-terminus of CXCR3 trafficked to the plasma membrane rather than to the Golgi [50].
To date, US27 remains orphan, with neither ligands nor specific signaling pathways identified yet. However, a recent study found that it was able to enhance signaling mediated by endogenous CXCR4, resulting in augmented calcium mobilization and chemotaxis [51]. Expression of US27 in transfected cells appears to boost cell proliferation and protect cells from the apoptotic stimuli [52, 53]. Conversely, an HCMV variant devoid of US27 was equally infective as the wild-type virus but produced significantly fewer progeny [54]. Collectively, this suggests a role for US27 in enhancing HCMV infectivity by promoting viral particle productivity and host cell survival.
HCMV US28
US28 is most highly homologous to CX3CR1 (35% identity). US28 is expressed early after HCMV infection and was detected by immunohistochemical analysis of tumor sections taken from glioblastoma patients [55]. US28 is known to be constitutively active, coupling via Gαq and Gαi proteins to a variety of signaling pathways. Several of these pathways are linked to cell proliferation and angiogenesis, notably phospholipase C (PLC) activation which is associated with the transcription factors NFAT, NF-κB, CRE, and SRF [56]. Studies led by the lab of Martine Smit have shown constitutive signaling of US28 in a variety of cell backgrounds, leading to the upregulation of several molecules associated with cell proliferation, notably cyclin D, VEGF, COX-2, and β-catenin [57, 58, 59, 60]. The ability of US28 to constitutively activate G proteins appears to rely on an intact DRY motif at the end of transmembrane helix III since US28 mutant R129A is unable to activate PLC or known transcription factors [55, 57, 58]. The role of G protein independent signaling (e.g., via β-arrestins) has not been fully elucidated for US28. However, since cells expressing the R129A mutant are still able to successfully form tumors in a nude mouse model, it would appear that these signaling pathways might be important in vivo [57].
The tumorigenic potential of the US28 constitutive activity has driven approaches to target this vCKR with small molecule antagonists. The prototypic US28 antagonist VUF2274 has been shown to act as an inverse agonist of US28, with an IC50 of 776 nM for the inhibition of PLC in human fibroblasts infected with the AD169 strain of HCMV [61]. Interestingly, VUF2274 was initially described as a specific antagonist of CCR1 which shares only 30% homology with US28 [62]. Similarly, compounds based on the structure of CXCR3 antagonists have been reported to also act as inverse agonists of US28 [63].
In addition to its constitutive signaling, US28 has also been shown to interact with several host chemokines, including the CC chemokines CCL2, CCL3, CCL4, CCL5, CCL7, CCL11, CCL13, CCL26 and CCL28, and, consistent with its CX3CR1 homology, CX3CL1 [64, 65]. Since US28 has been shown to be expressed on the viral envelope, it has the potential to aid the entry of HMCV into CX3CL1+ target cells like endothelial cells. Supportive of this, US28-deleted viral isolates have been shown to be defective in cell-to-cell spread in epithelial cells [66]. Also based in the US28 binding to CX3CL1, a novel strategy to inhibit HCMV infection in vivo has been designed using a CX3CL1 variant with enhanced affinity and specificity for US28 fused to a cytotoxic domain of Pseudomonas exotoxin A. This fusion molecule was shown to specifically target and kill HCMV infected cells expressing US28 in vitro and in a humanized SCID mouse model [67]. The recent derivation of a crystal structure of US28 in complex with CX3CL1 may serve to further hone the specificity of such a molecule [68].
In terms of a physiological consequence of chemokine binding, smooth muscle cells expressing US28 have been shown to migrate along gradients of CCL5, which is inhibited by CX3CL1 [69]. There appears to be an element of signaling bias, since in the same study, when US28 was expressed in macrophages, CX3CL1 was able to induce chemotaxis whereas CCL5 was devoid of activity. In the context of atherosclerosis, HCMV infection may be envisaged to perturb cell migration in the vessel wall. In addition, US28 has been shown to acts as a coreceptor for HIV-1 entry in vitro [70], although the relevance of this finding for HIV pathogenesis is unclear.
HCMV UL33
Expression of UL33 is reportedly restricted to the later stages of HCMV infection, suggesting that it may play a role in the dissemination of the virus [49]. UL33 can promiscuously couple to Gαq, Gαs, and Gαi/o proteins, constitutively activating inositol phosphate (IP) production and the transcription factor CREB (cyclic AMP responsive element binding) [71].
In contrast to its constitutive activity, Tschische et al. showed that in HEK 293T cells UL33 and UL78 could form heterodimers with US28, inhibiting the constitutive NF-κB activation mediated by US28 [72]. This modulation exhibited signaling bias since activation of the Gαq/PLC pathway was unaffected by the expression of either receptor. In a similar vein, Tadagaki et al. showed, using both HEK 293T and THP-1 expression systems, that UL33 and UL78 could form heterodimers with CCR5 or CXCR4 when coexpressed [73]. Coexpression of the vCKR with either human chemokine receptor had little effect on chemokine binding at either receptor but did negatively modulate downstream functions including chemotaxis and endocytosis. Notably, the ability of HIV to use CCR5 and CXCR4 as a coreceptor for viral entry was impaired, leading to the suggestion that inducing the formation of such heteromers could be a novel therapeutic approach to inhibit HIV infection [73].
HCMV UL78
UL78 is expressed early after HCMV infection, but it appears to be dispensable for viral replication in fibroblasts [74]. However, UL78 expression has been shown to impact virus growth in both endothelial and epithelial cells [75]. To date, no activating ligands or signaling properties have been attributed to UL78, although, as mentioned before, UL78 can negatively regulate host and vCKR signaling [72, 73].
HHV-6 and HHV-7 U12
U12 is a late gene expressed during lytic infection with HHV-6 [76]. Isegawa et al. [76] reported that expression of HHV-6 U12 in K562 erythroleukemia cells resulted in responses to CCL2, CCL3, CCL4, and CCL5 in intracellular calcium flux assays, mediated by pertussis toxin-insensitive G proteins. Although all chemokines bound to U12 with nanomolar affinity, calcium flux responses varied in potency and efficacy, with CCL3 and CCL4 requiring micromolar concentrations to elicit a response. In contrast, expression of HHV-7 U12 in K562 cells elicited calcium flux responses to CCL19, CCL17, CCL21, and CCL22 [77]. Surprisingly, while U12 itself is unable to respond to CCL19 or CCL22, coexpression of U12 with the host receptors CCR4 or CCR7 results in the cells becoming responsive to both CCL19 and CCL22, suggestive of U12 forming U12:CCR4 and U12:CCR7 heterodimers with modified functionality [78].
HHV-6 and HHV-7 U51
Infection of cord blood mononuclear cells with HHV6-A or HHV6-B revealed U51 to be expressed early after viral infection [79]. Expression of HHV-6 U51 in COS-7 cells showed that the receptor responds to CCL2, CCL5, and CCL11 in calcium mobilization assays [80]. Assays in which IP accumulation was measured in unstimulated cells revealed that U51 could also signal constitutively, which was enhanced by the addition of CCL5 [80]. Heterologous binding assays and receptor endocytosis assays carried out by the same group on HHV-6 U51 expressing cells revealed CX3CL1 and XCL1 to be additional U51 ligands [80]. In addition, it was shown that CCL2, CCL5, CCL11, CCL7, CCL13 and Kaposi's sarcoma-associated virus (KSHV)-encoded vCCL2 could all compete the binding of radiolabeled CCL5 to U51 [81]. On the other hand, HHV-7 U51 was shown to display the same chemokine-binding profile of HHV-7 U12, inducing calcium flux responses upon interaction with CCL19, CCL17, CCL21, and CCL22 [82]. Interestingly, unlike with HHV-7 U12, none of these chemokine interactions with HHV-7 U51 resulted in active cell chemotaxis [82]. This, plus the different expression kinetics of U12 and U51, suggests that, despite binding the same set of chemokines, these 2 vCKRs may play distinct roles in HHV-7 pathogenesis.
Gammaherpesviruses
Two human pathogens, i.e., EBV and KSHV, are included in the Gammaherpesvirinae subfamily. Both of these γ HHVs are oncogenic; however, while EBV is able to induce malignant cell transformation in cancers such as Burkitt's lymphoma, nasopharyngeal carcinoma, and Hodgkin's lymphoma [83, 84], the KSHV-induced eponymous tumor Kaposi's sarcoma (KS), is mostly polyclonal and angiogenic and inflammatory in nature [85]. Importantly, although KSHV is typically associated with AIDS patients, it has also been reported in HIV-free sub-Saharan and Mediterranean populations and as a secondary complication after allogeneic transplantation [86]. KSHV also causes primary effusion lymphoma as well as multicentric Castleman's disease, which like KS is also a noncancerous proliferative disease. Three distinct vCK - vCCL1, vCCL2 and vCCL3 - and 1 vCKR, i.e., ORF74, fully equip KSHV to hijack the host chemokine system at multiple levels. EBV is a relatively small herpesvirus, having only 84 ORF within its 184-kb genome, and encodes only 1 ORF encoding a putative GPCR, the orphan receptor BILF1.
vCK Encoded by Gammaherpesviruses
KSHV vCCL1
ORF K6 of KSHV encodes a vCK, i.e., vCCL1 (formerly named vMIP-I), that is most highly related to CCL18 (50% amino acid identity). Initially, it was shown that vCCL1 was able to induce calcium flux in CCR5-expressing cells and block HIV infection in cells transfected with the HIV coreceptors CCR5, CCR3, and CXCR6 [87, 88, 89]. However, consistent with its high CCL18 homology, 3 independent studies have now established vCCL1 as a specific agonist at the CCL18 receptor CCR8 [90, 91, 92]. Besides interfering with the antiviral response and immune cell migration to the site of infection, vCCL1 could benefit KSHV at other levels. For instance, vCCL1 is known to attract CCR8+ vascular endothelial cells [93], which could promote angiogenesis and vascular dissemination of the virus. In addition, like the human CCR8 ligand CCL1, vCCL1 binding to CCR8 induces antiapoptotic signaling [94]. This could prevent host cell apoptosis and ensure both productive KSHV replication and healthy neighboring cells for virus dissemination.
KSHV vCCL2
ORF K4 of KSHV encodes vCCL2 (formerly named vMIP-II), the best characterized vCK. vCCL2 is undoubtedly the most highly promiscuous of all known chemokines, capable of binding to chemokine receptors from all 4 structural subgroups. In particular, vCCL2 can interact with CCR1, CCR2, CCR3, CCR5, CCR8, CCR10, CXCR4, CX3CR1, and XCR1 [64, 92, 95]. Unlike most vCK described previously, the interaction of vCCL2 with its cellular receptors does not result in calcium flux or chemotaxis signals but instead blocks the binding of cognate human chemokine ligands. Therefore, vCCL2 is considered a broad-spectrum chemokine receptor antagonist that might serve KSHV by seriously impairing the recruitment of antiviral immune cells to the site of infection. Of note, vCCL2 has also been reported to be an agonist at CCR3 and CCR8 [96], which could potentially benefit KSHV by attracting CCR3+ or CCR8+ Th2 cells. Furthermore, vCCL2 is known to be a ligand for the atypical chemokine receptor ACKR3 (previously named CXCR7) [97], which might affect the availability of vCCL2 during KSHV infection, and for the vCKR HCMV US28, HHV-6 U51, and KSHV ORF74 (Fig. 3) [64, 81, 98]. In particular, it has been shown that vCCL2 is able to downregulate the constitutive signaling activity of ORF74 [99]. This has been proposed to foster immune evasion during specific phases of KSHV infection.
Besides its biological role in KSHV infection, a broad-spectrum inhibitor like vCCL2 may have potential applications as a pharmaceutical. For instance, peptides derived from vCCL2 have been designed to block CXCR4- and CCR5-mediated HIV cell entry [100, 101]. In addition, recombinant vCCL2 has been proven to have a significant therapeutic effect in multiple mouse models of inflammation [102, 103]. Furthermore, its anti-inflammatory and angiogenic activities might be used to improve graft survival after organ transplantation [104, 105, 106].
KSHV vCCL3
vCCL3 (also termed vMIP-III) encoded by ORF K4.1 of KSHV is most highly homologous to human XCL1. Consistent with this, vCCL3 is a potent agonist at the human XCL1 receptor XCR1 [92], which is mainly expressed by a subpopulation of dendritic cells that might be latently infected by KSHV. In addition, vCCL3 induces chemotaxis of Th2 cells by signaling through CCR4 [107]. This could presumably help KSHV to establish a proviral immunological environment at the site of infection. However, it is important to note that while micromolar doses (0.1–10 μM) of vCCL3 were required to induce CCR4 activation, nanomolar concentrations (0.1–10 nM) sufficed to activate XCR1, suggesting its greater relevance for XCR1.
Glycoprotein L
As for α HHV, gL of γ HHV was predicted to be related to chemokines [19]. This was confirmed by the crystal structure of the gH/gL heterodimer of EBV [108]. Interestingly, while α HHV gL is more highly homologous to C chemokines, γ HHV gL adopts a classical CC chemokine fold. gH/gL dimers in the γ HHV viral envelope mediate cell entry by their interaction with integrins and ephrin type A receptor 2 in EBV and KSHV, respectively [109, 110]. Whether a classical chemokine receptor could act as an entry coreceptor for gH/gL is currently unknown. It is also important to note that, while gH is a transmembrane protein, gL possesses an N-terminal signal peptide and is known to be secreted when transfected in the absence of gH [111]. Therefore, the idea of gL acting as a soluble vCK in α and γ HHV infections must be considered.
vCKR Encoded by Gammaherpesviruses
KSHV ORF74
ORF74, the only GPCR within the KSHV 160-kb genome, is undoubtedly the best-studied HHV vCKR. ORF74 has 27% homology with CXCR2 and has been shown to be expressed in KS lesions [112]. A pivotal role for ORF74 in KS was supported by a transgenic mouse expressing ORF74 under the direction of the human CD2 promoter. This mouse developed characteristic erythematous maculae that developed predominantly into skin tumors, surrounded by spindle cells and infiltrating leukocytes, similar to the lesions observed in human KS [113].
ORF74 is constitutively active, coupling to a variety of G proteins in transfected cells including Gαq, Gαi, and Gα12/13 [114, 115, 116]. In turn, this activates a variety of intracellular signaling pathways which likely accounts for the oncogenic nature of ORF74, including activation of ERK1/2, p38, JNK, RhoA, and Rac1 [114, 115, 117, 118]. The molecular basis for the constitutive activation of ORF74 is not fully elucidated, although an abundance of mutagenesis studies points to a role for mutations in what are normally highly conserved residues within GPCR, such as the DRY motif in helix III and conserved aspartate and glutamate residues in helices VI and VII. For a discussion of the finer points of these studies, the reader is pointed to an excellent review by de Munnik et al. [119]. As might be anticipated with promiscuous G protein coupling, signaling bias is in evidence. For example, ORF74 expression in COS-7 but not in HEK 293T cells resulted in constitutive activation of the ERK signaling pathway [115, 117].
In keeping with its homology to CXCR2, ORF74 binds the majority of CXCR2 ligands including CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8 (Fig. 2) [98]. ORF74 has also been shown to bind CXCL4, CXCL10, and CXCL12, which are agonists for CXCR3 and CXCR4 [98, 120]. Not shackled by class restriction, ORF74 also binds the CC chemokines CCL1 and CCL5 [121]. Chemokine binding to ORF74 is associated with modulation of its constitutive activity. CXCR2 ligands tend to display a broad range of agonist activities at ORF74, acting as both full and partial agonists in assays of IP accumulation and β-arrestin recruitment [98, 122]. Conversely, non-CXCR2 ligands such as CXCL10 and CXCL12 function as full inverse agonists, inhibiting the constitutive activity of ORF74 [98, 120, 123]. In addition to modulation by host chemokines, as mentioned previously, vCCL2 has been shown to act as a partial inverse agonist for ORF74 [99].
EBV BILF1
BILF1 possesses only 15% identity to the chemokine receptor CXCR4 but has been shown to form heterodimers with CXCR4, negatively regulating its function by scavenging Gαi proteins [124]. Expressed early in EBV cellular infection, BILF1 is a constitutively active orphan receptor, coupling to Gαi proteins and resulting in NF-κB activation in a COS-7 cell transfectant model [125, 126]. As in the case of HCMV US28, there appears to be tissue bias with respect to the BILF1 constitutive activity since, when expressed in Burkitt's lymphoma or lymphoblastoid B cells, NF-κB-independent CRE activation was observed [125]. BILF1 activity is dependent on the integrity of an EKT motif, analogous to the DRY motif commonly found in GPCR, since mutation of this motif resulted in reduced Gαi coupling [127]. Surprisingly, this signaling-deficient BILF1 mutant still induced tumor growth in a xenograft mouse model, which suggests that G protein-independent signaling pathways may be implicated [127].
BILF1 also forms an association with MHC-I molecules, enhancing the internalization of MHC-I from the cell surface and targeting MHC-I for lysosomal degradation, which is thought to play a role in the ability of EBV infected cells to evade CD8+ T cell responses [128, 129]. This immune-evasive function requires the C-terminus of BILF1 [130].
Concluding Remarks
About 3 decades ago, the first vCKR were identified in HCMV. This discovery established that DNA viruses could encode proteins to target and hijack specific components of the host immune response. With the subsequent identification of many additional vCK, vCKBP, and vCKR, it soon became evident that the host chemokine system is a preferred target for herpesvirus survival strategies. However, we are just beginning to understand how these vCK components might coordinate with other immune evasion mechanisms to facilitate viral spread and persistence. The specificity of HHV for human hosts impedes progress in defining biologically relevant functions for HHV-encoded chemokine systems in the context of a natural infection. In addition, not all HHV-encoded vCK, vCKBP and vCKR described in this review possess a clear orthologue in non-human herpesviruses, and those that do may display opposite activities (e.g., α HHV vs. nonhuman alphaherpesvirus gG). The development of new or humanized nonhuman herpesvirus models as well as recombinant nonhuman herpesviruses expressing HHV-encoded vCK, vCKR, and vCKBP will help to elucidate how the chemokine system modulates HHV pathogenesis and to identify new approaches for treating and preventing HHV infections.
Note Added in Proof
While this review was in press, neuropilin-2 was identified as the first bona fide cellular receptor for the HCMV pentamer gH/gL/UL128-UL130-UL131A [131].
Disclosure Statement
The authors declare that they have no conflict of interests.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
References
- 1.McGeoch DJ, Dolan A, Ralph AC. Toward a comprehensive phylogeny for mammalian and avian herpesviruses. J Virol. 2000;74:10401–10406. doi: 10.1128/jvi.74.22.10401-10406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pontejo SM, Murphy PM. Chemokines encoded by herpesviruses. J Leukoc Biol. 2017;102:1199–1217. doi: 10.1189/jlb.4RU0417-145RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vischer HF, Vink C, Smit MJ. A viral conspiracy: hijacking the chemokine system through virally encoded pirated chemokine receptors. In: Lane TE, Chemokines and Viral Infection, editor. Berlin: Springer; 2006. pp. pp 121–154. [DOI] [PubMed] [Google Scholar]
- 4.González-Motos V, Kropp KA, Viejo-Borbolla A. Chemokine binding proteins: an immunomodulatory strategy going viral. Cytokine Growth Factor Rev. 2016;30:71–80. doi: 10.1016/j.cytogfr.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Steiner I, Kennedy PGE, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6:1015–1028. doi: 10.1016/S1474-4422(07)70267-3. [DOI] [PubMed] [Google Scholar]
- 6.McGeoch DJ, Moss HW, McNab D, Frame MC. DNA sequence and genetic content of the HindIII l region in the short unique component of the herpes simplex virus type 2 genome: identification of the gene encoding glycoprotein G, and evolutionary comparisons. J Gen Virol. 1987;68:19–38. doi: 10.1099/0022-1317-68-1-19. [DOI] [PubMed] [Google Scholar]
- 7.Su HK, Eberle R, Courtney RJ. Processing of the herpes simplex virus type 2 glycoprotein gG-2 results in secretion of a 34,000-Mr cleavage product. J Virol. 1987;61:1735–1737. doi: 10.1128/jvi.61.5.1735-1737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viejo-Borbolla A, Martinez-Martín N, Nel HJ, Rueda P, Martín R, Blanco S, et al. Enhancement of chemokine function as an immunomodulatory strategy employed by human herpesviruses. PLoS Pathog. 2012;8:e1002497. doi: 10.1371/journal.ppat.1002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez-Martín N, Viejo-Borbolla A, Alcami A. Herpes simplex virus particles interact with chemokines and enhance cell migration. J Gen Virol. 2016;97:3007–3016. doi: 10.1099/jgv.0.000616. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Martin N, Viejo-Borbolla A, Martín R, Blanco S, Benovic JL, Thelen M, et al. Herpes simplex virus enhances chemokine function through modulation of receptor trafficking and oligomerization. Nat Commun. 2015;6:6163. doi: 10.1038/ncomms7163. [DOI] [PubMed] [Google Scholar]
- 11.González-Motos V, Jürgens C, Ritter B, Kropp KA, Durán V, Larsen O, et al. Varicella zoster virus glycoprotein C increases chemokine-mediated leukocyte migration. PLoS Pathog. 2017;13:e1006346. doi: 10.1371/journal.ppat.1006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moffat JF, Zerboni L, Kinchington PR, Grose C, Kaneshima H, Arvin AM. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J Virol. 1998;72:965–974. doi: 10.1128/jvi.72.2.965-974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chai Q, He WQ, Zhou M, Lu H, Fu ZF. Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J Virol. 2014;88:4698–4710. doi: 10.1128/JVI.03149-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heldwein EE, Krummenacher C. Entry of herpesviruses into mammalian cells. Cell Mol Life Sci. 2008;65:1653–1668. doi: 10.1007/s00018-008-7570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J Virol. 2010;84:12292–12299. doi: 10.1128/JVI.01700-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou M, Yu Q, Wechsler A, Ryckman BJ. Comparative analysis of gO isoforms reveals that strains of human cytomegalovirus differ in the ratio of gH/gL/gO and gH/gL/UL128–131 in the virion envelope. J Virol. 2013;87:9680–9690. doi: 10.1128/JVI.01167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianni T, Salvioli S, Chesnokova LS, Hutt-Fletcher LM, Campadelli-Fiume G. αvβ6- and αvβ8-integrins serve as interchangeable receptors for HSV gH/gL to promote endocytosis and activation of membrane fusion. PLoS Pathog. 2013;9:e1003806. doi: 10.1371/journal.ppat.1003806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat Struct Mol Biol. 2010;17:882–888. doi: 10.1038/nsmb.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malkowska M, Kokoszynska K, Dymecka M, Rychlewski L, Wyrwicz LS. Alphaherpesvirinae and Gammaherpesvirinae glycoprotein L and CMV UL130 originate from chemokines. Virol J. 2013;10:1. doi: 10.1186/1743-422X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Britt W. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol. 2008;325:417–470. doi: 10.1007/978-3-540-77349-8_23. [DOI] [PubMed] [Google Scholar]
- 21.Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang M-L, Wald A, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768–776. doi: 10.1056/NEJMoa042207. [DOI] [PubMed] [Google Scholar]
- 22.Streblow DN, Orloff SL, Nelson JA. Acceleration of allograft failure by cytomegalovirus. Curr Opin Immunol. 2007;19:577–582. doi: 10.1016/j.coi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leibovitch EC, Jacobson S. Evidence linking HHV-6 with multiple sclerosis: an update. Curr Opin Virol. 2014;9:127–133. doi: 10.1016/j.coviro.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham C, Gatherer D, Hilfrich B, Baluchova K, Dargan DJ, Thomson M, et al. Sequences of complete human cytomegalovirus genomes from infected cell cultures and clinical specimens. J Gen Virol. 2010;91:605–615. doi: 10.1099/vir.0.015891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Bresnahan W, Shenk T. Human cytomegalovirus encodes a highly specific RANTES decoy receptor. Proc Natl Acad Sci USA. 2004;101:16642–16647. doi: 10.1073/pnas.0407233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bresnahan WA, Shenk T. A subset of viral transcripts packaged within human cytomegalovirus particles. Science. 2000;288:2373–2376. doi: 10.1126/science.288.5475.2373. [DOI] [PubMed] [Google Scholar]
- 27.Lüttichau HR. The cytomegalovirus UL146 gene product vCXCL1 targets both CXCR1 and CXCR2 as an agonist. J Biol Chem. 2010;285:9137–9146. doi: 10.1074/jbc.M109.002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grundy JE, Lawson KM, MacCormac LP, Fletcher JM, Yong KL. Cytomegalovirus-infected endothelial cells recruit neutrophils by the secretion of C-X-C chemokines and transmit virus by direct neutrophil-endothelial cell contact and during neutrophil transendothelial migration. J Infect Dis. 1998;177:1465–1474. doi: 10.1086/515300. [DOI] [PubMed] [Google Scholar]
- 29.Yamin R, Lecker LSM, Weisblum Y, Vitenshtein A, Le-Trilling VTK, Wolf DG, et al. HCMV vCXCL1 binds several chemokine receptors and preferentially attracts neutrophils over NK cells by interacting with CXCR2. Cell Rep. 2016;15:1542–1553. doi: 10.1016/j.celrep.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 30.Hassan-Walker AF, Okwuadi S, Lee L, Griffiths PD, Emery VC. Sequence variability of the alpha-chemokine UL146 from clinical strains of human cytomegalovirus. J Med Virol. 2004;74:573–579. doi: 10.1002/jmv.20210. [DOI] [PubMed] [Google Scholar]
- 31.Heo J, Dogra P, Masi TJ, Pitt EA, de Kruijf P, Smit MJ, et al. Novel human cytomegalovirus viral chemokines, vCXCL-1s, display functional selectivity for neutrophil signaling and function. J Immunol. 2015;195:227–236. doi: 10.4049/jimmunol.1400291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cha TA, Tom E, Kemble GW, Duke GM, Mocarski ES, Spaete RR. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol. 1996;70:78–83. doi: 10.1128/jvi.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lurain NS, Fox AM, Lichy HM, Bhorade SM, Ware CF, Huang DD, et al. Analysis of the human cytomegalovirus genomic region from UL146 through UL147A reveals sequence hypervariability, genotypic stability, and overlapping transcripts. Virol J. 2006;3:4. doi: 10.1186/1743-422X-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryckman BJ, Rainish BL, Chase MC, Borton JA, Nelson JA, Jarvis MA, et al. Characterization of the human cytomegalovirus gH/gL/UL128–131 complex that mediates entry into epithelial and endothelial cells. J Virol. 2008;82:60–70. doi: 10.1128/JVI.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hahn G, Revello MG, Patrone M, Percivalle E, Campanini G, Sarasini A, et al. Human cytomegalovirus UL131–128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol. 2004;78:10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci USA. 2005;102:18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wille PT, Knoche AJ, Nelson JA, Jarvis MA, Johnson DC. A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells. J Virol. 2010;84:2585–2596. doi: 10.1128/JVI.02249-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D, Shenk T. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol. 2005;79:10330–10338. doi: 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wussow F, Chiuppesi F, Martinez J, Campo J, Johnson E, Flechsig C, et al. Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex. PLoS Pathog. 2014;10:e1004524. doi: 10.1371/journal.ppat.1004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kabanova A, Perez L, Lilleri D, Marcandalli J, Agatic G, Becattini S, et al. Antibody-driven design of a human cytomegalovirus gHgLpUL128L subunit vaccine that selectively elicits potent neutralizing antibodies. Proc Natl Acad Sci USA. 2014;111:17965–17970. doi: 10.1073/pnas.1415310111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiuppesi F, Wussow F, Johnson E, Bian C, Zhuo M, Rajakumar A, et al. Vaccine-derived neutralizing antibodies to the human cytomegalovirus gH/gL pentamer potently block primary cytotrophoblast infection. J Virol. 2015;89:11884–11898. doi: 10.1128/JVI.01701-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandramouli S, Malito E, Nguyen T, Luisi K, Donnarumma D, Xing Y, et al. Structural basis for potent antibody-mediated neutralization of human cytomegalovirus. Sci Immunol. 2017:2. doi: 10.1126/sciimmunol.aan1457. [DOI] [PubMed] [Google Scholar]
- 43.Straschewski S, Patrone M, Walther P, Gallina A, Mertens T, Frascaroli G. Protein pUL128 of human cytomegalovirus is necessary for monocyte infection and blocking of migration. J Virol. 2011;85:5150–5158. doi: 10.1128/JVI.02100-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao H, Hui-Hui G, Tao R, Ran T, Zheng Q, Qi Z, et al. Recombinant HCMV UL128 expression and functional identification of PBMC-attracting activity in vitro. Arch Virol. 2013;158:173–177. doi: 10.1007/s00705-012-1378-8. [DOI] [PubMed] [Google Scholar]
- 45.Dewin DR, Catusse J, Gompels UA. Identification and characterization of U83A viral chemokine, a broad and potent beta-chemokine agonist for human CCRs with unique selectivity and inhibition by spliced isoform. J Immunol. 2006;176:544–556. doi: 10.4049/jimmunol.176.1.544. [DOI] [PubMed] [Google Scholar]
- 46.Lüttichau HR, Clark-Lewis I, Jensen PØ, Moser C, Gerstoft J, Schwartz TW. A highly selective CCR2 chemokine agonist encoded by human herpesvirus 6. J Biol Chem. 2003;278:10928–10933. doi: 10.1074/jbc.M211329200. [DOI] [PubMed] [Google Scholar]
- 47.French C, Menegazzi P, Nicholson L, Macaulay H, DiLuca D, Gompels UA. Novel, nonconsensus cellular splicing regulates expression of a gene encoding a chemokine-like protein that shows high variation and is specific for human herpesvirus 6. Virology. 1999;262:139–151. doi: 10.1006/viro.1999.9875. [DOI] [PubMed] [Google Scholar]
- 48.Clark DJ, Catusse J, Stacey A, Borrow P, Gompels UA. Activation of CCR2+ human proinflammatory monocytes by human herpesvirus-6B chemokine N-terminal peptide. J Gen Virol. 2013;94:1624–1635. doi: 10.1099/vir.0.050153-0. [DOI] [PubMed] [Google Scholar]
- 49.Fraile-Ramos A, Pelchen-Matthews A, Kledal TN, Browne H, Schwartz TW, Marsh M. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic. 2002;3:218–232. doi: 10.1034/j.1600-0854.2002.030307.x. [DOI] [PubMed] [Google Scholar]
- 50.Stapleton LK, Arnolds KL, Lares AP, Devito TM, Spencer JV. Receptor chimeras demonstrate that the C-terminal domain of the human cytomegalovirus US27 gene product is necessary and sufficient for intracellular receptor localization. Virol J. 2012;9:42. doi: 10.1186/1743-422X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnolds KL, Lares AP, Spencer JV. The US27 gene product of human cytomegalovirus enhances signaling of host chemokine receptor CXCR4. Virology. 2013;439:122–131. doi: 10.1016/j.virol.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lares AP, Tu CC, Spencer JV. The human cytomegalovirus US27 gene product enhances cell proliferation and alters cellular gene expression. Virus Res. 2013;176:312–320. doi: 10.1016/j.virusres.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tu CC, Spencer JV. The DRY box and C-terminal domain of the human cytomegalovirus US27 gene product play a role in promoting cell growth and survival. PLoS One. 2014;9:e113427. doi: 10.1371/journal.pone.0113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Connor CM, Shenk T. Human cytomegalovirus pUS27 G protein-coupled receptor homologue is required for efficient spread by the extracellular route but not for direct cell-to-cell spread. J Virol. 2011;85:3700–3707. doi: 10.1128/JVI.02442-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slinger E, Maussang D, Schreiber A, Siderius M, Rahbar A, Fraile-Ramos A, et al. HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. Sci Signal. 2010;3:ra58. doi: 10.1126/scisignal.2001180. [DOI] [PubMed] [Google Scholar]
- 56.McLean KA, Holst PJ, Martini L, Schwartz TW, Rosenkilde MM. Similar activation of signal transduction pathways by the herpesvirus-encoded chemokine receptors US28 and ORF74. Virology. 2004;325:241–251. doi: 10.1016/j.virol.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 57.Maussang D, Verzijl D, van Walsum M, Leurs R, Holl J, Pleskoff O, et al. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci USA. 2006;103:13068–13073. doi: 10.1073/pnas.0604433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maussang D, Langemeijer E, Fitzsimons CP, Stigter-van Walsum M, Dijkman R, Borg MK, et al. The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res. 2009;69:2861–2869. doi: 10.1158/0008-5472.CAN-08-2487. [DOI] [PubMed] [Google Scholar]
- 59.de Wit RH, Mujić-Delić A, van Senten JR, Fraile-Ramos A, Siderius M, Smit MJ. Human cytomegalovirus encoded chemokine receptor US28 activates the HIF-1α/PKM2 axis in glioblastoma cells. Oncotarget. 2016;7:67966–67985. doi: 10.18632/oncotarget.11817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Langemeijer EV, Slinger E, de Munnik S, Schreiber A, Maussang D, Vischer H, et al. Constitutive β-catenin signaling by the viral chemokine receptor US28. PLoS One. 2012;7:e48935. doi: 10.1371/journal.pone.0048935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Casarosa P, Menge WM, Minisini R, Otto C, van Heteren J, Jongejan A, et al. Identification of the first nonpeptidergic inverse agonist for a constitutively active viral-encoded G protein-coupled receptor. J Biol Chem. 2003;278:5172–5178. doi: 10.1074/jbc.M210033200. [DOI] [PubMed] [Google Scholar]
- 62.Hesselgesser J, Ng HP, Liang M, Zheng W, May K, Bauman JG, et al. Identification and characterization of small molecule functional antagonists of the CCR1 chemokine receptor. J Biol Chem. 1998;273:15687–15692. doi: 10.1074/jbc.273.25.15687. [DOI] [PubMed] [Google Scholar]
- 63.Kralj A, Wetzel A, Mahmoudian S, Stamminger T, Tschammer N, Heinrich MR. Identification of novel allosteric modulators for the G-protein coupled US28 receptor of human cytomegalovirus. Bioorg Med Chem Lett. 2011;21:5446–5450. doi: 10.1016/j.bmcl.2011.06.120. [DOI] [PubMed] [Google Scholar]
- 64.Kledal TN, Rosenkilde MM, Coulin F, Simmons G, Johnsen AH, Alouani S, et al. A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science. 1997;277:1656–1659. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 65.Kledal TN, Rosenkilde MM, Schwartz TW. Selective recognition of the membrane-bound CX3 C chemokine, fractalkine, by the human cytomegalovirus-encoded broad-spectrum receptor US28. FEBS Lett. 1998;441:209–214. doi: 10.1016/s0014-5793(98)01551-8. [DOI] [PubMed] [Google Scholar]
- 66.Noriega VM, Gardner TJ, Redmann V, Bongers G, Lira SA, Tortorella D. Human cytomegalovirus US28 facilitates cell-to-cell viral dissemination. Viruses. 2014;6:1202–1218. doi: 10.3390/v6031202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spiess K, Jeppesen MG, Malmgaard-Clausen M, Krzywkowski K, Kledal TN, Rosenkilde MM. Novel chemokine-based immunotoxins for potent and selective targeting of cytomegalovirus infected cells. J Immunol Res. 2017;2017:4069260. doi: 10.1155/2017/4069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burg JS, Ingram JR, Venkatakrishnan AJ, Jude KM, Dukkipati A, Feinberg EN, et al. Structural biology: structural basis for chemokine recognition and activation of a viral G protein-coupled receptor. Science. 2015;347:1113–1117. doi: 10.1126/science.aaa5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vomaske J, Melnychuk RM, Smith PP, Powell J, Hall L, DeFilippis V, et al. Differential ligand binding to a human cytomegalovirus chemokine receptor determines cell type-specific motility. PLoS Pathog. 2009;5:e1000304. doi: 10.1371/journal.ppat.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pleskoff O, Tréboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 71.Casarosa P, Gruijthuijsen YK, Michel D, Beisser PS, Holl J, Fitzsimons CP, et al. Constitutive signaling of the human cytomegalovirus-encoded receptor UL33 differs from that of its rat cytomegalovirus homolog R33 by promiscuous activation of G proteins of the Gq, Gi, and Gs classes. J Biol Chem. 2003;278:50010–50023. doi: 10.1074/jbc.M306530200. [DOI] [PubMed] [Google Scholar]
- 72.Tschische P, Tadagaki K, Kamal M, Jockers R, Waldhoer M. Heteromerization of human cytomegalovirus encoded chemokine receptors. Biochem Pharmacol. 2011;82:610–619. doi: 10.1016/j.bcp.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tadagaki K, Tudor D, Gbahou F, Tschische P, Waldhoer M, Bomsel M, et al. Human cytomegalovirus-encoded UL33 and UL78 heteromerize with host CCR5 and CXCR4 impairing their HIV coreceptor activity. Blood. 2012;119:4908–4918. doi: 10.1182/blood-2011-08-372516. [DOI] [PubMed] [Google Scholar]
- 74.Michel D, Milotić I, Wagner M, Vaida B, Holl J, Ansorge R, et al. The human cytomegalovirus UL78 gene is highly conserved among clinical isolates, but is dispensable for replication in fibroblasts and a renal artery organ-culture system. J Gen Virol. 2005;86:297–306. doi: 10.1099/vir.0.80436-0. [DOI] [PubMed] [Google Scholar]
- 75.O'Connor CM, Shenk T. Human cytomegalovirus pUL78 G protein-coupled receptor homologue is required for timely cell entry in epithelial cells but not fibroblasts. J Virol. 2012;86:11425–11433. doi: 10.1128/JVI.05900-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isegawa Y, Ping Z, Nakano K, Sugimoto N, Yamanishi K. Human herpesvirus 6 open reading frame U12 encodes a functional beta-chemokine receptor. J Virol. 1998;72:6104–6112. doi: 10.1128/jvi.72.7.6104-6112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakano K, Tadagaki K, Isegawa Y, Aye MM, Zou P, Yamanishi K. Human herpesvirus 7 open reading frame U12 encodes a functional beta-chemokine receptor. J Virol. 2003;77:8108–8115. doi: 10.1128/JVI.77.14.8108-8115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tadagaki K, Yamanishi K, Mori Y. Reciprocal roles of cellular chemokine receptors and human herpesvirus 7-encoded chemokine receptors, U12 and U51. J Gen Virol. 2007;88:1423–1428. doi: 10.1099/vir.0.82665-0. [DOI] [PubMed] [Google Scholar]
- 79.Menotti L, Mirandola P, Locati M, Campadelli-Fiume G. Trafficking to the plasma membrane of the seven-transmembrane protein encoded by human herpesvirus 6 U51 gene involves a cell-specific function present in T lymphocytes. J Virol. 1999;73:325–333. doi: 10.1128/jvi.73.1.325-333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Catusse J, Spinks J, Mattick C, Dyer A, Laing K, Fitzsimons C, et al. Immunomodulation by herpesvirus U51A chemokine receptor via CCL5 and FOG-2 down-regulation plus XCR1 and CCR7 mimicry in human leukocytes. Eur J Immunol. 2008;38:763–777. doi: 10.1002/eji.200737618. [DOI] [PubMed] [Google Scholar]
- 81.Milne RS, Mattick C, Nicholson L, Devaraj P, Alcami A, Gompels UA. RANTES binding and down-regulation by a novel human herpesvirus-6 beta chemokine receptor. J Immunol. 2000;164:2396–2404. doi: 10.4049/jimmunol.164.5.2396. [DOI] [PubMed] [Google Scholar]
- 82.Tadagaki K, Nakano K, Yamanishi K. Human herpesvirus 7 open reading frames U12 and U51 encode functional beta-chemokine receptors. J Virol. 2005;79:7068–7076. doi: 10.1128/JVI.79.11.7068-7076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res. 2004;10:803–821. doi: 10.1158/1078-0432.ccr-0670-3. [DOI] [PubMed] [Google Scholar]
- 84.Carbone A, Gloghini A, Dotti G. EBV-associated lymphoproliferative disorders: classification and treatment. Oncologist. 2008;13:577–585. doi: 10.1634/theoncologist.2008-0036. [DOI] [PubMed] [Google Scholar]
- 85.Ensoli B. Kaposi's sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 1998;9:63–83. doi: 10.1016/s1359-6101(97)00037-3. [DOI] [PubMed] [Google Scholar]
- 86.Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190. doi: 10.1186/1471-2407-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakano K, Isegawa Y, Zou P, Tadagaki K, Inagi R, Yamanishi K. Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded vMIP-I and vMIP-II induce signal transduction and chemotaxis in monocytic cells. Arch Virol. 2003;148:871–890. doi: 10.1007/s00705-002-0971-7. [DOI] [PubMed] [Google Scholar]
- 88.Willey SJ, Reeves JD, Hudson R, Miyake K, Dejucq N, Schols D, et al. Identification of a subset of human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus strains able to exploit an alternative coreceptor on untransformed human brain and lymphoid cells. J Virol. 2003;77:6138–6152. doi: 10.1128/JVI.77.11.6138-6152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boshoff C, Endo Y, Collins PD, Takeuchi Y, Reeves JD, Schweickart VL, et al. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 90.Dairaghi DJ, Fan RA, McMaster BE, Hanley MR, Schall TJ. HHV8-encoded vMIP-I selectively engages chemokine receptor CCR8: agonist and antagonist profiles of viral chemokines. J Biol Chem. 1999;274:21569–21574. doi: 10.1074/jbc.274.31.21569. [DOI] [PubMed] [Google Scholar]
- 91.Endres MJ, Garlisi CG, Xiao H, Shan L, Hedrick JA. The Kaposi's sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J Exp Med. 1999;189:1993–1998. doi: 10.1084/jem.189.12.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lüttichau HR, Johnsen AH, Jurlander J, Rosenkilde MM, Schwartz TW. Kaposi sarcoma-associated herpes virus targets the lymphotactin receptor with both a broad spectrum antagonist vCCL2 and a highly selective and potent agonist vCCL3. J Biol Chem. 2007;282:17794–17805. doi: 10.1074/jbc.M702001200. [DOI] [PubMed] [Google Scholar]
- 93.Haque NS, Fallon JT, Taubman MB, Harpel PC. The chemokine receptor CCR8 mediates human endothelial cell chemotaxis induced by I-309 and Kaposi sarcoma herpesvirus-encoded vMIP-I and by lipoprotein(a)-stimulated endothelial cell conditioned medium. Blood. 2001;97:39–45. doi: 10.1182/blood.v97.1.39. [DOI] [PubMed] [Google Scholar]
- 94.Louahed J, Struyf S, Demoulin J-B, Parmentier M, Van Snick J, Van Damme J, et al. CCR8-dependent activation of the RAS/MAPK pathway mediates anti-apoptotic activity of I-309/ CCL1 and vMIP-I. Eur J Immunol. 2003;33:494–501. doi: 10.1002/immu.200310025. [DOI] [PubMed] [Google Scholar]
- 95.Lüttichau HR, Lewis IC, Gerstoft J, Schwartz TW. The herpesvirus 8-encoded chemokine vMIP-II, but not the poxvirus-encoded chemokine MC148, inhibits the CCR10 receptor. Eur J Immunol. 2001;31:1217–1220. doi: 10.1002/1521-4141(200104)31:4<1217::aid-immu1217>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 96.Sozzani S, Luini W, Bianchi G, Allavena P, Wells TN, Napolitano M, et al. The viral chemokine macrophage inflammatory protein-II is a selective Th2 chemoattractant. Blood. 1998;92:4036–4039. [PubMed] [Google Scholar]
- 97.Szpakowska M, Dupuis N, Baragli A, Counson M, Hanson J, Piette J, et al. Human herpesvirus 8-encoded chemokine vCCL2/vMIP-II is an agonist of the atypical chemokine receptor ACKR3/CXCR7. Biochem Pharmacol. 2016;114:14–21. doi: 10.1016/j.bcp.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 98.Rosenkilde MM, Kledal TN, Bräuner-Osborne H, Schwartz TW. Agonists and inverse agonists for the herpesvirus 8-encoded constitutively active seven-transmembrane oncogene product, ORF-74. J Biol Chem. 1999;274:956–961. doi: 10.1074/jbc.274.2.956. [DOI] [PubMed] [Google Scholar]
- 99.Geras-Raaka E, Varma A, Clark-Lewis I, Gershengorn MC. Kaposi's sarcoma-associated herpesvirus (KSHV) chemokine vMIP-II and human SDF-1alpha inhibit signaling by KSHV G protein-coupled receptor. Biochem Biophys Res Commun. 1998;253:725–727. doi: 10.1006/bbrc.1998.9557. [DOI] [PubMed] [Google Scholar]
- 100.Zhou N, Luo Z, Luo J, Hall JW, Huang Z. A novel peptide antagonist of CXCR4 derived from the N-terminus of viral chemokine vMIP-II. Biochemistry. 2000;39:3782–3787. doi: 10.1021/bi992750v. [DOI] [PubMed] [Google Scholar]
- 101.Luo Z, Fan X, Zhou N, Hiraoka M, Luo J, Kaji H, et al. Structure-function study and anti-HIV activity of synthetic peptide analogues derived from viral chemokine vMIP-II. Biochemistry. 2000;39:13545–13550. doi: 10.1021/bi000633q. [DOI] [PubMed] [Google Scholar]
- 102.Ghirnikar RS, Lee YL, Eng LF. Chemokine antagonist infusion attenuates cellular infiltration following spinal cord contusion injury in rat. J Neurosci Res. 2000;59:63–73. [PubMed] [Google Scholar]
- 103.Chen S, Bacon KB, Li L, Garcia GE, Xia Y, Lo D, et al. In vivo inhibition of CC and CX3C chemokine-induced leukocyte infiltration and attenuation of glomerulonephritis in Wistar-Kyoto (WKY) rats by vMIP-II. J Exp Med. 1998;188:193–198. doi: 10.1084/jem.188.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cherqui S, Kingdon KM, Thorpe C, Kurian SM, Salomon DR. Lentiviral gene delivery of vMIP-II to transplanted endothelial cells and endothelial progenitors is proangiogenic in vivo. Mol Ther. 2007;15:1264–1272. doi: 10.1038/sj.mt.6300183. [DOI] [PubMed] [Google Scholar]
- 105.DeBruyne LA, Li K, Bishop DK, Bromberg JS. Gene transfer of virally encoded chemokine antagonists vMIP-II and MC148 prolongs cardiac allograft survival and inhibits donor-specific immunity. Gene Ther. 2000;7:575–582. doi: 10.1038/sj.gt.3301128. [DOI] [PubMed] [Google Scholar]
- 106.Pillai RG, Beutelspacher SC, Larkin DFP, George AJT. Expression of the chemokine antagonist vMIP II using a non-viral vector can prolong corneal allograft survival. Transplantation. 2008;85:1640–1647. doi: 10.1097/TP.0b013e318172813f. [DOI] [PubMed] [Google Scholar]
- 107.Stine JT, Wood C, Hill M, Epp A, Raport CJ, Schweickart VL, et al. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood. 2000;95:1151–1157. [PubMed] [Google Scholar]
- 108.Matsuura H, Kirschner AN, Longnecker R, Jardetzky TS. Crystal structure of the Epstein-Barr virus (EBV) glycoprotein H/glycoprotein L (gH/gL) complex. Proc Natl Acad Sci USA. 2010;107:22641–22646. doi: 10.1073/pnas.1011806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proc Natl Acad Sci USA. 2009;106:20464–20469. doi: 10.1073/pnas.0907508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hahn AS, Kaufmann JK, Wies E, Naschberger E, Panteleev-Ivlev J, Schmidt K, et al. The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi's sarcoma-associated herpesvirus. Nat Med. 2012;18:961–966. doi: 10.1038/nm.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dubin G, Jiang H. Expression of herpes simplex virus type 1 glycoprotein L (gL) in transfected mammalian cells: evidence that gL is not independently anchored to cell membranes. J Virol. 1995;69:4564–4568. doi: 10.1128/jvi.69.7.4564-4568.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cesarman E, Nador RG, Bai F, Bohenzky RA, Russo JJ, Moore PS, et al. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang TY, Chen SC, Leach MW, Manfra D, Homey B, Wiekowski M, et al. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J Exp Med. 2000;191:445–454. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shepard LW, Yang M, Xie P, Browning DD, Voyno-Yasenetskaya T, Kozasa T, et al. Constitutive activation of NF-kappa B and secretion of interleukin-8 induced by the G protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus involve G alpha(13) and RhoA. J Biol Chem. 2001;276:45979–45987. doi: 10.1074/jbc.M104783200. [DOI] [PubMed] [Google Scholar]
- 115.Smit MJ, Verzijl D, Casarosa P, Navis M, Timmerman H, Leurs R. Kaposi's sarcoma-associated herpesvirus-encoded G protein-coupled receptor ORF74 constitutively activates p44/p42 MAPK and Akt via G(i) and phospholipase C-dependent signaling pathways. J Virol. 2002;76:1744–1752. doi: 10.1128/JVI.76.4.1744-1752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cannon ML, Cesarman E. The KSHV G protein-coupled receptor signals via multiple pathways to induce transcription factor activation in primary effusion lymphoma cells. Oncogene. 2004;23:514–523. doi: 10.1038/sj.onc.1207021. [DOI] [PubMed] [Google Scholar]
- 117.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, et al. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 118.Martín MJ, Tanos T, García AB, Martin D, Gutkind JS, Coso OA, et al. The Galpha12/13 family of heterotrimeric G proteins and the small GTPase RhoA link the Kaposi sarcoma-associated herpes virus G protein-coupled receptor to heme oxygenase-1 expression and tumorigenesis. J Biol Chem. 2007;282:34510–34524. doi: 10.1074/jbc.M703043200. [DOI] [PubMed] [Google Scholar]
- 119.de Munnik SM, Smit MJ, Leurs R, Vischer HF. Modulation of cellular signaling by herpesvirus-encoded G protein-coupled receptors. Front Pharmacol. 2015;6:40. doi: 10.3389/fphar.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gershengorn MC, Geras-Raaka E, Varma A, Clark-Lewis I. Chemokines activate Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor in mammalian cells in culture. J Clin Invest. 1998;102:1469–1472. doi: 10.1172/JCI4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 122.de Munnik SM, Kooistra AJ, van Offenbeek J, Nijmeijer S, de Graaf C, Smit MJ, et al. The viral G protein-coupled receptor ORF74 hijacks β-arrestins for endocytic trafficking in response to human chemokines. PLoS One. 2015;10:e0124486. doi: 10.1371/journal.pone.0124486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Geras-Raaka E, Varma A, Ho H, Clark-Lewis I, Gershengorn MC. Human interferon-gamma-inducible protein 10 (IP-10) inhibits constitutive signaling of Kaposi's sarcoma-associated herpesvirus G protein- coupled receptor. J Exp Med. 1998;188:405–408. doi: 10.1084/jem.188.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nijmeijer S, Leurs R, Smit MJ, Vischer HF. The Epstein-Barr virus-encoded G protein-coupled receptor BILF1 hetero-oligomerizes with human CXCR4, scavenges Gαi proteins, and constitutively impairs CXCR4 functioning. J Biol Chem. 2010;285:29632–29641. doi: 10.1074/jbc.M110.115618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beisser PS, Verzijl D, Gruijthuijsen YK, Beuken E, Smit MJ, Leurs R, et al. The Epstein-Barr virus BILF1 gene encodes a G protein-coupled receptor that inhibits phosphorylation of RNA-dependent protein kinase. J Virol. 2005;79:441–449. doi: 10.1128/JVI.79.1.441-449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Paulsen SJ, Rosenkilde MM, Eugen-Olsen J, Kledal TN. Epstein-Barr virus-encoded BILF1 is a constitutively active G protein-coupled receptor. J Virol. 2005;79:536–546. doi: 10.1128/JVI.79.1.536-546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lyngaa R, Nørregaard K, Kristensen M, Kubale V, Rosenkilde MM, Kledal TN. Cell transformation mediated by the Epstein-Barr virus G protein-coupled receptor BILF1 is dependent on constitutive signaling. Oncogene. 2010;29:4388–4398. doi: 10.1038/onc.2010.173. [DOI] [PubMed] [Google Scholar]
- 128.Zuo J, Currin A, Griffin BD, Shannon-Lowe C, Thomas WA, Ressing ME, et al. The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation. PLoS Pathog. 2009;5:e1000255. doi: 10.1371/journal.ppat.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zuo J, Quinn LL, Tamblyn J, Thomas WA, Feederle R, Delecluse H-J, et al. The Epstein-Barr virus-encoded BILF1 protein modulates immune recognition of endogenously processed antigen by targeting major histocompatibility complex class I molecules trafficking on both the exocytic and endocytic pathways. J Virol. 2011;85:1604–1614. doi: 10.1128/JVI.01608-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Griffin BD, Gram AM, Mulder A, Van Leeuwen D, Claas FHJ, Wang F, et al. EBV BILF1 evolved to downregulate cell surface display of a wide range of HLA class I molecules through their cytoplasmic tail. J Immunol. 2013;190:1672–1684. doi: 10.4049/jimmunol.1102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martinez-Martin N, Marcandalli J, Huang CS, Arthur CP, Perotti M, Foglierini M, et al. An unbiased screen for human cytomegalovirus identifies neuropilin-2 as a central vi ral receptor. Cell. 2018 doi: 10.1016/j.cell.2018.06.028. DOI: 10.1016/j.cell.2018.06.028. [DOI] [PubMed] [Google Scholar]