Abstract
Background
ADHD is a neurodevelopmental disorder with origins early in life. There is growing evidence that individual differences in temperament reactivity are predictive of ADHD symptoms, yet little is known about the relations between temperament reactivity in early infancy and later ADHD symptoms or the combined effect of reactivity with early environmental factors on ADHD symptom development. Using a nine-year prospective longitudinal design, this study tested the independent and interactive contributions of infant reactivity and maternal caregiving behaviors (MCB) on parent- and teacher-reported childhood ADHD symptoms.
Methods
Participants included 291 children (132 male; 159 female) who participated in a larger study of temperament and social-emotional development. Reactivity was assessed by behavioral observation of negative affect, positive affect, and motor activity during novel stimuli presentations at four months of age. MCB were observed during a series of semi-structured mother-infant tasks at nine months of age. Finally, ADHD symptoms were assessed by parent- and teacher-report questionnaires at seven and nine years, respectively.
Results
Reactivity was predictive of ADHD symptoms, but results were sex specific. For boys, infant motor activity was positively predictive of later ADHD symptoms, but only at lower-quality MCB. For girls, infant positive affect was positively predictive of later ADHD symptoms at lower-quality MCB, and—unexpectedly—infant positive affect and motor activity were negatively predictive of later ADHD symptoms at higher-quality MCB.
Conclusions
These results point to early parenting as a moderating factor to mitigate temperament-related risk for later ADHD, suggesting this as a potential intervention target to mitigate risk for ADHD among reactive infants.
Keywords: ADHD, parenting, temperament, infancy
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is classified as a neurodevelopmental disorder in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), and there is strong consensus that its origins lie early in life (American Psychiatric Association, 2013). ADHD also is a pervasive and impairing disorder (Kawabata, Tseng, & Gau, 2012), with a prevalence rate ranging from three to eight percent of children (Polanczyk, Salum, Sugaya, Caye, & Rohde, 2015). Although ADHD is a highly heritable disorder, with a substantial genetic contribution to its etiology, its pathogenesis is not well understood (Thapar et al., 2016). Better understanding of early etiological pathways underlying ADHD is critical for both the early identification of children at risk for developing ADHD, as well as the identification of mutable factors in these pathways that could be targeted for early intervention.
Temperament reactivity describes constitutionally-based individual differences in physiological and behavioral responses to the environment (Rothbart & Bates, 1998). These differences emerge within the first several months of life, can be reliably observed by four months of age, and, at least during early infancy, are thought to be primarily biologically-driven (i.e., shaped by genetic and pre-/peri-natal influences to a greater extent than environmental experiences) (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Goldsmith, Lemery, & Essex, 2004). Given these qualities, the examination of individual differences in reactivity is a promising avenue for furthering our understanding of the pathogenesis of ADHD; not only for the early identification of temperament-based risk profiles for developing ADHD symptoms, but also for the examination of how early risk profiles interact with environmental factors to shape the emergence of ADHD. Empirically, there is growing evidence supporting links between reactivity and ADHD symptoms (Arnett, MacDonald, & Pennington, 2013; Willoughby, Gottfredson, Stifter, & the Family Life Project Investigators, 2017). By contrast, we know almost nothing about the role of environmental processes in the translation of temperament risk into later ADHD symptoms.
Within the ADHD field, reactivity has commonly been operationalized in terms of motor and affective responses to stimuli (Johnson, Gliga, Jones, & Charman, 2015). Converging evidence from studies of infants at familial risk for ADHD, as well as retrospective and prospective longitudinal studies, suggests that higher levels of motor activity and negative affect (including expressions of distress, fear, and anger) during infancy are linked to higher risk for childhood ADHD symptoms (Arnett et al., 2013; Friedman, Watamura, & Robertson, 2005; Sullivan et al., 2015; Willoughby et al., 2017). Theoretically, higher levels of positive affect, especially in the context of exploring and approaching novel stimuli, also signal risk for later ADHD (Nigg, Goldsmith, & Sachek, 2004). Although no study has examined these links in infancy, several prospective longitudinal studies beginning in preschool support this position (e.g., Forbes, Rapee, Camberis, & McMahon, 2017; Stringaris, Maughan, & Goodman, 2010). However, during infancy and preschool higher levels of approach-related positive affect also are predictive of many adaptive outcomes, such as greater social competence and fewer conduct problems, suggesting this form of reactivity also acts as a protective factor (Degnan et al., 2011; Lahey et al., 2008).
In sum, there is evidence that higher levels of reactivity, especially involving motor activity and negative affect, are risk factors for later ADHD symptoms, and that positive affect may act as either a risk or a protective factor. There are limitations within the literature, however. First, aside from one study of motor behavior measured at three months (Friedman et al., 2005), all studies have measured reactivity at six months or older, and sometimes combined with measures from preschool age. In order to capture differences attributable primarily to genetic, pre-/perinatal influences, it is important to study these differences earlier in life, before they are more strongly shaped by environmental experiences. Second, conditions that are frequently co-morbid with ADHD symptoms, such as behavioral problems, have not been controlled in nearly all longitudinal studies of temperament predicting later ADHD symptoms, making it challenging to identify variation in temperament that is uniquely predictive of ADHD symptoms (see Stringaris et al., 2010 for an exception). In addition, this omission is problematic for interpreting findings of past studies because behavioral problems also are associated with higher levels of reactivity (Lahey et al., 2008), meaning that associations between reactivity and ADHD symptoms could be explained through shared variance with behavioral problems.
Although the genetic model is the predominant model for understanding ADHD etiology (Burt, 2009), many have argued that the translation of genetic liability into ADHD symptoms occurs through transactional processes between biological risk and environmental factors (Johnston & Chronis-Tuscano, 2015; Nigg, 2006; Sonuga-Barke & Halperin, 2010). However, relative to biological risk, much less is known about the contribution of environmental factors to ADHD pathogenesis. Due to the high heritability of ADHD, a challenge to this research has been that common psychosocial measures, such as parenting behaviors, are often confounded by shared genetic risk for ADHD. Nonetheless, recent studies of psychosocial factors using genetically-informed designs (e.g., covarying parental ADHD status or adoption design) provide insight into how these transactional processes unfold (Harold et al., 2013; Tung, Brammer, Li, & Lee, 2015). Tung and colleagues controlled for levels of parental ADHD symptoms and found that negative parenting behaviors (e.g., corporal punishment, inconsistent discipline) were predictive of changes in child ADHD symptom severity over time. Harold and colleagues found higher levels of impulsivity and activation at 4.5 years predicted higher levels hostile parenting among adoptive mothers, which in turn, contributed to higher levels of ADHD symptoms at six years. Taken together, these results suggest parenting behaviors can contribute to worsening ADHD symptoms, and that these processes unfold through reciprocal and ongoing effects between parent and child. But what is happening before symptoms manifest?
The early caregiving environment, and parenting behaviors in particular, are critical in supporting healthy infant neurodevelopment (Belsky & de Haan, 2011; Tottenham, 2012) and are likely involved in shaping etiological pathways for ADHD, a neurodevelopmental disorder. Excepting cases of extreme environmental deprivation (e.g., Kennedy et al., 2016), there is a paucity of research examining very early environment effects on ADHD pathogenesis. Although not examined in the context of ADHD, studies have investigated the combined effects of reactivity and parenting behaviors in infancy as predictors of child adjustment (Gueron-Sela, Atzaba-Poria, Meiri, & Marks, 2016; Poehlmann et al., 2012). For example, Poehlmann and colleagues found higher levels of reactivity (measured by proneness-to-distress) at nine months predicted lower cognitive skills and higher externalizing problems at 36 months in preterm infants—but only in combination with lower levels of maternal sensitivity and non-intrusiveness at nine months. Similarly, Gueron-Sela and colleagues found higher levels of reactivity at six months in pre- and fullterm infants were negatively predictive of cognitive functioning at 12 months, but only at lower levels of maternal structuring at six months. Results of these studies suggest lower-quality maternal caregiving behaviors (MCB) within the first year of life increase the likelihood that higher levels of reactivity translate into later maladjustment.
In sum, the transactional model of ADHD and evidence of the moderating effect of MCB on reactivity-to-maladjustment pathways point to potential that the combined effect of higher reactivity with lower-quality MCB—rather than higher levels of reactivity alone—are what increases risk for later ADHD symptoms. Although there is emerging evidence that higher levels of reactivity signal risk for later ADHD symptoms, no study has investigated the combined effects of infant temperament risk with the quality of the early caregiving environment. To address this gap, the current study used a nine-year prospective longitudinal design to examine the combined effects of reactivity, measured at four months of age, and quality of MCB (i.e., sensitive and less intrusive parenting), measured at nine months of age, on childhood ADHD symptoms, measured at seven and nine years. We hypothesized higher levels of negative affect, positive affect, and motor activity would each uniquely predict childhood ADHD symptoms, and higher-quality MCB would protect against ADHD risk conferred by high levels of reactivity. Given that behavioral problems frequently co-occur with ADHD and share some common pathways (Stringaris et al., 2010), we controlled for levels of behavioral problems (measured at seven years) to identify unique pathways between temperament reactivity and ADHD symptoms.
Because ADHD is marked by several sex differences, including differences in prevalence and symptom presentation (Owens, Cardoos, & Hinshaw, 2015), we investigated whether child sex moderated any of the predicted associations. Of the few studies that have tested the moderating role of child sex on the relation between temperament and ADHD, none have found a significant interaction effect (Arnett et al., 2013; Willoughby et al., 2017). Conversely, there is evidence that more severe cognitive endophenotypes (i.e., worse working memory, inhibition, and processing speed) underlie ADHD symptoms for boys versus girls—suggesting that developmental pathways leading to ADHD may differ by sex (Arnett et al., 2015). Given the paucity of research examining sex effects on reactivity-related ADHD risk, we explored the moderating role of child sex, but had no a priori predictions.
Method
Participants
Participants were 291 children (132 male; 159 female) recruited for a larger longitudinal study on temperament and child socioemotional development conducted in a large metropolitan Mid-Atlantic region of the United States beginning in 2001. 779 four-month-old infants participated in temperament screening tasks and a subgroup of infants were selected to participate based on their reactivity (see Hane, Fox, Henderson, & Marshall, 2008 for a detailed description of recruitment and screening methods). Infants with higher levels of reactivity were oversampled to represent a wider range of reactivity compared to a randomly selected community sample. Reported when infants were 4 months old, ethnic/racial demographics for mothers were: 69.1% Caucasian, 16.5% African American, 7.2% Hispanic, 3.1%, Asian, 3.4% other, and .7% missing. Ethnic/racial demographics for fathers were: 68.7% Caucasian, 18.6% African American, 5.5% Hispanic, 2.7% Asian, 3.1% other, and 1.4% missing. No information on family income was collected, however, 16.2% of mothers had a high school education, 41.9% had a two- or four-year college education, 35.7% had a graduate school education, 5.2% reported other forms of education, and 1% were missing.
There was attrition across assessment periods (see Table 1). However, there were no differences between participants with missing and non-missing data on demographic or key variables, excepting positive affect. Participants with missing data had higher levels of positive affect at four months compared to those without missing data (t(289) = 2.14, p = .03).
Table 1.
Descriptive statistics and correlations among variables of interest
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Negative affect (4m) | |||||||||
| 2. | Positive affect (4m) | −.25** | ||||||||
| 3. | Motor activity (4m) | .12* | −.003 | |||||||
| 4. | Maternal caregiving behaviors (9m) | −.04 | −.06 | .06 | ||||||
| 5. | Inattention (7y; P) | −.06 | .12 | .05 | −.05 | |||||
| 6. | Hyperactivity/impulsivity (7y; P) | −.01 | .10 | .05 | −.07 | .66** | ||||
| 7. | Inattention (9y; T) | −.09 | −.03 | .25* | −.16 | .66** | .52** | |||
| 8. | Hyperactivity/impulsivity (9y; T) | −.11 | −.05 | .34** | .10 | .20 | .27* | .46** | ||
| 9. | Behavioral problems (7y; P) | .03 | .04 | .03 | .003 | .52** | .60** | .38** | .07 | |
|
| ||||||||||
| M | 26.31 | 18.42 | 31.59 | .00 | 1.77 | 1.69 | 1.81 | 1.46 | 1.36 | |
| SD | 30.72 | 19.61 | 20.57 | 1.79 | .56 | .58 | .83 | .57 | .34 | |
| Skew (standard error) | 1.88 (.14) | 2.54 (.14) | 1.31 (.14) | −.75 (.16) | 1.00 (.18) | 1.02 (.18) | 1.04 (.28) | 2.04 (.28) | 1.12 (.18) | |
| Kurtosis (standard error) | 5.24 (.29) | 9.13 (.29) | 3.03 (.28) | .81 (.31) | 1.30 (.35) | .69 (.35) | .11 (.56) | 5.04 (.56) | .89 (.35) | |
| n | 291 | 291 | 291 | 241 | 193 | 193 | 72 | 71 | 193 | |
Note. 4m = 4 months; 9m = 9 months; 7y = 7 years; 9y = 9 years; P = parent-report; T = teacher-report;
p < .05,
p < .01
Measures
Temperament reactivity
Reactivity was measured during a lab visit when participants were four months old. Infants were presented with a series of novel auditory and visual stimuli, and their reactions were coded on dimensions of negative affect, positive affect, and motor activity in five-second intervals (Fox et al., 2001). Negative affect was operationalized by duration of crying and frequency of negative vocalizations. Positive affect was operationalized by frequency of smiling and positive vocalizations. Motor activity was operationalized by frequency of arm waves, leg kicks, back arches, and body hyperextensions. Codes were pro-rated based on number of five-second intervals coded. All coding was completed by four reliable coders (Hane et al., 2008).
Maternal Caregiving Behaviors (MCB)
MCB were measured during a home visit when participants were nine months old. Mothers interacted with their child in seven semi-structured tasks (e.g., feeding, tower building). MCB were coded using a modified version of Ainsworth’s Maternal Care Behavior rating scales (Ainsworth, 1976; Hane & Fox, 2006). For each task, raters provided a global rating (9-point Likert scale; 1 = low; 9 = high) of Acceptance, Sensitivity, Availability, Appropriateness, Delight, and Encouragement. Ratings were averaged for each task and combined into an omnibus sensitivity score for each mother-child dyad. Additionally, a composite of the global code of Interference-Cooperation (1 = interference; 9 = cooperation; reverse-scored) from the Ainsworth scales and a 4-point Likert scale of Intrusiveness (1 = not at all; 4 = highly; Park, Belsky, Putnam & Crnic, 1997) were each averaged across tasks and standardized mean scores for Interference-Cooperation and Intrusiveness were averaged together to create an omnibus intrusiveness score for each dyad. Omnibus sensitivity and intrusiveness scores were moderately negatively correlated (r(239) = −.61, p < .001). These scores were standardized before computing a single composite MCB score as sensitivity – intrusiveness, representing sensitive and non-intrusive MCB (Hane & Fox, 2006). All coding was completed by two trained, reliable coders who were blind to all other study data.
ADHD symptoms
ADHD symptoms were assessed using parent- and teacher-report on the Swanson, Nolan, and Pelham-IV (SNAP-IV; Swanson, 1992) nine-item inattention and nine-item hyperactivity/impulsivity scales. Raters reported how well each item described the child (1 = not at all; 4 = very much) and scores were calculated as the average of items within the scales. Due to constraints of the larger study, teacher-report data were collected at a different assessment period than parent-report data and for only a subsample of participants. Parent-report data were collected during the seven-year assessment period (M = 7.53 years old; SD = .97) and teacher-report data were collected later in the nine-year assessment period (M = 10.18 years; SD = .61). Reliability was excellent for parent- (α = .93) and teacher-report (α = .94). To maximize information from available data, ADHD symptoms were measured by a latent variable in the main analysis using parent- and teacher report of inattention and hyperactivity/impulsivity as indicators.
Confirmatory factor analysis of the measurement model of the latent variable revealed poor fit (RMSEA = .16, p = .01). Based on modification indices, we allowed residual covariances between teacher-report of inattention and hyperactivity/impulsivity, resulting in good model fit χ2(1) = 2.00, p = .16, CFI = .99, RMSEA = .07 (p = .21), SRMR = .04). Relative to parent-report inattention, loadings were strong for teacher-report inattention (λ = 1.00, p < .001) and weaker for parent- and teacher-report hyperactivity/impulsivity (λ = .69, p < .001 and λ = .23, p = .10 respectively). Measurement invariance testing indicated loadings did not significantly differ by child sex, χ2(3) = .44, p = .93.
Behavioral problems
Behavioral problems also were assessed using the age seven SNAP-IV parent-report by an average of 16 items reflecting oppositional behavior and conduct problems. Items were rated on the same four-point Likert scale as the ADHD items. Reliability for this scale was excellent (α = .90).
Analytic plan
The main hypotheses were tested using structural equation modeling with lavaan in RStudio 1.0.136. Predictor variables were mean-centered reactivity measures (i.e., negative affect, positive affect, motor activity), mean-centered MCB measure, and the three two-way interactions between reactivity measures and MCB. ADHD symptoms were modeled as a latent variable with four indicators including parent- and teacher-report of inattention and hyperactivity/impulsivity. Child behavioral problems were included as a predictor to isolate the unique contribution of reactivity, MCB, and their interactive effects on ADHD symptoms, specifically. Demographic variables (i.e., parent ethnicity and maternal education) were included in the model as covariates if they were significantly correlated with predictor or outcome variables. Significant two-way interaction effects were probed using simple slope analysis, which involved examining relations between reactivity variables and ADHD symptoms at one standard deviation above and below the mean of MCB (Aiken & West, 1991).
To account for missing data, we used a full information maximum likelihood estimator, which provides parameter estimates using all available data (Kline, 2010). We used robust standard errors to account for skew and kurtosis in our variables. To test model fit, we examined the Comparative Fit Index (CFI), Root Mean Square Error of Approximation (RMSEA), and the Standardized Root Mean Square Residual (SRMR); CFI values ≥ .95, RMSEA values ≤ .05, SRMR values ≤ .08 are indicative of excellent fit (Tabachnick & Fidell, 2013).
We examined the moderating role of child sex on both main and interactive effects in the model by modeling child sex as a grouping variable. Using chi-square difference testing, we compared the chi-square value from analyses where model parameters (regression coefficients, loadings) were constrained to be equal across sex groups, to the chi-square value from analyses allowing separate regression estimates for boys and girls. A significant difference between the chi-square values indicates the regression coefficients significantly differ between boys and girls and is evidence child sex moderates the relations between the main and interactive effects of reactivity and MCB on ADHD symptoms.
Results
Descriptive statistics
Table 1 summarizes descriptive statistics and bivariate correlations for the variables of interest. Of note, higher levels of four-month motor activity were correlated with nine-year teacher-reports of ADHD symptoms. We also divided the sample above and below the DSM-5 clinical threshold for ADHD by taking a symptom count from parent- and teacher-report of ADHD (i.e., six or more symptoms endorsed at quite a bit or very much on SNAP-IV inattention or hyperactivity subscale on parent- or teacher-report; Swanson et al., 2001). Thirty children (10 girls and 20 boys) scored above the clinical threshold; Table 2 includes predictor variable descriptive statistics for children above and below the ADHD clinical threshold. Parallel to the bivariate associations, children above the clinical threshold had marginally significantly higher levels of motor activity compared to those below the clinical threshold; effect size differences revealed a similar pattern for boys and girls (i.e., gs > .40). Although there were no significant differences for other predictor variables between children above and below the threshold, effect size differences indicated that girls above the clinical threshold also had higher levels of positive affect and lower-quality MCB compared to girls below the clinical threshold. In terms of symptom severity, boys above the clinical threshold scored marginally significantly higher on parent-report hyperactivity/impulsivity compared to girls above the clinical threshold (t(26) = 1.93, p = .06; g = .77); otherwise there were no differences in symptom severity between boys and girls above the clinical threshold—suggesting that boys and girls with high levels of ADHD symptoms were comparable in terms of symptom severity.
Table 2.
Comparison of temperament reactivity and quality of maternal caregiving behaviors (MCB) above and below the ADHD symptom clinical cut-off using parent- and/or teacher-report
| Boys | Girls | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 6 symptoms | ≥ 6 symptoms | t | g | < 6 symptoms | ≥ 6 symptoms | t | g | < 6 symptoms | ≥ 6 symptoms | t | g | ||
| Negative affect | M | 21.50 | 27.97 | −.90 | .23 | 28.58 | 18.19 | 1.09 | .36 | 25.65 | 24.71 | .17 | .03 |
| (SD) | (23.58) | (41.78) | (29.13) | (23.68) | (27.13) | (36.60) | |||||||
| n | 72 | 20 | 102 | 10 | 174 | 30 | |||||||
| Positive affect | M | 23.40 | 20.89 | .38 | .09 | 14.81 | 20.91 | −1.38 | .46 | 18.37 | 20.89 | −.62 | .12 |
| (SD) | (27.98) | (20.18) | (12.65) | (19.26) | (20.80) | (19.55) | |||||||
| n | 72 | 20 | 102 | 10 | 174 | 30 | |||||||
| Motor activity | M | 29.83 | 38.84 | −1.3a | .43 | 29.67 | 41.75 | −1.20a | .61 | 29.74 | 39.81 | −1.81a+ | .50 |
| (SD) | (18.23) | (29.47) | (18.32) | (31.40) | (18.23) | (29.61) | |||||||
| n | 72 | 20 | 102 | 10 | 174 | 30 | |||||||
| Quality of MCB | M | .19 | −.25 | .85 | .23 | .04 | −.65 | 1.11 | .41 | .10 | −.37 | 1.25 | .27 |
| (SD) | (1.82) | (2.11) | (1.70) | (1.42) | (1.74) | (1.90) | |||||||
| n | 62 | 18 | 92 | 8 | 154 | 26 | |||||||
Note. t = t-value for two-tailed, independent samples t-test; + p < .10; g = Hedges’ g effect size value with bolded values indicative of moderate to strong effect size (g > .40);
Levene’s test for equality of variances indicated unequal variances (ps < .05), therefore t-test conducted with adjusted degrees of freedom.
Regarding demographic variable associations with key variables, mother and father ethnicities (1 = non-Hispanic Caucasian; 0 = other ethnicities) were positively associated with MCB quality (mother: t(239) = −5.78, p < .001; father: t(239) = −6.15, p < .001) and negatively associated with teacher-report inattention (mother: t(70) = 1.76, p = .08; father: t(70) = 2.69, p = .009). Maternal high-school education was associated with lower-quality MCB (t(239) = 3.79, p < .001) and graduate education was associated with higher-quality MCB, (t(239) = −2.80, p = .006). Compared to girls, boys had significantly higher levels of positive affect (t(289) = −2.68, p = .008), parent-report of inattention (t(191) = −2.73, p = .007) and hyperactivity/impulsivity (t(191) = 4.17, p < .001), and teacher-report of inattention (t(70) = −3.32, p = .001). Therefore, we covaried parent ethnicities and maternal education with MCB quality and two-way interactions terms involving MCB quality. Parent ethnicities also were included as predictors of ADHD symptoms.
Group differences by child sex
Chi-square difference testing revealed significant differences between the partially constrained and the fully constrained model, χ2(10) = 28.79, p = .001. This result indicated regression coefficients for boys were significantly different than regression coefficients for girls. Therefore, we conducted main analyses using child sex as a grouping variable and allowed regression coefficients to vary between groups.
Interactive effects of temperament reactivity and MCB on ADHD symptoms
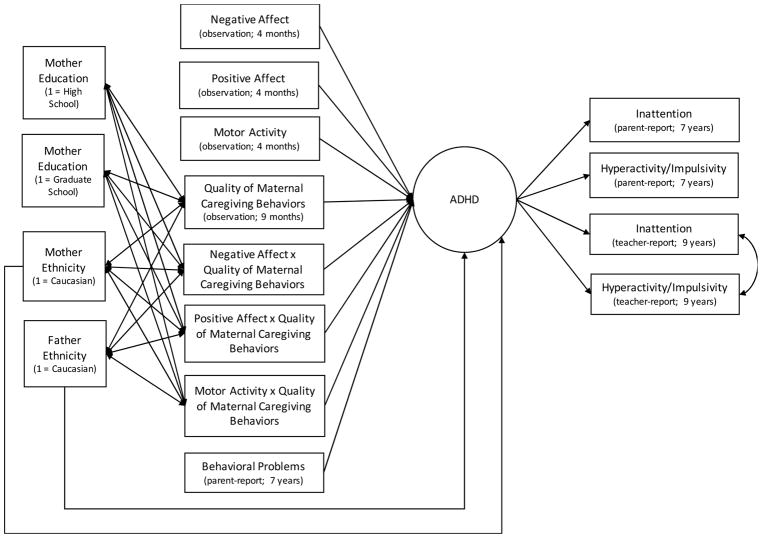
This model examined the main and interactive effects of four-month child temperament reactivity and nine-month MCB on childhood ADHD symptoms. Figure 1 depicts the structural model. Model fit was good (CFI = .95, RMSEA = .04 (p > .05), SRMR = .10, and χ2(113) = 145.04, p = .02 with Yuan-Bentler correction = .93).
Figure 1.
Structural model
Note. Modeled separately for boys and girls with loadings constrained to be equal across models. Covariances among predictor variables and covariances among demographic variables were modeled but not shown.
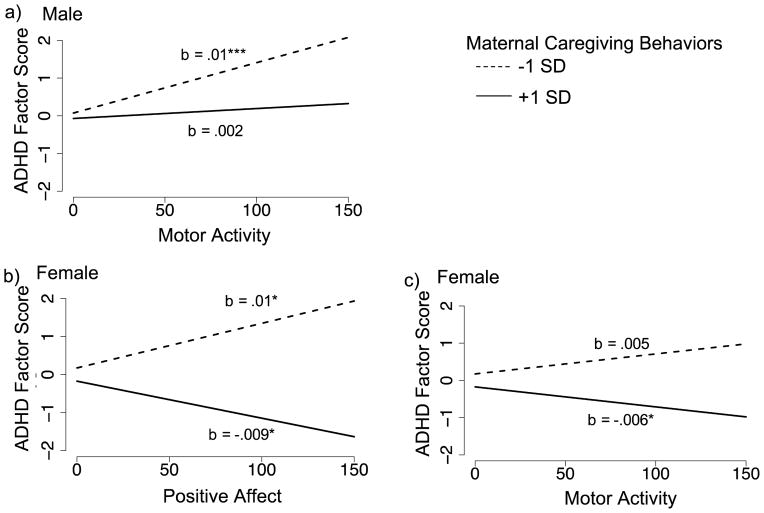
For boys, the model explained 63% of variance in ADHD symptoms (Table 3). There was a significant main effect of motor activity, such that higher levels of motor activity predicted higher levels of ADHD symptoms. There also was a significant two-way interaction between motor activity and MCB quality (Figure 2a). Simple slope analysis revealed that motor activity was positively related to ADHD symptoms at lower-quality MCB (i.e., −1SD below mean of MCB; b = .014, S.E. = .003, p < .001), but not significantly related to ADHD symptoms at higher-quality MCB (i.e., +1SD above mean of MCB; b = .002, S.E. = .004, p = .54).
Table 3.
Structural equation model for temperament reactivity and quality of maternal caregiving behaviors predicting latent factor of ADHD symptoms
| Boys | Girls | |||
|---|---|---|---|---|
|
| ||||
| b | S.E. | b | S.E. | |
| Negative affect (4m) | −.003 | .002 | −.002 | .002 |
| Positive affect (4m) | .002 | .002 | .001 | .003 |
| Motor activity (4m) | .008*** | .002 | < .001 | .002 |
| Maternal caregiving behavior (9m) | −.040 | .027 | −.097*** | .026 |
| Negative affect × Maternal caregiving | −.001 | .001 | .001 | .001 |
| Positive affect × Maternal caregiving | .001 | .002 | −.006** | .002 |
| Motor activity × Maternal caregiving | −.003* | .001 | −.003* | .001 |
| Behavioral problems (7y) | 1.079*** | .177 | .625*** | .131 |
| Father ethnicity (1 = Caucasian) | −.426*** | .123 | −.202 | .157 |
| Mother ethnicity (1 = Caucasian) | .465*** | .139 | .225 | .166 |
Note. 4m = 4 months; 9m = 9 months; 7y = 7 years;
p ≤. .05,
p ≤ .01,
p ≤ .001
Figure 2.
The effect of reactivity on childhood ADHD symptoms at different levels of maternal caregiving behaviors.
*p ≤ .05, ***p ≤ .001
For girls, the model explained 59% of variance in ADHD symptoms (Table 3). There were no main effects for reactivity; however, MCB quality was negatively related to ADHD symptoms. There was a significant two-way interaction between positive affect and MCB quality (Figure 2b). According to the simple slope analysis, positive affect was positively related to ADHD symptoms at lower-quality MCB (b = .011, S.E. = .005, p = .03) and negatively related to ADHD symptoms at higher-quality MCB (b = −.009, S.E. = .004, p = .02). There also was a significant two-way interaction between motor activity and MCB quality (Figure 2c). At lower-quality MCB motor activity was not related to ADHD symptoms (b = .005, S.E. = .004, p = .13), but at higher-quality MCB, motor activity was negatively related to ADHD symptoms (b = −.006, S.E. = .003, p = .05).
Discussion
Individual differences in temperament reactivity are predictive of variation in childhood ADHD symptoms (Arnett et al., 2013; Willoughby et al., 2017). However, not all children with high levels of reactivity develop ADHD, and beyond the zero-order relation between reactivity and ADHD symptoms, not much is known about the nature of these pathways. Better understanding of the heterogeneity of the temperament-related ADHD pathway is needed not only for early identification of children at risk for developing ADHD symptoms but also to identify potential prevention or early intervention targets involving mutable features. To address this gap, the current study used a prospective longitudinal design following children from four-months to nine-years to examine the combined effects of reactivity and MCB in infancy on childhood ADHD symptoms.
Replicating results of past studies (e.g., Arnett et al., 2013), we found higher levels of motor activity at four months—measured by response to novelty—were correlated with higher levels of teacher-reported childhood ADHD symptoms. We also found that infant motor activity was higher among boys and girls scoring above the clinical threshold for ADHD, measured by parent- or teacher-report measures. Conversely, neither negative affect nor positive affect reactivity were directly associated with childhood ADHD symptoms and there were no significant differences between children above and below the clinical threshold on these measures of affect reactivity. Together, these results suggest that higher levels of motor activity in response to novelty during early infancy may be an early indicator of risk for childhood ADHD symptoms.
When we examined the moderating role of MCB quality on the reactivity-to-ADHD pathways, we found that the direction and strength of these pathways depended not only on the quality of MCB, but also on child sex. Indeed, the extent that sex differences impacted the interactive effects of MCB quality with infant reactivity suggested that boys and girls may follow different pathways in terms of how reactivity translates into risk for ADHD symptoms. Echoing the bivariate associations, for boys, we found that higher levels of four-month motor activity predicted higher levels of ADHD symptoms; however, the strength of this relation decreased at higher-quality MCB suggesting a protective effect of caregiving environment. Similarly, for girls, we also found a two-way interaction between motor activity and MCB quality that suggested a protective effect of caregiving environment. However, in contrast to the results for boys, we found that—in combination with higher-quality caregiving— higher levels of four-month motor activity predicted fewer ADHD symptoms; this result was surprising as we did not expect reactivity to be negatively related to ADHD. Taken together, these results suggest that higher levels of motor activity signal risk for ADHD, but in the presence of higher-quality caregiving the effect of this risk on the development of ADHD symptoms is attenuated for boys and can even be reversed for girls.
Although affect reactivity was not a significant predictor of ADHD symptoms for either boys or girls, we found a significant interaction between positive affect—in response to novelty— and MCB quality for girls. As predicted, when girls were exposed to lower-quality MCB, higher positive affect predicted greater ADHD symptoms, indicating that positive affect in response to novelty may be a risk factor for ADHD for some girls. Surprisingly—although parallel to the motor activity-to-ADHD findings—the direction of this reactivity-to-ADHD relation reversed in the presence of higher-quality MCB, such that higher levels of positive affect predicted fewer ADHD symptoms. These results indicate that, for girls, positive affect in response to novelty can be either an early risk or early protective factor for later ADHD symptoms depending on the quality of the caregiving environment. In other words, higher levels of positive affect signal differential susceptibility to, rather than risk or protection for, later ADHD symptoms (Belsky & Pluess, 2009).
Contrary to prediction, four-month negative affect was not associated with childhood ADHD symptoms and this relation was not moderated by nine-month MCB for either boys or girls. This result was surprising given consistent support for the link between higher levels of negative affect during infancy/preschool and child ADHD symptoms (Arnett et al., 2013; Willoughby et al., 2017). In contrast to past studies, we examined negative affect in response to novel stimuli rather than to frustrating or distressing events. Consequently, it may be that our measure was not representative of the broader irritable phenotype hypothesized to contribute to some forms of ADHD (Nigg et al., 2004). Additionally, this is the first study to examine negative affect in such a young sample. Complex regulatory processes develop through infancy that modulate effects of reactivity on neurodevelopment (Rothbart, Sheese, Rueda, & Posner, 2011) and it may be the combination of negative affect reactivity with low regulation that increases risk for ADHD (Nigg et al., 2004). Future research is needed to examine the moderating effects of regulation on reactivity-related ADHD pathways.
Collectively, these results support a transactional model for ADHD involving a combination of genetic/pre-/peri-natal influences— indexed by temperament reactivity—with the effects of the early caregiving environment (Johnston & Chronis-Tuscano, 2015). The sex differences within these transactional models suggest that boys and girls may follow different developmental pathways towards ADHD symptoms involving different reactivity-related risk profiles in infancy with different susceptibility to early caregiving influences. For boys, lower-quality caregiving appears to exacerbate reactivity-related risk for ADHD symptoms; for girls, not only does lower-quality caregiving exacerbate reactivity-related risk, but also higher-quality caregiving appears to promote reactivity-related protection against ADHD symptoms. It is unclear why girls with higher levels of reactivity were protected by higher-quality caregiving and boys were not. These differences may reflect differential sensitivity to parenting (i.e., we found a main effect of caregiving on girls’ ADHD symptoms but not boys), differences in the neurodevelopmental phenotypes of ADHD (e.g., in community samples boys may have more severe cognitive endophenotype than girls; Arnett et al., 2015), or a combination of both (i.e., transactional effect of differential sensitivity with different phenotype on the development of ADHD). Future research examining sex differences in the pathogenesis of ADHD involving multiple levels of analysis—from genetic to psychosocial factors—is needed to address these questions.
This study had several notable strengths including a nine-year prospective longitudinal design and multiple methods of measurement. We are the first study to demonstrate a prospective relation between multiple dimensions of temperament reactivity in early infancy and childhood ADHD symptoms. Additionally, MCB, measured at nine months, was temporally separated and independent of our measure of reactivity, allowing better isolation of moderating effects. By using observational measures of temperament and MCB, and parent- and teacher-report for ADHD symptoms, we avoided the problem of shared method variance. We also addressed a limitation of previous studies by controlling for behavioral problems, allowing us to identify unique associations between early life predictors and childhood ADHD symptoms.
Notwithstanding these strengths, several limitations are noted. First, we used a community sample that was selectively recruited to represent the range of temperament reactivity. Although our measures of ADHD symptoms had variability, results may differ in a clinical or combined clinical/community sample. Furthermore, although at the time of recruitment our sample was representative of the population in the local area, we note the majority of parents in our sample were well-educated and Caucasian. Second, our measure of ADHD was derived from parent- and teacher-report collected at different assessment periods, and only a subsample of participants had data for teacher-report, although there were no demographic/key variable differences between participants with missing/non-missing teacher-report data. Third, as with any long-term prospective study, there was attrition across assessment periods. In particular, participants with higher levels of positive affect were more likely to drop out of the study compared to participants with lower levels of positive affect. Fortunately, the full information maximum likelihood estimator used in the analyses accounted for missing effects.
Conclusion
Our results highlight the importance of considering both infant and parenting characteristics in the early identification of risk for ADHD. That is, it is the combined effect of particular infant temperament reactivity and caregiving behaviors that translates into greater (or lesser) ADHD risk rather than either on its own. This is especially crucial for assessing reactivity-related risk for girls, where higher levels of positive affect or motor activity may actually signal lower risk for ADHD when combined with higher-quality caregiving. Clinically, our results suggest that screeners, such as those used in primary care settings during well child visits, that include temperament and parenting measures may optimize the early identification of ADHD risk better than temperament-only measures. To avoid high rates of false-positives, these screeners may be most helpful when used in conjunction with other information, such as family history of ADHD. Of course, future research is needed to evaluate the psychometrics and clinical utility of such measures. In terms of early intervention, our results suggest that early interventions targeting more sensitive and less intrusive parenting behaviors during infancy may buffer temperament-related risk for ADHD. Future research also is needed to identify the mechanisms-of-action operating in both the reactivity-related risk and reactivity-related protective ADHD pathways. Developmental pathways from temperament reactivity to higher levels of ADHD symptoms have received some research attention (Willoughby et al., 2017), yet understanding of reactivity-related pathways leading to fewer ADHD symptoms is limited. Increasing knowledge of these protected pathways would be beneficial in identifying potential parenting and child treatment targets within the at-risk pathways.
Key points.
ADHD is a neurodevelopmental disorder beginning early in life
Early identification of at-risk children is critical for informing early interventions
This is first study to demonstrate higher levels of temperament reactivity in early infancy —measured at 4 months of age—are associated with risk for childhood ADHD symptoms
We found the strength of reactivity-related risk for ADHD depends on the quality of maternal caregiving during infancy
For boys, higher-quality maternal caregiving buffers reactivity-related risk
For girls, higher-quality maternal caregiving not only buffers reactivity-related risk for ADHD but also enhances reactivity-related protection against ADHD
Interventions targeting early parenting may help prevent development of ADHD symptoms among infants with higher levels of temperament reactivity
Acknowledgments
This research was supported by grants from the National Institute of Health (MH093349 and HD17899) to Nathan Fox. Preparation of this manuscript was supported by Banting Postdoctoral Fellowship, awarded to Natalie Miller. The authors would like to thank the children and parents for their participation and our research assistants for their contributions. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: No conflict of interest.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. London, UK: Sage; 1991. [Google Scholar]
- Ainsworth MS. Technical manual for the systems for coding infant attachment and reciprocal maternal behaviors. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Arnett AB, MacDonald B, Pennington BF. Cognitive and behavioral indicators of ADHD symptoms prior to school age. Journal of Child Psychology and Psychiatry. 2013;54:1284–1294. doi: 10.1111/jcpp.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett AB, Pennington BF, Willcutt EG, DeFries JC, Olson RK. Sex differences in ADHD symptom severity. Journal of Child Psychology and Psychiatry. 2015;56:632–639. doi: 10.1111/jcpp.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, de Haan M. Annual research review: Parenting and children’s brain development: The end of the beginning. Journal of Child Psychology and Psychiatry. 2011;52:409–428. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: A meta-analysis of shared environmental influences. Psychological Bulletin. 2009;135:608–637. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- Degnan KA, Hane AA, Henderson HA, Moas OL, Reeb-Sutherland BC, Fox NA. Longitudinal stability of temperamental exuberance and social–emotional outcomes in early childhood. Developmental Psychology. 2011;47:765–780. doi: 10.1037/a0021316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes MK, Rapee RM, Camberis AL, McMahon CA. Unique associations between childhood temperament characteristics and subsequent psychopathology symptom trajectories from childhood to early adolescence. Journal of Abnormal Child Psychology. 2017;45:1221–1233. doi: 10.1007/s10802-016-0236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Friedman AH, Watamura SE, Robertson SS. Movement-attention coupling in infancy and attention problems in childhood. Developmental Medicine & Child Neurology. 2005;47:660–665. doi: 10.1017/S0012162205001350. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KS, Essex MJ. Temperament as a liability factor for childhood behavioral disorders: The concept of liability. In: DiLalla LF, editor. Behavior genetics principles: Perspectives in development, personality, and psychopathology. Washington, DC: American Psychological Association; 2004. pp. 19–39. [Google Scholar]
- Gueron-Sela N, Atzaba-Poria N, Meiri G, Marks K. Temperamental susceptibility to parenting among preterm and full-term infants in early cognitive development. Infancy. 2016;21:312–331. doi: 10.1111/infa.12120. [DOI] [Google Scholar]
- Hane AA, Fox NA. Ordinary variations in maternal caregiving influence human infants’ stress reactivity. Psychological Science. 2006;17:550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- Hane AA, Fox NA, Henderson HA, Marshall PJ. Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology. 2008;44:1491–1496. doi: 10.1037/a0012855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold GT, Leve LD, Barrett D, Elam K, Neiderhiser JM, Natsuaki MN, … Thapar A. Biological and rearing mother influences on child ADHD symptoms: Revisiting the developmental interface between nature and nurture. Journal of Child Psychology and Psychiatry. 2013;54:1038–1046. doi: 10.1111/jcpp.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Gliga T, Jones E, Charman T. Annual research review: Infant development, autism, and ADHD – early pathways to emerging disorders. Journal of Child Psychology and Psychiatry. 2015;56:228–247. doi: 10.1111/jcpp.12328. [DOI] [PubMed] [Google Scholar]
- Johnston C, Chronis-Tuscano A. Families and ADHD. In: Barkley RA, editor. Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment. New York: Guilford Press; 2015. pp. 191–209. [Google Scholar]
- Kawabata Y, Tseng WL, Gau SSF. Symptoms of attention-deficit/hyperactivity disorder and social and school adjustment: The moderating roles of age and parenting. Journal of Abnormal Child Psychology. 2012;40:177–188. doi: 10.1007/s10802-011-9556-9. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Kreppner J, Knights N, Kumsta R, Maughan B, Golm D, … Sonuga-Barke EJS. Early severe institutional deprivation is associated with a persistent variant of adult attention-deficit/hyperactivity disorder: Clinical presentation, developmental continuities and life circumstances in the English and Romanian adoptees study. Journal of Child Psychology and Psychiatry. 2016;57:1113–1125. doi: 10.1111/jcpp.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R. Principles and practice of structural equation modeling. 3rd edition. New York: Guilford; 2010. [Google Scholar]
- Lahey BB, Van Hulle CA, Keenan K, Rathouz PJ, D’Onofrio BM, Rodgers JL, Waldman ID. Temperament and parenting during the first year of life predict future child conduct problems. Journal of Abnormal Child Psychology. 2008;36:1139–1158. doi: 10.1007/s10802-008-9247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry. 2006;47:395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Goldsmith HH, Sachek J. Temperament and Attention Deficit Hyperactivity Disorder: The development of a multiple pathway model. Journal of Clinical Child and Adolescent Psychology. 2004;33:42–53. doi: 10.1207/S15374424JCCP3301_5. [DOI] [PubMed] [Google Scholar]
- Owens EB, Cardoos SL, Hinshaw SP. Developmental progression and gender differences among individuals with ADHD. In: Barkley RA, editor. Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment. New York: Guilford Press; 2015. pp. 223–255. [Google Scholar]
- Park SY, Belsky J, Putnam S, Crnic K. Infant emotionality, parenting, and 3-year inhibition: Exploring stability and lawful discontinuity in a male sample. Developmental Psychology. 1997;33:218–227. doi: 10.1037/0012-1649.33.2.218. [DOI] [PubMed] [Google Scholar]
- Poehlmann J, Hane A, Burnson C, Maleck S, Hamburger E, Shah PE. Preterm infants who are prone to distress: Differential effects of parenting on 36-month behavioral and cognitive outcomes. Journal of Child Psychology and Psychiatry. 2012;53:1018–1025. doi: 10.1111/j.1469-7610.2012.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. Journal of Child Psychology and Psychiatry. 2015;56:345–365. doi: 10.1111/jcpp.12381. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Eisenberg N, editor. Handbook of child psychology: Social, emotional, and personality development. Hoboken, NJ: John Wiley & Sons Inc; 1998. pp. 105–176. [Google Scholar]
- Rothbart MK, Sheese BE, Rueda MR, Posner MI. Developing mechanisms of self-regulation in early life. Emotion Review. 2011;3:207–213. doi: 10.1177/1754073910387943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM. School based assessments and interventions for ADD students. Irvine, CA: KC Publishing; 1992. [Google Scholar]
- Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conner CK, Abikoff HB, … Wu M. Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Halperin JM. Developmental phenotypes and causal pathways in attention deficit hyperactivity disorder: Potential targets for early intervention? Journal of Child Psychology and Psychiatry. 2010;51:368–389. doi: 10.1111/j.1469-7610.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Maughan B, Goodman R. What is a disruptive disorder? Temperamental antecedents of oppositional defiant disorder: Findings from the Avon Longitudinal Study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:474–483. doi: 10.1097/00004583-201005000-00008. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Holton KF, Nousen EK, Barling AN, Sullivan CA, Propper CB, Nigg JT. Early identification of ADHD risk via infant temperament and emotion regulation: A pilot study. Journal of Child Psychology and Psychiatry. 2015;56:949–957. doi: 10.1111/jcpp.12426. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 6. Boston: Pearson; 2013. [Google Scholar]
- Thapar A, Martin J, Mick E, Arias Vásquez A, Langley K, Scherer SW, … Holmans P. Psychiatric gene discoveries shape evidence on ADHD’s biology. Molecular Psychiatry. 2016;21:1202–1207. doi: 10.1038/mp.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. Human amygdala development in the absence of species-expected caregiving. Developmental Psychobiology. 2012;54:598–611. doi: 10.1002/dev.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung I, Brammer WA, Li JJ, Lee SS. Parenting behavior mediates the intergenerational association of parent and child offspring ADHD symptoms. Journal of Clinical Child and Adolescent Psychology. 2015;44:787–799. doi: 10.1080/15374416.2014.913250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby MT, Gottfredson NC, Stifter CA the Family Life Project Investigators. Observed temperament from ages 6 to 36 months predicts parent- and teacher-reported attention-deficit/hyperactivity disorder symptoms in first grade. Development and Psychopathology. 2017;29:107–120. doi: 10.1017/S0954579415001236. [DOI] [PubMed] [Google Scholar]