Abstract
Background & Aims:
Hepatic fibrosis is a primary risk factor for cirrhosis and hepatocellular carcinoma, which affect a disproportionate number of Hispanics in the United States. We aimed to determine the prevalence of significant fibrosis, measured by point shear-wave elastography (pSWE), and determine characteristics of hepatic fibrosis and simple steatosis in a population-based study of Mexican American Hispanics in south Texas.
Methods:
Liver stiffness was measured by pSWE, performed by 2 separate operators, for 406 participants in the Cameron County Hispanic Cohort from 2015 through 2017. Significant fibrosis (F2–F4) was defined as median stiffness > 1.34 m/s. Steatosis was determined by ultrasound. All participants underwent a clinical examination that included a comprehensive laboratory analysis and standardized interview about their medical and social history. We calculated weighted prevalence of fibrosis and determined clinical and demographic associations with significant fibrosis (with or without steatosis) and simple steatosis with no/minimal fibrosis using multinomial logistic regression.
Results:
Fifty-nine participants were excluded due to unreliable pSWE findings or inconclusive ultrasound results, for a final analysis of 347 participants. The prevalence of significant fibrosis was 13.8%; most of these participants (37/42, 88.1%) had no evidence of viral hepatitis or heavy drinking. Levels of liver enzymes were associated with fibrosis and simple steatosis. Indicators of metabolic health (insulin resistance, triglycerides, and cholesterol) were significantly associated with simple steatosis. Fibrosis, but not simple steatosis, was significantly associated with of antibodies against HCV in plasma (odds ratio, 18.9; P=.0138) and non-significantly associated with reduced platelet count (odds ratio, 0.8 per 50×103/μL; 95% CI, 0.5–1.1). Multivariable analyses, as well as sensitivity analyses removing F4 fibrosis and viral or alcoholic etiologies, confirmed our results.
Conclusion:
We estimated the prevalence of fibrosis in a large population of Mexican American Hispanics using pSWE measurements. We found Mexican American Hispanics to have a higher prevalence of fibrosis compared to European and Asian populations, primarily attributable to metabolic disease.
Keywords: CCHC, US, obesity, minority
Introduction
The incidence of hepatocellular carcinoma (HCC) is increasing in the United States (US), particularly among Hispanics1. In south Texas, Hispanics now have among the highest rates of HCC in the country (12.1 cases for 100,000 person-years vs 8.4 among Hispanics nationwide)1,2 However the prevalence and characteristics of liver fibrosis—a key risk factor for cirrhosis and HCC—in this region is unknown.
Biopsy remains the gold standard for staging of liver fibrosis, but is not suitable for population screening due to the potential for complications, patient discomfort, and expense.3 Therefore, point shear-wave elastography (pSWE), which has been validated against biopsy for the staging of liver fibrosis,4,5 is a promising method for population fibrosis screening. pSWE integrates easily into standard B-mode ultrasonography, has good inter-operator reliability,6,7 and has a lower failure rate than transient elastography in obese individuals.8 Validation studies of pSWE against biopsy in diverse populations have reported excellent accuracy to detect F2 fibrosis (meta-analysis sensitivity = 85.0%, specificity = 94.4)9 which is an ideal target for screening and HCC prevention.10
Despite robust clinical validation of pSWE, and the importance of early fibrosis detection, population-based applications of elastography are scant. Several European and Asian groups have used elastography in population-based studies11–13, but in the US no studies have employed elastography to determine the burden and distribution of fibrosis in the general population.
In the current study we applied pSWE screening to the Cameron County Hispanic Cohort (CCHC), a population-based study of Mexican Americans in south Texas, US. We assessed a cross-sectional sample of CCHC participants to (1) determine the prevalence of significant fibrosis, (2) determine the clinical and sociodemographic correlates of fibrosis and simple steatosis, and (3) identify clinical characteristics that distinguish individuals with fibrosis from those with simple steatosis.
Methods
Patient Population and Setting
The CCHC is a population-based cohort with stratified two-stage cluster sampling design in Brownsville, Texas, initiated in 2004. The Census blocks of Brownsville are stratified by socioeconomic quartiles based on US Census data; within each stratum, census tracts are selected randomly for invitation to the study; all members of selected households (≥ 18 years of age) are invited in-person at the home to participate in the CCHC studies.14 pSWE examinations began in 2015, with all participants invited to participate in the additional imaging studies (See Supplemental Figure 1). Greater than 95% of participants since 2015 have elected to undergo pSWE measurement. The study protocol was approved by the Committee for the Protection of Human Subjects of the University of Texas Health Science Center at Houston.
Clinical Examination
Participants arrived to the clinic 8 hours fasting and gave informed consent to participate in the clinical exam. Trained interviewers conducted in-depth interviews to capture sociodemographic parameters and social history, and completed a clinical exam, described elsewhere14. Hepatitis C Virus antibodies (anti-HCV) and Hepatitis B Virus surface antigen (HBsAg) were assayed using the Ortho HCV Version 3.0 ELISA Test System and Abnova Hepatitis B surface antigen Elisa Kit, respectively. Positive viral hepatitis results were sent to an independent CLIA-certified laboratory for confirmation, and those not confirmed positive were considered to be negative. Drinking history was obtained by interview. “Heavy drinking” was defined as regular consumption of > 21 drinks per week for men, and > 14 drinks per week for women.15
Ultrasonography and Elastography
Liver steatosis was determined by liver ultrasonography performed by trained operators and read by a single board-certified gastroenterologist. Next, pSWE liver stiffness measurements were taken using the Siemens Acuson S3000 (Siemens AG, Mountain View, CA). One operator captured shear wave speeds until a total of 10 readings were made (study 1). This process was repeated by a second operator (study 2), blind to the location of the previous 10 readings. The median shear wave velocity was recorded for each study, and the higher of the two medians is used to determine fibrosis stage.
Quality Control for pSWE Measurement
Participants were excluded (1) if either study had a shear-wave IQR-to-median ratio > 0.3;6 (2) if only one study was completed; (3) if either study had less than 7 valid readings;4 or (4) if the difference between medians in study 1 and study 2 was greater than two standard deviations of the distribution of differences. In addition, we excluded participants with an inconclusive liver ultrasound. Supplemental Figure 1 describes the development of the analytical data set.
Inter-operator reliability
There were four trained operators from which two completed the pSWE studies for each participant. We divided the sample into six strata (corresponding to the six pairwise combinations of operators) and calculated stratum-specific and overall kappa and tested homogeneity of kappa statistics over the strata for detection of significant fibrosis. In addition, we determined if the operator was associated with staging of significant fibrosis using logistic regression.
Statistical Analysis
All analyses take into account the sampling design of the CCHC. In addition, age- and gender-adjusted weights were calculated to estimate population prevalence, and we accounted for possible clustering effects by census block and household. First, we divided the participants into three disease groups: healthy (no steatosis and fibrosis < F2); steatosis (steatosis on ultrasound and fibrosis < F2); and fibrosis (fibrosis ≥ F2 with or without steatosis). In these groups, we calculated mean and standard error (SE), or frequency and proportion, of variables of interest. P-values for differences between the groups were calculated by one-way analysis of variance for continuous variables, and Rao-Scott χ2 test for categorical variables. Second, for parameters with a possible association with the disease groups (unadjusted p < 0.25), we used univariable multinomial logistic regression to estimate the odds ratio (OR) and 95% confidence interval (CI) for steatosis and fibrosis, relative to the healthy, for each parameter. Finally, we calculated the OR and 95% CI for fibrosis relative to steatosis in the multinomial framework, and repeated the univariable analyses after adjustment for age and sex. Results significant at the p < 0.05 level after controlling for false discovery rate (using the Benjamini-Hochberg procedure) are marked with an asterisk. Multivariable models were developed by including the variables that were predictive in univariable analysis as well as age and sex.
Sensitivity analyses
First we assessed changes to the multinomial results after removing participants with alcoholic or viral etiologies. Second, we repeated the multinomial analysis excluding those with F4 fibrosis (m/s > 2.55). Percent changes in ORs, changes in direction of association, and changes in significance were examined. Finally, we repeated analyses using a binomial parameterization of the outcome (significant fibrosis vs no/minimal fibrosis, irrespective of steatosis).
SAS 9.4 (Cary, NC) was used to complete all analyses. Figures were developed in the ggplot package16 of R Version 3.3.17
Results
Quality Control and Inter-Operator Reliability
Four hundred and six participants completed pSWE examinations between 2015 and 2017. We excluded 40 participants pursuant to our pSWE quality control criteria, and 19 participants with inconclusive liver ultrasound, for a final analytic sample of 347 (See supplementary Figure 1). For inter-operator reliability, the overall kappa statistic was 0.6, with 93.2% overall agreement between study 1 and study 2 for staging of significant fibrosis, and no evidence of heterogeneity across strata. There was no significant association between operator and fibrosis stage. Descriptive statistics of the analytic sample are given in Table 1; participant characteristics are similar to previous CCHC publications 14
Table 1.
Descriptive statistics of the analytic data set, Cameron County Hispanic Cohort
| Variable | Count | % |
|---|---|---|
| Age Group | ||
| <35 | 47 | 16.8 |
| 35 to <50 | 85 | 26.1 |
| 50 to < 65 | 134 | 28.9 |
| 65 + | 99 | 28.2 |
| Male Sex | 131 | 43.9 |
| No Insurance | 191 | 51.4 |
| No Insurance (< 65 yrs) [n = 265] | 174 | 67.9 |
| Place of Birth [n = 357] | ||
| United States | 102 | 27.6 |
| Mexico | 255 | 72.4 |
| Receives Public Assistance [n = 362] | 84 | 19.6 |
| Diabetesa | ||
| No | 251 | 71.4 |
| Yes, Diagnosed | 78 | 20.2 |
| Yes, Undiagnosed | 27 | 8.4 |
| Hypertensionb | 160 | 41.9 |
| Body Mass Index (BMI) Categories | ||
| Normal Weight (BMI < 25) | 63 | 16.1 |
| Overweight (25 ≤ BMI < 30) | 134 | 42.2 |
| Obese (30 ≤ BMI < 40) | 157 | 39.3 |
| Morbidly Obese (BMI ≥ 40) | 10 | 2.5 |
| Elevated waist circumferencec | 246 | 65.0 |
| Anti-HCV Positive | 9 | 1.8 |
| HBsAg Positive | 0 | 0 |
| Heavy Drinkingd | 8 | 3.5 |
Abbreviations. HCV, hepatitis C Virus. HBsAg, Hepatitis B Surface Antigen.
Diabetes status is determined according to the American Diabetes Association 2010 diagnostic guidelines.
Hypertension is defined as systolic blood pressure > 135 mmHg or diastolic blood pressure > 85 mmHg or taking antihypertensive medication
Defined as waist circumference > 102 cm for men, and > 88 cm for women
Defined as self-reported drinks > 21 for men and > 14 for women
Population Prevalence and Characteristics of Significant Liver Fibrosis
The overall prevalence of significant (F2+) fibrosis was 13.6% (95% CI 8.2—18.9%). By F-stage, we found that 5.6% (95% CI 2.3 – 8.9) had F2 fibrosis, 5.8% (95% CI 2.3 – 9.4) had F3 fibrosis, and 2.1% (95% CI 0.4 – 3.8) had F4 fibrosis. Among the 42 participants with significant fibrosis, two reported chronic heavy drinking and three were positive for anti-HCV (one participant had evidence of both risk factors). No participants had confirmed presence of HBsAg. The remaining cases were classified as NAFLD if they had at least one characteristic of NAFLD: steatosis, obesity, elevated waist circumference, or metabolic syndrome (n = 30, 66.9%). We considered those with no evidence of viral hepatitis, heavy drinking, or risk factors for NAFLD to have “unknown” etiology (n = 7, 18.6%). No participants were taking medications known or suspected of causing drug-induced liver disease. Figure 1 displays the liver stiffness and etiology of each participant by F score.
Figure 1. Liver stiffness measurements by point shear-wave elastography with etiologies, Cameron County Hispanic Cohort (2015-2017).
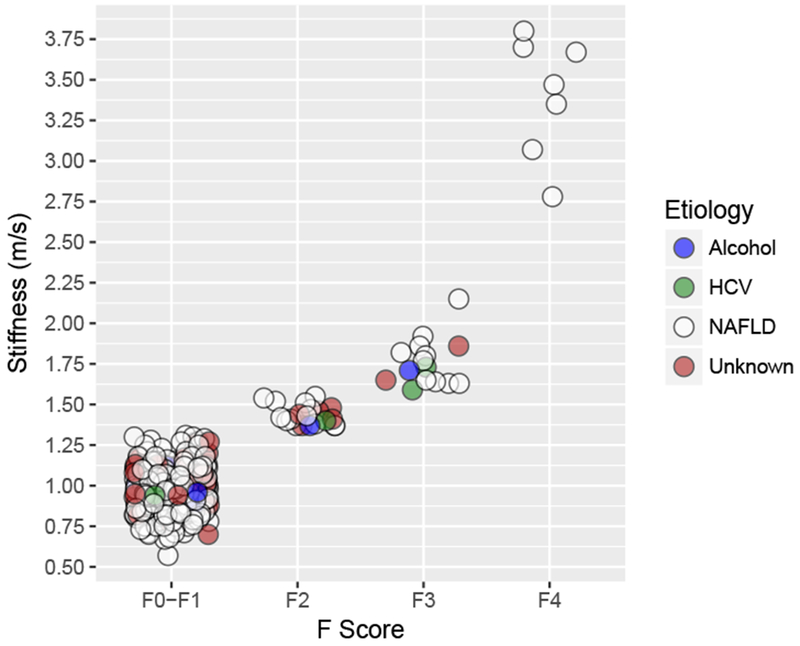
Disease etiology is represented by fill color. Most (78%) of participants with F2-F4 disease had non-viral, non-alcoholic etiologies.
The overall prevalence of fatty liver (irrespective of fibrosis) in the population was 43.4% (95% CI 37.6 – 49.2); among those with significant fibrosis, the prevalence of steatosis was 38.3% (95% CI 30.3 – 46.2), and among those with no/minimal fibrosis, it was 49.3% (95% CI 41.0 – 57.6). The 35 to 50 year age group had the greatest prevalence of both steatosis and fibrosis (Figure 2).
Figure 2. Prevalence of steatosis (without significant fibrosis) and significant fibrosis (with or without steatosis), Cameron County Hispanic Cohort (2015-2017).
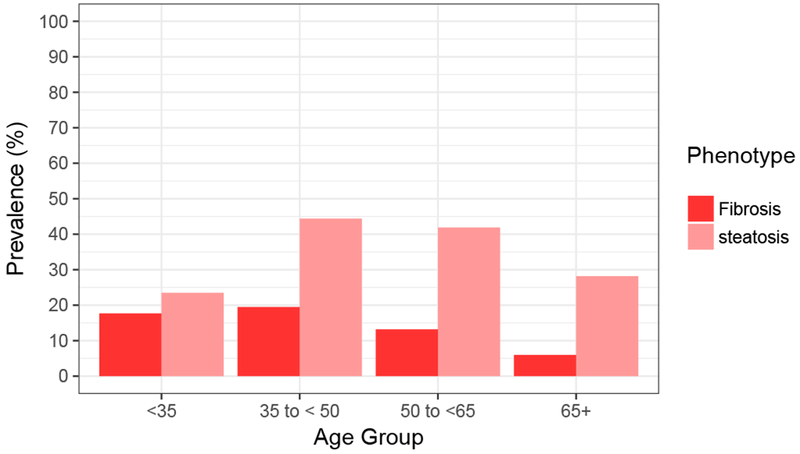
The prevalence of significant fibrosis (F2 – F4) was greatest in the younger two age groups. The presence of fibrosis and steatosis in young Mexican Americans highlights the need for effective community health interventions for younger individuals in the region.
Comparison of parameter values in each of the phenotypic groups
Table 2 presents participant characteristics in each phenotypic group. The prevalence of diabetes was highest in the steatosis group (37.5%), followed by fibrosis (27.5%), and healthy (23.2%; overall p = 0.1581). Measures of liver enzymes were associated with the liver phenotypes, with the highest levels of aspartate aminotransferase (AST) in the fibrosis group (p = 0.0228), and the highest levels of alanine aminotransferase (ALT) in the steatosis group (p = 0.0032). Measures of adiposity (obesity and waist circumference) were elevated in the steatosis group, with overall significant associations (p = 0.0006, p < 0.0001, respectively). There was an increasing number of mean drinks per week from healthy (1.8 drinks) to steatosis (2.1 drinks) to fibrosis (5.4 drinks), although this association did not reach nominal statistical significance (p = 0.1737).
Table 2.
Characteristics of participants with normal liver, steatosis, and F2 fibrosis, Cameron County Hispanic Cohort
| Parameters | Healthya Estimateb (95% CI) | Steatosis Estimate (95% CI) | Fibrosis Estimate (95% CI) | p-value |
|---|---|---|---|---|
|
| ||||
| Age (years) | 51.8 (48.0 - 55.6) | 52.5 (48.5 - 56.5) | 45.1 (38.9 - 51.4) | 0.1245 |
| Male Sex | 42.2 (33.4 - 51.0) | 39.5 (28.0 - 51.0) | 55.9 (39.6 - 72.3) | 0.2908 |
| No Insurance (< 65 years) | 57.5 (46.8 - 68.3) | 61.8 (49.9 - 73.7) | 68.6 (49.1 - 88.2) | 0.5859 |
| Born in US | 28.5 (20.3 - 36.7) | 22.9 (14.4 - 31.3) | 35.2 (15.5 - 55.0) | 0.4247 |
| Family History of Diabetes | 29.1 (20.5 - 37.7) | 33.7 (22.7 - 44.7) | 21.6 (6.6 - 36.6) | 0.4243 |
| Diabetesc | 23.2 (13.7 - 32.7) | 36.6 (26.8 - 46.5) | 27.5 (12.0 - 42.9) | 0.1581 |
| Fasting Glucose (mg/dL) | 104.0 (97.4 - 110.6) | 124.8 (107.8 - 141.7) | 111.9 (87.9 - 135.8) | 0.0839 |
| Hemoglobin A1C (%) | 6.1 (5.8 - 6.5) | 6.7 (6.2 - 7.2) | 6.4 (5.7 - 7.2) | 0.1951 |
| Insulin | 9.4 (8.3 - 10.5) | 14.7 (12.6 - 16.9) | 10.5 (7.6 - 13.4) | 0.0001* |
| HOMA-IR | 2.4 (2.1 - 2.7) | 4.4 (3.5 - 5.3) | 2.8 (1.8 - 3.8) | 0.0003* |
| Hypertensiond | 40.2 (30.1 - 50.2) | 43.0 (27.6 - 58.4) | 40.1 (20.1 - 60.1) | 0.9465 |
| Systolic BP (mmHg)e | 117.9 (114.4 - 121.3) | 120.2 (116.5 - 123.8) | 116.5 (108.0 - 125.1) | 0.5526 |
| Diastolic BP (mmHg) | 70.5 (68.8 - 72.3) | 73.1 (71.2 - 75.0) | 71.2 (66.6 - 75.9) | 0.0931 |
| Triglycerides (mg/dL) | 127.7 (116.4 - 139.0) | 161.9 (148.3 - 175.6) | 134.2 (94.5 - 173.9) | 0.0009* |
| Total Cholesterol (mg/dL) | 178.5 (170.0 - 187.0) | 180.6 (173.5 - 187.8) | 172.4 (155.5 - 189.4) | 0.6737 |
| LDL Cholesterol (mg/dL) | 107.3 (101.2 - 113.3) | 104.5 (97.9 - 111.1) | 97.4 (87.2 - 107.7) | 0.2879 |
| HDL Cholesterol, mean (mg/dL) | 47.7 (45.7 - 49.8) | 41.9 (40.1 - 43.6) | 46.1 (42.1 - 50.2) | <0.0001* |
| AST (units/L) | 21.3 (19.6 - 23.0) | 27.7 (22.0 - 33.5) | 34.2 (19.5 - 48.9) | 0.0313 |
| ALT (units/L) | 29.3 (26.2 - 30.3) | 44.5 (32.4 - 56.6) | 39.5 (28.7 - 50.4) | 0.0053* |
| AST/ALT Ratio | 0.77 (0.73 - 0.82) | 0.72 (0.61 - 0.83) | 0.83 (0.74 - 0.92) | 0.2464 |
| Obese (BMI ≥ 30) | 29.5 (21.4 - 37.5) | 56.2 .0(45 - 67.3) | 39.1 (22.9 - 55.3) | 0.0006* |
| BMI (kg/m2) | 28.6 (27.9 - 29.4) | 31.5 (30.7 - 32.4) | 28.1 (26.2 - 29.9) | <0.0001* |
| Elevated waist circumferencef | 57.2 (47.8 - 66.5) | 77.0 (65.8 - 88.2) | 58.5 (40.6 - 76.4) | 0.0234 |
| Waist Circumference (cm) | 97.9 (95.5 - 100.3) | 104.8 (102.6 - 106.9) | 98.7 (93.9 - 103.5) | <0.0001* |
| Platelet count (103/μL) | 236 (224 - 248) | 241.4 (228.3 - 254.4) | 219.2 (200.5 - 238.0) | 0.1374 |
| Albumin (mg/dL) | 3.9 (3.8 - 4.0) | 4.9 (3.9 - 4.0) | 3.9 (3.8 - 4.0) | 0.5477 |
| Total Bilirubin (mg/dL) | 2.2 (0.0 - 5.2) | 0.7 (0.4 - 1.0) | 0.7 (0.4 - 1.0) | 0.5936 |
| Anti-HCV Positive | 0.3 (0.0 - 1.0) | 2.6 (0.0 - 5.3) | 5.7 (0.0 - 12.5) | 0.0157 |
| HBsAg Positive | 0.0 (0.0 - 0.0) | 0.0 (0.0 - 0.0) | 0.0 (0.0 - 0.0) | N/A |
| Drinks per week | 1.8 (0.7 - 2.9) | 2.1 (0.8 - 3.4) | 5.4 (1.8 - 8.9) | 0.1737 |
| Heavy Drinkingg | 2.5 (0.0 - 6.9) | 1.8 (0.0 - 4.5) | 12.4 (0.0 - 27.1) | 0.0639 |
Abbreviations: CI, confidence interval; HOMA-IR, homeostatis model of insulin resistance; LDL, low-density lipoprotein; HDL, high-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, Body Mass Index; Anti-HCV, hepatitis C Virus antibody; HBsAg, Hepatitis B Surface Antigen.
Note. Statistical tests significant at the 5% level after correction for false discovery rate are marked with an asterisk.
Healthy indicates no evidence of steatosis and liver stiffness ≤ 1.34; steatosis indicates evidence of steatosis and liver stiffness ≤ 1.34 m/s; fibrosis indicates liver stiffness > 1.34 m/s with or without steatosis
For continuous variables, mean; for categorical variables, proportion. P-values calculated from ANOVA for continuous variables and from Rao-Scott χ2 test for categorical variables
According to American Diabetes Association 2010 Diagnostic Guidelines
Systolic Blood Pressure > 135 or Diastolic Blood Pressure > 85 or taking antihypertensive medication.
Blood pressure analyses are adjusted for self-reported use of antihypertensive medication
Defined as waist circumference > 102 for men and > 88 for women
Defined as self-reported consumption of >21 drinks per week for men, and >14 drinks per week for women.
Univariable analysis comparing steatosis and fibrosis to healthy participants
After controlling the false discovery rate, several clinical variables were associated significantly only with steatosis (Table 3), including fasting blood glucose [odds ratio (OR) 1.1 per 10 mg/dL, 95% CI 1.0 – 1.2], triglycerides (OR 1.4 per 50 mg/dL, 95% CI 1.1 – 1.8), ALT levels (OR 1.5 per 10 units/L, 95% CI 1.2 – 1.8), obesity (OR 2.3, 95% CI 1.5 – 5.0) and insulin (OR 2.8 per 10 mg/dL, 95% CI 1.6 – 4.7). Both triglyceride levels and ALT levels were independently associated with steatosis, but not fibrosis, in the multivariable model (OR 1.3 per 10 mg/dL, 95% CI 1.0 – 1.7; and OR 1.5 per 10 units/L, 95% CI 1.0 – 2.2, respectively) after adjustment for age, sex, fasting glucose, waist circumference, platelet count, HCV positivity and number of drinks per week (Supplementary Table 5).
Table 3.
Unadjusted association of sociodemographic and clinical parameters with healthy liver, steatosis, and liver fibrosis, Cameron County Hispanic Cohort
| Steatosis vs Healthya | Fibrosis vs Healthy | Fibrosis vs Steatosis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | OR (95% CI)b | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Age (years) | 1.0 (0.8 - 1.3) | 0.7975 | 0.8 (0.6 - 1.0) | 0.0671 | 0.8 (0.6 - 1.0) | 0.0475 |
| Male Sex | 0.9 (0.5 - 1.7) | 0.7313 | 1.7 (0.8 - 3.7) | 0.1473 | 1.9 (0.8 - 4.6) | 0.1259 |
| Diabetesc | 1.9 (1 - 3.8) | 0.0654 | 1.3 (0.5 - 3.2) | 0.6361 | 0.7 (0.3 - 1.6) | 0.3371 |
| Fasting Glucose (per 10 mg/dL) | 1.1 (1.0 - 1.2) | 0.0156 | 1.1 (0.9 - 1.2) | 0.4311 | 1.0 (0.8 - 1.1) | 0.4818 |
| Hemoglobin A1c (%) | 1.2 (0.9 - 1.5) | 0.3242 | 1.1 (0.9 - 1.5) | 0.4220 | 1.0 (0.8 - 1.2) | 0.7178 |
| Insulin (10 mg/dL) | 2.8 (1.6 - 4.7) | 0.0002* | 1.4 (0.7 - 2.6) | 0.3294 | 0.5 (0.2 - 1.1) | 0.0832 |
| HOMA-IR | 1.4 (1.2 - 1.7) | <0.0001* | 1.1 (0.9 - 1.4) | 0.3072 | 0.8 (0.6 - 1.1) | 0.1047 |
| Diastolic BP (mmHg)d | 1.4 (1.0 - 2.0) | 0.0375 | 1.1 (0.6 - 2.0) | 0.7770 | 0.8 (0.4 - 1.5) | 0.4540 |
| Hypertensione | 1.1 (0.5 - 2.4) | 0.7585 | 1.0 (0.4 - 2.6) | 0.9979 | 0.9 (0.3 - 2.5) | 0.8260 |
| Triglycerides (per 50 mg/dL) | 1.4 (1.1 - 1.8) | 0.0033* | 1.1 (0.6 - 1.9) | 0.7381 | 0.8 (0.4 - 1.4) | 0.3992 |
| HDL Cholesterol (mg/dL) | 0.6 (0.4 - 0.8) | 0.0003* | 0.9 (0.6 - 1.3) | 0.5098 | 1.5 (1.0 - 2.3) | 0.0421 |
| AST (per 10 units/L) | 1.4 (1.1 - 1.9) | 0.0176 | 1.5 (1.1 - 2.0) | 0.0080 | 1.1 (1.0 - 1.1) | 0.2640 |
| ALT (per 10 units/L) | 1.5 (1.2 - 1.8) | 0.0005* | 1.4 (1.2 - 1.8) | 0.0014* | 1.0 (0.9 - 1.1) | 0.5715 |
| AST/ALT Ratio | 0.5 (0.1 - 3.5) | 0.4546 | 1.9 (0.7 - 5.6) | 0.2227 | 4.2 (0.5 - 38.4) | 0.2016 |
| Obesef | 3.0 (1.7 - 5.5) | 0.0001* | 1.5 (0.7 - 3.4) | 0.2828 | 0.5 (0.2 - 1.2) | 0.1039 |
| Waist Circumference (per 10 cm) | 1.0 (1.0 - 1.0) | 0.0002* | 1.0 (1.0 - 1.0) | 0.7556 | 0.7 (0.5 - 1.0) | 0.0408 |
| Platelets (per 50×103/μL) | 1.1 (0.8 - 1.4) | 0.5600 | 0.8 (0.6 - 1.1) | 0.1680 | 0.7 (0.4 - 1.0) | 0.0649 |
| Anti-HCV | 8.4 (0.9 - 79.4) | 0.0646 | 18.9 (1.8 - 196.4) | 0.0138 | 2.3 (0.4 - 11.7) | 0.3285 |
| Drinks per week | 1.0 (0.9 - 1.1) | 0.7294 | 1.1 (1.0 - 1.2) | 0.0358 | 1.1 (1.0 - 1.1) | 0.0505 |
| Heavy Drinkingg | 0.7 (0.1 - 7.6) | 0.7913 | 5.5 (0.6 - 52.3) | 0.1368 | 7.6 (1.0 - 56.8) | 0.0493 |
Abbreviations: OR, Odds Ratio; CI, confidence interval; HOMA-IR, homeostasis model of insulin resistance ; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; NAFLD, non-alcoholic fatty liver disease;
Note. Statistical tests significant at the 5% level after correction for false discovery rate are indicated with an asterisk.
Healthy indicates no evidence of steatosis and liver stiffness ≤ 1.34; steatosis indicates evidence of steatosis and liver stiffness ≤ 1.34 m/s; fibrosis indicates liver stiffness > 1.34 m/s
OR, 95% CI and P-value obtained from survey-based multinomial logistic regression
According to American Diabetes 2010 Diagnostic Guidelines
Blood pressure analyses are adjusted for self-reported use of antihypertensive medication
Systolic Blood Pressure > 135 or Diastolic Blood Pressure > 85 or taking antihypertensive medication
BMI ≥ 30
For men, greater than 21 self-reported drinks per week; for women, greater than 14 drinks per week.
In contrast, few associations reached 5% significance only for fibrosis vs healthy. Fasting glucose had a similar (but non-significant) association with fibrosis (OR 1.1 per 10 mg/dL, 95% CI 1.0 – 1.2). There was a suggestive negative association with platelet count (OR 0.8 per 50×103/μL, 95% CI 0.6 – 1.1). Anti-HCV presence was strongly associated with fibrosis relative to healthy (OR 18.9, 95% CI 1.8 – 196.4), but did not reach corrected significance.
When comparing fibrosis to the steatosis reference, we found that reduced platelet count was borderline significantly associated with fibrosis relative to steatosis [OR 0.7 per 50×103/μL, 95% CI 0.4 – 1.0]. We also found that HDL cholesterol was slightly higher in the fibrosis group (OR per 10 mg/dL = 1.5, 95% CI 1.0 – 2.3). Neither association was significant after controlling the false discovery rate. Results were similar after adjusting for age and sex (Supplementary Table 1).
Sensitivity analyses
We then repeated the analysis in Table 3 excluding those with evidence of HCV infection or heavy alcohol consumption (n = 5; Supplementary Table 2), revealing only minor differences in measures of association and significance. Similar results were found after removing participants with F4 fibrosis (cirrhosis; Supplementary Table 3), and when using a binomial parameterization of the outcome (significant fibrosis vs no/minimal fibrosis; Supplementary Table 4).
Discussion
We have estimated the population prevalence of significant liver fibrosis in a Mexican American population using an accurate and non-invasive method, pSWE.18,19 Our results show that a large proportion (13.8%) of the Mexican American population in Texas, which is known to have a high incidence of liver cancer, has significant liver fibrosis.
Over 88% of participants had no evidence of viral or alcoholic etiologies of disease. Among these non-alcoholic, non-viral cases, 81% likely had NAFLD, but the remaining 19% had no known risk factors for fibrosis. This is expected as non-alcoholic steatohepatitis is the fastest-growing indication for liver transplantation in the US20, and obesity and diabetes are common in south Texas.14 In addition, liver fibrosis is significantly heritable, suggesting possible genetic predisposition to fibrosis among those with no known risk factors.21 Therefore, baseline susceptibility to NAFLD among Hispanic populations in Texas,22 combined with the rise of obesity and diabetes, may explain the high rates of HCC in south Texas1. However, to examine the hypothesis that aflatoxin B1 exposure contributes substantially to HCC in south Texas, we previously sequenced the P53 R249S mutation (which is characteristic of aflatoxin B1 exposure) in the CCHC, and found no participants with the mutation23. These data call into question the assertion by Ramirez et al. that aflatoxin is an important etiology of disease in south Texas24.
There are few population-based studies (none in the Americas) with which to compare our prevalence estimates. Two population-based studies using TE in European populations found prevalence of significant fibrosis equal to 5.6% and 9.0%.11,25 Another group applied TE in a randomly selected Hong Kong Chinese population and found a 3.7% prevalence of significant fibrosis.12 Many others have applied TE to selected clinical populations whose estimates are not generalizable to general populations. Despite the limited studies employing pSWE or TE in a population-based context, our results suggest that the burden of significant liver fibrosis is substantially greater in Mexican American Hispanic groups than in Europe or Asia.
Clinical characteristics of participants with steatosis only and those with significant fibrosis did not differ appreciably, highlighting the importance of imaging for liver disease risk stratification. Surprisingly, the younger age groups had a greater prevalence of significant fibrosis (Figure 2), possibly reflecting a cohort effect related to US nativity26, which is associated with younger age in the CCHC (data not shown). This finding echoes previous studies in the CCHC which emphasized the need for chronic disease intervention in younger Mexican Americans, particularly men.27,28 Indeed, the overall prevalence of fibrosis was higher in men (17.3%) than women (10.6%) in this study.
This study has several limitations. First, unlike clinical studies, this community-recruited study does not perform liver biopsy. This prevents an independent validation of pSWE against biopsy in this population. However, a recent meta-analysis showed that pSWE had the greatest area under receiver operating characteristic curve for F2+ fibrosis compared to TE and biomarker measures, so misclassification is likely to be minor and nondifferential with respect to most clinical measures. Finally, our ultrasound measurement of liver steatosis may have limited sensitivity for the detection of steatosis < 30% of the liver, leading to an under-estimate of the prevalence of steatosis, particularly in the presence of fibrosis.29
In conclusion, hepatic fibrosis is a crucial phenotype for HCC risk stratification in Mexican American populations in the US. Published data show that liver fibrosis, regardless of NAFLD activity score or degree of steatohepatitis, is a primary predictor of liver-related mortality;30,31 therefore, the detection of and intervention on liver fibrosis in this population has the potential to reduce morbidity and mortality of both cirrhosis and HCC. Overall, our results urge increased community health efforts to stem the burden of NAFLD among Mexican Americans, particularly in younger groups. Elastography—whether pSWE or TE—is an affordable, rapid, and practical screening modality for early liver disease in the general population, and has broad potential for detection and prevention of advanced liver disease in health disparity groups.
Supplementary Material
Acknowledgments
We would like to thank our cohort team, particularly Rocío Uribe and her team, who not only recruited and interviewed the participants, but also performed the ultrasound and elastography studies under expert supervision; Marcela Morris, BS, and Israel Hernandez, AAS, and their teams for laboratory and data support; Norma Pérez-Olazarán, BBA, and Christina Villarreal, BA for administrative support; Valley Baptist Medical Center, Brownsville, Texas, for providing us space for our Center for Clinical and Translational Science Clinical Research Unit; and the community of Brownsville and the participants who so willingly participated in this study in their city.
Grant Support
Center for Clinical and Translational Sciences, National Institutes of Health Clinical and Translational Award no.UL1 TR000371 from the National Center for Advancing Translational Sciences.
Predoctoral Fellowship, University of Texas School of Public Health Cancer Education and Career Development Program – National Cancer Institute/NIH Grant R25 CA057712.
Abbreviations:
- ALT
alanine aminotransferase
- ARFI
acoustic radiation force impulse elastography
- AST
aspartate aminotransferase
- AUROC
area under receiver operator curve
- CCHC
Cameron County Hispanic Cohort
- TE
transient elastography
- NAFLD
non-alcoholic fatty liver disease
- OR
odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Disclosures:
Gordon P. Watt Nothing to disclose.
Jen-Jung Pan Nothing to disclose.
Michael B. Fallon Nothing to disclose.
Miryoung Lee Nothing to disclose.
Rohit Loomba Nothing to disclose.
Laura Beretta Nothing to disclose.
Joseph B. McCormick Nothing to disclose.
Susan P. Fisher-Hoch Nothing to disclose.
Contributor Information
Gordon P. Watt, The University of Texas Health Science Center at Houston School of Public Health, Brownsville Regional Campus, Brownsville, TX, USA
Miryoung Lee, The University of Texas Health Science Center at Houston School of Public Health, Brownsville Regional Campus, Brownsville, TX, USA.
Jen-Jung Pan, The University of Arizona College of Medicine Tuscon, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Tuscon, Arizona, USA.
Michael B. Fallon, The University of Arizona College of Medicine Phoenix, Department of Medicine, Phoenix, Arizona, USA
Rohit Loomba, The University of California San Diego, Division of Gastroenterology, San Diego, CA, USA.
Laura Beretta, The University of Texas MD Anderson Cancer Center, Department of Molecular and Cellular Oncology, Houston, TX, USA.
Joseph B. McCormick, The University of Texas Health Science Center at Houston School of Public Health, Brownsville Regional Campus, Brownsville, TX, USA
Susan P. Fisher-Hoch, The University of Texas Health Science Center at Houston School of Public Health, Brownsville Regional Campus, Brownsville, TX, USA
REFERENCES
- 1.Steele CB. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity — United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2017;66 doi: 10.15585/mmwr.mm6639e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramirez AG, Munoz E, Holden AE, Adeigbe RT, Suarez L. Incidence of Hepatocellular Carcinoma in Texas Latinos, 1995-2010: An Update: e99365. PLoS One San Franc. 2014;9(6) doi: 10.1371/journal.pone.0099365. doi: http://dx.doi.org.ezproxyhost.library.tmc.edu/10.1371/journal.pone.0099365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bravo AA Sheth SG Chopra S Liver Biopsy N Engl J Med 2001. 344 7 495–500 10.1056/NEJM200102153440706 [DOI] [PubMed] [Google Scholar]
- 4. Cui J Heba E Hernandez C et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study: STEATOHEPATITIS/METABOLIC LIVER DISEASE Hepatology 2016. 63 2 453–461 10.1002/hep.28337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedrich-Rust M Nierhoff J Lupsor M et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis J Viral Hepat 2012. 19 2 e212–219 10.1111/j.1365-2893.2011.01537.x [DOI] [PubMed] [Google Scholar]
- 6. Boursier J Isselin G Fouchard-Hubert I et al. Acoustic radiation force impulse: a new ultrasonographic technology for the widespread noninvasive diagnosis of liver fibrosis Eur J Gastroenterol Hepatol 2010. 22 9 1074–1084 10.1097/MEG.0b013e328339e0a1 [DOI] [PubMed] [Google Scholar]
- 7. Ferraioli G Tinelli C Lissandrin R et al. Point shear wave elastography method for assessing liver stiffness World J Gastroenterol WJG 2014. 20 16 4787–4796 10.3748/wjg.v20.i16.4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jaffer OS Lung PFC Bosanac D Shah A Sidhu PS Is ultrasound elastography of the liver ready to replace biopsy? A critical review of the current techniques Ultrasound 2012. 20 1 24–32 10.1258/ult.2011.011043 [DOI] [Google Scholar]
- 9. Xiao G Zhu S Xiao X Yan L Yang J Wu G Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis Hepatology 2017. 66 5 1486–1501 10.1002/hep.29302 [DOI] [PubMed] [Google Scholar]
- 10. Thoma C Day CP Trenell MI Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: A systematic review J Hepatol 2012. 56 1 255–266 10.1016/j.jhep.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 11.Caballeria L, Pera G, Arteaga I. et al. High Prevalence of Liver Fibrosis Among European Adults with Unknown Liver Disease. A Population-Based Study. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2018 Feb; doi: 10.1016/j.cgh.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 12. Wong VW-S Chu WC-W Wong GL-H et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography Gut 2012. 61 3 409–415 10.1136/gutjnl-2011-300342 [DOI] [PubMed] [Google Scholar]
- 13. van der Voort E Koehler E Nijsten T et al. Increased Prevalence of Advanced Liver Fibrosis in Patients with Psoriasis: A Cross-sectional Analysis from the Rotterdam Study Acta Derm Venereol 2016. 96 2 213–217 10.2340/00015555-2161 [DOI] [PubMed] [Google Scholar]
- 14.Fisher-Hoch SP, Rentfro AR, Gaines Wilson J. et al. [July 27, 2014];Socioeconomic Status and Prevalence of Obesity and Diabetes in a Mexican American Community, Cameron County, Texas, 2004-2007. Prev Chronic Dis. 2010 7(3) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2879985/ [PMC free article] [PubMed] [Google Scholar]
- 15. Chalasani N Younossi Z Lavine JE et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology Gastroenterology 2012. 142 7 1592–1609 10.1053/j.gastro.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 16.Wickham H. Ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York; 2009. http://ggplot2.org. [Google Scholar]
- 17.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. https://www.R-project.org/ [Google Scholar]
- 18. Perez A Anzaldua M Mccormick J Fisher-Hoch S High frequency of chronic end-stage liver disease and hepatocellular carcinoma in a Hispanic population J Gastroenterol Hepatol 2004. 19 3 289–295 10.1111/j.1440-1746.2003.03277.x [DOI] [PubMed] [Google Scholar]
- 19.Jiao J, Watt GP, Lee M. et al. Cirrhosis and Advanced Fibrosis in Hispanics in Texas: The Dominant Contribution of Central Obesity: e0150978. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150978. doi: http://dx.doi.org.utsph.idm.oclc.org/10.1371/journal.pone.0150978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong RJ Cheung R Ahmed A Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S Hepatology 2014. 59 6 2188–2195 10.1002/hep.26986 [DOI] [PubMed] [Google Scholar]
- 21. Loomba R Schork N Chen C-H et al. Heritability of Hepatic Fibrosis and Steatosis Based on a Prospective Twin Study Gastroenterology 2015. 149 7 1784–1793 10.1053/j.gastro.2015.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Browning JD Szczepaniak LS Dobbins R et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity Hepatology 2004. 40 6 1387–1395 10.1002/hep.20466 [DOI] [PubMed] [Google Scholar]
- 23.Jiao J, Niu W, Wang Y. et al. Prevalence of Aflatoxin-associated TP53R249S mutation in Hepatocellular Carcinoma in Hispanics in South Texas. Cancer Prev Res (Phila Pa) 2017 Jan; doi: 10.1158/1940-6207.CAPR-17-0235. canprevres.0235.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramirez AG Muñoz E Parma DL et al. Lifestyle and Clinical Correlates of Hepatocellular Carcinoma in South Texas: A Matched Case-control Study Clin Gastroenterol Hepatol 2017. 15 8 1311–1312 10.1016/j.cgh.2017.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koehler EM Plompen EPC Schouten JNL et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study Hepatology 2016. 63 1 138–147 10.1002/hep.27981 [DOI] [PubMed] [Google Scholar]
- 26. Hernández-Valero MA Bustamante-Montes LP Hernández M et al. Higher risk for obesity among Mexican-American and Mexican immigrant children and adolescents than among peers in Mexico J Immigr Minor Health 2012. 14 4 517–522 10.1007/s10903-011-9535-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salinas J McCormick JB Rentfro A et al. The Missing Men: High Risk and low use of health care in Men of Mexican Origin Am J Mens Health 2011. 5 4 332–340 10.1177/1557988310379390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watt GP, Vatcheva KP, Griffith DM, Reininger BM, Beretta L, Fallon MB. The Precarious Health of Young Mexican American Men in South Texas, Cameron County Hispanic Cohort, 2004-2015. Prev Chronic Dis. 2016;13:E113. doi: 10.5888/pcd13.160020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takahashi Y Fukusato T Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis World J Gastroenterol 2014. 20 42 15539–15548 10.3748/wjg.v20.i42.15539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Le MH Devaki P Ha NB et al. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States PLoS ONE 2017. 12 3 1–13 10.1371/journal.pone.0173499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Angulo P Kleiner DE Dam-Larsen S et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease Gastroenterology 2015. 149 2 389–397.e10 10.1053/j.gastro.2015.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


