Abstract
Spinal cord injury (SCI) results in paralysis below the injury and strategies are being developed that support axonal regrowth, yet recovery lags, in part, because many axons are not re-myelinated. Herein, we investigated strategies to increase myelination of regenerating axons by over-expression of platelet-derived growth factor-AA (PDGF) and noggin either alone or in combination in a mouse SCI model. Noggin and platelet-derived growth factor aa (PDGF) have been identified as factors that enhance recruitment and differentiation of endogenous progenitors to promote myelination. Lentivirus encoding for these factors were delivered from a multi-channel bridge, which we have previously shown creates a permissive environment and supports robust axonal growth through channels. The combination of noggin + PDGF enhanced total myelination of regenerating axons relative to either factor alone, and importantly, enhanced functional recovery relative to the control condition. The increase in myelination was consistent with an increase in oligodendrocyte-derived myelin, which was also associated with a greater density of cells of an oligodendroglial lineage relative to each factor individually and control conditions. These results suggest enhanced myelination of regenerating axons by noggin + PDGF that act on oligodendrocyte-lineage cells post-SCI, which ultimately led to improved functional outcomes.
Keywords: Spinal Cord Injury, Gene Therapy, Myelination, Tissue Engineering
Introduction
Spinal cord injury (SCI) causes paralysis below the level of injury, which, at the cellular level, results from neuron and oligodendrocyte cell death, axonal loss, and demyelination(L. De Laporte et al., 2009; P. M. Richardson, U. M. Mcguinness, & A. J. Aguayo, 1980; H. M. Tuinstra et al., 2012). Though spinal cord neurons have an innate capacity to regenerate, they are limited by a microenvironment that features an insufficient supply of factors that promote regeneration and an abundance of inhibitory factors including the glial scar(M. S. Beattie, G. E. Hermann, R. C. Rogers, & J. C. Bresnahan, 2002; M. B. Bunge, 2001; D. J. Donnelly & P. G. Popovich, 2008; M. T. Fitch & J. Silver, 2008; K. Kadoya et al., 2009; C. E. Schmidt & J. B. Leach, 2003; M. E. Schwab, 2002). Post-mitotic oligodendrocytes, the myelinating cells native to the central nervous system (CNS), infrequently myelinate regenerating axons as oligodendrocytes undergo apoptosis due to excitotoxicity and the inflammatory milieu(S. Casha, W. R. Yu, & M. G. Fehlings, 2001; G. L. Li, M. Farooque, A. Holtz, & Y. Olsson, 1999; J. M. Lytle & J. R. Wrathall, 2007). Remyelination by Schwann cell of the peripheral nervous system is observed, though it is expected that Schwann cell-derived myelin is less effective for CNS function(S.-X. Zhang, F. Huang, M. Gates, & E. G. Holmberg, 2013). Surviving oligodendrocytes are inefficient at proliferation or extensive migration that is necessary for the cells to myelinate the majority of regenerating axons(A. Almad, F. R. Sahinkaya, & D. M. Mctigue, 2011; J. M. Lytle & J. R. Wrathall, 2007). Thus, strategies are needed to overcome the inhibitory microenvironment to enhance the number of available oligodendrocytes, such as through the recruitment of endogenous progenitors, and supporting their capacity for myelination(A. Almad et al., 2011; F. Barnabe-Heider et al., 2010; S. Mi et al., 2009; D. L. Sellers, D. O. Maris, & P. J. Horner, 2009).
Modulating the microenvironment following injury has proven to be difficult, with a multitude of cell, gene, and biomaterial approaches having been evaluated. Transplantation of Schwann cells, stem cells, or cells genetically engineered to secrete inductive factors have been used to shift the microenvironment towards a pro-regenerative phenotype(B. K. Chen et al., 2017; J. Fortun, C. E. Hill, & M. B. Bunge, 2009; D. D. Pearse & D. J. Barakat, 2006). However, the impact of cell transplantation is frequently limited by the extent of survival and engraftment(J. Li & G. Lepski, 2013; W. Tetzlaff et al., 2011), which may not provide factors for times that are necessary to promote regeneration. Alternatively, injection of proteins into the lesion space or the spinal cord parenchyma has been employed to deliver trophic factors, yet these strategies cannot sustain the presence of these factors due to their clearance or degradation. Gene delivery represents a versatile strategy in which transduced cells function as bioreactors for the localized production of trophic factors to create a permissive environment for regeneration. We have previously reported that poly (lactide-co-glycolide) (PLG) multi-channel bridges are an effective vehicle for localized, sustained lentiviral gene therapy capable of altering the post-injury microenvironment and promoting regeneration(H. M. Tuinstra et al., 2012). PLG has been widely used as a material for spinal cord repair or peripheral nerve conduits. PLG is also biodegradable, bioresorbable, and its degradation products are cleared by the body (M. Wang et al., 2011). Lentiviral expression is highest at the site of the implant and decreases as a function of distance. The lentivirus transduces cells in the surrounding area including astrocytes, macrophages, fibroblasts, and invading Schwann cells (H. M. Tuinstra et al., 2012). Additionally, these bridges feature an architecture that encourages axon growth through channels and infiltration of supporting cells into interconnected pores(H. M. Tuinstra et al., 2012; H. M. Tuinstra et al., 2014). Regenerating axons have been observed growing through the bridge and into tissue caudal to the injury(K. Pawar et al., 2015). However, the bridge alone is limited in its ability to foster remyelination. We have previously reported on the combinatorial delivery of sonic hedgehog (SHH) and neurotrophin 3 (NT3) to improve myelination of regenerating axons. SHH and NT3 are reported to promote neurite extension that is dependent on the concentration and spatial/temporal distribution(A. C. Delgado et al., 2014; E. Marti & P. Bovolenta, 2002), with SHH also influencing neuronal and oligodendrocyte differentiation during development and following injury(A. Gritli-Linde, P. Lewis, A. P. Mcmahon, & A. Linde, 2001; N. Lowry et al., 2012). While SHH alone, but not its combination with NT3, was able to enhance the percentage of axons myelinated by oligodendrocytes, the overall percentage of myelinated axons was lower than normally found in the contralateral tissue. This could be attributed to NT3 promoting Schwann cell recruitment and progenitor quiescence(A. C. Delgado et al., 2014). SHH has also been associated with ventral patterning, but has not been shown to enhance progenitor recruitment necessary to generate myelinating oligodendrocytes(V. Ribes & J. Briscoe, 2009). Factors that more effectively recruit the endogenous progenitor pool toward an oligodendrocyte lineage may further enhance myelination.
Noggin and platelet-derived growth factor aa (PDGF) have been identified as factors that enhance recruitment and differentiation of endogenous progenitors to promote myelination in vitro and in vivo. Noggin is a bone morphogenetic protein (BMP) receptor antagonist(A. Liu & L. A. Niswander, 2005). BMPs, which are upregulated after SCI, promote astrocyte differentiation of proliferating progenitor cells. Although noggin inhibits the BMP pathway, it is not sufficient to increase the overall differentiation of progenitor cells to myelinating oligodendrocytes(G. U. Enzmann et al., 2005). PDGF specifically, has been noted for its capacity to enhance proliferation and recruitment of progenitor cells both in vitro and in vivo(Y. Chen et al., 2007; R. A. Hill, K. D. Patel, J. Medved, A. M. Reiss, & A. Nishiyama, 2013; R. H. Woodruff, M. Fruttiger, W. D. Richardson, & R. J. Franklin, 2004; H. Zhang, L. Vutskits, V. Calaora, P. Durbec, & J. Z. Kiss, 2004). PDGF is a potent mitogen for progenitor cell proliferation(K. Asakura, S. F. Hunter, & M. Rodriguez, 1997; R. H. Woodruff et al., 2004) and is a required signaling molecule for differentiation of embryonic and adult neural stem cells into O4+ oligodendrocytes(M. Bradl & H. Lassmann, 2010; G. L. Hinks & R. J. Franklin, 1999; J. G. Hu et al., 2008). These reports elucidated how inductive factors can increase progenitor activity after SCI and even encourage spontaneous remyelination of spared axons(J. Lasiene, L. Shupe, S. Perlmutter, & P. Horner, 2008; B. E. Powers et al., 2012; B. E. Powers et al., 2013); however, the ability of these endogenous progenitors to potentiate myelination of large numbers of newly regenerating axons has not been determined.
In this report, we investigated noggin and PDGF individually or in combination for their ability to enhance myelination of regenerating axons growing through a biomaterial bridge implanted into an acute spinal cord lesion. A mouse lateral hemisection model was used, with immediate intervention with PLG bridges inserted into the injury to deliver lentiviral vectors for sustained and localized expression noggin and/or PDGF (D. J. Margul et al., 2016; A. M. Thomas et al., 2014). Immunohistochemistry (IHC) was initially employed to quantify the extent of axon growth and myelination, and functional recovery was quantified using the Basso Mouse Scale (BMS) scale. As myelination can occur from CNS-derived oligodendrocytes or PNS-derived Schwann cells, we quantified the source of myelinating cells, as well as the density of cells within the oligodendrocyte lineage. Collectively, these studies demonstrate the potential for synergy between biomaterials to guide tissue growth and the localized expression of factors to modulate the local environment and enhance the recruitment of endogenous progenitors that can restore function.
Materials and Methods
Virus production and validation
HEK-293FT cells (80–90% confluent, American Type Culture Collection (ATCC), Manassas, VA, USA) were transfected with third generation lentiviral packaging vectors and pLenti-CMV-Luciferase, pLenti-CMV-noggin, or pLenti-CMV-PDGF. Correct insertion was validated via DNA sequencing. Plasmids were incubated in OptiMEM (Life Technologies, Carlsbad, CA, USA) with Lipofectamine 2000 (Life Technologies) for 20 minutes prior to being added to cells. After 48 hours of incubation, supernatant was collected, centrifuged to remove cellular debris, and then incubated with PEG-It (System Biosciences, Palo Alto, CA, USA) for 16–24 hours at 4°C. Virus was centrifuged at 1500g at 4°C for 30 min, supernatant was removed, and the pellet was re-suspended in sterile phosphate buffered saline (PBS; Life Technologies). Viral solution was aliquoted and frozen at −80°C until use. Viral titers used throughout the study were 3E9 IU/mL as determined by the Lentivirus qPCR Titer Kit (Applied Biological Materials, Richmond, BC, Canada).
Fabrication of multi-channel bridges
Bridges were fabricated using a sacrificial template variation(J. Li, T. A. Rickett, & R. Shi, 2009) of the gas foaming/particulate leaching technique, as previously described(A. Thomas et al., 2013; H. M. Tuinstra et al., 2012). Briefly, PLG (75:25 lactide:glycolide; i.v. 0.76 dL/g; Lakeshore Biomaterials, Birmingham, AL, USA) was dissolved in dichloromethane (6% w/w) and emulsified in 1% poly (ethylene-alt-maleic anhydride) using a homogenizer (PolyTron 3100; Kinematica AG, Littau, Switzerland) to create microspheres (z-average diameter ~1μm). D-sucrose (Sigma Aldrich), D-glucose (Sigma Aldrich), and dextran MW 100,000 (Sigma Aldrich) were mixed at a ratio of 5.3:2.5:1 respectively by mass. The mixture was caramelized, cooled, and drawn from solution with a Pasteur pipette to make sugar fibers. Fibers were drawn to 150 – 250 μm, coated with a 1:1 mixture of PLG microspheres and salt (63–106 μm) and pressed into a salt-lined aluminum mold. The sugar strands were used to create 7 channels and the salt create a porous structure. The materials were then equilibrated with CO2 gas (800 psi) for 16 h and then gas foamed in a custom-made pressure vessel. Bridges were subsequently cut into 2.25 mm sections and leached for 2 h to remove porogen. The bridges are dried overnight and stored in a desiccator.
Virus loading into bridges
Viruses were adsorbed onto bridges, with multiple steps to increase lentiviral loading. Prior to virus addition, bridges were disinfected in 70% ethanol and washed with sterile water. Bridges were then dried by touching sterile filter paper to the bridge. Bridges were then saturated with 2 μL of virus. After 2 minutes of incubation, sterile filter paper was touched to the surface of the bridge to remove excess moisture. This process was then repeated until a total of 8 μL of virus was added. After the final addition, the bridges were not dried with filter paper and were stored on ice until use. Bridges were used within 3 hours of coating with lentivirus. Lentivirus loading conditions included FLuc, PDGF, noggin, and noggin + PDGF. Lentiviral loading was done to ensure the same number of lentiviral particles per bridge.
Mouse spinal cord hemisection
All animal procedures were approved and in accordance with the Institutional Animal Care and Use Committee at the University of Michigan. A hemisection model of SCI was performed as previously described(A. Thomas et al., 2013) on female C57BL/6 mice (6–8 weeks old; Jackson Laboratories). After administration of bupivacaine (.8 ml/kg), a laminectomy was performed at T9-T10 to allow for a 2.25 mm lateral hemisection for immediate bridge implantation. The injury site was covered using Gelfoam (Pfizer, New York, NY, USA) followed by suturing together of the muscle and stapling of skin. Postoperative care consisted of administration of enrofloxacin (2.5 mg/kg; daily for 2 weeks), buprenorphine (0.1 mg/kg; twice daily for 3 days), and Lactated Ringer’s solution (5 mL/100 g; daily for 5 days). Bladders were expressed twice daily until function recovered.
Immunohistochemistry and quantitative analysis of nerve regeneration, myelination, and NPC differentiation
Spinal cords were extracted 8 weeks after SCI and flash frozen in isopentane. For immunofluorescence, spinal cord segments were embedded in Tissue Tek O.C.T. Compound (Sakura Finetek, Torrance, CA, USA) with 30% sucrose. Cords were cryo-sectioned transversely in 18-μm-thick sections. Antibodies against the following antigens were used for immunofluorescence: neurofilament 200 (NF-200, Sigma Aldrich), myelin basic protein (MBP, Santa Cruz Biotech, Dallas, TX, USA), Protein-zero myelin protein (P0, Aves Labs, Tigard, OR, USA), Sox2 (Abcam), Olig2 (Millipore), NG2 (Millipore), and O4 (Millipore). Tissues were imaged on an Axio Observer Z1 (Zeiss, Oberkochen, Germany) using a 10×/0.45 M27 apochromatic objective and an ORCA-Flash 4.0 V2 Digital CMOS camera (C11440–22CU, Hamamatsu Photonics, Hamamatsu City, Shizuoka, Japan) or Nikon A1+ (Nikon Inc., Garden City, NY) using a 60×/1.4 apochromatic objective.
To assess the numbers of regenerated and myelinated axons within the PLG bridge area, NF-200 was used to identify axons, NF-200+/MBP+ to determine the number of myelinated axons, and NF-200+/MBP+/P0+ to determine the amount of myelin derived from infiltrating Schwann cells(A. M. Thomas et al., 2014). Twenty-one tissues distributed between conditions were counted by 2 blinded counters to calibrate software for automated counting developed by McCreedy et al(D. A. Mccreedy et al., 2016). In short, images were imported into MATLAB and the area of the section corresponding to PLG bridge was outlined. A Hessian matrix was created by convolution filtering using second derivative of the Gaussian function in the x, y, and xy directions. Eigenvalues were determined by extracted from the Hessian matrix and used to reconstruct the original image. Following filtering, positive NF-200 events were identified by intensity threshold, single pixel events were removed, and the number of connected objects were identified. This ensures highly branching axons are counted as one object and long axons are also counted as one object. For calibration, the software will output a matrix of potential axon counts based on this method. These values are directly compared to manual counts for the initial 22 tissues used for calibrating the software. The appropriate filter size and threshold sensitivity are selected based on the correlation between the manual and automated counts to properly calibrate the software for use in quantifying the remaining tissues that had not been manually counted. To obtain axon densities, total NF-200 counts were divided by the area of the PLG bridge. MBP and P0 events were identified similarly. NF-200 objects containing pixel locations overlapping with positive MBP or P0 staining were counted and compared to total NF-200 counts. To ensure proper calibration, Pearson’s coefficients were compared between counters and between the software and the counter averages.
Nine tissues were selected randomly from the rostral, middle, and caudal section of the bridge implant from each animal (n=6) of each condition to be counted for progenitors and differentiated cells. Immunopositive cells within the PLG bridges were counted manually by four researchers independently. Due to PLG material generally exhibiting high background, cells were counted as Olig2 or Sox2 positive only when appearance of these markers spatially overlapped Hoechst 33342 counterstaining. Co-staining for multiple markers was always assessed by evaluating overlap of pixels above a set threshold in images acquired over identical sample areas. Co-localization of Sox2 and Olig2, was evaluated to determine the numbers of NPCs and OPCs. To quantify densities, total immunopositive cells were divided by the area of PLG outlined.
Behavioral analysis
The Basso mouse scale (BMS) open-field locomotor test was used to evaluate functional recovery over period of 8 weeks after SCI as previously described(D. M. Basso et al., 2006) for FLuc (n=12) and noggin + PDGF (n=12) conditions. A baseline was determined prior to SCI, and the mice were tested at 1, 2, 4, 6, and 8 weeks. Observations and BMS scoring were performed by two blinded observers for 4 minutes per animal.
Statistical analysis
For multiple comparisons, statistical significance between groups was determined by one-way or two-way ANOVA with Bonferroni’s post-hoc. For single comparisons, the statistical significance between pairs was determined by unpaired t-test. All statistics test significance using a α value of 0.05. Error bars represent standard error in all figures. Prism 7 (GraphPad Software, La Jolla, CA, USA) software was used for all data analysis.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Lentiviral construct validation
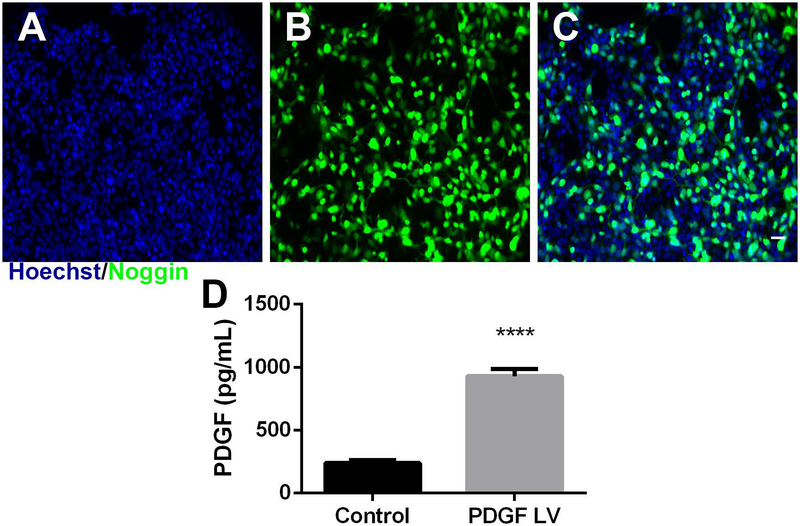
Initial studies validated the lentiviral constructs encoding noggin and PDGF. HEK-293FT cells were transduced with a multiplicity of infection (MOI) of 10 viral particles per cell with noggin-encoding lentivirus. After 3 days, expression of noggin was assessed with anti-noggin antibody with Hoechst 33342 counterstaining (Fig. 1A–C). There was substantial staining with anti-noggin antibodies throughout cells transduced with lentivirus. There is also substantial staining of noggin in the cytosol. PDGF overexpression was validated by transducing HEK-293FT cells with MOI 10 of PDGF lentivirus and quantifying protein level with an ELISA kit. PDGF protein expression increased 4-fold over cell transduced with FLuc lentivirus.
Figure 1.
Noggin and PDGF expression. (A) Hoechst (blue), (B) Noggin (green), and (C) overlaid images of lentiviral expression of noggin. (D) Quantification of PDGF secretion protein from cells transfected with no virus (Control) or PDGF lentivirus using ELISA. Data presented as mean +/− SEM. Scale: 50 μm **** denotes p<0.0001 v. control.
Increased axon numbers and myelination post injury
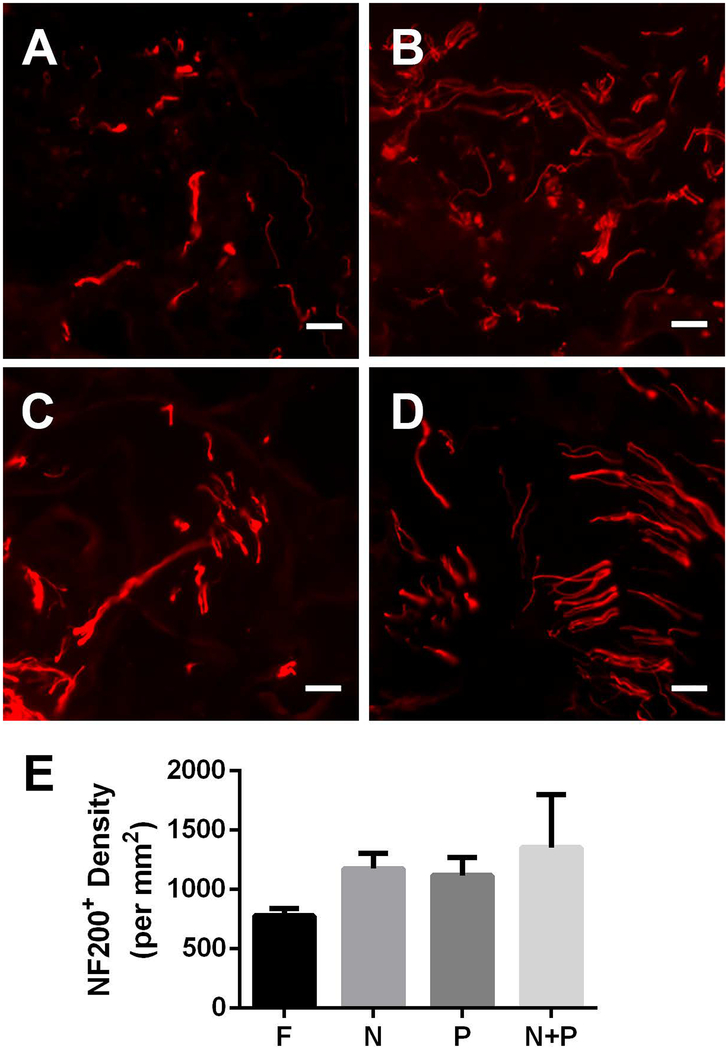
We quantified axonal density as a function of FLuc, noggin, PDGF, or co-delivery of both factors from the bridge. Axons (NF-200+) were present throughout the bridges (Fig. 2A–D) 8 weeks after SCI in all experimental conditions. NF-200+ axons were typically observed in small groups or bundles as previously reported for multi-channel PLG bridges(D. A. Mccreedy et al., 2016; H. M. Tuinstra et al., 2012). FLuc bridges had a mean of approximately 800 axons/mm2, single lentiviral conditions had approximately 1100 axons/mm2, and noggin + PDGF had approximately 1200 axons/mm2 (Fig. 2E). However, these differences were not statistically significant.
Figure 2.
Axonal Growth at 8 weeks post injury. NF-200+ (red) immunofluorescence from bridge implants delivering (A) FLuc, (B) Noggin, (C) PDGF, or (D) Noggin + PDGF. Brightness and contrast were adjusted for clarity. (E) Quantification of axon density in FLuc, Noggin, PDGF, and Noggin + PDGF conditions. Data presented as mean +/− SEM. Scale: 20 μm. N = 6 per condition.
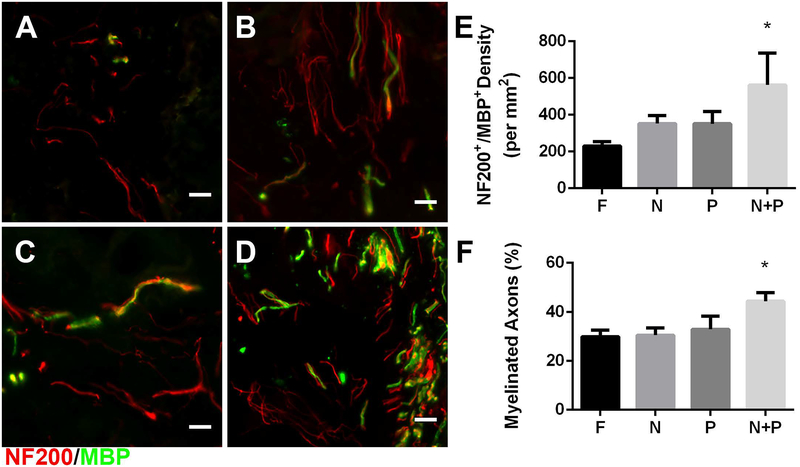
Myelinated axons (NF-200+/MBP+; Fig. 3A–D) were present throughout bridge implants and typically appeared in bundles of multiple axons as previously reported. Myelinated axon density in the bridge implants was significantly enhanced, approximately 3-fold by co-delivery of noggin + PDGF relative to FLuc controls (Fig 3E). While single lentiviral vector delivery increased the density of myelinated axons compared to the FLuc control, it was not significant. The percentage of axons myelinated, which was determined from the ratio of NF-200+/MBP+ axons divided by total number of NF-200+ axons, was approximately 30% with no differences between groups for the control and individual factor expression (Fig. 3F); however, combined noggin + PDGF delivery resulted in a significantly higher percentage of myelinated axons at 44%.
Figure 3.
Myelinated axons 8 weeks post injury. NF-200+ (red) /MBP+ (green) immunofluorescence from bridge implants delivering (A) FLuc, (B) Noggin, (C) PDGF, or (D) Noggin + PDGF. Brightness and contrast were adjusted for clarity. Quantification of (E) myelinated axon density and (F) percentage of myelinated axons in FLuc, Noggin, PDGF, and Noggin + PDGF conditions. Data presented as mean +/− SEM. Scale: 20 μm. * denotes p<0.05 v. FLuc. N = 6 per condition.
Enhanced motor function recovery with combinatorial delivery
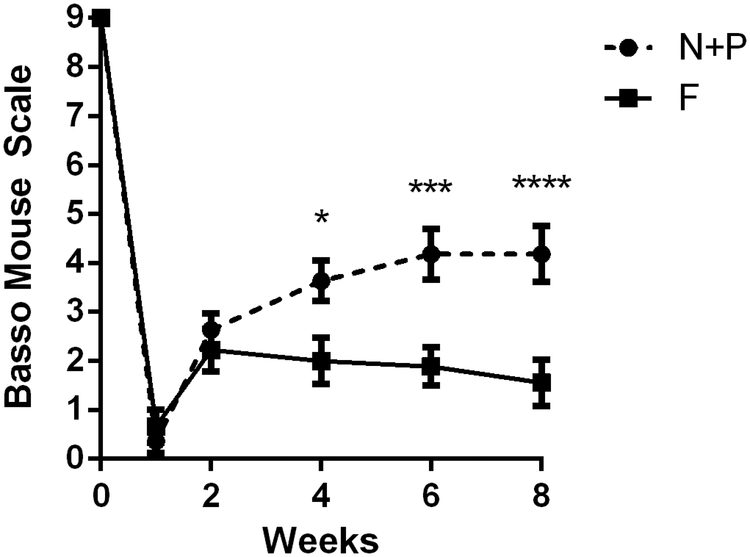
We subsequently investigated the capacity for the combination of PDGF + noggin, which enhanced myelinated, to improve functional recovery. Bridges loaded with noggin + PDGF were implanted into the lateral hemisection, with a control cohort receiving FLuc, and motor function was evaluated for 8 weeks post-SCI using the BMS (Fig. 4). Prior to surgery, all mice were fully functional with perfect scores (BMS = 9). At 1-week post-SCI, all mice had no movement in the ipsilateral hindlimb. From week 4 onward, mice receiving noggin + PDGF co-delivery had significantly improved function in comparison to mice that received bridges with FLuc lentivirus. Mice receiving noggin + PDGF lentivirus earned an average BMS score of approximately 4.2, with a score of 4 indicating occasional stepping. By comparison, mice from the control condition mice scored at an average of approximately 1.5, which indicates ankle movement, yet an inability to achieve paw placement or perform stepping.
Figure 4.
Functional recovery induced by Noggin + PDGF co-delivery. The Basso Mouse Scale was used to determine differences in motor recovery in the ipsilateral hindlimb. Data presented as mean +/− SEM. ** denotes p<0.05 v. FLuc, *** denotes p<0.001 v. FLuc, **** denotes p<0.0001 v. FLuc. N = 12 per condition.
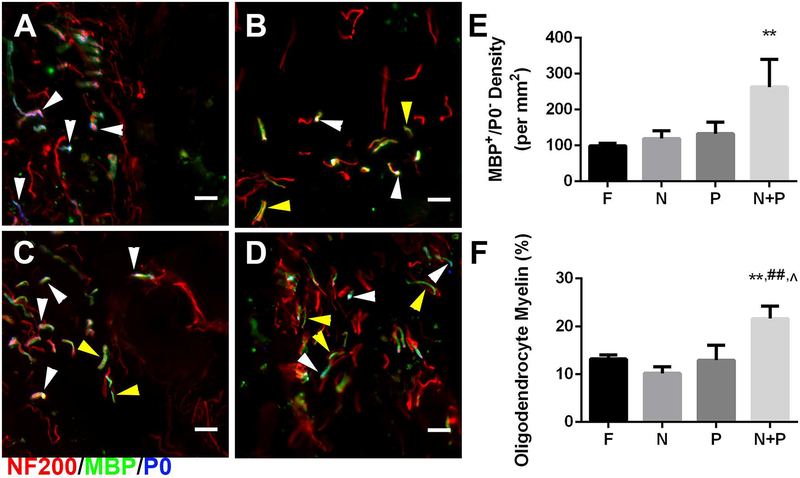
Source of myelination post injury
The source of myelination in the bridge was subsequently characterized to further investigate the correlation between increased myelination and motor function recovery. Histological sections were immunostained to identify Schwann cell (NF-200+/MBP+/P0+) or oligodendrocyte-derived myelin (NF-200+/MBP+/P0−) (Fig. 5A–D). No significant difference in density of Schwann cell myelinated axons between conditions was observed. In control, noggin, and PDGF conditions, the density of oligodendrocyte-derived myelin was approximately 100 neurites/mm2. Overexpression of noggin + PDGF resulted in approximately 250 neurites/mm2, a significant 2.5-fold increase (Fig. 5E). The percentage of oligodendrocyte-derived myelin was determined as the ratio of number of NF-200+/MBP+/P0− axons divided by the total number of NF-200+ axons. Overexpression of noggin or PDGF alone resulted in similar percentages of oligodendrocyte-derived myelin as control animals at 11–13%. Combined overexpression of noggin + PDGF significantly increased the level of total axons myelinated by oligodendrocytes to 22% relative to all experimental conditions (Fig. 5F).
Figure 5.
Source of myelination 8 weeks post injury. Immunofluorescence from bridges of Schwann cell (NF-200+/MBP+/P0+: red/green/blue, respectively) and oligodendrocyte (NF-200+/MBP+/P0−) derived myelin fibers from bridge implants delivering lentivirus encoding (A) FLuc, (B) Noggin, (C) PDGF, or (D) Noggin + PDGF. White arrows show fibers wrapped by Schwann cell myelin. Yellow arrows show fibers wrapped by Oligodendrocyte myelin. Brightness and contrast were adjusted for clarity. Quantification of (E) oligodendrocyte myelin density and (F) percentage of oligodendrocyte-derived myelinated axons in FLuc, Noggin, PDGF, and Noggin + PDGF conditions. Data presented as mean +/− SEM. Scale: 20 μm. ** denotes p<0.01 v. FLuc, ## denotes p<0.01 v. Noggin, ^ denotes p<0.05 v. PDGF. N = 6 per condition.
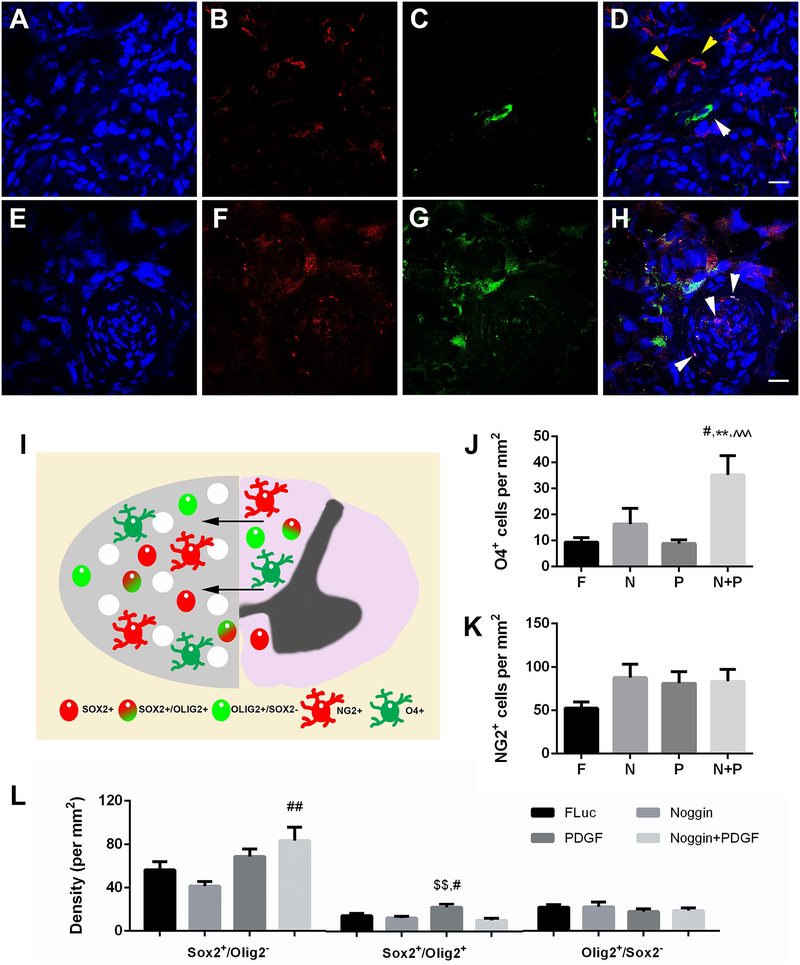
Recruitment and differentiation of endogenous progenitors
The increase in CNS-derived oligodendrocyte myelination of regenerating axons at 8 weeks post-injury within the bridge was subsequently interrogated by quantifying the density of cells within the oligodendroglial lineage. The presence of oligodendrocytes was evaluated by staining for O4 (Fig. 6A–D). Few O4+ pre-oligodendrocytes were observed in controls (Fig. 6J). A significant increase in O4+ cells for noggin + PDGF overexpression was observed compared to all other experimental conditions (Fig. 6J).
Figure 6.
Oligodendrocyte-lineage cells in bridge implants at 8 weeks post injury. Images are representative of positive cell counts. (A-D) Oligodendrocyte-lineage cells at 8 weeks post injury. (A) Hoechst, (B) NG2+, and (C) O4+ expression in bridge implants. (D) Merged image. Yellow arrows denote NG2+ cells. White arrows denote O4+ cells. Scale: 20 μm. (E-H) Neural progenitor cells at 8 weeks post injury. (E) Hoechst, (F) Sox2+, and (G) Olig2+ expression in bridge implants. (H) Merged image shows single expression and co-expression of Sox2 and Olig2. White arrows indicate positive nuclei for co-expression. Brightness and contrast were adjusted for clarity. Scale: 20 μm. (I) Schematic of infiltration of cells into bridge implants from uninjured contralateral tissue following injury. Cells nearest the midline migrate into the bridge to support regenerating axons. (J) Quantification of O4+ cells. (K) Quantification of NG2+ cells. (F) Quantification of neural progenitor cell phenotype densities. Data presented as mean +/− SEM. ** denotes p<0.01 v. FLuc, # denotes p<0.05 v. Noggin, ## denotes p<0.01 v. Noggin, $ $ denotes p<0.01 v. Noggin + PDGF, ^^^ denotes p<0.001 v. PDGF. N = 6 per condition.
Cells in the oligodendrocyte lineage (O4+) can arise from either neural progenitor cells (NPCs) that are Sox2+, or from glial-restricted progenitors (Olig2+/Sox2− or NG2+), which were subsequently analyzed. This analysis was performed at the 8-week time point, which would provide enough time for the progenitors to develop along the multiple lineages. For glial-restricted OPCs (Fig 6B), no significant differences in the density of Olig2+/Sox2− cells or NG2+ cells across experimental conditions were observed (Fig. 6L). However, the density trended toward an increase in NG2+ stained cells for all conditions compared to control (Fig. 6K). Similar levels of NG2+ cells levels were present for noggin, PDGF, and combination of noggin + PDGF overexpression.
For NPCs (Sox2+), noggin over-expression resulted in no significant difference in number of Sox2+ cells relative to FLuc delivery (Fig. 6L). In contrast, PDGF over-expression trended towards elevated numbers of Sox2+ cells relative to noggin over-expression. Interestingly, the combination of noggin + PDGF had an additive effect, producing significantly more Sox2+ cells relative to noggin over-expression alone. We subsequently assessed the co-localization of Sox2 and Olig2 markers, which represents Sox2+ NPCs in the process of differentiating along oligodendrocyte lineages that leads to nuclear expression of Olig2(F. Barnabe-Heider et al., 2010; H. J. Lee, J. Wu, J. Chung, & J. R. Wrathall, 2013; H. Sabelstrom, M. Stenudd, & J. Frisen, 2014). A significant increase in density of Sox2+/Olig2+ cells was observed with PDGF overexpression compared to noggin and noggin + PDGF overexpression (Fig. 6L), indicating a greater number of NPCs differentiating into OPCs. Collectively, these studies suggest that the combination of PDGF + noggin enhances the recruitment of both glial-restricted progenitors and NPCs toward an oligodendrocyte lineage.
Discussion
We investigated remyelination of regenerating axons by endogenous cells responding to lentiviral induced trophic factor production by cells recruited into PLG multi-channel bridges implanted into a T9–10 mouse lateral hemisection SCI. We have previously reported axonal growth post-SCI through an aligned linear multichannel bridge which also provides a porous structure for cellular infiltration(H. M. Tuinstra et al., 2012; H. M. Tuinstra et al., 2014; Y. Yang et al., 2009). These bridges are a valuable tool to study the spinal cord microenvironment post-injury and investigate treatments in a controlled and defined manner. The bridges are acellular, indicating that any cells, extracellular matrix, or proteins present in the bridge at the time of extraction must have originated from the host tissue. Similarly, any axons entering the implant must be attributed to either regeneration of injured axons or sprouting of new axons from spared or contralateral tissue. This bridge provides a defined space for histological analysis, analysis of cell populations at and near the lesion site, and treatment outcomes. The bridge alone has supported robust axon ingrowth, myelination, and recovery of some motor function(K. Pawar et al., 2015). These bridges also provide a vehicle for lentiviral delivery resulting in long-term, localized transgene expression with delivery of multiple factors which is difficult to achieve and generally requires the use of osmotic pumps(A. M. Thomas et al., 2014; H. M. Tuinstra et al., 2012). Osmotic pumps can clog, require surgery for removal, and can cause further tissue damage. Other reports have used direct injection of vectors, which may not localize delivery to the injury. Unlike other viral vectors, lentivirus does not influence the phenotype of progenitors(S. M. Hughes, F. Moussavi-Harami, S. L. Sauter, & B. L. Davidson, 2002) or cause significant inflammation(A. A. Abdellatif et al., 2006). The physical properties of lentiviral vectors are independent of the encoding gene, which allows for the exchange of vectors or the potential to deliver multiple vectors encoding various inductive factors without modification to the base biomaterial creating a high-throughput system. In these studies, we delivered two distinct transgenes from PLG bridges alone and in combination. We have previously demonstrated sustained expression for a minimum of 12 weeks, with peak expression localized within the bridge and decreased expression both rostral and caudal to the bridge(A. M. Thomas & L. D. Shea, 2013). This expression pattern ensures the delivered factors can have prolonged, targeted effects on cells within the intact tissue and infiltrating cells within the bridge.
Co-delivery of noggin + PDGF significantly increased the density of myelinated axons and achieved the largest percentage of myelinated axons (44%) and oligodendrocyte-derived myelin (22%) that we have observed. While, co-delivery of noggin + PDGF did not significantly increase axon density versus control, the combination did enhance myelination relative to individual factors or control. For comparison to the extent of myelination, delivery of SHH and NT3 in our previous studies only resulted in ~30% of myelinated axons and ~13% of oligodendrocyte-derived myelin when compared to total axon counts(A. M. Thomas et al., 2014). Current estimates of myelinated axons in healthy spinal cord of rodent models range from 40 – 60%(K. Chung & R. E. Coggeshall, 1983a, 1983b; J.-Y. C. Hsu, S. A. Stein, & X.-M. Xu, 2006). Therefore, this represents a significant result in the enhancement of axon myelination. Reports from other systems have demonstrated varying degrees of remyelination via delivery of single factors(A. Alizadeh et al., 2017; B. T. Tan et al., 2017) or cell transplantation(Q. Cao et al., 2010; B. K. Chen et al., 2017; L. X. Deng et al., 2013; S. Karimi-Abdolrezaee, E. Eftekharpour, J. Wang, C. M. Morshead, & M. G. Fehlings, 2006), with these reports not achieving the myelination levels reported herein. Lack of myelination has been demonstrated to be a significant hindrance to recovery of function to regenerating axons(I. D. Duncan, A. Brower, Y. Kondo, J. F. Curlee, & R. D. Schultz, 2009; I. D. Duncan, R. L. Marik, A. T. Broman, & M. Heidari, 2017), and we employed the BMS and identified a significant increase in motor function improvement after SCI compared to control. This result suggests the difference in myelination density and percentage may contribute in part to the improved functional outcomes. This result is consistent with other research suggesting remyelination contributes to normal function recovery(I. D. Duncan et al., 2009). Another interesting outcome is that no difference in Schwann cell myelin between groups was observed, yet only the noggin/PDGF combination resulted in improved functional outcome, potentially due to the differences in oligodendrocyte-derived myelination. Oligodendrocyte-derived myelin has been reported to be thicker and more supportive of axonal growth. This suggests that oligodendrocyte-derived myelin is necessary for return of function and Schwann cell myelin is inefficient.
The increased myelination and functional recovery were associated with a greater recruitment of progenitor cells into the oligodendrocyte lineage. Post injury, oligodendrocyte-lineage cells have been reported to migrate from the spared tissue to repopulate lost cell populations(G. W. J. Hawryluk & M. G. Fehlings, 2008). These progenitor cells proliferate extensively between 24 hours to 2 weeks post injury but these populations are reduced at later timepoints (L. L. Horky, F. Galimi, F. H. Gage, & P. J. Horner, 2006; D. L. Sellers et al., 2009). Therefore, many progenitor cell populations would not be expected to be present in the bridges at 8 weeks post-injury without trophic factor expression. Our results with the control bridge (i.e., FLuc expression) are consistent with the relatively low density of progenitor cells populations. However, the delivery of lentivirus encoding for noggin, PDGF, or noggin/PDGF combination for sustained transgene expression altered the recruitment and differentiation of progenitor cells. At 8 weeks post-injury, endogenous progenitor pools were present within the bridge with trophic factor delivery. NPCs (Sox2+) can differentiate into OPCs (Olig2+, NG2+) by exposure to various factors, and OPCs can differentiate into multiple other cell types including oligodendrocytes, astrocytes, Schwann cells, and neurons(V. E. Miron, T. Kuhlmann, & J. P. Antel, 2011). At the O4+ stage, the cells are lineage locked into becoming mature, myelinating oligodendrocytes. Noggin expression at the bridge would be expected to block the receptors for BMP 2/4/7, which would normally act to inhibit NPC differentiation and migration (A. Liu & L. A. Niswander, 2005; J. K. Sabo, T. D. Aumann, D. Merlo, T. J. Kilpatrick, & H. S. Cate, 2011; Q. Xiao, Y. Du, W. Wu, & H. K. Yip, 2010). The decreased presence of NPCs relative to control with noggin expression is consistent with inhibiting the action of BMPs, which would allow differentiation of NPCs toward an oligodendrocyte lineage. Although noggin alone increased the presence of O4+ oligodendrocytes, noggin alone was insufficient to induce myelination of large numbers of regenerating axons.
Delivery of PDGF-encoding lentiviral vectors from the bridge significantly increased the presence of OPCs (Sox2+/Olig2+). PDGF in the spinal cord elicits multiple effects on OPCs, such as increasing the proliferation(Ying Chen et al., 2007; R. A. Hill et al., 2013; R. H. Woodruff et al., 2004) and differentiation(M. Bradl & H. Lassmann, 2010; J. G. Hu et al., 2008; C. Lutton et al., 2012) of OPCs at lesions. In contrast, in vitro culture studies with oligodendrocytes indicated an inhibition of myelinating properties(Z. Wang, H. Colognato, & C. Ffrench-Constant, 2007), and has been reported to delay oligodendrocyte differentiation and axonal myelination in vivo during development(A. M. Butt, M. F. Hornby, S. Kirvell, & M. Berry, 1997). However, the distinct effects of PDGF may depend on its temporal availability during proliferation, differentiation, and myelination(A. Barateiro & A. Fernandes, 2014), as withdrawal of this growth factor triggers cell-cycle exit and differentiation(J. J. Boulanger & C. Messier, 2014). Herein, lentivirus was used for the sustained expression of PDGF for the 8-week study resulting in increased OPC density. However, these increases in OPC density did not contribute to increased density of O4+ pre-oligodendrocytes, which is consistent with the lack of increased myelination and oligodendrocyte-derived myelin relative to control. Conditional expression systems such as the tetracycline system have been used for temporal control of lentiviral expression (X. Zhou, M. Vink, B. Klaver, B. Berkhout, & A. T. Das, 2006). This type of viral delivery system could allow for PDGF to be expressed transiently to encourage further maturation of OPCs.
Interestingly, combined delivery of noggin + PDGF encoding lentivirus significantly increased the presence of O4+ pre-oligodendrocytes. The noggin + PDGF overexpression significantly increased Sox2+/Olig2− cell density compared to noggin alone and had similar density compared to PDGF. This result suggests the decrease in Sox2+/Olig2− caused by noggin delivery may have been offset by PDGF co-delivery. Co-delivery also resulted in significantly lower densities of Olig2+ cells compared to other conditions. However, the density of O4+ pre-oligodendrocytes was increased 4-fold relative to control and PDGF conditions and 2-fold relative to noggin alone. Noggin alone increased the density of immature oligodendrocytes, yet when paired with PDGF, the increase was further enhanced. Although these cells were O4+, many cells did not exhibit a typical oligodendrocyte morphology. The O4 marker for differentiation is expressed at many stages of oligodendrocyte lineage so positive cells may not resemble the classical mature oligodendrocyte morphology. Furthermore, biomaterials and SCI have varying effects on the morphology of cells dependent on stiffness, modulus, and severity of injury (Y. Aizawa, N. Leipzig, T. Zahir, & M. Shoichet, 2008; T. Lourenço & M. Grãos, 2016; S. R. Mciver et al., 2010; L. N. Russell & K. J. Lampe, 2017), thus cells may not exhibit classical morphology due to biomaterial interactions and injury. However, we note that O4+ cells are lineage locked to becoming myelinating oligodendrocytes (A. Nishiyama, M. Komitova, R. Suzuki, & X. Zhu, 2009). These findings suggest that combinatorial delivery of inductive factors can considerably enhance the recruitment and differentiation of endogenous OPCs that persist at long time points.
Collectively, we report the ability of noggin + PDGF to promote remyelination by endogenous progenitor cells post-SCI. Co-delivery of noggin + PDGF encoding lentivirus significantly increased total myelinated axon density and percentage. Co-delivery also promoted greater myelination by oligodendrocytes compared to all other conditions (22% vs 11%). This result was consistent with the increased density of O4+ pre-oligodendrocytes via co-delivery. Overall, we have demonstrated that lentivirus-based expression of multiple factors, such as noggin and PDGF, from multichannel PLG bridges provides a strategy for identifying synergistic actions with the potential to target multiple barriers to regeneration.
Bridges are increasingly being considered for both penetrating wounds as well as for chronic injuries in which the scar is surgically resected that creates a defect (Z. Xiao et al., 2016). While the bridge provides a path and support for axon regeneration, it is insufficient alone to promote regeneration. As we have shown, PDGF and noggin may be used to recruit and differentiate endogenous progenitors after spinal cord injury to encourage remyelination. Lentivirus represents an effective strategy to increase and sustain levels of these target proteins at the injury. Lentiviral vectors are currently in clinical trials (M. C. Milone & U. O’doherty, 2018) and, at a minimum, represent a tool to identify factors or combinations of factors that enhance myelination. An alternative to lentivirus delivery would be the direct delivery of these proteins, which is being attempted by various delivery strategies (C. M. Walthers & S. K. Seidlits, 2015). This combination of a bridge to support and direct axon growth with a strategy to enhance their myelination represents a potential clinically translatable treatment for SCI.
Acknowledgments
This work was supported by the NIH (R01EB005678).
References
- Abdellatif AA, Pelt JL, Benton RL, Howard RM, Tsoulfas P, Ping P, … Whittemore SR (2006). Gene delivery to the spinal cord: comparison between lentiviral, adenoviral, and retroviral vector delivery systems. J Neurosci Res, 84(3), 553–567. doi: 10.1002/jnr.20968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa Y, Leipzig N, Zahir T, & Shoichet M (2008). The effect of immobilized platelet derived growth factor AA on neural stem/progenitor cell differentiation on cell-adhesive hydrogels. Biomaterials, 29(35), 4676–4683. doi: https://doi.org/10.1016/j.biomaterials.2008.08.018 [DOI] [PubMed] [Google Scholar]
- Alizadeh A, Dyck SM, Kataria H, Shahriary GM, Nguyen DH, Santhosh KT, & Karimi-Abdolrezaee S (2017). Neuregulin-1 positively modulates glial response and improves neurological recovery following traumatic spinal cord injury. Glia, 65(7), 1152–1175. doi: 10.1002/glia.23150 [DOI] [PubMed] [Google Scholar]
- Almad A, Sahinkaya FR, & McTigue DM (2011). Oligodendrocyte fate after spinal cord injury. Neurotherapeutics, 8(2), 262–273. doi: 10.1007/s13311-011-0033-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura K, Hunter SF, & Rodriguez M (1997). Effects of transforming growth factor-beta and platelet-derived growth factor on oligodendrocyte precursors: insights gained from a neuronal cell line. J Neurochem, 68(6), 2281–2290. [DOI] [PubMed] [Google Scholar]
- Barateiro A, & Fernandes A (2014). Temporal oligodendrocyte lineage progression: In vitro models of proliferation, differentiation and myelination. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1843(9), 1917–1929. doi: http://doi.org/10.1016/j.bbamcr.2014.04.018 [DOI] [PubMed] [Google Scholar]
- Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, & Frisen J (2010). Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell, 7(4), 470–482. doi: 10.1016/j.stem.2010.07.014 [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, & Popovich PG (2006). Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma, 23(5), 635–659. doi: 10.1089/neu.2006.23.635 [DOI] [PubMed] [Google Scholar]
- Beattie MS, Hermann GE, Rogers RC, & Bresnahan JC (2002). Cell death in models of spinal cord injury. Prog Brain Res, 137, 37–47. [DOI] [PubMed] [Google Scholar]
- Boulanger JJ, & Messier C (2014). From precursors to myelinating oligodendrocytes: Contribution of intrinsic and extrinsic factors to white matter plasticity in the adult brain. Neuroscience, 269, 343–366. doi: http://doi.org/10.1016/j.neuroscience.2014.03.063 [DOI] [PubMed] [Google Scholar]
- Bradl M, & Lassmann H (2010). Oligodendrocytes: biology and pathology. Acta Neuropathol, 119(1), 37–53. doi: 10.1007/s00401-009-0601-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge MB (2001). Bridging areas of injury in the spinal cord. Neuroscientist, 7(4), 325–339. doi: 10.1177/107385840100700409 [DOI] [PubMed] [Google Scholar]
- Butt AM, Hornby MF, Kirvell S, & Berry M (1997). Platelet-derived growth factor delays oligodendrocyte differentiation and axonal myelination in vivo in the anterior medullary velum of the developing rat. J Neurosci Res, 48(6), 588–596. [PubMed] [Google Scholar]
- Cao Q, He Q, Wang Y, Cheng X, Howard RM, Zhang Y, … Whittemore SR (2010). Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci, 30(8), 2989–3001. doi: 10.1523/jneurosci.3174-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casha S, Yu WR, & Fehlings MG (2001). Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience, 103(1), 203–218. [DOI] [PubMed] [Google Scholar]
- Chen BK, Madigan NN, Hakim JS, Dadsetan M, McMahon SS, Yaszemski MJ, & Windebank AJ (2017). GDNF Schwann cells in hydrogel scaffolds promote regional axon regeneration, remyelination and functional improvement after spinal cord transection in rats. doi: 10.1002/term.2431 [DOI] [PubMed] [Google Scholar]
- Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, & Lu QR (2007). Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat. Protocols, 2(5), 1044–1051. [DOI] [PubMed] [Google Scholar]
- Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, & Lu QR (2007). Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc, 2(5), 1044–1051. doi: 10.1038/nprot.2007.149 [DOI] [PubMed] [Google Scholar]
- Chung K, & Coggeshall RE (1983a). Numbers of axons in lateral and ventral funiculi of rat sacral spinal cord. The Journal of Comparative Neurology, 214(1), 72–78. doi: 10.1002/cne.902140107 [DOI] [PubMed] [Google Scholar]
- Chung K, & Coggeshall RE (1983b). Propriospinal fibers in the rat. The Journal of Comparative Neurology, 217(1), 47–53. doi: 10.1002/cne.902170105 [DOI] [PubMed] [Google Scholar]
- De Laporte L, Yang Y, Zelivyanskaya ML, Cummings BJ, Anderson AJ, & Shea LD (2009). Plasmid releasing multiple channel bridges for transgene expression after spinal cord injury. Mol Ther, 17(2), 318–326. doi: 10.1038/mt.2008.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado AC, Ferron SR, Vicente D, Porlan E, Perez-Villalba A, Trujillo CM, … Farinas I (2014). Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron, 83(3), 572–585. doi: 10.1016/j.neuron.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Deng LX, Deng P, Ruan Y, Xu ZC, Liu NK, Wen X, … Xu XM (2013). A novel growth-promoting pathway formed by GDNF-overexpressing Schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury. J Neurosci, 33(13), 5655–5667. doi: 10.1523/jneurosci.2973-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, & Popovich PG (2008). Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol, 209(2), 378–388. doi: 10.1016/j.expneurol.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan ID, Brower A, Kondo Y, Curlee JF, & Schultz RD (2009). Extensive remyelination of the CNS leads to functional recovery. Proceedings of the National Academy of Sciences of the United States of America, 106(16), 6832–6836. doi: 10.1073/pnas.0812500106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan ID, Marik RL, Broman AT, & Heidari M (2017). Thin myelin sheaths as the hallmark of remyelination persist over time and preserve axon function. Proc Natl Acad Sci U S A, 114(45), E9685–e9691. doi: 10.1073/pnas.1714183114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzmann GU, Benton RL, Woock JP, Howard RM, Tsoulfas P, & Whittemore SR (2005). Consequences of noggin expression by neural stem, glial, and neuronal precursor cells engrafted into the injured spinal cord. Exp Neurol, 195(2), 293–304. doi: 10.1016/j.expneurol.2005.04.021 [DOI] [PubMed] [Google Scholar]
- Fitch MT, & Silver J (2008). CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol, 209(2), 294–301. doi: S0014–4886(07)00213–0 [pii] 10.1016/j.expneurol.2007.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortun J, Hill CE, & Bunge MB (2009). Combinatorial strategies with Schwann cell transplantation to improve repair of the injured spinal cord. Neurosci Lett, 456(3), 124–132. doi: 10.1016/j.neulet.2008.08.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A, Lewis P, McMahon AP, & Linde A (2001). The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev Biol, 236(2), 364–386. doi: 10.1006/dbio.2001.0336 [DOI] [PubMed] [Google Scholar]
- Hawryluk GWJ, & Fehlings MG (2008). The Center of the Spinal Cord May Be Central to Its Repair. Cell Stem Cell, 3(3), 230–232. doi: http://dx.doi.org/10.1016/j.stem.2008.08.009 [DOI] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Medved J, Reiss AM, & Nishiyama A (2013). NG2 cells in white matter but not gray matter proliferate in response to PDGF. J Neurosci, 33(36), 14558–14566. doi: 10.1523/JNEUROSCI.2001-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks GL, & Franklin RJ (1999). Distinctive patterns of PDGF-A, FGF-2, IGF-I, and TGF-beta1 gene expression during remyelination of experimentally-induced spinal cord demyelination. Mol Cell Neurosci, 14(2), 153–168. doi: 10.1006/mcne.1999.0771 [DOI] [PubMed] [Google Scholar]
- Horky LL, Galimi F, Gage FH, & Horner PJ (2006). Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol, 498(4), 525–538. doi: 10.1002/cne.21065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J-YC, Stein SA, & Xu X-M (2006). Development of the corticospinal tract in the mouse spinal cord: A quantitative ultrastructural analysis. Brain Research, 1084(1), 16–27. doi: http://dx.doi.org/10.1016/j.brainres.2006.02.036 [DOI] [PubMed] [Google Scholar]
- Hu JG, Fu SL, Wang YX, Li Y, Jiang XY, Wang XF, … Xu XM (2008). Platelet-derived growth factor-AA mediates oligodendrocyte lineage differentiation through activation of extracellular signal-regulated kinase signaling pathway. Neuroscience, 151(1), 138–147. doi: 10.1016/j.neuroscience.2007.10.050 [DOI] [PubMed] [Google Scholar]
- Hughes SM, Moussavi-Harami F, Sauter SL, & Davidson BL (2002). Viral-mediated gene transfer to mouse primary neural progenitor cells. Mol Ther, 5(1), 16–24. doi: 10.1006/mthe.2001.0512 [DOI] [PubMed] [Google Scholar]
- Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT, … Tuszynski MH (2009). Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron, 64(2), 165–172. doi: 10.1016/j.neuron.2009.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, & Fehlings MG (2006). Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci, 26(13), 3377–3389. doi: 10.1523/jneurosci.4184-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiene J, Shupe L, Perlmutter S, & Horner P (2008). No evidence for chronic demyelination in spared axons after spinal cord injury in a mouse. J Neurosci, 28(15), 3887–3896. doi: 10.1523/JNEUROSCI.4756-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Wu J, Chung J, & Wrathall JR (2013). SOX2 expression is upregulated in adult spinal cord after contusion injury in both oligodendrocyte lineage and ependymal cells. J Neurosci Res, 91(2), 196–210. doi: 10.1002/jnr.23151 [DOI] [PubMed] [Google Scholar]
- Li GL, Farooque M, Holtz A, & Olsson Y (1999). Apoptosis of oligodendrocytes occurs for long distances away from the primary injury after compression trauma to rat spinal cord. Acta Neuropathol, 98(5), 473–480. [DOI] [PubMed] [Google Scholar]
- Li J, & Lepski G (2013). Cell transplantation for spinal cord injury: a systematic review. Biomed Res Int, 2013, 786475. doi: 10.1155/2013/786475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Rickett TA, & Shi R (2009). Biomimetic nerve scaffolds with aligned intraluminal microchannels: a “sweet” approach to tissue engineering. Langmuir, 25(3), 1813–1817. doi: 10.1021/la803522f [DOI] [PubMed] [Google Scholar]
- Liu A, & Niswander LA (2005). Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci, 6(12), 945–954. doi: 10.1038/nrn1805 [DOI] [PubMed] [Google Scholar]
- Lourenço T, & Grãos M (2016). Modulation of Oligodendrocyte Differentiation by Mechanotransduction. Frontiers in Cellular Neuroscience, 10(277). doi: 10.3389/fncel.2016.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry N, Goderie SK, Lederman P, Charniga C, Gooch MR, Gracey KD, … Temple S (2012). The effect of long-term release of Shh from implanted biodegradable microspheres on recovery from spinal cord injury in mice. Biomaterials, 33(10), 2892–2901. doi: 10.1016/j.biomaterials.2011.12.048 [DOI] [PubMed] [Google Scholar]
- Lutton C, Young YW, Williams R, Meedeniya AC, Mackay-Sim A, & Goss B (2012). Combined VEGF and PDGF treatment reduces secondary degeneration after spinal cord injury. J Neurotrauma, 29(5), 957–970. doi: 10.1089/neu.2010.1423 [DOI] [PubMed] [Google Scholar]
- Lytle JM, & Wrathall JR (2007). Glial cell loss, proliferation and replacement in the contused murine spinal cord. Eur J Neurosci, 25(6), 1711–1724. doi: 10.1111/j.1460-9568.2007.05390.x [DOI] [PubMed] [Google Scholar]
- Margul DJ, Park J, Boehler RM, Smith DR, Johnson MA, McCreedy DA, … Seidlits SK (2016). Reducing neuroinflammation by delivery of IL-10 encoding lentivirus from multiple-channel bridges. Bioengineering & Translational Medicine, n/a–n/a. doi: 10.1002/btm2.10018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E, & Bovolenta P (2002). Sonic hedgehog in CNS development: one signal, multiple outputs. Trends Neurosci, 25(2), 89–96. [DOI] [PubMed] [Google Scholar]
- McCreedy DA, Margul DJ, Seidlits SK, Antane JT, Thomas RJ, Sissman GM, … Shea LD (2016). Semi-automated counting of axon regeneration in poly(lactide co-glycolide) spinal cord bridges. J Neurosci Methods, 263, 15–22. doi: 10.1016/j.jneumeth.2016.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver SR, Muccigrosso M, Gonzales ER, Lee J-M, Roberts MS, Sands MS, & Goldberg MP (2010). OLIGODENDROCYTE DEGENERATION AND RECOVERY AFTER FOCAL CEREBRAL ISCHEMIA. Neuroscience, 169(3), 1364–1375. doi: 10.1016/j.neuroscience.2010.04.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Miller RH, Tang W, Lee X, Hu B, Wu W, … Pepinsky B (2009). Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol, 65(3), 304–315. doi: 10.1002/ana.21581 [DOI] [PubMed] [Google Scholar]
- Milone MC, & O’Doherty U (2018). Clinical use of lentiviral vectors. Leukemia, 32(7), 1529–1541. doi: 10.1038/s41375-018-0106-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Kuhlmann T, & Antel JP (2011). Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim Biophys Acta, 1812(2), 184–193. doi: 10.1016/j.bbadis.2010.09.010 [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, & Zhu X (2009). Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci, 10(1), 9–22. doi: 10.1038/nrn2495 [DOI] [PubMed] [Google Scholar]
- Pawar K, Cummings BJ, Thomas A, Shea LD, Levine A, Pfaff S, & Anderson AJ (2015). Biomaterial bridges enable regeneration and re-entry of corticospinal tract axons into the caudal spinal cord after SCI: Association with recovery of forelimb function. Biomaterials, 65, 1–12. doi: http://dx.doi.org/10.1016/j.biomaterials.2015.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse DD, & Barakat DJ (2006). Cellular repair strategies for spinal cord injury. Expert Opin Biol Ther, 6(7), 639–652. doi: 10.1517/14712598.6.7.639 [DOI] [PubMed] [Google Scholar]
- Powers BE, Lasiene J, Plemel JR, Shupe L, Perlmutter SI, Tetzlaff W, & Horner PJ (2012). Axonal thinning and extensive remyelination without chronic demyelination in spinal injured rats. The Journal of Neuroscience, 32(15), 5120–5125. doi: 10.1523/JNEUROSCI.0002-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers BE, Sellers DL, Lovelett EA, Cheung W, Aalami SP, Zapertov N, … Horner PJ (2013). Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proc Natl Acad Sci U S A, 110(10), 4075–4080. doi: 10.1073/pnas.1210293110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes V, & Briscoe J (2009). Establishing and Interpreting Graded Sonic Hedgehog Signaling during Vertebrate Neural Tube Patterning: The Role of Negative Feedback. Cold Spring Harbor Perspectives in Biology, 1(2), a002014. doi: 10.1101/cshperspect.a002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PM, McGuinness UM, & Aguayo AJ (1980). Axons from CNS neurons regenerate into PNS grafts. Nature, 284(5753), 264–265. [DOI] [PubMed] [Google Scholar]
- Russell LN, & Lampe KJ (2017). Oligodendrocyte Precursor Cell Viability, Proliferation, and Morphology is Dependent on Mesh Size and Storage Modulus in 3D Poly(ethylene glycol)-Based Hydrogels. ACS Biomaterials Science & Engineering, 3(12), 3459–3468. doi: 10.1021/acsbiomaterials.7b00374 [DOI] [PubMed] [Google Scholar]
- Sabelstrom H, Stenudd M, & Frisen J (2014). Neural stem cells in the adult spinal cord. Exp Neurol, 260, 44–49. doi: 10.1016/j.expneurol.2013.01.026 [DOI] [PubMed] [Google Scholar]
- Sabo JK, Aumann TD, Merlo D, Kilpatrick TJ, & Cate HS (2011). Remyelination is altered by bone morphogenic protein signaling in demyelinated lesions. J Neurosci, 31(12), 4504–4510. doi: 10.1523/jneurosci.5859-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CE, & Leach JB (2003). Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng, 5, 293–347. doi: 10.1146/annurev.bioeng.5.011303.120731 [DOI] [PubMed] [Google Scholar]
- Schwab ME (2002). Repairing the injured spinal cord. Science, 295(5557), 1029–1031. doi: 10.1126/science.1067840 [DOI] [PubMed] [Google Scholar]
- Sellers DL, Maris DO, & Horner PJ (2009). Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. J Neurosci, 29(20), 6722–6733. doi: 10.1523/jneurosci.4538-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BT, Jiang L, Liu L, Yin Y, Luo ZR, Long ZY, … Yu LH (2017). Local injection of Lenti-Olig2 at lesion site promotes functional recovery of spinal cord injury in rats. 23(6), 475–487. doi: 10.1111/cns.12694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, … Kwon BK (2011). A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma, 28(8), 1611–1682. doi: 10.1089/neu.2009.1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Kubilius L, Holland S, Seidlits S, Boehler R, Anderson A, … Shea L (2013). Channel density and porosity of degradable bridging scaffolds on axon growth after spinal injury. Biomaterials, 34(9), 2213–2220. doi: 10.1016/j.biomaterials.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, Seidlits SK, Goodman AG, Kukushliev TV, Hassani DM, Cummings BJ, … Shea LD (2014). Sonic hedgehog and neurotrophin-3 increase oligodendrocyte numbers and myelination after spinal cord injury. Integr Biol (Camb), 6(7), 694–705. doi: 10.1039/c4ib00009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, & Shea LD (2013). Polysaccharide-modified scaffolds for controlled lentivirus delivery in vitro and after spinal cord injury. J Control Release, 170(3), 421–429. doi: 10.1016/j.jconrel.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinstra HM, Aviles MO, Shin S, Holland SJ, Zelivyanskaya ML, Fast AG, … Shea LD (2012). Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury. Biomaterials, 33(5), 1618–1626. doi: 10.1016/j.biomaterials.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinstra HM, Margul DJ, Goodman AG, Boehler RM, Holland SJ, Zelivyanskaya ML, … Shea LD (2014). Long-Term Characterization of Axon Regeneration and Matrix Changes Using Multiple Channel Bridges for Spinal Cord Regeneration. Tissue Engineering. Part A, 20(5–6), 1027–1037. doi: 10.1089/ten.tea.2013.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walthers CM, & Seidlits SK (2015). Gene delivery strategies to promote spinal cord repair. Biomark Insights, 10(Suppl 1), 11–29. doi: 10.4137/BMI.S20063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhai P, Chen X, Schreyer DJ, Sun X, & Cui F (2011). Bioengineered Scaffolds for Spinal Cord Repair. Tissue Engineering Part B: Reviews, 17(3), 177–194. doi: 10.1089/ten.teb.2010.0648 [DOI] [PubMed] [Google Scholar]
- Wang Z, Colognato H, & Ffrench-Constant C (2007). Contrasting effects of mitogenic growth factors on myelination in neuron-oligodendrocyte co-cultures. Glia, 55(5), 537–545. doi: 10.1002/glia.20480 [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Fruttiger M, Richardson WD, & Franklin RJ (2004). Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol Cell Neurosci, 25(2), 252–262. doi: 10.1016/j.mcn.2003.10.014 [DOI] [PubMed] [Google Scholar]
- Xiao Q, Du Y, Wu W, & Yip HK (2010). Bone morphogenetic proteins mediate cellular response and, together with Noggin, regulate astrocyte differentiation after spinal cord injury. Experimental Neurology, 221(2), 353–366. doi: http://dx.doi.org/10.1016/j.expneurol.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Xiao Z, Tang F, Tang J, Yang H, Zhao Y, Chen B, … Dai J (2016). One-year clinical study of NeuroRegen scaffold implantation following scar resection in complete chronic spinal cord injury patients. Science China Life Sciences, 59(7), 647–655. doi: 10.1007/s11427-016-5080-z [DOI] [PubMed] [Google Scholar]
- Yang Y, De Laporte L, Zelivyanskaya ML, Whittlesey KJ, Anderson AJ, Cummings BJ, & Shea LD (2009). Multiple channel bridges for spinal cord injury: cellular characterization of host response. Tissue Eng Part A, 15(11), 3283–3295. doi: 10.1089/ten.TEA.2009.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Vutskits L, Calaora V, Durbec P, & Kiss JZ (2004). A role for the polysialic acid-neural cell adhesion molecule in PDGF-induced chemotaxis of oligodendrocyte precursor cells. J Cell Sci, 117(Pt 1), 93–103. doi: 10.1242/jcs.00827 [DOI] [PubMed] [Google Scholar]
- Zhang S. x., Huang F, Gates M, & Holmberg EG (2013). Role of endogenous Schwann cells in tissue repair after spinal cord injury. Neural Regeneration Research, 8(2), 177–185. doi: 10.3969/j.issn.1673-5374.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Vink M, Klaver B, Berkhout B, & Das AT (2006). Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene Ther, 13(19), 1382–1390. doi: 10.1038/sj.gt.3302780 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.