Abstract
Interleukin 1 (IL1) is a cytokine that plays a role in inflammation and is a potential contributor to the inflammation present in tendinopathy. Its inhibition may be of use in the treatment of tendinopathy and has been a target for treatment. To evaluate how an IL1-receptor antagonist (IL1-RA) reverses pathologic changes associated with established patellar tendinopathy we randomized 48 Sprague-Dawley retired-breeder rats into three groups having weekly bilateral patellar tendon injections for six weeks. The control group received 0.1ml saline for six weeks. The intervention groups were treated with 0.1ml 2% carrageenan for four weeks. Beginning at week three, the IL1-RA group received 0.94mg of the IL1-RA (2.5mg/kg) added to the 0.1ml 2% carrageenan and 0.94mg of the IL1-RA alone for the final two weeks, while the CAR received 0.1ml saline for the final two weeks. Animals were euthanized six weeks after initial injection. The CAR group demonstrated significantly (P<0.05) shorter tendon lengths (7.81± 0.44mm) than the control (8.25 ± 0.58mm) and IL1-RA (8.34 ± 0.52mm) group (p<0.05). Macroscopically, plaque-like formations were reduced and margins of the tendon were more evident in the IL1-RA group compared to the CAR group. CAR group demonstrated significantly greater histopathologic changes (inflammatory cell density, disorganization of collagen, nuclear rounding, angiogenesis) than the control and IL1-RA group. No significant difference in mechanical properties of the tendon were noted. These findings demonstrate IL1-RA can reduce pathologic changes in the patellar tendon in an established tendonitis model although did not demonstrate a difference in mechanical properties.
Keywords: inflammatory cytokine, patellar tendon, IL1, carrageenan, tendon inflammation
Introduction
Cytokines and their contribution to inflammation is becoming an increasing target for therapy in orthopaedics. One cytokine that has been investigated as a potential target in the treatment of tendinopathy is IL1. Anakinra, an IL1 receptor antagonist (IL1-RA) is a medication created for use in inflammatory conditions such as rheumatoid arthritis. IL1-RA is a competitive inhibitor of IL1 that prevents the downstream effects of IL1 that lead to inflammation1. This inflammatory effect has been demonstrated in the setting of tendinopathy. When tendons are exposed to mechanical loads, IL1 production is up-regulated and can contribute to further inflammation2. Previous studies have shown cytokines such as TNF-α, MMP-13 and IL-β also demonstrate increased levels in tendinopathy3–7. This has also been demonstrated in patellar tendons with increased production of MMP-13 and IL1- β after fatigue loading of a rat patellar tendon8. Tsuzaki et al. demonstrated that this effect could be exogenously produced with IL1-β, inducing PGE2, MMP-1 and MMP-3 as well as IL1-β gene expression in cultured human tendon cells9. If inflammatory cytokines do play a role in tendinopathy, IL1-RA may play a potential role in treatment.
In order to evaluate the role of cytokines in tendon inflammation, several methods have been used to establish tendinopathy10,11. Carrageenan, which has been shown to increase inflammatory cytokines, is a reproducible means of developing inflammation and tendinopathy 5,12–16. A previous study performed by this research group used carrageenan to show that the use of an IL1-RA prevented some of the changes associated with tendinopathy16. However, in this prior study, carrageenan was administered at the same time of the IL1-RA without established tendinopathy. This limits the translation of these findings to common clinical scenarios as this model prevents rather than treats the tendonitis. Therefore, our goal was to develop a model that more closely replicates the more common scenario of established tendinopathy. Given previous success in creating tendinopathy with a carrageenan injection model we developed a similar model where tendinopathy would be established before administration of the IL1-RA.
The objective of this study was to determine if the use of an IL1-RA would reverse the pathologic changes that have been demonstrated in established tendinopathy. We hypothesized that the IL1-RA would decrease the histological and mechanical changes that were previously identified in established tendinopathy compared to a placebo treatment.
Methods and Materials
The local Institutional Animal Care and Use Committee approved this study. 48 female Sprague-Dawley retired-breeder rats were randomized into three groups and given weekly bilateral patella tendon injections for 6 weeks: a control (CON) group (n=16), a carrageenan (CAR) only injection group (n=16), and an intervention (IL1-RA) group (n=16). 0.1ml phosphate-buffered saline (PBS) was injected in the CON group for six weeks. The intervention groups were each administered 0.1 ml 2% carrageenan for four weeks. Beginning at week three, the IL1-RA group received 0.94mg of the IL1-RA (2.5mg/kg) added to the 0.1ml 2% carrageenan and 0.94mg of the IL1-RA alone for the final two weeks, while the CAR received 0.1ml PBS for the final two weeks (Table 1). Animals were randomly distributed to give an average weight within each group of 282g. The average age of the retired breeder rats provided by our supplier is 24 weeks.
Table 1.
Injection Schedule for the 3 groups
| Group | CON | CAR | IL-1RA |
|---|---|---|---|
| Week 1 | 0.1ml Saline | 0.1ml 2% Carrageenan |
0.1ml 2% Carrageenan |
| Week 2 | 0.1ml Saline | 0.1ml 2% Carrageenan |
0.1ml 2% Carrageenan |
| Week 3 | 0.1ml Saline | 0.1ml 2% Carrageenan |
0.1ml 2% Carrageenan |
| Week 4 | 0.1ml Saline | 0.1ml 2% Carrageenan |
0.1ml 2% Carrageenan w/ 0.94 mg Anakinra |
| Week 5 | 0.1ml Saline | 0.1ml Saline | 0.94mg Anakinra |
| Week 6 | 0.1ml Saline | 0.1ml Saline | 0.94mg Anakinra |
| Week 7 | Euthanized | Euthanized | Euthanized |
Prior to injection, the animals were anesthetized with isoflurane and their bilateral hind limbs were disinfected with alcohol and betadine. Each animal’s knee was flexed and the patellar tendon was injected using a 27-guage needle inserted halfway between the origin and insertion of the patellar tendon while aiming for the proximal aspect of the tendon. This method has previously been performed by this study group and practiced with dye on cadaver limbs to ensure correct placement17. The IL1-RA dosage used in this study corresponds with dosages previously administered locally in rat inflammation models as well as in the previous study by this group18. The carrageenan solution was prepared with 0.6g of -Carrageenan (22049, Sigma-Aldrich; St. Louis MO) in 30 ml of PBS with a measured pH of 7.3 1.5. The carrageenan solutions were made fresh weekly and were placed into separate injection vials that were autoclaved for sterilization.
The animals were given acetaminophen (150mg/kg) in their drinking water for analgesia beginning prior to initial injection and until termination. They were housed on a 12-hour dark-light cycle with free access to standard rat chow and water. The animals were weighed at pre-injection, at 2 weeks, 4 weeks and at euthanasia. Animal activity was monitored in 4 animals from each group with the use of a photoelectric sensor that tracked cage crossings.
At the completion of the study, the animals were euthanized by an overdose of carbon dioxide followed by thoracotomy. Lateral radiographs of the right knee were taken with a cabinet radiograph unit (Faxitron 8050, FEC, McMinnville, OR) and size 3 dental x-ray films for evaluation of patellar tendon length. Radiographs were deemed adequate when the posterior femoral condyles were superimposed and the anatomic axis of the femur and tibia was 90°. The films were digitally scanned and patellar tendon length was measured with image analysis software (Image J, NIH, Bethesda, MD) from the distal aspect of the inferior pole of the patella to the most proximal aspect of the tibial insertion of the tendon. The measurements were performed by two authors a total of three times each and averaged to produce a single value. Each of the 48 pairs of limbs were harvested with 18 (6 from each group) of the left limbs collected for histological analysis and the remaining 30 (10 from each group) snap-frozen in liquid nitrogen and stored at −80° C for subsequent evaluation of collagenase activity. The 48 right limbs (16 from each group) were used for evaluation of the patellar tendon mechanical properties followed by quantification of glycosaminoglycan (GAG) content.
Histology Evaluation
18 Patellar tendons (6 from each group) were cut sharply from their tibial insertion, while the insertion on the patella was left intact. Histology specimens were fixed in 10% neutral buffered formalin for 48 hours under tension and then decalcified with Immunocal (Decal Chemical Corp., Tallman, NY) over two weeks. Specimens were dehydrated and cleared using methods customized to produce high fidelity tendon histology and then embedded in paraffin19. Two mid-sagittal, 5µm sections from two depths of the tendon separated by 200µm were cut from each tendon and stained with hematoxylin and eosin. Slides were scanned with the Aperio ScanScope XT slide scanning system and viewed with Imagescope viewing software (Aperio Technologies, Vista, CA).
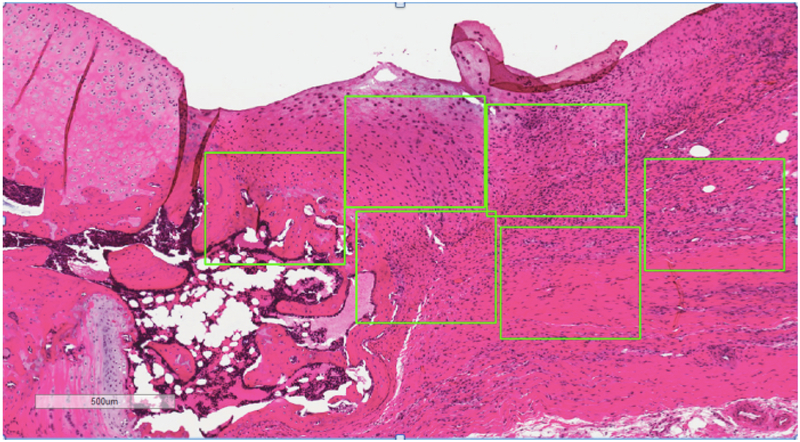
Three blinded observers graded twelve rectangular regions of interest (ROI) within the tendon distributed over the two tendon sections. ROIs were uniformly set to 500×400μm unique portions of the patellar tendon (Figure 1). Using a modified Movin grading system, ROI were graded (score: 0–3, each parameter) for the following four parameters: cell density, fiber arrangement, nuclear rounding, and angiogenesis20. Therefore, a normal tendon would receive a 0 while a tendon with maximally increased disorganization of fiber arrangement, rounded nuclei, increased angiogenesis and cell density would receive 3 for each group for a total of 12. A previously published atlas of tendon histology scores for these parameters was used as a reference during grading21. Scores were averaged across ROIs and then across observers to compute an average score for each tendon.
Figure 1.
Example of histologic image of CAR tendon used for microscopic grading. (Patella to the left of the image).
Mechanical Testing
To obtain cross-sectional areas, all 48 right patellar tendons (16 from each group) were dissected out by removing all non-tensile tissue and inflammatory adhesions. Dissection of non-tensile adhesive tissue was determined to be complete when all adherent tissue that could be bluntly dissected had been removed. Cross-sectional area of the patella tendon was then measured in triplicate and averaged using an area micrometer with a standard compression pressure of 0.12 MPa applied to the mid-substance22. Tensile tests were performed with a material testing system (Instron 8500 Plus, Instron Corporation, Norwood, MA). For testing, the tibia was aligned with the loading axis of the system using a cylindrical fixture with triangulating screws. A sliding bracket was used to apply compression to the tibial plateau and help stress protect the tibia from epiphyseal plate failures during the tensile test. A cryoclamp was used to grip the quadriceps tendon approximately 2mm above its insertion to the patella.
All samples were set to 2N of pretension and preconditioned to 2.5% strain for 10 cycles. Tendons were then tensioned to failure at 0.5mm/sec while load and actuator displacement data were recorded at 100Hz. From the load-displacement curve the ultimate load and stiffness were measured. Stiffness values were calculated by fitting a linear regression equation to the slope of the load-displacement curves between 20% and 60% of the ultimate load. Utilizing the cross-sectional area and tendon length of the specimens, the corresponding material properties were computed from the structural properties (i.e. elastic modulus= stiffness*length/ area, tensile strength= ultimate load/area). The site of failure was recorded for all specimens and data analysis only included specimens where failure occurred along the patellar tendon.
GAG Content Evaluation
All 48 tendons (16 from each group) were thawed and subject to papain digestion at 65C for 24 hours. GAG content of the digest was quantified by the dimethylmethylene blue assay23,24. Sample absorbance was read on a plate reader at 525nm with sulphated-GAG concentration deduced from standard curves developed from known concentrations of chondroitin sulfate from shark cartilage (C4384, Sigma-Aldrich, St. Louis, MO).
Collagenase Activity Evaluation
27 tendons (9 from each group) were homogenized in liquid nitrogen with a cryogrinder (OPS Diagnostics, Lebanon, NJ). The homogenate was centrifuged at 10,000g for 15 minutes and the recovered supernatant was suspended in an Azocoll buffer solution and incubated at 37°C for 24 hours. Collagenase activity was quantified using an Azocoll assay described by Chavira et al25. Sample absorbance was read on a plate reader at 520nm with background subtracted.
Statistical Analysis
Statistical differences (P<0.05) in the groups were evaluated by one-way analysis of variance (ANOVA) followed by Holm-Sidak mean-comparison testing. Histology grades were analyzed with Kruskal-Wallis ANOVA on ranks. A pre-power analysis for the ultimate tensile load had indicated that an N of 12 rats per group could detect a 40% difference between the 3 groups with an expected standard deviation of 30% of the control mean with a power of 0.8 and significance level of 0.05. Given the expected loss of some specimens per group based on previous experience, 16 rats per group were determined.
Results
There were no significant differences in initial weight (CON: 282 ± 49, CAR: 282 ± 40, IL1-RA: 283 ± 40) or in change in weight across the study (CON: 24 ± 18, CAR: 14 ± 14, IL1-RA: 17 ± 17). All rats completed the study without deviation from the protocol. There were no significant differences found between the groups in activity pre or post-injection.
The effect on the downstream action of IL1 was evaluated through the examination of collagenase activity and GAG content. The CAR (0.058 ± 0.017) did not show a statistically significant difference in collagenase activity compared to the CON (0.053 ± 0.008) and IL1-RA (0.051 ± 0.006) (Table 2). This was also observed in GAG content, with the CAR (28.8 ± 4.9 µg/ml) compared to the IL1-RA (27.9 ± 4.7 µg/ml) groups and CON (27.3 ± 3.8µg/ml) (Table 2).
Table 2.
Properties of the patellar tendon (Mean ± Standard Deviation)
| Group | GAG Content (µg/ml) |
Collagenase Activity (absorbance) |
|---|---|---|
| Tendons Evaluated |
48 | 30 |
| CON | 27.3 ± 3.8 | 0.053 ± 0.008 |
| CAR | 28.8 ± 4.9 | 0.058 ± 0.017 |
| IL1-RA | 27.9 ± 4.7 | 0.051 ± 0.006 |
No Statistically significant difference
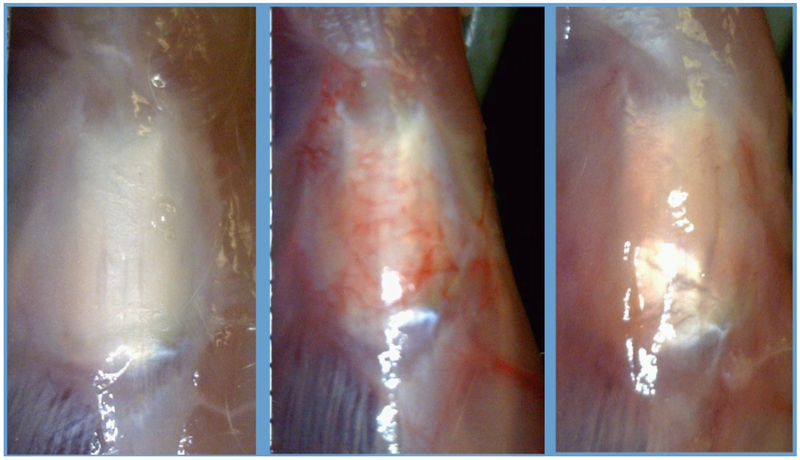
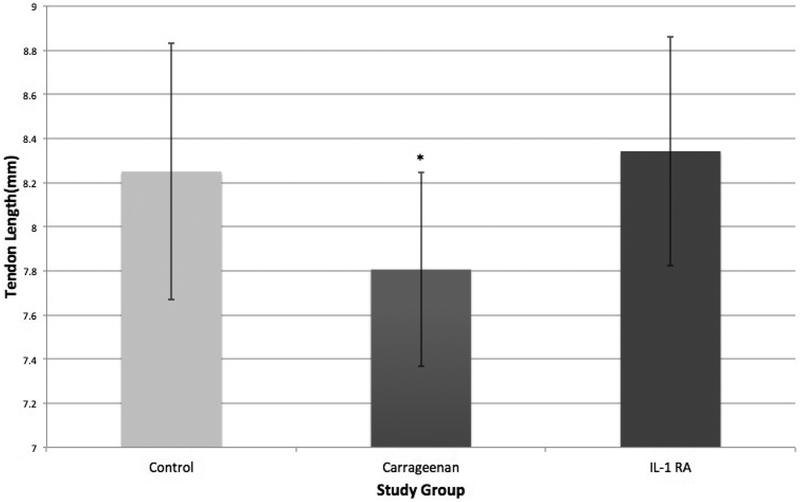
There was a notable difference in macroscopic changes between treatment groups (Figure 2). Although subjective, the CON group demonstrated less thickening and tissue proliferation and the CAR group showed an increased proliferative and hypervascular response surrounding the tendon. This was also demonstrated by differences in patellar tendon length (Figure 3). The CAR group (7.81 ± 0.44mm) was shortened compared to the CON (8.25± 0.58mm) and IL1-RA group (8.34 ± 0.52mm) (P=0.011). The patellar tendon length of the CON group was not significantly different compared to the IL1-RA group.
Figure 2.
From Left to Right (CON, CAR, IL1-RA)
Macroscopic images in situ of the patellar tendon and surrounding tissues of the three groups prior to dissection for cross-sectional area. The CAR shows greater vascularity compared to the other groups.
Figure 3.
(Left) Statistically significant differences were found with patellar tendon length when comparing CAR to CON and IL1-RA. No significant difference between CON and IL1-RA.
Significant differences in histologic factors including fiber arrangement, angiogenesis, nuclear rounding, cell density grades were demonstrated when comparing the CAR and CON/IL1-RA groups. For all four factors that were evaluated a statistical difference was found between the CAR and CON (P<0.05) and between the CAR and IL1-RA in all four categories (P<0.05). The difference between IL1-RA and CAR tendons did not reach statistical significance in the angiogenesis category (Table 3).
Table 3.
Histologic changes in 18 tendons (6 from each group) averaged between three blinded and independent observers on a 0-3 Scale. (Mean ± Standard Deviation)
| Group | Cell Density |
Fiber Arrangement |
Angiogenesis | Nuclear Rounding |
|---|---|---|---|---|
| CON | 0.5 ± 0.38 | 0.33 ± 0.25 | 0.17 ± 0.16 | 0.68 ± 0.37 |
| CAR | 2.0 ± 0.67* | 2.0 ± 0.30* | 0.92 ± 0.38* | 2.10 ± 0.50* |
| IL1-RA | 1.08 ± 0.60# | 0.83 ± 0.41# | 0.33 ± 0.20 | 1.26 ± 0.52# |
Significant difference relative to CON (P<0.05).
Significant difference relative to CAR (P<0.05).
The differences in mechanical factors did not reach statistical significance for stiffness, ultimate tensile load, or cross sectional area (Table 4). The mechanical analysis was limited due to seven specimens (two CON and two IL1-RA and three CAR) out of 48 not being included due to failure of the specimen at the quadriceps tendon or through a fracture as opposed to failure within the patellar tendon. However, given our previous experience with failures in this testing we obtained data from over 12 specimens in each group determined to be sufficient for our prior power analysis.
Table 4.
Mechanical Data of all 48 patellar tendons minus the 7 tendons that were not included do to failure at the quadriceps. (Mean ± Standard Deviation)
| Group | Stiffness (N/mm) |
Ultimate Tensile Load(N) |
Cross sectional Area(mm2) |
|---|---|---|---|
| CON | 56.31 ± 14.5 | 70.39 ± 19.1 | 3.43 ± 0.7 |
| CAR | 44.14 ± 16.7 | 66.36 ± 13.9 | 3.20 ± 1.0 |
| IL1-RA | 51.32 ± 16.8 | 73.36 ± 18.0 | 3.87 ± 1.7 |
No finding reached statistical significance.
Discussion
IL1-RA reduced some features of tendinopathy including tendon shortening, histologic appearance, and macroscopic changes. Overall, this established tendinopathy model demonstrated some evidence for the reduction of the inflammatory effects of the carrageenan, however with less effect than in a preventative model. Histologic and macroscopic evaluation demonstrated a significant difference between the IL1-RA to CAR groups and between the CON and IL1-RA groups except for the angiogenesis category. A significant difference was also noted with greater tendon shortening in the CAR group. However, a similar response was not seen in cross sectional area or in mechanical and chemical testing. These findings demonstrate that the IL1-RA provided some reduction of established tendinopathy, however with decreased effectiveness of the IL1-RA compared to the tendinopathy prevention model.
However, as opposed to this research group’s previously published preventative tendinitis model, chemical and mechanical testing did not show any significant difference16. In a preventative model without established tendinopathy, downstream cytokines are prevented from being upregulated, limiting their contributions to inflammation. This appears to have a more substantial effect in preventing tendinopathy as opposed to attempting to reverse these changes once tendinopathy has been established. It is likely that our results showing no difference in collagenase and GAG content can be attributed to the impact of these previously upregulated cytokines and the intervention not providing a means for reversal.
This study’s main strength is the applicability of this model to a clinically relevant established tendinopathy as opposed to a preventative model. However, it also has several limitations. This model utilized carrageenan in order to create the established tendinopathy and this may not create a similar chemical response to the tendinopathy seen in clinical practice. IT is also limited by variation in dissection. We attempted to remove all non load-bearing tissue that could be removed from the tendon but any variation in this may have altered the mechanical evaluation. However, this would occur across all groups and the risk was not different between each group.
Perspective
This study demonstrates that an IL-1 RA can reduce some of the changes associated with tendinopathy. However, there was less reduction when compared to a previously performed preventative tendinopathy model16. This demonstrates that an IL1-RA may be of use in the treatment of tendinopathy, however with likely greater impact in a preventative model than in established tendinopathy.
Acknowledgements
We appreciate the assistance from the histology and immunotechnologies cores of the Center for Gastrointestinal Biology and Disease, which is supported by NIH - P30 DK34987. The authors have not declared any conflict of interest involved with this study. Financial support provided by the UNC-Chapel Hill Orthopaedic Research Fund.
References
- 1.Ma Y, Yan X, Zhao H, Wang W. Effects of interleukin-1 receptor antagonist on collagen and matrix metalloproteinases in stress-shielded achilles tendons of rats. Orthopedics 2012;35:e1238–1244. [DOI] [PubMed] [Google Scholar]
- 2.Uchida H, Tohyama H, Nagashima K, Ohba Y, Matsumoto H, Toyama Y, Yasuda K. Stress deprivation simultaneously induces over-expression of interleukin-1beta, tumor necrosis factor-alpha, and transforming growth factor-beta in fibroblasts and mechanical deterioration of the tissue in the patellar tendon. J Biomech 2005;38:791–798. [DOI] [PubMed] [Google Scholar]
- 3.Archambault J, Tsuzaki M, Herzog W, Banes AJ. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res Off Publ Orthop Res Soc 2002;20:36–39. [DOI] [PubMed] [Google Scholar]
- 4.Barbe MF, Elliott MB, Abdelmagid SM, Amin M, Popoff SN, Safadi FF, Barr AE. Serum and tissue cytokines and chemokines increase with repetitive upper extremity tasks. J Orthop Res Off Publ Orthop Res Soc 2008;26:1320–1326. [DOI] [PubMed] [Google Scholar]
- 5.Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br J Pharmacol 2000;130:1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko JY, Wang FS, Huang HY, Wang CJ, Tseng SL, Hsu C. Increased IL-1beta expression and myofibroblast recruitment in subacromial bursa is associated with rotator cuff lesions with shoulder stiffness. J Orthop Res Off Publ Orthop Res Soc 2008;26:1090–1097. [DOI] [PubMed] [Google Scholar]
- 7.Voloshin I, Gelinas J, Maloney MD, O’Keefe RJ, Bigliani LU, Blaine TA. Proinflammatory cytokines and metalloproteases are expressed in the subacromial bursa in patients with rotator cuff disease. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc 2005;21:1076.e1–1076.e9. [DOI] [PubMed] [Google Scholar]
- 8.Sun HB, Li Y, Fung DT, Majeska RJ, Schaffler MB, Flatow EL. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin Orthop 2008;466:1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuzaki M, Guyton G, Garrett W, Archambault JM, Herzog W, Almekinders L, Bynum D, Yang X, Banes AJ. IL-1 beta induces COX2, MMP-1, −3 and −13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res Off Publ Orthop Res Soc 2003;21:256–264. [DOI] [PubMed] [Google Scholar]
- 10.Lake SP, Ansorge HL, Soslowsky LJ. Animal models of tendinopathy. Disabil Rehabil 2008;30:1530–1541. [DOI] [PubMed] [Google Scholar]
- 11.Warden SJ. Animal models for the study of tendinopathy. Br J Sports Med 2007;41:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadeghi H, Hajhashemi V, Minaiyan M, Movahedian A, Talebi A. A study on the mechanisms involving the anti-inflammatory effect of amitriptyline in carrageenan-induced paw edema in rats. Eur J Pharmacol 2011;667:396–401. [DOI] [PubMed] [Google Scholar]
- 13.Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, Mitchell D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain Off J Am Pain Soc 2007;8:127–136. [DOI] [PubMed] [Google Scholar]
- 14.Marsolais D, Duchesne E, Côté CH, Frenette J. Inflammatory cells do not decrease the ultimate tensile strength of intact tendons in vivo and in vitro: protective role of mechanical loading. J Appl Physiol Bethesda Md 1985 2007;102:11–17. [DOI] [PubMed] [Google Scholar]
- 15.Tillander B, Franzén LE, Nilsson E, Norlin R. Carrageenan-induced subacromial bursitis caused changes in the rat’s rotator cuff. J Orthop Res Off Publ Orthop Res Soc 2001;19:441–447. [DOI] [PubMed] [Google Scholar]
- 16.Berkoff DJ, Kallianos SA, Eskildsen SM, Weinhold PS. Use of an IL1-receptor antagonist to prevent the progression of tendinopathy in a rat model. J Orthop Res Off Publ Orthop Res Soc 2016;34:616–622. [DOI] [PubMed] [Google Scholar]
- 17.Ferry ST, Afshari HM, Lee JA, Dahners LE, Weinhold PS. Effect of prostaglandin E2 injection on the structural properties of the rat patellar tendon. Sports Med Arthrosc Rehabil Ther Technol SMARTT 2012;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.qiu Zheng Y, Wei W, Dai M, Zhu L, yi Jia X, Wang Y. Interleukin-1 receptor antagonist intervenes in signaling between different types of synoviocytes in rats with adjuvant arthritis. Acta Pharmacol Sin 2006;27:111–118. [DOI] [PubMed] [Google Scholar]
- 19.Laudier D, Schaffler MB, Flatow EL, Wang VM. Novel procedure for high-fidelity tendon histology. J Orthop Res Off Publ Orthop Res Soc 2007;25:390–395. [DOI] [PubMed] [Google Scholar]
- 20.Movin T, Gad A, Reinholt FP, Rolf C. Tendon pathology in long-standing achillodynia. Biopsy findings in 40 patients. Acta Orthop Scand 1997;68:170–175. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Yu Q, Wu B, Lin Z, Pavlos NJ, Xu J, Ouyang H, Wang A, Zheng MH. Autologous tenocyte therapy for experimental Achilles tendinopathy in a rabbit model. Tissue Eng Part A 2011;17:2037–2048. [DOI] [PubMed] [Google Scholar]
- 22.Butler DL, Grood ES, Noyes FR, Zernicke RF, Brackett K. Effects of structure and strain measurement technique on the material properties of young human tendons and fascia. J Biomech 1984;17:579–596. [DOI] [PubMed] [Google Scholar]
- 23.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 1986;883:173–177. [DOI] [PubMed] [Google Scholar]
- 24.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Glycosaminoglycans of human rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis 1994;53:367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chavira R, Burnett TJ, Hageman JH. Assaying proteinases with azocoll. Anal Biochem 1984;136:446–450. [DOI] [PubMed] [Google Scholar]
- 26.Kurtz CA, Loebig TG, Anderson DD, DeMeo PJ, Campbell PG. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am J Sports Med 1999;27:363–369. [DOI] [PubMed] [Google Scholar]