Abstract
TCPOBOP (1,4-Bis [2-(3,5-Dichloropyridyloxy)] benzene) is a constitutive androstane receptor (CAR) agonist that induces robust hepatocyte proliferation and hepatomegaly without any liver injury or tissue loss. TCPOBOP-induced direct hyperplasia has been considered to be CAR-dependent with no evidence of involvement of cytokines or growth factor signaling. Receptor tyrosine kinases (RTKs), MET and EGFR, are known to play a critical role in liver regeneration after partial hepatectomy, but their role in TCPOBOP-induced direct hyperplasia, not yet explored, is investigated in the current study. Disruption of the RTK-mediated signaling was achieved utilizing MET KO mice along with Canertinib treatment for EGFR inhibition. Combined elimination of MET and EGFR signaling [MET KO + EGFRi], but not individual disruption, dramatically reduced TCPOBOP-induced hepatomegaly and hepatocyte proliferation. TCPOBOP-driven CAR activation was not altered in [MET KO + EGFRi] mice, as measured by nuclear CAR translocation and analysis of typical CAR target genes. However, TCPOBOP induced cell cycle activation was impaired in [MET KO + EGFRi] mice due to defective induction of cyclins, which regulate cell cycle initiation and progression. TCPOBOP-driven induction of FOXM1, a key transcriptional regulator of cell cycle progression during TCPOBOP-mediated hepatocyte proliferation, was greatly attenuated in [MET KO + EGFRi] mice. Interestingly, TCPOBOP treatment caused transient decline in HNF4α expression concomitant to proliferative response; this was not seen in [MET KO + EGFRi] mice. Transcriptomic profiling revealed vast majority (~40%) of TCPOBOP-dependent genes mainly related to proliferative response, but not to drug metabolism, were differentially expressed in [MET KO + EGFRi] mice.
Conclusion
Taken together, combined disruption of EGFR and MET signaling lead to dramatic impairment of TCPOBOP-induced proliferative response without altering CAR activation.
Keywords: Constitutive androstane receptor (CAR), Cyclin D1, Forkhead box protein M1 (FOXM1), Hepatocyte Nuclear Factor 4 alpha (HNF4α) and Yes associated protein (YAP)
INTRODUCTION
Liver has an extraordinary capacity to regenerate upon tissue loss. Liver regeneration can occur as a compensatory response to toxin-induced liver injury, surgical resection or infection (1). Interestingly, many xenobiotics, retinoic acids and thyroid hormone can stimulate hepatocyte proliferation and cause liver enlargement in absence of liver injury/tissue loss via activation of nuclear receptors belonging to steroid/thyroid superfamily (such as constitutive androstane receptor (CAR), peroxisome proliferator-activated receptor-α (PPAR-α), retinoic acid receptor (RAR), thyroid hormone receptor (TR)) (2). 1,4-Bis [2-(3,5-Dichloropyridyloxy)] benzene (TCPOBOP) is a CAR agonist which induces robust hepatocyte proliferation and hepatomegaly after acute administration and is one of the strongest known chemical mitogens to mouse liver (3). TCPOBOP directly binds to CAR leading to its nuclear translocation and activation of its target genes. TCPOBOP-induced proliferative response is very rapid and comparable to proliferative response after 2/3 partial hepatectomy (PHx) (4). The regenerative response induced by treatment with TCPOBOP can protect liver from failure even after massive tissue loss (91% hepatectomy), indicating great clinical potential (5). However, signaling mechanisms of cell proliferation induced by TCPOBOP or any other nuclear receptor agonists have not been extensively explored.
MET and epidermal growth factor receptor (EGFR) are receptor tyrosine kinases (RTKs) and play an important role in liver pathobiological processes including development, proliferation and hepatocellular cancer. Ligands for EGFR (such as EGF and transforming growth factor alpha (TGF-α)) and MET (hepatocyte growth factor (HGF)) are the only known direct mitogens for liver which can cause hepatocyte proliferation in vitro (in chemically defined serum free media) and in vivo (1). Our recent study demonstrated that combined elimination of MET and EGFR signaling results in complete inhibition of liver regeneration after PHx (6). No other study has previously shown complete abolition of liver regeneration in any model after inhibition of any extracellular signaling pathways (either alone or in combination), highlighting that availability of combined MET and EGFR signaling pathways is indispensable for liver regeneration.
Previous study utilizing CAR-KO mice have shown that TCPOBOP-induced hyperplasia is completely dependent on CAR (7). However, proliferative signaling pathways underlying this CAR-initiated response are not well characterized. The mechanisms underlying TCPOBOP-induced direct hyperplasia are considered to be unrelated to classical regenerative pathways, with studies showing no role of cytokines such as IL-6 and TNF-α (4, 8–12). Recent studies have indicated emerging role of various signaling mediators in this context such as β-catenin, Hippo signaling/Yes-associated protein (YAP), cMyc, Forkhead box protein M1 (FOXM1) and mir122 (13–17). However, the role of RTKs (specifically MET and EGFR) is not yet explored. Considering the indispensable role of MET and EGFR for liver regeneration, we hypothesized that these receptors are also important for TCPOBOP-induced hepatocyte proliferation and hepatomegaly. Here we report, that combined MET KO and EGFR inhibition results in dramatic attenuation of TCPOBOP-induced proliferative response in liver without altering CAR activation. No previous studies, to our knowledge, have shown such a remarkable effect on TCPOBOP-induced proliferative response by altering any mediator other than its direct interacting nuclear receptor, CAR. Our study changes the paradigm that nuclear receptor agonist mediated direct hyperplasia is unrelated to signaling of liver regeneration.
MATERIALS AND METHODS
Animals, treatments and tissue harvesting
METfl/fl: TamCre+/+ mice with a targeted deletion for exon 16 were generated and characterized as described previously (6). These mice were injected i.p. for five consecutive days with tamoxifen (100 μl of a 10 mg/ml solution in corn oil) to obtain MET KO mice. Control group consisted of littermates injected with vehicle (corn oil) only. Single dose of TCPOBOP (3 mg/kg; prepared in corn oil) was administered by oral gavage at-least 5 days after last tamoxifen injection. For EGFR inhibition, Canertinib, an EGFR inhibitor (EGFRi) was administered to mice in diet at 80 mg/kg/day as described previously (6). Canertinib is a very potent and highly selective irreversible EGFRi, which have been used previously to effectively inhibit EGFR signaling in liver (6, 18, 19). Canertinib diet was initiated one day prior to TCPOBOP injection. For combined inhibition of MET and EGFR [MET KO + EGFRi], Canertinib diet was administered to MET KO mice at least 4 days after tamoxifen injection. Combined MET KO + EGFR inhibition strategy was previously used by our group to successfully block both RTKs signaling in PHx model (6). For initial studies, mice from control, EGFRi, MET KO and [MET KO + EGFRi] groups (n = 6–8) were sacrificed at 2 day (48 hr) after TCPOBOP administration (experimental design shown in Fig. 1A). For time-course analysis, mice from control and [MET KO + EGFRi] group (n = 6– 10) were euthanized at various time points (0, 1, 2, 5 and 10 days) after TCPOBOP administration (experimental design shown in Fig. 2A).
Figure 1. Effect of MET KO, EGFR inhibition or combination on TCPOBOP-induced hepatomegaly and hepatocyte proliferation.
(A) Schematics showing experimental design and treatment groups. (B) Bar graphs showing liver to body weight ratio. (C) Percentage of hepatocytes in DNA synthesis (Ki67 positive nuclei). (D) Representative photomicrographs of Ki67 stained (brown nuclei) liver sections (magnification: 400x). All liver samples were collected from various groups of mice (Control: vehicle-treated wild type; EGFRi: Canertinib-treated (80 mg/kg/day) wild type; MET KO; and [MET KO + EGFRi]: Canertinib–treated MET KO) at 48 hr after TCPOBOP treatment. No TCPOBOP group in (B) was similar to control group but without TCPOBOP treatment. # and * indicate significant difference w.r.t. no TCPOBOP and control group, respectively, at P < 0.05.
Figure 2. TCPOBOP-induced hepatomegaly and hepatocyte proliferation attenuated after combined MET KO and EGFR inhibition.
(A) Schematics showing experimental design and treatment groups. (B) Bar graphs showing liver to body weight ratio. (C) Representative photomicrographs of Ki67 stained (brown nuclei) liver sections (magnification: 400x). (D) Percentage of hepatocytes in DNA synthesis (Ki67 positive nuclei). All samples were collected from control and Canertinib-treated MET KO mice [MET KO + EGFRi] at various time-points after TCPOBOP treatment. * indicates significant difference w.r.t. control group at particular time-point; P < 0.05.
Other methods details are provided in supplementary information.
RESULTS
TCPOBOP-induced hepatomegaly and hepatocyte proliferation remarkably attenuated after combined MET KO and EGFR inhibition
MET KO mice or EGFR inhibitor-treated mice (EGFRi) were administered TCPOBOP. Since these RTKs are known to compensate for each other (6, 20), MET KO mice were treated with EGFR inhibitor [MET KO + EGFRi], for combined disruption of MET and EGFR signaling. Female mice were used in all these studies, unless specified, as TCPOBOP-induced proliferative response is known to be higher in females (21). Liver to body weight ratio and hepatocyte proliferation were analyzed at 48 hr after TCPOBOP treatment (Fig. 1A–D). As expected, TCPOBOP-treated control mice showed remarkable (1.6 fold vs no TCPOBOP control) increase in liver to body weight (LW/BW) ratio at 48 hr (Fig. 1B). EGFRi mice had similar increase and MET KO displayed slightly lower increase, but not significantly different compared to control. Strikingly, [MET KO + EGFRi] mice did not show any increase in LW/BW ratio (1.008 fold vs no TCPOBOP control) (Fig. 1B). Robust hepatocyte proliferation was observed in control, EGFRi and MET KO groups with no significant difference between these groups. However, proliferation was completely abolished in [MET KO + EGFRi] mice with almost no observable Ki67 positive cells (Fig. 1C and D). No effect of tamoxifen was observed on TCPOBOP-induced hepatocyte proliferation or hepatomegaly in METfl/fl: TamCre−/− mice (Suppl. Fig. 1A–C).
Since major difference was observed only in [MET KO + EGFRi] mice, this group was analyzed further. A time-course analysis (0 – 10 days after TCPOBOP treatment) was performed in these mice comparing to control mice (Fig. 2A–D). At Day 0 (prior to TCPOBOP treatment), LW/BW ratio was similar in [MET KO + EGFRi] and control mice indicating basal liver size is not altered. LW/BW ratio increased consistently with time in control group and was about 2.2 fold higher at Day 5 vs Day 0 (Fig. 2B). [MET KO + EGFRi] mice did not show any increase in LW/BW ratio at Day 1 and Day 2, but displayed slight increase at Day 5 (1.3 fold). LW/BW ratios in both the groups at Day 10 remain similar to respective Day 5 values indicating plateauing of hepatomegaly response. LW/BW ratio remained significantly lower in [MET KO + EGFRi] mice compared to control mice at all the analyzed time points after TCPOBOP treatment (1, 2, 5 and 10 days) (Fig. 2B). Similarly, hepatocyte proliferation increased in control mice with time, peaking at Day 2 and decreasing sharply at Day 5 (Fig. 2C and D). In contrast, proliferation was significantly lower in [MET KO + EGFRi] mice at Day 1 and 2, with no obvious Ki67 positive cells; some Ki67 positive cells were observed only at Day 5 (Fig. 2C and D). Similar protein expression profile was also observed for PCNA, another marker for proliferation (Fig. 4A). All these experiments were also repeated in male mice with similar findings (Suppl. Fig. 2A–C).
Figure 4. Impairment of cell cycle activation in [MET KO + EGFRi] mice.
(A) Western blot analysis of PCNA, Cyclin D1, phospho-Rb, p27, p21 and GAPDH. mRNA expression of (B) Cyclin A2, (C) Cyclin B1 and (D) Cyclin E1 in liver. All samples were collected at 0, 1, 2 and 5 days after TCPOBOP treatment in control and [MET KO + EGFRi] mice. * indicates significant difference w.r.t. control group at particular time-point; P < 0.05.
CAR activation after TCPOBOP treatment remained unaffected by combined MET KO and EGFR inhibition
TCPOBOP-induced hepatocyte proliferation is known to be CAR-dependent process; therefore, the activation status of CAR was analyzed (7). Upon TCPOBOP-binding, CAR translocates to nucleus and activates its target genes. Total CAR levels were not altered in [MET KO + EGFRi] mice (Fig. 3A). Nuclear CAR levels remarkably increased at Day 1 and 2 after TCPOBOP treatment in both control and [MET KO + EGFRi] mice compared to Day 0 (Fig. 3A). Further, induction of classical CAR target genes (Cyp2b10, Cyp2c55 and UGT1a1) was comparable in both control and [MET KO + EGFRi] mice after TCPOBOP treatment (Fig. 3B–D). Notably, Cyp2b10 was induced more than 4000 fold at all the time points in both the groups (Fig. 3B). Next, microarray analysis was performed on samples from control and [MET KO + EGFRi] groups at various time points (0, 1, 2 and 5 days) and data were analyzed using Ingenuity Pathway Analysis (IPA) to look at global picture of CAR target genes. Overall CAR target genes were induced similarly in both the groups at Day 2, as evidenced by comparable z-score for CAR activation in both the groups (Fig. 3E). High order of p-value (~2–3×10−21) of overlap between CAR target genes induced in both the experimental groups vs. CAR target genes in the reference genome demonstrate robust activation of CAR in both the groups (Fig. 3E). In fact, CAR turned out to be the topmost transcription factor predicted to be activated at all the time points (Day 1, 2 and 5) in both the groups compared to basal levels (Day 0) based on upstream regulator analysis using IPA (Suppl. Fig. 4B, CAR is represented as NR1I3). All these data clearly demonstrate that despite defective proliferative response, CAR activation remained unaltered in [MET KO + EGFRi] mice after TCPOBOP treatment.
Figure 3. CAR activation after TCPOBOP treatment remained unaltered in [MET KO + EGFRi] mice.
(A) Western blot analysis of nuclear CAR, total CAR and HDAC1 (nuclear loading control). mRNA expression of CAR target genes: (B) Cyp2b10, (C) Cyp2c55 and (D) UGT1a1 in liver. All samples were collected at 0, 1, 2 and 5 day after TCPOBOP treatment in control or [MET KO + EGFRi] mice. (E) Ingenuity pathway analysis (IPA) of microarray data showing activation of CAR downstream gene network in both control and [MET KO + EGFRi] group at Day 2 after TCPOBOP treatment compared to Day 0. Positive z-score represents predicted activation of transcription factor activity (absolute z-score > 2 considered as significant) based on expression profile of downstream genes. p-values signifies extent of overlap between set of downstream target genes of a given transcription factor in dataset compared to all known downstream target genes of that transcription factor in the reference genome. (red shapes: upregulated genes; green shapes: downregulated genes with intensity of color reflecting extent of upregulation or downregulation)
Impaired cell cycle activation in [MET KO + EGFRi] mice
At downstream level, both MET and EGFR are known to activate core cell cycle machinery and induce cyclins including Cyclin D1, which controls entry into the cell cycle. To further elucidate the mechanisms for attenuated proliferative response in [MET KO + EGFRi] mice, status of core cell cycle machinery was assessed. CyclinD1 protein expression increased at all the time points (1, 2 and 5 days) after TCPOBOP treatment in control mice compared to basal levels (0 day) (Fig. 4A). Cyclin D1 did not increase at all in [MET KO + EGFRi] mice at Day 1 and 2 and was remarkably lower compared to control group, increasing only at Day 5 (Fig. 4A). Cyclin D1 binds to Cyclin Dependent Kinase 4 triggering its activation, which then phosphorylates retinoblastoma (Rb) protein, ultimately resulting in induction of several cell cycle genes. Phosphorylation of Rb protein increased in control group at Day 1 compared to Day 0 and gradually decreased later on. However, phosphorylation of Rb protein remained very low at all the time points (including Day 5) in [MET KO + EGFRi] mice (Fig. 4A). Next, we investigated gene expression of Cyclin E1, Cyclin A2 and Cyclin B1 (Fig. 4B–D), which sequentially regulate progression through cell cycle. Apart from cyclin E1, which appeared not to be induced after TCPOBOP treatment, both of the other later stage cyclins (Cyclin A2 and Cyclin B1) were induced after TCPOBOP treatment in control group and were significantly lower at Day 1 and 2 in [MET KO + EGFRi] mice (Fig. 4B–D). Additionally, protein levels of major cell cycle inhibitory proteins (p21 and p27) were measured (Fig. 4A). p21 levels were similar between two groups across time-course, but induced in both the groups at Day 5. p27 levels moderately decreased at the time point of peak proliferation (Day 2) in control mice and return to basal levels at Day 5. However, p27 expression remained constant in [MET KO + EGFRi] mice across time-course and appeared to be higher compared to control mice at Day 2 (Fig. 4A).
Downregulation of FOXM1 transcription factor and its downstream gene network in [MET KO + EGFRi] mice
We also studied various signaling pathways downstream of MET and EGFR involved in hepatocyte proliferation. There was no apparent alteration of ERK, AKT and mTOR activation that could be consistent with decreased proliferation in [MET KO + EGFRi] mice (Suppl. Fig. 3A). β-catenin signaling is another pathway regulated by MET and reported to be involved in TCPOBOP-induced proliferation (13, 17, 22), was not altered in our model (Suppl. Fig. 3B). Our previous data and several other reports indicated that both MET and EGFR can regulate an important transcription factor, FOXM1, which governs transcription of genes important for DNA replication and mitosis (6, 23–27). FOXM1 is considered critical for inducing TCPOBOP-mediated hepatocyte proliferation (3, 5, 14, 16, 22, 28). Consistent with these previous reports, FOXM1 was remarkably induced by TCPOBOP at all the time points in control mice with peak induction (10- fold) coinciding with the time point of peak proliferation (Day 2) (Fig. 5A). Interestingly, there was a significant delay in FOXM1 induction in [MET KO + EGFRi] mice. FOXM1 induction was significantly attenuated in [MET KO + EGFRi] at Day 1 and 2, but was higher at Day 5 compared to control (Fig. 5A). IPA analysis of microarray data further substantiated downregulation of FOXM1 in [MET KO + EGFRi] mice compared to control; several FOXM1 inducible genes which were upregulated in control mice were greatly downregulated in [MET KO + EGFRi] mice at Day 2 (Fig. 5B and Suppl. Table 1). In fact, FOXM1 was among the top transcription factors predicted to be inhibited (activation z-score: −3.164) in [MET KO + EGFRi] mice compared to control based on upstream regulator analysis using IPA (Fig. 5B and Fig. 7D). Further, downregulation of FOXM1 was confirmed by investigating expression of several FOXM1 target genes (Birc5/Survivin, Aurora B Kinase, Polo-like Kinase 1, Cyclin B2, Cdc20, Cenpa, Kif20a and Cdk1) using real time PCR; all these genes were significantly downregulated at Day 1 and 2 in [MET KO + EGFRi] mice (Fig. 5C). Previous studies indicated cMyc as an important transcriptional regulator for induction of FOXM1 during TCPOBOP-mediated proliferation (16). cMyc is also a known downstream effector of both MET and EGFR. Although cMyc expression was increased at RNA and protein level after TCPOBOP treatment in both the groups, cMyc expression was not lower in [MET KO + EGFRi] group (Fig. 5D and Suppl. Figure 3A).
Figure 5. Downregulation of FOXM1 transcription factor and its network genes in [MET KO + EGFRi] mice.
mRNA expression of (A) FOXM1, (C) FOXM1 target genes (Birc5/Survivin, AurkB/Aurora B Kinase, Plk1/Polo-like Kinase 1, Cyclin B2, Cdc20, Cenpa, Kif20a and Cdk1) and (D) cMyc at 0, 1, 2 and 5 days after TCPOBOP treatment as analyzed using real-time PCR. (B) Ingenuity Pathway Analysis (IPA) of microarray data showing inhibition of FOXM1 downstream gene network in [MET KO + EGFRi] group vs control at Day 2 after TCPOBOP treatment. Negative z-score in (B) represents predicted inhibition of transcription factor activity (absolute z-score > 2 considered as significant) based on expression profile of downstream genes. p-values signifies extent of overlap between set of downstream target genes of a given transcription factor in dataset compared to all known downstream target genes of that transcription factor in the reference genome. (red shapes: upregulated genes; green shapes: downregulated genes with intensity of color reflecting extent of upregulation or downregulation) * indicates significant difference w.r.t. control group at particular time-point; P < 0.05.
Figure 7. Global changes in gene expression profile after combined MET KO and EGFR inhibition.
(A) Venn diagram with overlap showing subset of genes altered after TCPOBOP treatment in control mice at Day 2 vs Day 0 and also altered in [MET KO + EGFRi] group vs control at Day 2. (B) Enrichment analysis using DAVID showing biological processes (GO terms) altered in [MET KO + EGFRi] mice based on genes induced by TCPOBOP in control mice (compared to basal levels), but downregulated in [MET KO + EGFRi] mice compared to control mice at Day 2. (C) Canonical signaling pathways and (D) transcription factors predicted to be altered in [MET KO + EGFRi] group vs control at Day 2 based on set of genes shown as overlap in (A), analyzed using IPA. The orange (positive z-score) and blue (negative z-score) colored bars in (C) indicate predicted pathway activation, or predicted inhibition, respectively, based on z-scores. Gray bars indicate pathways where no prediction on directionality of activity can be made. Threshold for –log(p-value) to be considered significant in (C) was 1.3.
Effect on HNF4α expression after combined MET KO and EGFR inhibition
Transcription factor, HNF4α, is important for maintaining differentiated state and inhibiting proliferation in quiescent liver (29–32). Several studies indicate crosstalk between CAR and HNF4α involving direct competition (14, 33–35). Here we report that HFN4α protein expression decreases dramatically after TCPOBOP treatment in control mice (Fig. 6A). HNF4α expression was lowest at Day 2 after TCPOBOP treatment in control mice, coinciding with the time point of peak proliferation, and increased later on at Day 5 (Fig. 6A). In contrast, HNF4α expression was maintained in MET KO +EGFRi mice after TCPOBOP treatment and was strikingly higher compared to control mice at Day 1 and 2 (Fig. 6A). Similar changes were also observed at transcriptional level; HNF4α mRNA levels were significantly higher at Day 1 and 2 in MET KO +EGFRi mice compared to control mice (Fig. 6B). However, these changes were not as striking as observed at protein level.
Figure 6. Effect on (A–B) HNF4α expression, (C) Hippo signaling, and (D) Gadd45β after combined MET KO and EGFR inhibition.
(A) Protein and (B) mRNA expression of HNF4α. (C) Western blot analysis showing protein expression of p-MST1, MST1, p-LATS1, LATS1, p-YAP, YAP (total and nuclear) and GAPDH. (D) mRNA and (E) protein expression of Gadd45β with western blot densitometry shown in (F). All samples were collected at various time-points after TCPOBOP treatment in control and [MET KO + EGFRi] mice. * indicates significant difference w.r.t. control group at particular time-point; P < 0.05.
Effect on Hippo signaling pathway after combined MET KO and EGFR inhibition
Organ size including that of liver is known to be tightly controlled by transcriptional regulator, yes-associated protein (YAP). Overexpression of YAP in a normal mouse liver results in hepatocyte proliferation and hepatomegaly (36). Recent studies also indicate a role of YAP in controlling TCPOBOP-induced hepatocytes proliferation (15). Hippo signaling pathway mediators (MST1/2 and LATS1/2) negatively regulates nuclear YAP levels (via its phosphorylation leading to degradation), which were studied in our model (Fig. 6C). Activated phospho-MST1 and phospho-LATS1 were higher in [MET KO + EGFRi] mice compared to control mice at Day 2, along with slightly higher phospho-YAP levels at the same time (Fig. 6C). However, these changes were not reflected at nuclear YAP levels as nuclear YAP was not decreased in [MET KO + EGFRi] mice compared to control (Fig. 6C). Nuclear YAP levels increased at Day 1 and 2 compared to Day 0 both in control and [MET KO + EGFRi] mice. Total YAP levels were not altered at any time point (Fig. 6C). Overall these data suggest that the inhibitory effect of combined MET KO + EGFR inhibition on TCPOBOP-induced proliferative response is not mediated by Hippo pathway and YAP.
Gadd45β is another transcription factor that is reported to control liver size after TCPOBOP treatment and also known to be induced after PHx (37). Gadd45β KO mice have marked growth delay without reduced proliferation after TCPOBOP treatment (37). Further, our previous study showed that Gadd45β gene expression is altered remarkably after combined MET KO and EGFR inhibition in PHx model (lower induction during peak regenerative phase and increased induction during termination compared to wild-type mice) (6). Therefore, Gadd45β expression was analyzed in our model (Fig. 6D–F). Gadd45β gene expression was notably upregulated after TCPOBOP-treatment in control mice (compared to basal levels). Gadd45β induction appear to be lower in [MET KO + EGFRi] mice compared to control at Day 1 and 2, but did not attain statistical significance (Fig. 6D). Gadd45β protein expression displayed similar pattern with moderate but significant downregulation in [MET KO + EGFRi] mice compared to control at Day 1 (Fig. 6E and F). Analysis of microarray data using IPA revealed Gadd45β signaling as one of the top canonical pathway predicted to be altered in [MET KO + EGFRi] mice compared to control (Fig. 7C and Suppl. Fig. 5A).
Global changes in gene expression profile after combined MET KO and EGFR inhibition
Next, we investigated changes in global gene expression profile using microarray. Both intra-group (temporal) and inter-group analysis was performed using IPA. Based on gene signature analysis, SREBF1, SREBF2, and SREBF cleavage-activating protein (SCAP) were among some of the top upstream regulators that were predicted to be inhibited consistently at all the time points (except Day 5) in [MET KO + EGFRi] group compared to control (Suppl. Fig. 4A). SREBF1 has previously reported as co-transcriptional regulator affecting CAR-driven transcriptional activity (38). Similar alteration was observed in some of the canonical signaling pathways related to cholesterol biosynthesis and LXR/RXR activation in [MET KO + EGFRi] mice compared to control mice (Suppl. Fig. 5A). Temporal analysis revealed, as mentioned previously, CAR (NR1I3) as the topmost upstream regulator predicted to be activated in both groups at all the time points compared to basal levels (Suppl. Fig. 4B). However, several upstream regulators were predicted to be altered only in [MET KO + EGFRi] mice (but not in control mice) consistently at all the time points after TCPOBOP treatment compared to basal levels (Suppl. Fig. 4B). One of such transcription factors was TRIM24 (a.k.a. transcriptional intermediary factor 1 alpha), which was predicted to be activated only in [MET KO + EGFRi] mice (Suppl. Fig. 4B). A recent study demonstrated TRIM24 to directly interact with CAR as a coregulator at DNA binding site and alter its transcriptional activity (39). TRIM24 is also known co-repressor of RARs and inhibits proliferation in hepatocytes (40).
To filter out genes relevant to TCPOBOP treatment, we specifically looked at genes which were altered (upregulated/downregulated at least 2-fold) after TCPOBOP treatment compared to basal levels in control mice and were, at the same time, also differentially altered (at least 2-fold) in [MET KO + EGFRi] mice compared to control mice. Interestingly, out of 1248 genes which were altered by TCPOBOP treatment at Day 2 compared to Day 0, around 40% (497 genes) were differentially expressed in [MET KO + EGFRi] mice compared to control mice at Day 2 (Fig. 7A). This demonstrates significant genome-wide impact of combined MET KO and EGFR inhibition on TCPOBOP response. These mostly included genes which were induced by TCPOBOP and downregulated in [MET KO + EGFRi] mice (249 genes; top 50 listed in Suppl. Table 2), along with genes which were repressed by TCPOBOP but upregulated in [MET KO + EGFRi] mice compared to controls (224 genes; top 50 listed in Suppl. Table 3). Analysis of the specific genes which were induced by TCPOBOP and downregulated in [MET KO + EGFRi] mice using DAVID (Fig. 7B) revealed that these genes were significantly enriched for biological processes mainly related to cell proliferation such as cell cycle (specific genes pertaining to this GO term are listed in Suppl. Table 4), cell division, mitotic nuclear division, mitotic sister chromatid segregation and DNA replication. Analysis of all the 497 genes (shown as overlap in Fig. 7A) using IPA, taking directionality of change into account, predicted several canonical pathways to be significantly altered in [MET KO + EGFRi] mice vs control mice, including activation of ATM signaling, G2/M DNA damage check point regulation, acute phase signaling, p53 signaling and inhibition of signaling related to mitotic role of polo-like kinase (Fig. 7C). Further, based on downstream gene signatures, IPA predicted alteration of several transcriptional factors relevant to cell proliferation (Fig. 7D), including significant inhibition of E2F1, E2F2, E2F3, FOXM1 and activation of CDKN2A or p16 in [MET KO + EGFRi] mice. TBX2 and NR1I2 (PXR) were the top two transcription factors predicted to be inhibited in [MET KO + EGFRi] mice (Fig. 7D). Whereas, TBX2 is known to promote cell proliferation and tumorigenesis in several organ systems (41), PXR is reported to potentiate TCPOBOP/CAR-mediated hepatocyte proliferation (42).
DISCUSSION
Signaling via MET and EGFR is considered critical for regenerative response in liver with HGF (ligand for MET) and EGFR ligands as only known direct mitogens for hepatocytes (1). Numerous previous studies in the PHx model of liver regeneration show that inhibition/elimination of any single extracellular signaling mediator can only delay regenerative response but cannot permanently abolish liver regeneration due to induction of compensatory pathways (1). However, our previous work demonstrated that combined inhibition of MET and EGFR results in complete inhibition of liver regeneration after PHx (6). Here, we report that combined MET KO and EGFR inhibition results in dramatic attenuation of hepatocyte proliferation and hepatomegaly induced by CAR agonist, TCPOBOP. No previous studies, to our knowledge, have shown such a remarkable effect on TCPOBOP-induced proliferative response by altering any mediator other than its direct interacting nuclear receptor, CAR. Several previous studies have suggested that the proliferative response of TCPOBOP was dependent on CAR and not sharing or dependent on signaling pathways operative during liver regeneration after PHx (4, 8–12). Our current findings in this study change this paradigm. The data presented in this study demonstrate that whereas CAR activation alone can trigger all responses related to activation of drug metabolism, hepatomegaly is dependent on pathways which are controlled by MET and EGFR, requiring cooperative signaling of the two RTKs in order for CAR to exert its effects on hepatocyte proliferation. These results along with our previous data in PHx model (6) indicate that combined functioning of EGFR and MET is quintessential for inciting any proliferative response in hepatocytes and may be very critical for maintaining proliferative environment in liver. This is not surprising considering that quiescent liver is constantly exposed to “tonic” effects of both HGF (embedded in hepatic extracellular matrix in high amount) and EGF (constantly available via portal circulation from Brunner’s glands of the duodenum) (1). Thus, both these growth factors and their receptors constantly affect the protein interactome of quiescent liver. It is of interest, however, that each one of the two RTKs was sufficient to allow TCPOBOP proliferative response, indicating that EGFR and MET can compensate for each other in this process as reported previously in the PHx model (6, 20).
One of the most interesting finding of this study was that neither CAR nuclear translocation nor induction of its classical targets (drug metabolic enzymes such as Cyp2b10) were affected in [MET KO + EGFRi] mice, despite remarkable effects on proliferative genes and hepatocyte proliferation. This differential effect suggests that TCPOBOP-driven detoxification and proliferative response, although dependent on initial CAR activation, require diverse co-transcriptional regulators or downstream mediators. Similar differential response has been reported previously, where repeated administration of TCPOBOP did not result in further increase hepatocyte proliferation and liver size, but classic CAR target gene involved in drug metabolism (Cyp2b10) was remarkably upregulated (15). This differential attenuation of proliferation was attributed to decrease in YAP activity, which appeared not to be altered in our model. Inverse effect was observed in female β-catenin KO and Gadd45β KO mice, which displayed lower level of drug metabolic enzymes but increased proliferative response to TCPOBOP treatment (22, 37).
FOXM1 is one of the major transcription factors that regulate cell cycle at multiple levels including progression to S-phase and mitosis. It regulates transcription of cyclins (such as cyclin B1), Cdc25 phosphatases (that activate cyclin-dependent kinases), several mitotic regulators (such as Polo Like Kinase 1, Aurora B kinase and Survivin) and also negatively regulate expression of cell cycle inhibitors such as p27. FOXM1 is considered a critical mediator of TCBOBOP-induced proliferative response (3, 5, 14, 16, 22, 28). FOXM1 was reported to be required for improved regenerative response triggered by TCPOBOP treatment in extensive liver resection (86%) model and also important for maintaining TCPOBOP-induced proliferative response in aged-mice (5, 28). Our previous microarray data and various other studies indicated that both EGFR and MET can regulate FOXM1 expression (6, 23–27). Here we report that TCPOBOP-driven FOXM1 induction and its downstream gene network activation was remarkably impaired in [MET KO + EGFRi] mice, which might contribute to attenuated proliferation in our model. Conversely, inverse phenotype to our model (i.e. increased proliferation but reduced expression of drug metabolic enzymes) previously reported in β-catenin KO mice upon TCPOBOP treatment was attributed to increased FOXM1 levels (22).
Another important transcription factor that was differentially altered in our model was HNF4α. Sharp decline in HNF4α expression was associated with robust proliferative response upon TCPOBOP treatment in control mice. This decline was not observed in [MET KO + EGFRi] mice, which might also contribute to proliferative defect in these mice. This is supported by the fact that liver-specific HNF4α KO mice show hyper-proliferative liver phenotype with hepatomegaly similar to TCPOBOP treatment (29–31). Further, apart from its role in liver differentiation, HNF4α is known to negatively regulate a myriad of proliferative genes in liver (29–32). Several studies have demonstrated crosstalk between CAR and HNF4α showing competition for same DR1 DNA binding sites and co-transcription factors and regulation of genes in opposite direction (14, 33–35). Role of HNF4α in CAR induced proliferation is also supported by a recent study demonstrating that activated CAR after TCPOBOP treatment can displace HNF4α from Pri-miR-122 binding site, suppressing expression of miR-122, which was associated with proliferative response via cMyc/FOXM1 axis (14).
It was impressive to see that 40% of the genes altered by TCPOBOP treatment in control mice were differentially regulated in [MET KO + EGFRi] mice. These genes were mostly enriched for biological processes regulating various aspects of cell proliferation including DNA replication, mitotic nuclear division, mitotic cytokinesis and check point regulation. This demonstrates enormous global impact of combined MET KO + EGFR inhibition on TCPOBOP-induced proliferative response in mice. Several canonical signaling pathways and transcription factors activity (some of them are known co-transcriptional regulators of CAR) were also predicted to be altered in [MET KO + EGFRi] mice, which was relevant to impaired proliferative response. Overall, TCPOBOP-induced proliferative response involves complex cybernetics of several signaling mediators, not merely CAR activation, and combined elimination of MET and EGFR signaling appears to disrupt this proliferative gene expression algorithm and protein interactome, without having major impact on CAR target genes associated with drug metabolism (illustrated by schematics in Fig. 8).
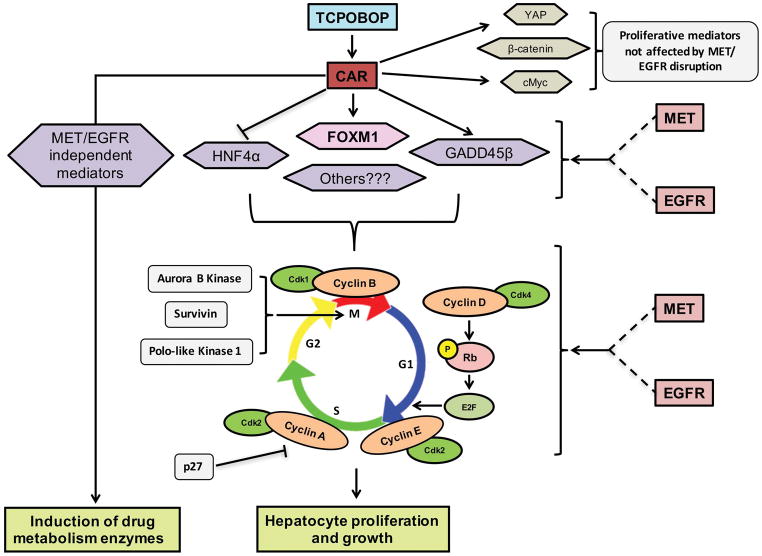
Figure 8. Schematics showing modulation of CAR-initiated proliferative response by MET and EGFR.
TCPOBOP-induced hepatocyte proliferation and liver growth, but not initial CAR activation and induction of drug metabolism enzymes, were dependent on normal functioning of MET and EGFR. Combined disruption of MET and EGFR signaling, but not individual inhibition, resulted in abolition of CAR-initiated hepatocyte proliferation and hepatomegaly. Several crucial transcription factors downstream of CAR, including FOXM1, HNF4α and GADD45β, which are involved in hepatocyte proliferation and growth were dependent on MET and EGFR signaling. Some other CAR-interacting transcription factors involved in proliferative response such as YAP, β-catenin and c-Myc were not affected by MET and EGFR disruption. Activation of cell cycle machinery and expression of important cell cycle proteins, including cyclins (cyclin D, A, B), cyclin-dependent kinase (cdk1), cyclin-dependent kinase inhibitor (p-27) and mitotic regulators (Aurora B Kinase, Survivin and Polo-like Kinase 1), were dependent on MET and EGFR. Overall, MET and EGFR govern entire cellular protein network required for hepatocyte proliferation and maintain proliferative environment in liver, which is severely disrupted by combined blockage of these receptors signaling.
In conclusion, our study revealed that combined disruption of signaling via receptor tyrosine kinases, MET and EGFR results in remarkable attenuation of TCPOBOP-induced hepatocyte proliferation and hepatomegaly by without altering CAR activation. Our result supports the hypothesis that these RTKs are quintessential for maintaining proliferative responses in liver not only after tissue loss but also in augmentative hepatomegaly induced by chemical mitogens.
Supplementary Material
Acknowledgments
Financial Support:
Support for this study was provided by the Program Project Grant NIH/NIDDK P01 DK096690 (P.I. David Perlmutter), Cleveland Foundation and the Menten Endowment Foundation.
List of Abbreviations
- TCPOBOP
1,4-Bis [2-(3,5-Dichloropyridyloxy)] benzene
- CAR
Constitutive androstane receptor
- RTK
Receptor tyrosine kinase
- EGFR
Epidermal growth factor receptor
- EGFRi
EGFR inhibitor (Canertinib)
- FOXM1
Forkhead box protein M1
- HNF4α
Hepatocyte Nuclear Factor 4 alpha
- HGF
Hepatocyte Growth Factor
- PHx
Partial hepatectomy
- YAP
Yes associated protein
- LW/BW
Liver to body weight ratio
- IPA
Ingenuity Pathway Analysis
References
- 1.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Columbano A, Ledda-Columbano GM. Mitogenesis by ligands of nuclear receptors: an attractive model for the study of the molecular mechanisms implicated in liver growth. Cell Death Differ. 2003;10(Suppl 1):S19–21. doi: 10.1038/sj.cdd.4401113. [DOI] [PubMed] [Google Scholar]
- 3.Costa RH, Kalinichenko VV, Tan Y, Wang IC. The CAR nuclear receptor and hepatocyte proliferation. Hepatology. 2005;42:1004–1008. doi: 10.1002/hep.20953. [DOI] [PubMed] [Google Scholar]
- 4.Ledda-Columbano GM, Pibiri M, Loi R, Perra A, Shinozuka H, Columbano A. Early increase in cyclin-D1 expression and accelerated entry of mouse hepatocytes into S phase after administration of the mitogen 1, 4-Bis[2-(3,5-Dichloropyridyloxy)] benzene. Am J Pathol. 2000;156:91–97. doi: 10.1016/S0002-9440(10)64709-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tschuor C, Kachaylo E, Limani P, Raptis DA, Linecker M, Tian Y, Herrmann U, et al. Constitutive androstane receptor (Car)-driven regeneration protects liver from failure following tissue loss. J Hepatol. 2016;65:66–74. doi: 10.1016/j.jhep.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 6.Paranjpe S, Bowen WC, Mars WM, Orr A, Haynes MM, DeFrances MC, Liu S, et al. Combined systemic elimination of MET and epidermal growth factor receptor signaling completely abolishes liver regeneration and leads to liver decompensation. Hepatology. 2016;64:1711–1724. doi: 10.1002/hep.28721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 8.Leoni VP, Ledda-Columbano GM, Pibiri M, Saliba C, Perra A, Kowalik MA, Grober OM, et al. Expression of c-jun is not mandatory for mouse hepatocyte proliferation induced by two nuclear receptor ligands: TCPOBOP and T3. J Hepatol. 2011;55:1069–1078. doi: 10.1016/j.jhep.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Ledda-Columbano GM, Curto M, Piga R, Zedda AI, Menegazzi M, Sartori C, Shinozuka H, et al. In vivo hepatocyte proliferation is inducible through a TNF and IL-6-independent pathway. Oncogene. 1998;17:1039–1044. doi: 10.1038/sj.onc.1202018. [DOI] [PubMed] [Google Scholar]
- 10.Columbano A, Ledda-Columbano GM, Pibiri M, Piga R, Shinozuka H, De Luca V, Cerignoli F, et al. Increased expression of c-fos, c-jun and LRF-1 is not required for in vivo priming of hepatocytes by the mitogen TCPOBOP. Oncogene. 1997;14:857–863. doi: 10.1038/sj.onc.1200891. [DOI] [PubMed] [Google Scholar]
- 11.Columbano A, Ledda-Columbano GM, Pibiri M, Cossu C, Menegazzi M, Moore DD, Huang W, et al. Gadd45beta is induced through a CAR-dependent, TNF-independent pathway in murine liver hyperplasia. Hepatology. 2005;42:1118–1126. doi: 10.1002/hep.20883. [DOI] [PubMed] [Google Scholar]
- 12.Columbano A, Shinozuka H. Liver regeneration versus direct hyperplasia. FASEB J. 1996;10:1118–1128. doi: 10.1096/fasebj.10.10.8751714. [DOI] [PubMed] [Google Scholar]
- 13.Dong B, Lee JS, Park YY, Yang F, Xu G, Huang W, Finegold MJ, et al. Activating CAR and beta-catenin induces uncontrolled liver growth and tumorigenesis. Nat Commun. 2015;6:5944. doi: 10.1038/ncomms6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazantseva YA, Yarushkin AA, Mostovich LA, Pustylnyak YA, Pustylnyak VO. Xenosensor CAR mediates down-regulation of miR-122 and up-regulation of miR-122 targets in the liver. Toxicol Appl Pharmacol. 2015;288:26–32. doi: 10.1016/j.taap.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Kowalik MA, Saliba C, Pibiri M, Perra A, Ledda-Columbano GM, Sarotto I, Ghiso E, et al. Yes-associated protein regulation of adaptive liver enlargement and hepatocellular carcinoma development in mice. Hepatology. 2011;53:2086–2096. doi: 10.1002/hep.24289. [DOI] [PubMed] [Google Scholar]
- 16.Blanco-Bose WE, Murphy MJ, Ehninger A, Offner S, Dubey C, Huang W, Moore DD, et al. C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology. 2008;48:1302–1311. doi: 10.1002/hep.22475. [DOI] [PubMed] [Google Scholar]
- 17.Ganzenberg K, Singh Y, Braeuning A. The time point of beta-catenin knockout in hepatocytes determines their response to xenobiotic activation of the constitutive androstane receptor. Toxicology. 2013;308:113–121. doi: 10.1016/j.tox.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Maillet V, Boussetta N, Leclerc J, Fauveau V, Foretz M, Viollet B, Couty JP, et al. LKB1 as a Gatekeeper of Hepatocyte Proliferation and Genomic Integrity during Liver Regeneration. Cell Rep. 2018;22:1994–2005. doi: 10.1016/j.celrep.2018.01.086. [DOI] [PubMed] [Google Scholar]
- 19.Bhushan B, Chavan H, Borude P, Xie Y, Du K, McGill MR, Lebofsky M, et al. Dual Role of Epidermal Growth Factor Receptor in Liver Injury and Regeneration after Acetaminophen Overdose in Mice. Toxicol Sci. 2017;155:363–378. doi: 10.1093/toxsci/kfw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Luque J, Caballero-Diaz D, Martinez-Palacian A, Roncero C, Moreno-Caceres J, Garcia-Bravo M, Grueso E, et al. Dissecting the role of epidermal growth factor receptor catalytic activity during liver regeneration and hepatocarcinogenesis. Hepatology. 2016;63:604–619. doi: 10.1002/hep.28134. [DOI] [PubMed] [Google Scholar]
- 21.Ledda-Columbano GM, Pibiri M, Concas D, Molotzu F, Simbula G, Cossu C, Columbano A. Sex difference in the proliferative response of mouse hepatocytes to treatment with the CAR ligand, TCPOBOP. Carcinogenesis. 2003;24:1059–1065. doi: 10.1093/carcin/bgg063. [DOI] [PubMed] [Google Scholar]
- 22.Braeuning A, Heubach Y, Knorpp T, Kowalik MA, Templin M, Columbano A, Schwarz M. Gender-specific interplay of signaling through beta-catenin and CAR in the regulation of xenobiotic-induced hepatocyte proliferation. Toxicol Sci. 2011;123:113–122. doi: 10.1093/toxsci/kfr166. [DOI] [PubMed] [Google Scholar]
- 23.Cui J, Xia T, Xie D, Gao Y, Jia Z, Wei D, Wang L, et al. HGF/Met and FOXM1 form a positive feedback loop and render pancreatic cancer cells resistance to Met inhibition and aggressive phenotypes. Oncogene. 2016;35:4708–4718. doi: 10.1038/onc.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francica P, Nisa L, Aebersold DM, Langer R, Bladt F, Blaukat A, Stroka D, et al. Depletion of FOXM1 via MET Targeting Underlies Establishment of a DNA Damage-Induced Senescence Program in Gastric Cancer. Clin Cancer Res. 2016;22:5322–5336. doi: 10.1158/1078-0432.CCR-15-2987. [DOI] [PubMed] [Google Scholar]
- 25.Stoll SW, Stuart PE, Swindell WR, Tsoi LC, Li B, Gandarillas A, Lambert S, et al. The EGF receptor ligand amphiregulin controls cell division via FoxM1. Oncogene. 2016;35:2075–2086. doi: 10.1038/onc.2015.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Zhang W, Wen L, Yang H, Wen M, Yun Y, Zhao L, et al. FOXM1 confers resistance to gefitinib in lung adenocarcinoma via a MET/AKT-dependent positive feedback loop. Oncotarget. 2016;7:59245–59259. doi: 10.18632/oncotarget.11043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarrouki B, Benterki I, Fontes G, Peyot ML, Seda O, Prentki M, Poitout V. Epidermal growth factor receptor signaling promotes pancreatic beta-cell proliferation in response to nutrient excess in rats through mTOR and FOXM1. Diabetes. 2014;63:982–993. doi: 10.2337/db13-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledda-Columbano GM, Pibiri M, Cossu C, Molotzu F, Locker J, Columbano A. Aging does not reduce the hepatocyte proliferative response of mice to the primary mitogen TCPOBOP. Hepatology. 2004;40:981–988. doi: 10.1002/hep.20403. [DOI] [PubMed] [Google Scholar]
- 29.Bonzo JA, Ferry CH, Matsubara T, Kim JH, Gonzalez FJ. Suppression of hepatocyte proliferation by hepatocyte nuclear factor 4alpha in adult mice. J Biol Chem. 2012;287:7345–7356. doi: 10.1074/jbc.M111.334599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walesky C, Edwards G, Borude P, Gunewardena S, O’Neil M, Yoo B, Apte U. Hepatocyte nuclear factor 4 alpha deletion promotes diethylnitrosamine-induced hepatocellular carcinoma in rodents. Hepatology. 2013;57:2480–2490. doi: 10.1002/hep.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walesky C, Gunewardena S, Terwilliger EF, Edwards G, Borude P, Apte U. Hepatocyte-specific deletion of hepatocyte nuclear factor-4alpha in adult mice results in increased hepatocyte proliferation. Am J Physiol Gastrointest Liver Physiol. 2013;304:G26–37. doi: 10.1152/ajpgi.00064.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walesky C, Apte U. Role of hepatocyte nuclear factor 4alpha (HNF4alpha) in cell proliferation and cancer. Gene Expr. 2015;16:101–108. doi: 10.3727/105221615X14181438356292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kachaylo EM, Yarushkin AA, Pustylnyak VO. Constitutive androstane receptor activation by 2,4,6-triphenyldioxane-1,3 suppresses the expression of the gluconeogenic genes. Eur J Pharmacol. 2012;679:139–143. doi: 10.1016/j.ejphar.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Miao J, Fang S, Bae Y, Kemper JK. Functional inhibitory cross-talk between constitutive androstane receptor and hepatic nuclear factor-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1alpha. J Biol Chem. 2006;281:14537–14546. doi: 10.1074/jbc.M510713200. [DOI] [PubMed] [Google Scholar]
- 35.Yarushkin AA, Kachaylo EM, Pustylnyak VO. The constitutive androstane receptor activator 4-[(4R,6R)-4,6-diphenyl-1,3-dioxan-2-yl]-N,N-dimethylaniline inhibits the gluconeogenic genes PEPCK and G6Pase through the suppression of HNF4alpha and FOXO1 transcriptional activity. Br J Pharmacol. 2013;168:1923–1932. doi: 10.1111/bph.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian J, Huang H, Hoffman B, Liebermann DA, Ledda-Columbano GM, Columbano A, Locker J. Gadd45beta is an inducible coactivator of transcription that facilitates rapid liver growth in mice. J Clin Invest. 2011;121:4491–4502. doi: 10.1172/JCI38760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roth A, Looser R, Kaufmann M, Meyer UA. Sterol regulatory element binding protein 1 interacts with pregnane X receptor and constitutive androstane receptor and represses their target genes. Pharmacogenet Genomics. 2008;18:325–337. doi: 10.1097/FPC.0b013e3282f706e0. [DOI] [PubMed] [Google Scholar]
- 39.Kanno Y, Kure Y, Kobayashi S, Mizuno M, Tsuchiya Y, Yamashita N, Nemoto K, et al. Tripartite Motif Containing 24 Acts as a Novel Coactivator of the Constitutive Active/Androstane Receptor. Drug Metab Dispos. 2018;46:46–52. doi: 10.1124/dmd.117.077693. [DOI] [PubMed] [Google Scholar]
- 40.Khetchoumian K, Teletin M, Tisserand J, Herquel B, Ouararhni K, Losson R. Trim24 (Tif1 alpha): an essential ‘brake’ for retinoic acid-induced transcription to prevent liver cancer. Cell Cycle. 2008;7:3647–3652. doi: 10.4161/cc.7.23.7123. [DOI] [PubMed] [Google Scholar]
- 41.Wansleben S, Peres J, Hare S, Goding CR, Prince S. T-box transcription factors in cancer biology. Biochim Biophys Acta. 2014;1846:380–391. doi: 10.1016/j.bbcan.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Shizu R, Benoki S, Numakura Y, Kodama S, Miyata M, Yamazoe Y, Yoshinari K. Xenobiotic-induced hepatocyte proliferation associated with constitutive active/androstane receptor (CAR) or peroxisome proliferator-activated receptor alpha (PPARalpha) is enhanced by pregnane X receptor (PXR) activation in mice. PLoS One. 2013;8:e61802. doi: 10.1371/journal.pone.0061802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.