Abstract
Short sleep duration has been widely linked to increased cardiovascular morbidity and mortality. We performed a post hoc analysis of 24‐hour ambulatory blood pressure monitoring (ABPM) in the Lifestyle Modification in Blood Pressure Lowering Study (LIMBS) and Penn Icelandic Sleep Apnea (PISA) Study. The 24‐hour mean systolic blood pressure (BP) was 12.7 mm Hg higher in LIMBS (P < 0.001; n = 66) and 4.7 mm Hg higher in PISA (P = 0.005; n = 153) among participants with shorter sleep duration (less than 7 hours) compared to those with longer sleep duration (at least 7 hours). In multivariable adjusted models, shorter sleep duration was strongly associated with higher systolic BP on 24‐hour ABPM, independent of nocturnal BP and in‐office BP. There was no effect modification by obstructive sleep apnea. Adults with shorter sleep duration may benefit from screening with 24‐hour ABPM to promote earlier detection of hypertension and potentially mitigate their increased risk for future cardiovascular disease.
Keywords: ambulatory, blood pressure monitoring, circadian rhythm, hypertension, obstructive sleep apnea, sleep deprivation, sleep disorders
1. INTRODUCTION
Circadian variations in blood pressure (BP) play an integral role in long‐term vascular outcomes. In 1988, O'Brien et al noted that both systolic BP (SBP) and diastolic BP (DBP) during sleep are typically 10% lower than waking BPs, a phenomenon he called “dipping.”1 Several subsequent studies demonstrated strong associations between the absence of nocturnal dipping and increased risk of cardiovascular disease and stroke in both normotensive and hypertensive patients.2 Multiple studies have also shown a strong association between sleep disorders, including short sleep duration (often defined as anywhere from less than 5 hours of sleep3, 4, 5 to less than 7 hours of sleep6, 7, 8, 9, 10), and increased risk of cardiovascular disease.10, 11, 12 The increased cardiovascular risk appreciated in patients with short sleep duration and other sleep disorders suggests a potential relationship between abnormal sleep patterns and alterations in circadian BP control.
While previous studies have demonstrated a link between short sleep duration and a diagnosis of hypertension,3, 5, 13, 14 data evaluating the relationship between short sleep duration and parameters related to circadian control of BP (such as 24‐hour BP control, dipping, and 24‐hour BP variability) are limited. In a study of 4810 patients in the National Health and Nutrition Examination Survey, Gangwisch et al found that less than 5 hours of self‐reported sleep duration was significantly associated with subsequent diagnosis with incident hypertension (hazard ratio 2.10; 95% confidence interval 1.58‐2.79),3 with a similar relationship between shorter sleep duration and hypertension corroborated in other cohorts.5, 9, 14 A study by Mezick et al, performed in adolescents, demonstrated an association between short sleep duration with an absence of nocturnal dipping and with increased 48‐hour BPs on ambulatory BP monitoring (ABPM).4 To the best of our knowledge, these results have not been corroborated in an adult population.
In this study, our goal was to examine the association between sleep duration and 24‐hour BP parameters that would not otherwise be captured by in‐office BP measurements, including 24‐hour BP control, nocturnal dipping, and 24‐hour BP variability. To achieve this, we evaluated data from two distinct cohorts of adults not on antihypertensive medications, including one cohort of otherwise healthy, prehypertensive individuals and one cohort of individuals assessed for obstructive sleep apnea (OSA). Based on existing mechanistic and epidemiologic evidence, we hypothesized that shorter sleep duration would be significantly associated with increased 24‐hour ambulatory SBP and increased prevalence of nondipping, particularly among obese adults and adults with OSA.
2. METHODS
We performed post hoc, cross‐sectional analyses of baseline measurements from the Lifestyle Modification in BP Lowering Study II (Lifestyle Modification in Blood Pressure Lowering Study [LIMBS]; NCT00964847) and the Penn Iceland Sleep Apnea (PISA; NCT03176732) study. Both protocols were approved by the University of Pennsylvania institutional review board; PISA was also approved by the Landspitali – The National University Hospital of Iceland institutional review board. All participants provided informed consent.
2.1. LIMBS study design
Lifestyle Modification in Blood Pressure Lowering Study was a randomized, parallel group, nonblinded, prospective, controlled trial evaluating the effects of yoga and BP education on subsequent development of hypertension among subjects recruited from the University of Pennsylvania.15 The study population included adults age 18‐80 years with prehypertension who were not taking any antihypertensive medications for at least 3 months prior to screening (Figure S1, Table S1). Subjects were excluded with BMI >40 kg/m2, history of kidney diseases (estimated glomerular filtration rate <60 mL/min/1.73m2), diabetes mellitus, cardiovascular disease, active smoking status, or heavy alcohol use (defined as more than seven drinks per week among women, and >14 drinks per week among men). Pregnant women were also excluded from the study.
2.2. PISA study design
PISA is an observational prospective trial evaluating clinical and molecular characteristics responsible for BP changes seen in adults with OSA. The study screened adults age 40‐65 years with no previous history of treatment for OSA; individuals were included in the interventional arm of the study if they were found to have moderate‐severe OSA (apnea‐hypopnea index ≥15 events per hour based on full‐night diagnostic polysomnography; Figure S2, Table S1). The method of OSA diagnosis in PISA was described in detail previously.16 Subjects were excluded with any unstable or new medical condition in the month prior to screening, severe and uncontrolled arterial hypertension (SBP > 180 mm Hg; DBP > 110 mm Hg), BMI > 40 kg/m2, routine consumption of more than two alcoholic beverages per day, and excessive caffeine use (>10 cups/d). Pregnant women were also excluded. Subjects were recruited from The University of Pennsylvania and Landspitali – The National University Hospital of Iceland.
Subjects were included in the primary analyses if they underwent ABPM during screening for eligibility for PISA, whether or not they were found to have OSA, and if they were not on antihypertensive treatment for ≥2 months prior to screening.
2.3. Blood pressure measurement
Arm circumference was measured to determine the appropriate cuff size. In‐office readings were obtained using the validated Datascope Accutorr Plus17 (Soma Technology, Paramus, NJ) in LIMBS and the OMRON HEM‐907XL18 (Omron Healthcare, Lake Forest, IL) in PISA by calculating mean of three observed automated office BP readings taken at 1‐minute intervals following 5 minutes seated at rest. In both studies, 24‐hour ABPM was performed at the baseline study visit using Spacelabs model 90207 and 90217 monitors19 (Spacelabs Healthcare, Snoqualmie, WA). In LIMBS, BP s were measured every 20 minutes during the day and every 30 minutes at night; the minimum acceptable number of readings were 28 from 6 AM to midnight, and six from midnight to 6 AM. In PISA, BPs were measured every 30 minutes over the 24‐hour period; the minimum acceptable number of readings was 16 from 6 AM to midnight, and six from midnight to 6 AM. If the minimum number of readings for a valid ABPM interpretation was not achieved, the participant was asked to repeat the study. Sleep times were self‐reported, determined by direct subject query. Every subject completed a sleep diary indicating the time they fell asleep and the time they woke up for determination of sleep and wake times. Sleep duration was calculated as the difference between self‐reported time to sleep and time of awakening. Subjects were also instructed to report any unusual events in the diaries, such as atypical symptoms or sleep disruption. Subjects were excluded from the analyses if they did not complete a sleep diary. In PISA, sleep times were also corroborated with wrist actigraphy measurements, which record sleep and wake patterns using physical movement.20
2.4. Statistical analyses
Statistical analyses were performed using STATA version 15 (Statacorp LP, College Station, TX) with 2‐sided hypothesis testing and P‐value of <0.05 as the criteria for statistical significance. All analyses were stratified by cohort (PISA or LIMBS) to account for heterogeneity across the two study populations. Means and proportions were used to describe baseline characteristics of individuals with shorter sleep duration (less than 7 hours) compared to individuals with longer sleep duration (at least 7 hours), based on thresholds used most frequently in existing studies on sleep duration and adverse outcomes.6, 7, 8 Continuous variables were compared using the Student t test. Categorical and binary variables were compared using chi‐square test.
We used in‐office BP and ABPM readings to identify individuals with controlled hypertension (BP < 140/90 in‐office and <135/85 on ABPM), white‐coat hypertension (BP ≥ 140/90 in‐office and <135/85 on ABPM), masked hypertension (BP < 140/90 in‐office and ≥135/85 on ABPM), and sustained hypertension (BP ≥ 140/90 in‐office and ≥135/85 on ABPM).21 We evaluated dipping status, defined by a decline in SBP >10% during sleep.1 We also evaluated 24‐hour, daytime (self‐reported time awake), and nocturnal (self‐reported time asleep) mean SBP, DBP, pulse pressure, and heart rate, as well as 24‐hour SBP variability (using average real variability, calculated as the sum of the absolute differences between each respective BP reading divided by the total number of readings minus one,22 as well as coefficient of variation, calculated as the standard deviation of the BP divided by the mean BP).
Multivariable linear regression was used to evaluate the association of 24‐hour mean SBP, heart rate, dipping status, and in‐office BP with sleep duration, adjusting for key covariates identified a priori based on known associations with elevated BP, including age, Black or African American race, and BMI.2, 23, 24, 25
2.5. Sensitivity analyses
In the subgroup of subjects in the PISA study, we assessed for a correlation between self‐reported sleep duration by sleep diary while undergoing ABPM and sleep duration extrapolated from the actigraphy data. We performed sensitivity analyses replacing the self‐reported sleep durations with actigraphy sleep duration (mean sleep duration by actigraphy over 1 week) in each of the multivariable analyses. We performed sensitivity analyses omitting participants in PISA who had key exclusion criteria from LIMBS, including diabetes mellitus, cardiovascular disease, and congestive heart failure, for each of the multivariable analyses. Additionally, we performed sensitivity analyses including only participants in PISA on antihypertensive medications. We also stratified and assessed for effect modification of the association between sleep duration and each of the outcomes by OSA (only available in PISA) and obesity (BMI ≥30 vs <30 kg/m2).23, 25, 26, 27, 28
3. RESULTS
3.1. Characteristics among participants with shorter vs longer sleep duration
In LIMBS, the mean sleep duration among participants with shorter sleep (less than 7 hours, N = 21) was 5.4 (standard deviation [SD] 1.5) hours, and the mean sleep duration among patients with longer sleep (at least 7 hours, N = 45) was 8.6 (SD 1.0) hours. Compared to participants with longer sleep duration, individuals with shorter sleep duration were similar in age (Table 1; mean 50, SD 13 vs 49, SD 11 years, P = 0.736), sex (62% vs 44% male, P = 0.186), race/ethnicity (43% vs 36% Black, P = 0.816), and BMI (mean 31, SD 5 vs 29, SD 6 kg/m2, P = 0.274). Participants with longer sleep duration were more likely to drink alcohol (40% vs 76%, P = 0.006). None of the LIMBS participants had a history of tobacco use, OSA, diabetes mellitus, coronary artery disease, or congestive heart failure. Participants with shorter sleep duration had similar in‐office SBP (mean 140, SD 12 vs 137, SD 11 mm Hg, P = 0.368) and DBP (mean 88, SD 11 vs 87, SD 9 mm Hg, P = 0.856) compared to participants with longer sleep duration.
Table 1.
Baseline participant characteristics by sleep duration in LIMBS and PISA
| LIMBS study, N = 66 | PISA study, N = 153 | |||||
|---|---|---|---|---|---|---|
| Short sleep (<7 h), N = 21 | Long sleep (≥7 h), N = 45 | P‐value | Short sleep (<7 h), N = 84 | Long sleep (≥7 h), N = 69 | P‐value | |
| Mean sleep duration, hours (SD) | 5.4 (1.5) | 8.6 (1.0) | <0.001 | 5.8 (1.2) | 8.3 (0.9) | <0.001 |
| Mean age, years (SD) | 50 (13) | 49 (11) | 0.736 | 52 (7) | 52 (7) | 0.581 |
| Male sex, n (%) | 13 (62) | 20 (44) | 0.186 | 74 (88) | 58 (84) | 0.470 |
| Race, n (%) | ||||||
| Caucasian | 10 (48) | 23 (51) | 0.816 | 66 (79) | 50 (74) | 0.768 |
| Black | 9 (43) | 16 (36) | 16 (19) | 16 (23) | ||
| Asian, Pacific Islander, or other | 2 (9) | 6 (13) | 2 (2) | 2 (3) | ||
| Mean BMI, kg/m2 (SD) | 31 (5) | 29 (6) | 0.274 | 31 (4) | 30 (4) | 0.038 |
| Obese, n (%) | 11 (55) | 19 (42) | 0.340 | 50 (60) | 35 (51) | 0.276 |
| Alcohol use, n (%) | 8 (40) | 34 (76) | 0.006 | 65 (77) | 56 (81) | 0.567 |
| Tobacco use, n (%) | 0 (0) | 0 (0) | 1.000 | 16 (19) | 17 (25) | 0.448 |
| Obstructive sleep apnea, n (%) | 0 (0) | 0 (0) | 1.000 | 60 (71) | 47 (72) | 0.811 |
| Diabetes mellitus, n (%) | 0 (0) | 0 (0) | 1.000 | 8 (10) | 2 (3) | 0.091 |
| Coronary artery disease, n (%) | 0 (0) | 0 (0) | 1.000 | 1 (1) | 1 (1) | 0.921 |
| Congestive heart failure, n (%) | 0 (0) | 0 (0) | 1.000 | 1 (1) | 0 (0) | 0.364 |
| Mean in‐office SBP, mm Hg (SD) | 140 (12) | 137 (11) | 0.368 | 136 (14) | 130 (13) | 0.007 |
| Mean in‐office DBP, mm Hg (SD) | 88 (11) | 87 (9) | 0.856 | 85 (10) | 81 (10) | 0.043 |
| Mean in‐office pulse pressure, mm Hg (SD) | 52 (11) | 50 (9) | 0.392 | 52 (11) | 49 (13) | 0.148 |
DBP, diastolic blood pressure; LIMBS, Lifestyle Modification in Blood Pressure Lowering Study; PISA, Penn Iceland Sleep Apnea Study; SBP, systolic blood pressure; SD, standard deviation.
In PISA, participants with shorter sleep duration (N = 84) had a mean of 5.8 (SD 1.2) hours of sleep, and participants with longer sleep duration (N = 69) had a mean of 8.3 (SD 0.9) hours of sleep. There was no clinically significant difference in age (Table 1; mean 52, SD 7 vs 52, SD 7 years, P = 0.581), sex (88% vs 84% male, P = 0.470), race (19% vs 23% black, P = 0.768), BMI (mean 31, SD 4 vs 30, SD 4 kg/m2, P = 0.038), or alcohol use (77% vs 81%, P = 0.567) among subjects with shorter sleep duration (less than 7 hours) compared to subjects with longer sleep duration (at least 7 hours). There were similar rates of tobacco use (19% vs 25%, P = 0.448), moderate‐severe OSA (71% vs 72%, P = 0.811), diabetes mellitus (10% vs 3%, P = 0.091), coronary artery disease (1% vs 1%, P = 0.921), and congestive heart failure (1% vs 0%, P = 0.364) among participants with shorter sleep duration compared to those with longer sleep duration. Subjects with shorter sleep duration had significantly higher in‐office SBP (mean 136, SD 14 vs 130, SD 13 mm Hg, P = 0.007) and DBP (mean 85, SD 10 vs 81, SD 10 mm Hg, P = 0.043) compared to those with longer sleep duration.
3.2. ABPM characteristics and sleep duration
In LIMBS, the 24‐hour mean SBP was 12.7 mm Hg higher among participants with shorter sleep duration compared to those with longer sleep duration (Table 2, Table S2, Figure 1A; mean 24‐hour SBP 146.3, SD 14.0 vs 133.6, SD 10.1 mm Hg, P < 0.001); the greatest difference was appreciated in the daytime mean SBP (148.1 vs 136.0 mm Hg, P < 0.001), although there was also a significant difference in nocturnal mean SBP (133.4 vs 124.1 mm Hg, P = 0.029). Participants with shorter sleep duration were also more likely to have uncontrolled, or sustained hypertension (47% vs 20%, P = 0.033) and faster mean 24‐hour heart rate (65.8 vs 57.8 beats per minute [bpm], P = 0.027) compared to patients with longer sleep duration. There was no significant difference in 24‐hour SBP variability (mean average real variability 9.3, SD 1.9 vs 8.6, SD 1.6 mm Hg, P = 0.121), frequency of nocturnal dipping (65% vs 47%, P = 0.205), frequency of white coat hypertension (0% vs 6%, P = 0.275), or frequency of masked hypertension (24% vs 22%, P = 0.913).
Table 2.
Ambulatory blood pressure monitoring characteristics by sleep duration in LIMBS and PISA
| LIMBS study, N = 66 | PISA study, N = 153 | |||||
|---|---|---|---|---|---|---|
| Short sleep (<7 h), N = 21 | Long sleep (≥7 h), N = 45 | P‐value | Short sleep (<7 h), N = 84 | Long sleep (≥7 h), N = 69 | P‐value | |
| 24‐h mean SBP (SD), mm Hg | 146.3 (14.0) | 133.6 (10.1) | <0.001 | 131.5 (10.6) | 126.8 (9.6) | 0.005 |
| 24‐h SBP variability (SD), mm Hg | 9.3 (1.9) | 8.6 (1.6) | 0.121 | 8.9 (2.0) | 9.0 (1.9) | 0.777 |
| 24‐h heart rate (SD), bpm | 65.8 (11.6) | 57.8 (12.4) | 0.027 | 76.2 (9.1) | 74.3 (8.6) | 0.182 |
| Dippers, n (%) | 11 (65) | 21 (47) | 0.205 | 55 (65) | 45 (65) | 0.973 |
| White coat hypertension, n (%) | 0 (0) | 3 (6) | 0.275 | 9 (11) | 5 (7) | 0.459 |
| Masked hypertension, n (%) | 4 (24) | 10 (22) | 0.913 | 15 (18) | 18 (26) | 0.218 |
| Uncontrolled hypertension, n (%) | 8 (47) | 9 (20) | 0.033 | 21 (25) | 9 (13) | 0.064 |
bpm, beats per minute; LIMBS, Lifestyle Modification in Blood Pressure Lowering Study; PISA, Penn Iceland Sleep Apnea Study; SBP, systolic blood pressure; SD, standard deviation.
Figure 1.
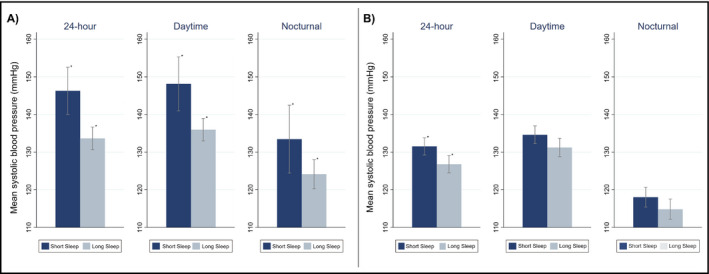
Mean 24‐h systolic blood pressure (SBP) in (A) LIMBS and (B) PISA. The graphs represent mean 24‐h SBP (bars) and 95% confidence intervals (capped lines) during the full 24‐h period, self‐reported wake time (“Daytime”), and self‐reported sleep time (“Nocturnal”). Mean SBP was stratified by sleep duration. Shorter sleep duration was defined as <7 h; longer sleep duration was defined as ≥7 h. *Indicative of a statistically significant difference (P < 0.05) between the shorter and longer sleep duration groups
In PISA, the 24‐hour mean SBP was 4.7 mm Hg higher among shorter sleepers compared to longer sleepers (Table 2, Table S2, Figure 1B; mean 24‐hour SBP 131.5, SD 10.6 vs 126.8, SD 9.6 mm Hg, P = 0.005). Both daytime SBP (134.6 vs 131.2 mm Hg, P = 0.053) and nocturnal SBP (118.0 vs 114.8 mm Hg, P = 0.098) were not significantly different in participants with shorter compared to long sleep duration. There was no significant difference in 24‐hour SBP variability (mean average real variability 8.9, SD 2.0 vs 9.0, SD 1.9 mm Hg, P = 0.777), 24‐hour mean heart rate (76.2 vs 75.3 bpm, P = 0.182), or in frequency of nocturnal dipping (65% vs 65%, P = 0.973), white coat hypertension (11% vs 7%, P = 0.459), masked hypertension (18% vs 26%, P = 0.218), or uncontrolled hypertension (25% vs 13%, P = 0.064). After excluding participants with tobacco use, diabetes, coronary artery disease, or congestive heart failure, there was a 5.5‐ mm Hg higher 24‐hour SBP among participants with shorter sleep duration compared to those with longer sleep duration (Table S3; 131.0 mm Hg vs 126.5 mm Hg, P = 0.009), and a higher frequency of participants with uncontrolled hypertension (Table S3; 28% vs 13%, P = 0.028).
There was no significant effect modification between obesity (Table S4, Figure S3) or moderate‐severe OSA (Table S5) and 24‐hour mean SBP with regard to sleep duration.
3.3. Multivariable models evaluating ABPM and sleep duration
In multivariable linear regression models adjusting for age, race, and BMI, there was a 1‐ mm Hg higher 24‐hour mean SBP for every 2.57 minute shorter sleep duration (Table 3, Figure 2A; P = 0.012) and a 1‐bpm higher 24‐hour mean heart rate for every 2.50 minute shorter sleep duration (P = 0.017) among participants of LIMBS. Among PISA participants, there was a 1‐mm Hg higher 24‐hour mean SBP for every 1.99 minute shorter sleep duration (Table 3, Figure 2B; P = 0.009) and a 1‐bpm higher 24‐hour mean heart rate for every 1.68 minute shorter sleep duration (P = 0.007). There was no significant association between sleep duration and nocturnal dipping (LIMBS P = 0.163; PISA P = 0.548) or in‐office SBP (LIMBS P = 0.942; PISA P = 0.064). The relationship between 24‐hour mean SBP and sleep duration persisted in both cohorts after additional adjustment for nocturnal dipping and in‐office SBP (Table 3).
Table 3.
Age, race, and BMI‐adjusted models evaluating the association between sleep duration (minutes) and ambulatory blood pressure parameters in LIMBS and PISA
| LIMBS study, N = 66 | PISA study, N = 153 | |||
|---|---|---|---|---|
| β coefficient (95% CI) | P‐value | β coefficient (95% CI) | P‐value | |
| 1 mm Hg increase in 24‐h mean SBP | −2.57 (−4.56 to −0.58) | 0.012 | −1.99 (−3.48 to −0.50) | 0.009 |
| 1 bpm increase in 24‐h heart rate | −2.50 (−4.54 to −0.46) | 0.017 | −1.68 (−2.89 to −0.47) | 0.007 |
| Nocturnal dipping | −0.60 (−1.45 to 0.25) | 0.163 | −10.64 (−45.60 to 24.31) | 0.548 |
| 1 mm Hg increase in in‐office SBP | −0.11 (−3.09 to 2.87) | 0.942 | −1.11 (−2.25 to 0.02) | 0.064 |
| 24‐h mean SBP model also adjusted for nocturnal dipping | ||||
| 1 mm Hg increase in 24‐h mean SBP | −2.74 (−4.63 to −0.85) | 0.005 | −2.10 (−3.57 to −0.63) | 0.005 |
| Nocturnal dipping | −0.48 (−0.97 to 0.35) | 0.072 | −8.54 (−40.51 to 23.44) | 0.599 |
| 24‐h mean SBP model also adjusted for in‐office SBP | ||||
| 1 mm Hg increase in 24‐h mean SBP | −3.92 (−7.00 to −0.85) | 0.013 | −1.65 (−2.66 to −0.03) | 0.046 |
| 1 mm Hg increase in in‐office SBP | 2.68 (−0.90 to 6.25) | 0.138 | −0.74 (−1.93 to 0.45) | 0.224 |
bpm, beats per minute; CI, confidence interval; LIMBS, Lifestyle Modification in Blood Pressure Lowering Study; PISA, Penn Iceland Sleep Apnea; SBP, systolic blood pressure.
Figure 2.
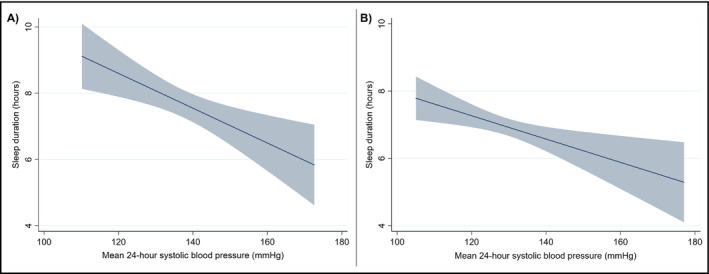
Adjusted 24‐h systolic blood pressure (SBP) and sleep duration in (A) LIMBS and (B) PISA. The graphs represent the linear association (solid lines) and 95% confidence intervals (shading) of the multivariable models evaluating the association between mean 24‐h SBP and sleep duration, adjusted for age, race, and BMI
There was a similar inverse relationship between sleep duration and 24‐hour mean SBP in sensitivity analyses excluding PISA participants who did not meet eligibility criteria for LIMBS (Table S6; regression coefficient −1.85, P = 0.034), and after replacing the PISA self‐reported sleep durations with sleep duration measured by actigraphy (Table S6; regression coefficient −1.54, P = 0.003. Figure S4; correlation between self‐reported and actigraphy sleep duration Pearson's Rho 0.23, P = 0.008). There was no significant association between 24‐hour mean SBP and sleep duration among participants in PISA who were on antihypertensive medications (Table S6; regression coefficient 1.28, P = 0.158).
4. DISCUSSION
In this analysis of two distinctive cohorts of adults not on antihypertensive medications, there was a considerable association between shorter sleep duration and higher SBP on 24‐hour ABPM. There was no association between sleep duration and nocturnal dipping, countering existing evidence that shorter sleep duration is associated with nondipping in adolescents.4 In fact, in LIMBS, we observed a greater increase in daytime SBP readings than in nocturnal readings when comparing patients with shorter sleep duration to those with longer sleep duration. Thus, our study demonstrates the novel finding that, in adults, the association between shorter sleep duration and 24‐hour elevated SBP is robust, and seems to be independent of nocturnal variations in BP.
A rich body of evidence links shorter sleep duration with several comorbidities and all‐cause mortality.7, 8 Many of the comorbidities associated with shorter sleep duration are end organ effects of hypertension, including progressive decline in renal function6 and cardiovascular disease.10, 11 Additionally, both short sleep duration29, 30 and circadian abnormalities in BP31, 32, 33 have been associated with neurocognitive decline and dementia. Taking this evidence into account, elevated ambulatory BP may be a mediator or early indicator of the association between shorter sleep duration and adverse outcomes. In this study, we found that 24‐hour ABPM had a stronger, more consistent association with shorter sleep duration than in‐office BP; additionally, the association of 24‐hour mean SBP with sleep duration persisted after adjusting for in‐office SBP. Given the prognostic superiority of ABPM over in‐office BP measurement,34, 35 use of ABPM in premorbid, normotensive and prehypertensive patients with shorter sleep duration may help identify many patients with shorter sleep duration at particularly high risk for adverse renal, neurocognitive, and cardiovascular outcomes.
The existing studies that demonstrated an association between shorter sleep duration and subsequent hypertension or circadian dysregulation in BP identified this relationship in individuals already on antihypertensive medications36 (making ABPM results difficult to interpret in the absence of information on duration of action or nocturnal dosing of antihypertensive medications), or in healthy, relatively young individuals.3, 4, 5, 9 Corresponding to the latter, we found the strongest association between higher 24‐hour SBP and shorter sleep duration in the LIMBS study, which targeted relatively healthy, prehypertensive subjects, and excluded individuals with other medical comorbidities such as diabetes, chronic kidney disease, congestive heart failure, and cardiovascular disease. PISA, on the other hand, which aimed to recruit higher risk adults, demonstrated a significant, but less robust relationship between 24‐hour SBP and sleep duration. Correspondingly, we did not find a significant association between sleep duration and BP control in sensitivity analyses of PISA patients on antihypertensive medications. Although we performed subgroup analyses of PISA excluding patients who were ineligible for LIMBS (which demonstrated a greater unadjusted difference in 24‐hour SBP between shorter and longer sleepers than in the primary analyses), we suspect these subgroup analyses were unable to sufficiently control for the higher morbidity in the patients selected for the PISA study. We hypothesize that the stronger relationship between SBP and sleep duration in the younger, healthier cohort may be related to younger vascular age, resulting in more sensitive vascular response to sympathetic stimuli.37
Both short sleep duration38, 39 and nondipping23, 40 are particularly prevalent in patients with OSA and obesity. The underlying pathophysiology of hypertension, OSA, and obesity are closely interrelated, with increased rates of cardiovascular morbidity and mortality observed in these patient populations.26, 41 OSA, which is commonly seen in obese patients, gives rise to a surge in sympathetic tone during apneic events, followed by an elevation in BP that persists for hours after awakening.27, 28 Based on this physiologic mechanism, we expected that decreased sleep duration in obese patients and patients with OSA would be associated with higher risk of nondipping and elevated 24‐hour SBP readings. However, OSA and obesity did not significantly modify the association between 24‐hour ABPM and sleep duration. Thus, we hypothesize that the sympathetic mechanisms driving hypertension in OSA may contribute similarly to elevated 24‐hour SBP in patients with shorter sleep duration, regardless of OSA status; this is further supported by our finding of higher 24‐hour heart rate with shorter sleep duration. The lack of association of dipping with shorter sleep duration in our study may be related to differences in continuous sleep and sleep quality between subjects with shorter sleep duration and those with OSA. Accordingly, the physiologic mechanisms linking 24‐hour BP and sleep duration require further investigation.
While the current study is strengthened by validation of the results across two distinctive cohorts that benefited from prospective data collection, there are also important limitations. Self‐reported sleep duration may be prone to misclassification of sleep duration due to differences in sleep quality, which is known to be associated with nocturnal dipping,42 and recall bias. We attempted to address this by assessing for correlation between self‐reported sleep duration and objective sleep duration by actigraphy data obtained in PISA; we also performed sensitivity analyses using the sleep duration determined by the actigraphy data for the multivariable analyses, which corroborated our findings. Additionally, we did not have information available on the reason for shorter sleep duration (eg, poor sleep hygiene, insomnia, anxiety, sleep disorder), besides the diagnosis of OSA in many PISA patients, which would potentially provide insight for future areas of intervention. As this was a cross‐sectional study, we also did not have information on the length of time over which the subjects were exposed to shorter sleep duration (eg, days, months, years); nonetheless, we are not aware of any literature that has identified if differing lengths of exposure to short sleep duration alter the association of short sleep duration with adverse outcomes. Lastly, the cross‐sectional nature of the study limits the ability to draw conclusions regarding temporal relationship between sleep duration and 24‐hour ABPM measurements. Furthermore, there may have been environmental and demographic differences across the Philadelphia and Iceland groups that could not be accounted for by statistical adjustment; for example, it is possible that differential seasonal exposure to light may impact circadian variations in BP across the two geographic groups.43
In conclusion, the association between shorter sleep duration and hypertension seems to be most strongly discernable by 24‐hour ABPM. With an increasing emphasis on out‐of‐office BP measurement when screening for hypertension,44, 45 patients with self‐reported shorter sleep duration in particular may benefit from 24‐hour ABPM to promote earlier detection of elevated BP readings and perhaps attenuate longitudinal adverse outcomes. Future studies should evaluate the effects of interventions to address elevated BP and sleep duration (such as lifestyle modifications, pharmacologic management of hypertension, and therapies to improve sleep duration) on mitigating end organ damage in patients with shorter sleep duration.
CONFLICT OF INTEREST
The authors of this manuscript declare no relevant conflicts of interest.
Supporting information
ACKNOWLEDGMENTS
None
Shulman R, Cohen DL, Grandner MA, et al. Sleep duration and 24‐hour ambulatory blood pressure in adults not on antihypertensive medications. J Clin Hypertens. 2018;20:1712–1720. 10.1111/jch.13416
Funding information
This research was supported in part by the NIH (Bethesda, MD) grant numbers K23‐HL133843 (NHLBI, PI: Cohen JB), R01‐AT004921 (NCCAM, PI: Cohen DL), and P01‐HL094307 (NHLBI, PI: Pack AI). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the NIH
REFERENCES
- 1. O'Brien E, Sheridan J, O'Malley K. Dippers and non‐dippers. Lancet. 1988;2(8607):397. [DOI] [PubMed] [Google Scholar]
- 2. Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24‐h blood pressure: the Ohasama study. J Hypertens. 2002;20(11):2183‐2189. [DOI] [PubMed] [Google Scholar]
- 3. Gangwisch JE, Heymsfield SB, Boden‐Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833‐839. [DOI] [PubMed] [Google Scholar]
- 4. Mezick EJ, Hall M, Matthews KA. Sleep duration and ambulatory blood pressure in black and white adolescents. Hypertension. 2012;59(3):747‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cappuccio FP, Stranges S, Kandala NB, et al. Gender‐specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50(4):693‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McMullan CJ, Curhan GC, Forman JP. Association of short sleep duration and rapid decline in renal function. Kidney Int. 2016;89(6):1324‐1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kojima M, Wakai K, Kawamura T, et al. Sleep patterns and total mortality: a 12‐year follow‐up study in Japan. J Epidemiol. 2000;10(2):87‐93. [DOI] [PubMed] [Google Scholar]
- 8. Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all‐cause mortality: a systematic review and meta‐analysis of prospective studies. Sleep. 2010;33(5):585‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169(11):1055‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sabanayagam C, Shankar A. Sleep duration and cardiovascular disease: results from the National Health Interview Survey. Sleep. 2010;33(8):1037‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hermann DM, Bassetti CL. Role of sleep‐disordered breathing and sleep‐wake disturbances for stroke and stroke recovery. Neurology. 2016;87(13):1407‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grandner MA, Alfonso‐Miller P, Fernandez‐Mendoza J, Shetty S, Shenoy S, Combs D. Sleep: important considerations for the prevention of cardiovascular disease. Curr Opin Cardiol. 2016;31(5):551‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta‐analysis of prospective cohort studies. Hypertens Res. 2013;36(11):985‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fernandez‐Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60(4):929‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen DL, Bowler A, Fisher SA, et al. Lifestyle Modification in Blood Pressure Study II (LIMBS): study protocol of a randomized controlled trial assessing the efficacy of a 24 week structured yoga program versus lifestyle modification on blood pressure reduction. Contemp Clin Trials. 2013;36(1):32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim J, Mohler ER 3rd, Keenan BT, et al. Carotid artery wall thickness in obese and nonobese adults with obstructive sleep apnea before and following positive airway pressure treatment. Sleep. 2017;40(9); zsx126. 10.1093/sleep/zsx126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anwar YA, Tendler BE, McCabe EJ, Mansoor GA, White WB. Evaluation of the Datascope Accutorr Plus according to the recommendations of the Association for the Advancement of Medical Instrumentation. Blood Press Monit. 1997;2(2):105–110. [PubMed] [Google Scholar]
- 18. El Assaad MA, Topouchian JA, Darne BM, Asmar RG. Validation of the Omron HEM‐907 device for blood pressure measurement. Blood Press Monit. 2002;7(4):237‐241. [DOI] [PubMed] [Google Scholar]
- 19. O'Brien E, Mee F, Atkins N, O'Malley K. Accuracy of the SpaceLabs 90207 determined by the British Hypertension Society protocol. J Hypertens. 1991;9(6):573‐574. [DOI] [PubMed] [Google Scholar]
- 20. Marino M, Li Y, Rueschman MN, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747‐1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen JB, Cohen DL. Integrating out‐of‐office blood pressure in the diagnosis and management of hypertension. Curr Cardiol Rep. 2016;18(11):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mena L, Pintos S, Queipo NV, Aizpurua JA, Maestre G, Sulbaran T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23(3):505‐511. [DOI] [PubMed] [Google Scholar]
- 23. Kotsis V, Stabouli S, Bouldin M, Low A, Toumanidis S, Zakopoulos N. Impact of obesity on 24‐hour ambulatory blood pressure and hypertension. Hypertension. 2005;45(4):602‐607. [DOI] [PubMed] [Google Scholar]
- 24. Sakhuja A, Textor SC, Taler SJ. Uncontrolled hypertension by the 2014 evidence‐based guideline: results from NHANES 2011‐2012. J Hypertens. 2015;33(3):644‐651; discussion 652. [DOI] [PubMed] [Google Scholar]
- 25. Cohen JB. Hypertension in obesity and the impact of weight loss. Curr Cardiol Rep. 2017;19(10):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378‐1384. [DOI] [PubMed] [Google Scholar]
- 27. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia–influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15(12 Pt 2):1593‐1603. [DOI] [PubMed] [Google Scholar]
- 29. Spira AP, Chen‐Edinboro LP, Wu MN, Yaffe K. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psych. 2014;27(6):478‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lutsey PL, Misialek JR, Mosley TH, et al. Sleep characteristics and risk of dementia and Alzheimer's disease: the atherosclerosis risk in communities study. Alzheimer Dementia. 2018;14(2):157‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McDonald C, Pearce MS, Kerr SR, Newton JL. Blood pressure variability and cognitive decline in older people: a 5‐year longitudinal study. J Hypertens. 2017;35(1):140‐147. [DOI] [PubMed] [Google Scholar]
- 32. Yano Y, Butler KR, Hall ME, et al. Associations of nocturnal blood pressure with cognition by self‐identified race in middle‐aged and older adults: the GENOA (Genetic Epidemiology Network of Arteriopathy) Study. J Am Heart Assoc. 2017;6(11): e007022. 10.1161/JAHA.117.007022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yano Y, Ning H, Muntner P, et al. Nocturnal blood pressure in young adults and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Hypertens. 2015;28(10):1240‐1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of "masked" hypertension and "white‐coat" hypertension detected by 24‐h ambulatory blood pressure monitoring 10‐year follow‐up from the Ohasama study. J Am Coll Cardiol. 2005;46(3):508–515. [DOI] [PubMed] [Google Scholar]
- 35. Banegas JR, Ruilope LM, de la Sierra A, et al. Relationship between Clinic and ambulatory blood‐pressure measurements and mortality. N Engl J Med. 2018;378(16):1509‐1520. [DOI] [PubMed] [Google Scholar]
- 36. Friedman O, Shukla Y, Logan AG. Relationship between self‐reported sleep duration and changes in circadian blood pressure. Am J Hypertens. 2009;22(11):1205‐1211. [DOI] [PubMed] [Google Scholar]
- 37. Bruno RM, Ghiadoni L, Seravalle G, Dell'oro R, Taddei S, Grassi G. Sympathetic regulation of vascular function in health and disease. Front Physiol. 2012;3:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stranges S, Cappuccio FP, Kandala NB, et al. Cross‐sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: the Whitehall II Study. Am J Epidemiol. 2008;167(3):321‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim NH, Lee SK, Eun CR, et al. Short sleep duration combined with obstructive sleep apnea is associated with visceral obesity in Korean adults. Sleep. 2013;36(5):723‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suzuki M, Guilleminault C, Otsuka K, Shiomi T. Blood pressure "dipping" and "non‐dipping" in obstructive sleep apnea syndrome patients. Sleep. 1996;19(5):382‐387. [DOI] [PubMed] [Google Scholar]
- 41. Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237‐245. [DOI] [PubMed] [Google Scholar]
- 42. Loredo JS, Nelesen R, Ancoli‐Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in normal adults. Sleep. 2004;27(6):1097‐1103. [DOI] [PubMed] [Google Scholar]
- 43. Obayashi K, Saeki K, Iwamoto J, Ikada Y, Kurumatani N. Association between light exposure at night and nighttime blood pressure in the elderly independent of nocturnal urinary melatonin excretion. Chronobiol Int. 2014;31(6):779‐786. [DOI] [PubMed] [Google Scholar]
- 44. Piper MA, Evans CV, Burda BU, et al. Screening for high blood pressure in adults: a Systematic Review for the U.S. Preventative Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality (US); 2014. [PubMed] [Google Scholar]
- 45. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13‐e115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


