Abstract
Background:
Alcohol use disorders (AUDs) are often accompanied by co-morbid physiologic and psychosocial conditions, including sleep disturbances. Sleep disturbances in these individuals may be associated with increased risk of relapse to drinking following detoxification and rehabilitation.
Participants:
The sample of inpatient treatment-seeking individuals with AUDs (N =164) was 70.1% male and 47.6% African American with a mean age of 45.6 years (± 9.5 years).
Methods:
Latent class analysis (LCA) was used to identify unmeasured class membership based on seven indicators: maximum Clinical Institute Withdrawal Assessment (CIWA) scores; sleep efficiency (actigraphy); sleep disturbances (Pittsburgh Sleep Quality Index-PSQI); anxiety/depression (Comprehensive Psychopathological Rating Scale-CPRS); and current and lifetime post-traumatic stress disorder (PTSD).
Results:
The average number of drinking days in the 90 days preceding admission was 72.0 (± 22.0 days), with an average of 13.16 drinks per day (± 5.70 drinks). Nearly one-quarter (24.4%) of respondents reported lifetime PTSD. Three latent classes were identified: Sleep Disturbance (SD); Sleep Disturbance, Anxiety and Depression (SD/AD); and Sleep Disturbance, Anxiety and Depression, and PTSD (SD/AD/PTSD). Members of the SD/AD/PTSD group were more likely to be female and had the highest withdrawal and sleep disturbance scores of all three groups.
Conclusion:
Findings support the use of LCA to identify subgroups of individuals with AUDs and accompanying sleep disturbances. Class identification may provide clinicians with insight into the integrative tailoring of interventions that meet the varied needs of individuals with AUDs, accompanying co-morbidities, and sleep disturbances.
Keywords: sleep disturbance, alcohol use disorder, latent class analysis, PTSD, Anxiety, Depression
Introduction
Preventing the excessive use of alcohol is a major public health priority (CDC, 2016). In 2014 alone, 16.3 million adults 18 years of age and older had an alcohol use disorder (NIAAA, 2016). The Diagnostic and Statistical Manual (DSM-V) now integrates what was formerly known as alcohol abuse/dependence into a single diagnostic criterion labeled “alcohol use disorder” (AUD) with mild, moderate, and severe classifications (NIAAA, 2016). Severe AUD is classified by six or more symptoms outlined in the DSM-V (APA, 2013).
Alcohol use disorders (AUDs) are often accompanied by co-morbid physiologic and psychosocial conditions, including but not limited to sleep disturbances (Benca, 1996; WHO, 2013). Alcohol use can negatively affect sleep via increased nightmares, snoring, and other interruptions (Landolt & Gillin, 2001). Sleep disturbances are common during phases of drinking and recovery (Gillin & Drummond, 2000) and can persist for months or years during the process of recovery (Landolt & Gillin, 2001). These sleep disturbances are often associated with a decrease in an individual’s health-related quality of life (Ayas, White, Al-Delaimy, et al., 2003; Stine & Chapman, 2005; Chaudhary, Kampman, Kranzler, Grandner, Debbarma, & Chakravorty, 2015; Brower, Aldrich, Robinson, Zucker, & Greden, 2001). Among treatment-seeking individuals who are alcohol-dependent, insomnia symptoms may increase psychosocial consequences related to alcohol (Foster & Peters, 1999). Sleep disturbances in these individuals may be associated with increased risk of relapse to drinking following detoxification and rehabilitation (Landolt & Gillin, 2001; Brower, Aldrich, Robinson, Zucker, & Greden, 2001; Foster & Peters, 1999). Additionally, there are established, complex associations between alcohol dependence, anxiety, depression and post-traumatic stress disorder (PTSD) (Klimkiewicz, Klimkiewicz, Jakubczyk, Kieres-Salomonski, & Wojnar, 2015). Understanding the nature of how physiologic and psychosocial co-morbidities present and whether they occur in predictable clusters is important in the assessment and management of sleep disorders in individuals with severe alcohol use disorders throughout their treatment and recovery.
Mounting evidence suggests that individuals diagnosed with mental health disorders would benefit from a “person-centered” approach, not only in their treatment, but also in the assessment and analysis of their symptoms. Many studies, however, use approaches that focus on relationships between variables (e.g. regression, factor analysis, and structured equation monitoring). This approach limits translation of findings to individuals since the information obtained by the statistical method is variable-oriented, not individual-oriented (Bergman & Magnusson, 1997). The person-centered focus is useful in symptom research where data often include heterogeneous groups of individuals with multiple symptoms. The goal of person-centered approaches is to divide heterogeneous group units into homogenous subgroups, in which members are similar to each other while different from individuals in other subgroups (Muthén & Muthén, 2000). These approaches may be particularly relevant for individuals with chronic conditions (Miaskowski et al., 2016).
Increasingly, studies utilize latent class analysis (LCA) to identify “unobservable” subgroups in populations of interest including individuals with AUD. The fundamental latent class model postulates that there are unobservable (i.e., latent) subgroups, which are called classes, within a population (Chung, Lanza, & Loken, 2008). When examining alcohol abuse and dependence specifically, researchers have used LCA to examine nosology/diagnoses, treatment-seeking behavior, and outcomes (Cranford, Krentzman, Mowbray, & Robinson, 2014; Jacob, Blonigen, Koenig, Wachsmuth, & Price, 2010; Moss, Goldstein, Chen, & Yi, 2015; Mowbray, Glass, & Grinnell-Davis, 2015; Schuler, Puttaiah, Mojtabai, & Crum, 2015). A number of these LCA studies have utilized the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) dataset to establish behavior clusters and predictors of concurrent substance abuse in individuals with alcohol dependence (Jacob, Blonigen, Koenig, Wachsmuth, & Price, 2010; Moss, Goldstein, Chen, & Yi, 2015; Mowbray, Glass, & Grinnell-Davis, 2015; Schuler, Puttaiah, Mojtabai, & Crum, 2015). Among individuals with alcohol use disorders, withdrawal, sleep, and psychiatric disorders are often not independent of each other. LCA takes into account the relationships between these variables in identifying groups of people who are similar to each other, and, thus, offers more utility than univariate approaches. Our study builds on these and other previous studies examining varied symptoms and psychopathologies in treatment-seeking individuals with AUD by focusing on sleep disturbance as one of the primary indicators of interest.
Sleep disturbance is a modifiable co-morbid condition in individuals with severe AUD which when addressed could have implications for reduced drinking behaviors and improved health outcomes during recovery. The purpose of the latent class analysis presented in this paper was to identify subgroups of treatment-seeking individuals undergoing inpatient alcohol rehabilitation based on eight indicators of interest: alcohol withdrawal, wake after sleep onset, sleep efficiency, sleep disturbances, anxiety and depression, current post-traumatic stress disorder (PTSD), and lifetime PTSD. The selection of these eight indicators was based on the prevalence of the comorbidities including self-reported sleep disturbance both in our sample and in individuals with alcohol use disorders in general. Specifically, alcohol withdrawal was included as a measure of the severity of alcohol dependence assessed within the first four days after admission. Sleep efficiency (SE) was chosen as the objective measures of sleep quality based on our interest in the full study published results that showed improvement in this measure over four weeks of inpatient treatment time (Wallen, Brooks, Whiting, et al., 2014). We also examined demographic factors associated with each subgroup to further delineate the possibility of designing tailored interventions in targeted populations.
Methods
This analysis is part of a larger study approved by the Addictions Institutional Review Board (IRB) at the National Institutes of Health (NIH; NCT#001060903). All participants included in this analysis (n=164) were first admitted to a clinical research facility providing inpatient detoxification and treatment under a screening and assessment protocol, which enrolls adults over 18 years of age seeking treatment for alcohol dependence. Individuals who had medical problems that could not be adequately managed at the NIH Clinical Center, had serious neuro-psychiatric conditions which impair judgment or cognitive function, were unlikely or unable to complete the treatment program because of incarceration, and/or who were required to receive treatment by a court of law were ineligible for participation. Sleep measures were added to this ongoing assessment and treatment study to explore the prevalence of sleep disturbances among a consecutive subset of participants enrolled in the treatment seeking study. Data for this analysis were collected between 2011 and 2013 and all participants enrolled onto the screening and assessment protocol elected to participate. All participants received continued physical evaluations, inpatient treatment of alcohol withdrawal, psychosocial management, and an educational treatment program.
Measures.
Both objective and subjective measures of sleep quantity and quality were collected as part of a sleep prevalence cohort in the screening and assessment protocol (Wallen, Brooks, Whiting, et al., 2014). Participants were asked to wear a Philips Actiwatch 2 (Philips Respironics) for the length of their inpatient stay (approximately 30 days), however for the purposes of this analysis we only used the first week of actigraphy data. Actiwatches are small actigraphy-based wristband data loggers that record a digitally-integrated measure of gross motor activity and ambient light. This provided an objective measure of sleep schedule variability, sleep quantity, and sleep efficiency; raw data were analyzed using computerized sleep scoring software (Respironics). Investigators reviewed each sleep period prior to analysis to screen for malfunctioning watches, corrupt data, and make adjustments using bedtimes and wake times from diary self-reports when necessary. Sleep efficiency is defined as the proportion of time the individual spent sleeping versus how much time they spent in bed during the major rest interval. Studies have shown that actigraphy has high sensitivity with moderate accuracy in detecting sleep both in populations with normal and disturbed sleep compared to polysomnography (Kushida et al., 2001; Paquet et al., 2007).
Participants completed the Pittsburgh Sleep Quality Index (PSQI), a self-reported questionnaire that provides a measure of sleep disturbances over the previous 30-day time interval, on Day 2 of their admission. Nineteen items generate seven “component” scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The sum of scores for these components yields one PSQI global score for which a score greater than five yields a diagnostic sensitivity of 89.6% and specificity of 86.5% (p ≤ 0.001) in distinguishing between good and poor sleepers (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989).
Alcohol withdrawal was assessed on days one through four of the inpatient admission using the Clinical Institute Withdrawal Assessment (CIWA-Ar) (Sullivan, Sykora, Schneiderman, Naranjo, & Sellers, 1989). The maximum score during the first four days of the inpatient stay was used for the analysis. This validated tool was used to evaluate the severity of alcohol withdrawal based on symptoms and physical signs of withdrawal (Sullivan, Sykora, Schneiderman, Naranjo, & Sellers, 1989). The CIWA-Ar is a validated measure that serves as a gold standard for clinical assessment of alcohol withdrawal in both inpatient and outpatient settings. It consists of 10 signs and symptoms (nausea, tremor, autonomic hyperactivity, anxiety, agitation, tactile, visual and auditory disturbances, headache and disorientation) on numeric scales to evaluate the severity of the signs/symptoms (Saitz, Mayo-Smith, Roberts, Redmond, Bernard, & Calkins, 1994). Total scores range from 0-67, with any score over 18 indicating severe withdrawal (Elliott, Geyer, Lionetti and Doty, 2012). Alcohol dependence was measured using the Alcohol Dependence Scale (ADS). This scale is a valid and reliable tool to assess the severity of alcohol dependence (Doyle & Donovan, 2009) in a variety of clinical settings and consists of 25 questions that take approximately 5-10 minutes to complete (Skinner & Allen, 1982).
Baseline anxiety and depression (during the first week of the inpatient stay) were assessed using the Comprehensive Psychopathological Rating Scale (CPRS) on day two after admission (Svanborg & Asberg, 1994). The CPRS includes 19 self-assessed variables corresponding to three CPRS-based subscales for affective and anxiety syndromes. Two subscales were included in this analysis: the Montgomery Åsberg Depression Rating Scale (MADRS) and the Brief Scale for Anxiety (BSA). Scores higher than 11 were indicative of depression (MADRS) and/or anxiety (BSA) of clinical relevance, based on other validated studies (Zimmerman, Posternak, & Chelminski, 2004; Jedel, Waern, Gustafson, et al., 2010; Lichstein, Stone, Donaldson, et al., 2006).
Post-traumatic stress disorder (PTSD) diagnoses were based on the Structured Clinical Interview for the DSM-IV (SCID-I). The SCID-I interview is used to evaluate criteria for a psychiatric diagnosis, including alcohol dependence and disorders that are frequently co-morbid with alcohol dependence. The interview consists of 11 modules with between 35-292 items per module and takes about 120-180 minutes (First, Spitzer, Gibbon, & Williams, 2002).
Statistical analysis.
Latent class analysis (LCA), a type of finite mixture model, was used to identify unmeasured class membership based on the following seven indicators of interest: maximum CIWA scores, sleep efficiency (Actiwatches), sleep disturbances (PSQI), anxiety/depression (CPRS), current and lifetime PTSD. This LCA is designed to examine subgroups (i.e., latent classes) of patients with unique clustered experiences (Muthen & Muthen, 2015; Muthen & Shedden, 1999). The analysis was conducted using Mplus Version 7.2 (Muthen & Muthen, 2015). Estimation was carried out with robust Maximum-Likelihood and the Expectation-Maximization algorithm (Muthen & Shedden, 1999). Selection of the optimal number of latent classes (best fitting model) was determined by several criteria: Akaike information criterion (AIC) (Akaike, 1974); Bayesian information criterion (BIC) (Schwartz, 1978); Vuong-Lo-Mendell Rubin likelihood ratio test (VLMR); and the parametric bootstrapped likelihood ratio test (BLRT). In addition, the entropy R-square provides an indication of the overall degree of classification uncertainty in the solution (Celeux & Soromenho, 1996). Among the competing models, the model that fits the data best has the lowest BIC and a VLMR and/or BLRT and higher entropy value. The BIC performed the best among the information criteria for the model selection, however further simulations demonstrated that the BLRT served as a better indicator of classes across all of the models considered (Jung & Wickrama, 2008; Nylund, Asparouhov, & Muthen, 2007). In addition, demographic characteristics of each subgroup were examined using analysis of variance (ANOVA). The statistical analyses were performed with SPSS version 22.0 (IBM, 2013).
Results
The sample was 70.1% male and 47.6% African American with an average age of 45.6 years (S.D. ± 9.5 years). The average number of drinking days in the 90 days preceding inpatient admission was 72.0 (± 22.0 days), with an average of 13.16 drinks per day (± 5.70 drinks). Nearly one-quarter (24.4%) of the sample reported being diagnosed with PTSD in their lifetimes (Table 1). Of the 164 participants, 138 (84.1%) had six or more full nights of actigraphy recorded during the first seven days of their inpatient stay, 14 participants (8.5% provided between one and five nights’ worth of data. Ten participants (6.1% of the sample) did not have any actigraphy data (the watch either malfunctioned or was not returned to the study team). Thus, the average number of days of actigraphy available during the first week of inpatient treatment was period was 6.45 days. The mean sleep efficiency for the sample was 75.78% (±SD = 14.15%) as measured by actigraphy and calculated as an average from the first seven days of the admission.
Table 1:
Participant Demographics, Clinical, and Actigraphy Variables (N=164)
| Demographics | Mean (Standard Deviation) | |
|---|---|---|
| Age (years) (range 22-64) | 45.6 ( 9.5) | |
| n | % | |
| Gender | ||
| Male | 115 | 70.1 |
| Female | 49 | 29.9 |
| Race | ||
| Black / African American | 78 | 47.6 |
| White | 72 | 43.9 |
| Other | 9 | 5.4 |
| Unknown | 6 | 3.7 |
| Ethnicity | ||
| Non-Hispanic | 157 | 95.7 |
| Hispanic | 5 | 3.0 |
| Missing | 2 | 1.2 |
| PTSD (Current) | ||
| Yes | 27 | 16.5 |
| No | 126 | 76.8 |
| Missing | 11 | 6.7 |
| PTSD (Ever - lifetime) | ||
| Yes | 40 | 24.4 |
| No | 113 | 68.9 |
| Missing | 11 | 6.7 |
| Anxiety Disorders (SCID-I) | ||
| 0 | 74 | 45.1 |
| 1 | 52 | 31.7 |
| 2 | 15 | 9.1 |
| 3+ | 12 | 7.3 |
| Missing | 11 | 6.7 |
| Mood Disorders (SCID-I) | ||
| 0 | 64 | 39.0 |
| 1 | 76 | 46.3 |
| 2 | 13 | 7.9 |
| Missing | 11 | 6.7 |
| Clinical Variables | Mean (Standard Deviation) | |
| Alcohol Dependence Scale (n=150, range 1-37) | 20.0 ( 7.0) | |
| Number of drinking days (n=153, range 7-90) | 72.0 ( 22.0) | |
| Average drinks per day (n=153, range 3-27) | 13.2 ( 5.7) | |
| Maximum CIWA Days 1-4 (n=153, range 0-26) | 8.0 ( 6.0) | |
| Baseline Anxiety - BSA (n=161, range 0-32) | 11.0 ( 7.0) | |
| Baseline Depression - MADRS (n=161, range 0-42) | 16.0 ( 9.0) | |
| Actigraphy Variables (Week 1 averages)* (n=152) | Mean (Standard Deviation) | |
| Sleep efficiency (%) (range 20.7-90.6) | 75.8 ( 14.2) | |
| Duration (hours) (range 1.8-11.0) | 6.4 ( 2.0) | |
| Wake bouts (range 9.2-40.4) | 24.1 ( 10.2) | |
| Wake after sleep onset (minutes) (range 21.1-150.9) | 68.0 ( 38.9) | |
| Total sleep time (hours) (range 1.4-9.9) | 5.3 ( 1.8) | |
| Sleep-onset latency (minutes) (range 0-53.7) | 14.3 ( 23.1) | |
BSA, Brief Scale for Anxiety; CIWA, Clinical Institute Withdrawal Assessment; MADRS (Montgomery Asberg Depression Rating Scale; PTSD, post-traumatic stress disorder; SCID-I, Structured Clinical Interview for the DSM-IV.
On the inpatient unit, patients were required to be in their rooms by midnight Sunday through Thursday and by 1:00 am on weekend nights, but there was no official “lights out” policy.
As shown in Table 2, three distinct latent classes were identified based on having smaller BIC of 1213.013 than the two-class and four-class model, and non-significant VLMR and BLRT in the four-class model. The VLMR and BLRT were not significant in the four-class model, indicating that the three-class model fit the data better than the four-class model (Nylund, Asparouhov, & Muthen, 2007).
Table 2:
Model Fit Information for LCA Models Fit to Data
| Class | N. of parameters |
AIC | BIC | Entropy | VLMRa | BLRTa |
|---|---|---|---|---|---|---|
| 2 | 15 | 1182.173 | 1228.671 | 0.910 | p < .001 | p < .001 |
| 3b | 23 | 1141.716 | 1213.013 | 0.908 | p < .001 | p < .001 |
| 4 | 31 | 1148.395 | 1244.491 | 0.798 | p = 0.2757 | p = 0.6150 |
AIC, Akaike information criterion; BIC, Bayesian information criterion; VLMR, Vuong-Lo-Mendell-Rubin; BLRT, Bootstrapped likelihood ratio test.
Chi-square statistic for the VLMR and the BLRT. When non-significant (p > .05), the VLMR and the BLRT test provide evidence that K-1 class model fits the data better than the K-class model.
3-class model was selected, based on its having smaller BIC than the 2-class and 4-class model, and non-significant VLBR and BLRT in the 4-class model.
Figure 1 presents the three distinct classes of treatment seeking individuals with alcohol dependence and sleep disturbance. Class 1 (27%) included individuals who were alcohol-dependent with sleep disturbance (SD), Class 2 (52%) included individuals who were alcohol-dependent with sleep disturbance, anxiety and depression (SD/AD), and Class 3 (21%) included individuals who were alcohol-dependent with sleep disturbance, anxiety and depression and PTSD (SD/AD/PTSD). Of note, those in the SD/AD/ PTSD class also experienced higher symptoms of withdrawal and reported higher scores reflecting sleep disturbances.
Figure 1:
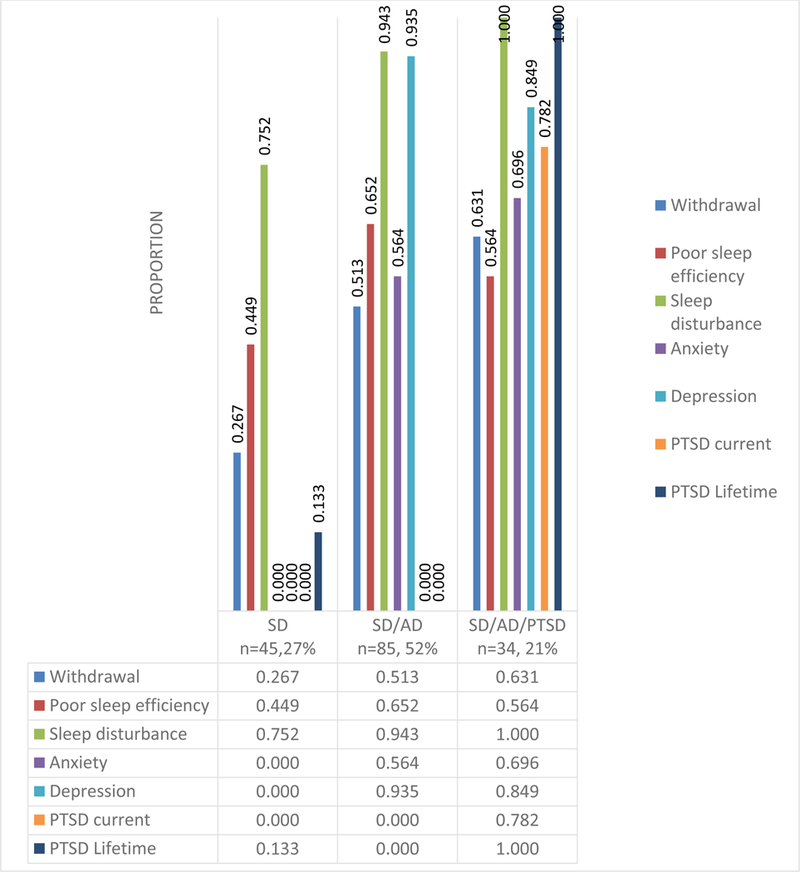
Three latent classes in treatment seeking individuals with alcohol dependence with sleep disturbance
Table 3 displays the socio-demographic characteristics of the three latent classes. Among demographic factors examined, there was a statistically significant difference (p <.01) in gender across the three different classes with females more likely to be in the SD/AD/PTSD group. Despite the fact that the sample was nearly half African American, race was not statistically different across classes. Additionally, age and ethnicity were not statistically different across classes.
Table 3:
Characteristics of each of the three latent classes
| Variables | Mean (Standard Deviation) n (%) |
p-value | ||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| SD (n=45, 27%) |
SD/AD (n=85, 52%) |
SD/AD/PTSD (n=34, 21%) |
||
| Age | 45.9 (9.6) | 46.0 (9.3) | 44.4 (10.1) | .682 |
| Gender | ||||
| Male | 37 (82.2) | 62 (72.9) | 16 (47.1) | .002* |
| Female | 8 (17.8) | 23 (27.1) | 18 (52.9) | |
| Race | ||||
| Black / African American | 26 (57.8) | 39 (45.9) | 13 (38.2) | .091 |
| White | 14 (31.1) | 40 (47.1) | 18 (52.9) | |
| Other | 1 ( 2.2) | 4 ( 4.7) | 3 ( 8.8) | |
| Unknown | 4 ( 8.9) | 2 ( 2.4) | 0 ( 0.0) | |
| Ethnic | ||||
| Non-Hispanic | 43 (97.7) | 82 (97.6) | 32 (94.1) | .570 |
| Hispanic | 1 ( 2.3) | 2 ( 2.4) | 2 ( 5.9) | |
Numbers may not sum to total due to missing data.
AD, anxiety and depression; PTSD, post-traumatic stress disorder; SD, sleep disturbance.
p < .05
Discussion
Our results highlight three distinct profiles and the importance of examining subgroups of individuals undergoing alcohol rehabilitation treatment with accompanying sleep disturbances that may differ on potentially unobserved (“latent”) but modifiable indices including depression and anxiety. Furthermore, the results indicate possible profiles, which may require close clinical management of additional symptoms (withdrawal and PTSD) so as not to further disrupt sleep. If replicated, these identified classes could aid clinicians in categorizing newly admitted patients for more focused treatment. Because we found a significant difference in the number of women attributed to group three, multimodal gender specific interventions may be particularly important in women who are in recovery given the presence of these co-morbidities including higher alcohol withdrawal scores and presence of PTSD in Class 3 (SD/AD/PTSD). Clinical interventions that focus on sleep alone such as the use of cognitive behavioral sleep for insomnia (CBT-I) without taking into account the need for interventions that also address anxiety, depression and PTSD in the groups that are at highest risk may make sustained sobriety much more difficult in these individuals. Our findings support the findings of Moss and colleagues and Vermeulen-Smit and colleagues by suggesting the need for integrative, tailored interventions that account for the presentation of behavior clusters and co-morbidities, which in our sample include sleep disturbance, anxiety, depression and PTSD (Moss, Goldstein, Chen, & Yi, 2015; Vermeulen-Smit, Ten Have, Van Laar, & De Graaf, 2015). However, our findings differ from Cranford and colleagues who explored drinking behavior over time and found that being male predicted membership in their heaviest at baseline to stable heavy class of individuals with alcohol dependence (Cranford, Krentzman, Mowbray, & Robinson, 2014). It is also interesting to note that despite other studies such as that by Mulia and colleagues (2014) suggesting that alcohol treatment is lower among racial/ethnic minorities compared to Caucasians, our sample was 47.6% Black/African American. One might speculate that this is due to the demographics of the population where we recruit for research participants with AUD in our surrounding community in the Washington metropolitan areas as reflected in the U.S. Census data for Washington DC reporting 47.7% Black or African Americans in their sample (https://www.census.gov/quickfacts/fact/table/DC/RHI225216).
Limitations of this study include the cross-sectional nature of the data that prohibit conclusions regarding causality between the three identified classes. Participants in this study were treatment-seeking individuals and thus findings are not generalizable to all individuals who are alcohol-dependent. Future studies should examine alcohol treatment-related outcomes between these or similar classes.
Conclusion
The findings from this analysis support the use of LCA to assess behavior and psychopathology clusters and identify subgroups of individuals with AUDs and accompanying sleep disturbances. Sleep disturbance was present in all three classes and thus did not serve as a distinguishing factor between the three latent classes, yet these results suggest it may be important to evaluate/treat insomnia complaints during early recovery for the majority of patients. Furthermore, class identification may provide clinicians with insight into the integrative tailoring of interventions that meet the varied needs of individuals with AUDs and accompanying co-morbidities and sleep disturbances.
Acknowledgements:
This project was funded in whole or in part with federal funds from the National Institutes of Health, Clinical Center intramural research program. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services. We gratefully acknowledge Dr. Vijay Ramchandani, Dr. David “Ted” George, and the patients who participated in this research as well as the nursing staff from the 1SE clinic.
Footnotes
Conflict of Interests: The authors have no financial or other competing interests to declare.
References
- Akaike H A new look at the statistical model identification. IEEE Transactions on Automatic Control 1974; 19: 716–23. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders. 5th ed Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Asberg M, Montgomery SA, Perris C, Schalling D, & Sedvall G (1987). A comprehensive psychopathological rating scale. Acta Psychiat Scand, S271:5–27. [DOI] [PubMed] [Google Scholar]
- Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer…Hu FB (2003). A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care, 26(2): 380–84. [DOI] [PubMed] [Google Scholar]
- Benca RM (1996). Sleep in psychiatric disorders. Neurologic Clinics, 14(4): 739–64. [DOI] [PubMed] [Google Scholar]
- Bergman LR & Magnusson D (1997). A person-oriented approach in research on developmental psychopathology. Development and Psychopathology, 9, 291–319. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, & Greden JF (2001). Insomnia, self-medication, and relapse to alcoholism. American Journal of Psychiatry, 158(3): 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DL, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research, 28: 193–213. [DOI] [PubMed] [Google Scholar]
- Celeux G & Soromenho G (1996). An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification, 13: 195–212. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2015). “Excessive drinking” Retrieved from http://www.cdc.gov/alcohol/data-stats.htm 18 Oct. 2016.
- Chaudhary NS, Kampman KM, Kranzler HR, Grandner MA, Debbarma S, & Chakravorty S (2015). Insomnia in alcohol dependent subjects is associated with greater psychosocial problem severity. Addictive Behaviors, 50: 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Lanza ST, & Loken E (2008). Latent transition analysis: inference and estimation. Statistics in Medicine, 27, 1834–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford JA, Krentzman AR, Mowbray O, & Robinson EA (2014). Trajectories of alcohol use over time among adults with alcohol dependence. Addictive Behaviors, 39(5): 1006–11. [DOI] [PubMed] [Google Scholar]
- Doyle SR & Donovan DM (2009). A validation study of the Alcohol Dependence Scale. J Stud Alcohol Drugs, 70(5), 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DY, Geyer C, Lionetti T & Doty L (2012). Managing alcohol withdrawal in hospitalized patients. Nursing 2012, 22–30. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (2002). User’s Guide for the SCID-I: Structured Clinical Interview for DSM-IV-TR Axis I Disorders- Research Version- SCID-I for DSM-IV-TR, November 2002 Revision. Biometrics Research, New York. [Google Scholar]
- Foster JH & Peters TJ (1999). Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcoholism: Clinical and Experimental Research, 23(6): 1044–51. [PubMed] [Google Scholar]
- Gillin JC & Drummond SP Medication and substance abuse (2000). In Kryger MH, Roth T, and Dement WC (eds), Principles and Practice of Sleep Medicine, 3rd edition Philadelphia, PA: WB Saunders. [Google Scholar]
- IBM Corp. (2013). SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp; 2013. [Google Scholar]
- Jackson KM, Bucholz KK, Wood PK, Steinley D, Grant JD, & Sher KJ (2014). Towards the characterization and validation of alcohol use disorder subtypes: integrating consumption and symptom data. Psychological Medicine, 44(1): 143–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob T, Blonigen DM, Koenig LB, Wachsmuth W, & Price RK (2010). Course of alcohol dependence among Vietname combat veterans and nonveteran controls. Journal of Studies on Alcohol and Drugs, 71(5): 629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedel E, Waern M, Gustafson D, Landén M, Eriksson E, Holm G, Nilsson L…Stener-Victorin E (2010). Anxiety and depression symptoms in women with polycystic ovary syndrome compared with controls matched for body mass index. Human Reproduction, 25(2): 450–56. [DOI] [PubMed] [Google Scholar]
- Jung T, & Wickrama KAS (2008). An Introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass, 2: 302–17. [Google Scholar]
- Klimkiewicz A, Klimkiewicz J, Jakubczyk A, Kieres-Salomonski I, & Wojnar M (2015). Comorbidity of alcohol dependnence with other psychiatric disorders. Part I. Epidemiology of dual diagnosis. Psychiatr Pol, 49(2): 265–75. [DOI] [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Medicine. 2001;2:389–396. [DOI] [PubMed] [Google Scholar]
- Landolt HP & Gillin JC (2001). Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiology and management. CNS Drugs, 15(5): 413–25. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Stone KC, Donaldson J, Nau SD, Soeffing J, Murray D, Lester KW, & Aguillard N (2006). Actigraphy validation with insomnia. SLEEP, 29(2): 232–39. [PubMed] [Google Scholar]
- Miaskowski C, Barsevick A, Berger A, Casagrande R, Grady PA, Jacobsen P, Kutner J, Patrick D, Zimmerman L, Xiao C, Matocha M, & Marden M (2016). Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. J Natl Cancer Inst, 109(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Goldstein RB, Chen CM, & Yi HT (2015). Patterns of use of other drugs among those with alcohol dependence: associations with drinking behavior and psychopathology. Addictive Behaviors, 50: 192–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowbray O, Glass JE, & Grinnell-Davis CL (2015). Latent class analysis of alcohol treatment utilization patterns and 3-year alcohol related outcomes. Journal of Substance Abuse Treatment, 54: 21–8. [DOI] [PubMed] [Google Scholar]
- Muthén B & Shedden K (1999). Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics, 55: 463–69. [DOI] [PubMed] [Google Scholar]
- Muthén B, & Muthén LK (2000). Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcoholism: Clinical and Experimental Research, 24(6): 882–891. [PubMed] [Google Scholar]
- Muthén LK & Muthén BO (2015). Mplus User’s Guide Seventh Edition Los Angeles, CA: 1998–2015. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). (2016). “Overview of Alcohol Consumption” http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics; 18 Oct. 2016.
- Newtown-Howes G & Boden JM (2016). Relation between age of first drinking and mental health and alcohol and drug disorders in adulthood: evidence from a 35-year cohort study. Addiction, 111(4): 637–44. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, & Muthén BO (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling a Monte Carlo simulation study. Structural Equation Modeling, 14: 535–69. [Google Scholar]
- Olino TM, Klein DN, Farmer RF, Seeley JR, & Lewinsohn PM Examination of the structure of psychpathology using latent class analysis. Comprehensive Psychiatry, 53(4): 323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet J, Kawinska A, Carrier J. Wake detection capacity of actigraphy during sleep. Sleep. 2007;30: 1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR & Calkins DR (1994). Individualized treatment for alcohol withdrawal. JAMA. 272(7): 519–523. [PubMed] [Google Scholar]
- Schuler MS, Puttaiah S, Mojtabai R, & Crum RM (2015). Perceived barriers to treatment for alcohol problems: a latent class analysis. Psychiatric Services (Washington, D.C.), 66(11): 1221–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G (1978). Estimating the dimension of a model. The Annals of Statistics, 6: 461–64. [Google Scholar]
- Skinner HA & Allen BA (1982). Alcohol dependence syndrome: measurement and validation. Journal of Abnormal Psychology, 91(3):199–209. [DOI] [PubMed] [Google Scholar]
- Stine TW & Chapman DP (2005). Associations of frequent sleep insufficiency with health-related quality of life and health behaviors. Sleep Medicine, 6(1): 23–7. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, & Sellers EM (1989). Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). British Journal of Addiction, 84: 1353–57. [DOI] [PubMed] [Google Scholar]
- Svanborg P & Åsberg M (1994). A new self-rating scale for depression and anxiety states based on the Comprehensive Psychopathological Rating Scale. Acta Psychiatrica Scandinavica, 89: 21–8. [DOI] [PubMed] [Google Scholar]
- Vermeulen-Smit E, Ten Have M, Van Laar M, & De Graaf R (2015). Clustering of health risk behaviours and the relationship with mental disorders. Journal of Affective Disorders, 171: 111–19. [DOI] [PubMed] [Google Scholar]
- Vermunt JK & Magidson J (2002). Latent class cluster analysis In Hagenaars JA & McCutcheon AL (Eds.), Applied Latent Class Analysis. Cambridge: Cambridge University Press; p. 89–106. [Google Scholar]
- Wallen GR, Brooks AT, Whiting B, Clark R, Krumlauf MC, Yang L, Schwandt ML…Ramchandani VA (2014). The prevalence of sleep disturbance in alcoholics admitted for treatment: A Target for Chronic Disease Management. Family & Community Health, 37(4):288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington D.C.: U.S. Census Data (2016, July 1) Retrieved on July 19, 2017 from https://www.census.gov/quickfacts/fact/table/DC/RHI225216. [Google Scholar]
- World Health Organization (WHO). (2013). “Alcohol” Retrieved from http://www.who.int/substance_abuse/facts/alcohol/en/index.html. 18 Oct. 2016.
- Zimmerman M, Posternak MA, Chelminski I (2004). Derivation of a definition of remission on the Montgomery-Asberg depression rating scale corresponding to the definition of remission on the Hamilton rating scale for depression. Journal of Psychiatric Research, 38(6): 577–82. [DOI] [PubMed] [Google Scholar]


