Abstract
Objectives
Disease activity has been considered as independent cardiovascular risk factor in rheumatoid arthritis (RA) patients. We aimed to evaluate the effect of RA disease activity on left ventricular (LV) and right ventricular (RV) functions by speckle tracking echocardiography (STE).
Methods
120 patients with RA without evidence of cardiovascular disease and 40 healthy control subjects were included. Disease activity was evaluated according to Simplified Disease Activity Index (SDAI) score and Disease Activity Score 28 (DAS28). LV and RV functions were assessed using conventional echocardiography and global longitudinal strain (GLS) technique measured by STE.
Results
81 patients had active disease while 39 patients were in remission. The LV and RV GLS value for active RA patients was reduced compared to RA patients in remission and control group (p = <0.001). There was a significant correlation between RA disease activity scores level and LV GLS value, increasing levels of disease activity was associated with worse LV GLS (r = −0.802, p value = <0.001) and r = −0.824, p value = <0.001) for SDAI and DAS28 scores respectively. Also, there were significant correlations between RA disease activity scores level and RV GLS value as the disease activity level increases the RV GLS value become worse (r = −0.682, p value = <0.001) and r = −0.731, p value = <0.001) for SDAI and DAS28 scores respectively Receiver operating characteristic (ROC) curve analysis showed that SDAI score and DAS28 were predictive for reduced LV GLS with a cut off value of >7 and >2.8 respectively with sensitivity of 77.6%, specificity of 85.0% and area under ROC curve = 90.4 for SDAI score and with sensitivity of 89.7%, specificity of 71.7% and area under ROC curve = 89.4 for DAS28 score. Also, SDAI score and DAS28 were predictive for reduced RV GLS with a cut off value of >11 and >3 respectively with sensitivity of 73.1%, specificity of 93.5% and area under ROC curve = 91.6 for SDAI score and with sensitivity of 84.6%, specificity of 80.4% and area under ROC curve = 90.8 for DAS28 score.
Conclusion
Disease activity in patients with rheumatoid arthritis is associated with lower left and right ventricular function. Disease activity scores can predict subclinical left and right ventricular dysfunction.
Abbreviations
- CVD
cardiovascular disease
- CAD
coronary artery disease
- RA
rheumatoid arthritis
- IHD
ischemic heart disease
- HF
heart failure
- GLS
global longitudinal strain
- STE
speckle tracking echocardiography
- SDAI
Simplified Disease Activity Index
- SJC28 and TJC28
swollen and tender joints counts in 28 joints
- VAS
visual analogue scale
- CRP
C reactive protein
- DAS28
Disease Activity Score in 28 Joints
- ACPA
anti-citrullinated peptide antibody
- EF
ejection fractions
- RF
rheumatoid factor
- LVESV
LV end-systolic volume
- LVEDV
LV end diastolic volume were evaluated
- TDI
Tissue Doppler imaging
- IVRT
Isovolumetric relaxation time
- ET
ejection time
- e′
early diastolic wave
- RVFAC
Right ventricular fractional area change
- TAPSE
Tricuspid annular plane systolic excursion
- MPI
myocardial performance index
- IVCT
isovolumetric contraction time
- SD
standard deviation
- ROC
Receiving operator characteristics
- DMARD
disease modifying antirheumatic drugs
- TNFI
tumor necrosis factor inhibitor
1. Introduction
It is well known that risk of development of cardiovascular disease (CVD) and especially coronary artery disease (CAD) is increased in patients with rheumatoid arthritis (RA) [1]. Data from previous studies showed double fold elevation in the risk of development of heart failure (HF) in RA patients [2].
The increased risk of ischemic heart disease (IHD) cannot explain HF development in RA patients. It was found that non ischemic HF is more pronounced in RA patients and the risk of HF development was associated with RA severity [3]. RA disease activity is characterized by high level of Inflammation and this could contribute to the development of HF through production of cytokines and direct toxic effect on cardiomyocytes and microvasculature and this could explain the missing pathophysiological mechanism of HF development [4], [5].
Most of the patients with RA who presented with HF have impaired diastolic function with preserved systolic function which assessed by left ventricular ejection fraction [6]. However, recent studies using strain imaging technique, which is a sensitive tool in detecting early stages of impairment of systolic function, showed impairment in global longitudinal strain (GLS) in RA patients with normal ejection fraction [7], [8].
In the current study we will assess the effect of RA disease activity on systolic function of the left and right ventricles using GLS technique measured by speckle tracking echocardiography (STE) in patients with normal systolic function by conventional two-dimensional echocardiography.
2. Patients and methods
-
•
Study population:
From the outpatient clinic of the rheumatology department consecutive 120 patients with rheumatoid arthritis who were diagnosed according to the American College of Rheumatology classification for RA [9] during period of September 2015 to June 2017 were prospectively included in the study. Patients were included in the study if they have normal left and right ventricular function documented by two-dimensional echocardiography.
Exclusion criteria were as follow: evidence of coronary artery disease (detected by clinical history, electrocardiogram and echocardiographic evidence of wall motion abnormalities), valvular or congenital heart disease, diabetes mellitus, systemic arterial hypertension, pulmonary arterial hypertension, arrhythmia, advanced renal and liver diseases, and inadequate image quality.
Forty healthy subjects with similar age and sex were recruited as a control group.
Informed consent was taken from all participants and the study was approved by the local ethical committee.
-
•
RA disease activity:
Simplified Disease Activity Index (SDAI) score was calculated as the sum of the swollen and tender joints counts in 28 joints (SJC28 and TJC28) + physician’s assessment of disease activity on a visual analogue scale (VAS) (cm) + patient’s assessment of global health on a VAS (cm) + C reactive protein (CRP) (mg/dL) [10].
Patients were considered to be in remission if SDAI score is ≤3.3, while active disease was defined as SDAI score >3.3 [11]. Accordingly, patients were divided into two groups the active RA group (n = 81) and remission RA group (n = 39).
We also calculated Disease Activity Score in 28 Joints (DAS28) by recording the number of swollen and tender joints and ESR [12].
-
•
Data collection:
The history and physical examination were done in all participants with special emphasis on the disease duration and current medications received in patients with RA.
Routine laboratory measurements were obtained. In all patients and control, measurements of total serum cholesterol, serum creatinine, CRP and ESR were obtained. In patients with RA, rheumatoid factor (RF) and anti-citrullinated peptide antibody (ACPA) were also obtained.
-
•
Echocardiography:
Echocardiograms were performed with (M5S probe, GE Vivid E9 echocardiographic system) to all participants according to standard guidelines [12].
2.1. LV systolic function
LV septum and LV posterior wall end-diastolic thickness were measured by M-mode echocardiography. LV ejection fractions (EF), LV end-systolic volume (LVESV), LV end diastolic volume were evaluated (LVEDV) using biplane method of discs [13]. From the trans-mitral flow profile, the E and A waves peak velocities were calculated. Tissue Doppler imaging (TDI) of the mitral annulus was performed in the apical 4 chamber view using 1- to 2- mm sample volume placed in the septal mitral valve annulus. The values of s′ and è were measured and E/è was obtained. Isovolumetric relaxation time (IVRT) is the time between the end of ejection time (ET) and the beginning of early diastolic wave (e′), was also measured [14].
2.2. Right ventricular function
Tricuspid annular plane systolic excursion (TAPSE) was measured in the apical 4-chamber view by M-mode echocardiography with the cursor placed through the tricuspid lateral annulus and measuring the amount of longitudinal motion of the annulus at peak systole [15].
Right ventricular fractional area change (RVFAC) was defined as (RV end-diastolic area-RV end-systolic area)/RV end-diastolic area. The RV endocardium was traced both in systole and diastole along the free wall to the apex and then back to annulus along the inter-ventricular septum in the apical 4-chamber view [15].
TDI derived RV-myocardial performance index (MPI) was calculated. Pulsed TDI analysis was obtained from apical 4-chamber view with TDI cursor placed at the level of tricuspid annulus. One major positive velocity (S′) was recorded with movement of the annulus towards the apex during systole. Two major negative waves were recorded, one during early diastole (E′) and one during late diastole (A′). (S′) duration was measured as ejection time. The time between the end of (S′) and beginning of (E′) was measured as IVRT. The time between the end of (A′) and beginning of (S′) was measured as isovolumetric contraction time (IVCT). MPI was calculated as (IVRT + IVCT)/ET (ejection time) [15].
Pulsed wave derived tricuspid E wave, A wave, early diastolic wave velocity by TDI (e′) and E/e′ ratio were measured to evaluate RV diastolic function [15].
2.3. Two D-speckle tracking echocardiography
For evaluation of the global LV longitudinal strain, apical views including apical long axis, apical 4 and 2 chamber views were obtained using conventional 2-D gray scale imaging, during breath hold with a stable ECG recording and an adequate gray scale images to allow optimal delineation of myocardial tissue and extra-cardiac structures, 3 consecutive heart cycles were recorded and averaged at frame rate of at least 50 frames/second (Fig. 1). LV endocardial surface was traced manually at the end-systolic frame and the width and shape of the region of interest was adjusted manually. For analysis of RV strain, the RV was traced in the apical 4-chamber view (Fig. 2) [16]. Recordings were processed using a software (Echo Pac, GE Vivid E9 echocardiography system version 113), for off-line measurements of speckle-based strain.
Figure 1.
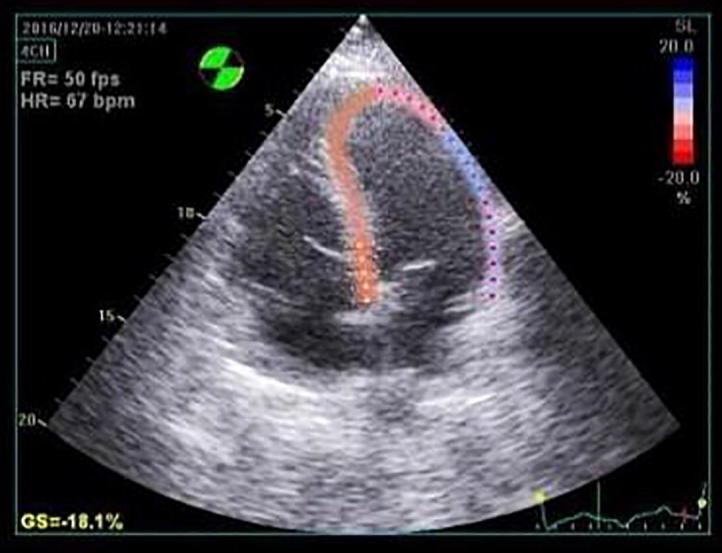
Left ventricular longitudinal strain from the apical 4-chamber view in rheumatoid arthritis patient.
Figure 2.
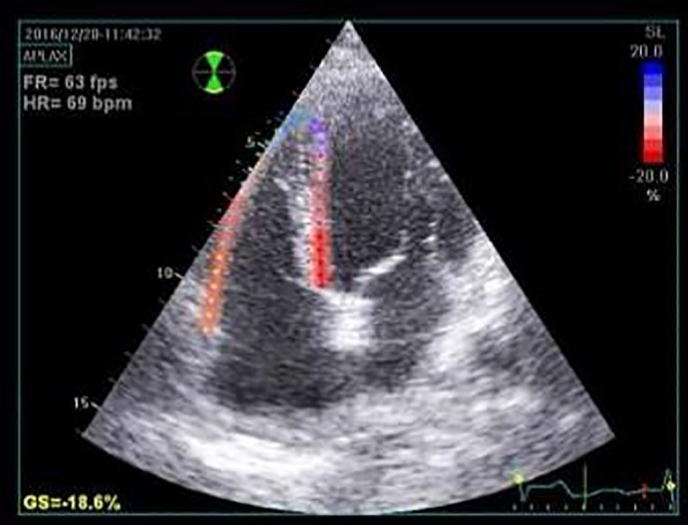
Right ventricular longitudinal strain from the apical 4-chamber view in rheumatoid arthritis patient.
An absolute value of peak LV GLS below −20% value was considered to be abnormal [13].
All measurement were performed by an experienced echocardiographer (L.K). Intraobserver variability was assessed in 15 randomly selected patients by repeated analysis on the same cine loop.
2.4. Statistical study
All statistical studies were carried out using Statistical Package for Social Sciences software (SPSS 18.0 for Windows, SPSS Inc., Chicago, Illinois).
The quantitative variables are expressed as mean ± standard deviation (SD). The qualitative data are expressed as counts and percentage. To compare values student’s t-test and chi-square were used for quantitative and qualitative values respectively. Correlation analysis was calculated using Pearson’s correlation coefficient. Receiving operator characteristics (ROC) curve is used to detect optimal cut-off values of SDAI and DSA28 scores for predicting reduced LV GLS and RV GLS. A p value <0.05 is considered as statistically significant.
3. Results
Of the 120 patients with rheumatoid arthritis who were included in the present study, 81 patients had active disease who constituted the first group and 39 patients were in remission constituted the second group in addition to 40 healthy subjects who constituted the control group.
3.1. Baseline demographic and clinical characteristics
The Baseline demographic and clinical characteristics are summarized in Table 1. There were no statistically significant differences between the three groups as regards age, sex, smoking status, systolic and diastolic blood pressure, heart rate, total serum cholesterol level and serum creatinine level. Also, there was no statistically significant difference regarding the disease duration between the active RA group and those in remission.
Table 1.
Baseline demographic and clinical characteristics of the studied groups.
| Active RA (n = 81) | Remission RA (n = 39) | Control | p-Value | |
|---|---|---|---|---|
| Age | 54.52 ± 7.31 | 55.08 ± 6.86 | 53.08 ± 7.45 | 0.436 |
| Female% | 55 (67.9%) | 27 (69.2%) | 25 (62.5%) | 0.788 |
| Current smoker | 12 (14.8%) | 7 (17.9%) | 9 (22.5%) | 0.584 |
| Systolic blood pressure (mm Hg) | 129.63 ± 9.87 | 132.18 ± 10.18 | 128.38 ± 6.64 | 0.176 |
| Diastolic blood pressure (mm Hg) | 85.17 ± 6.94 | 85.77 ± 7.39 | 83.38 ± 5.24 | 0.240 |
| Heart rate (beats/min) | 77.75 ± 9.05 | 77.18 ± 8.86 | 76.25 ± 8.75 | 0.684 |
| RA disease characteristics | ||||
| Disease duration (years) | 8.47 ± 3.83 | 7.92 ± 3.47 | 0.453 | |
| SDAI score | 11.18 ± 7.59 | 2.12 ± 0.46 | <0.001 | |
| DAS28 | 3.42 ± 0.80 | 1.98 ± 0.25 | <0.001 | |
| Laboratory | ||||
| Total serum cholesterol (mg/dl) | 195.68 ± 28.58 | 200.26 ± 32.75 | 197.58 ± 31.34 | 0.739 |
| RF positive | 55 (67.9%) | 19 (48.7%) | <0.001 | |
| Anti CCP positive | 52 (64.1%) | 15 (38.4%) | <0.001 | |
| Serum creatinine (mg/dl) | 1.13 ± 0.21 | 1.11 ± 0.29 | 1.17 ± 0.20 | 0.523 |
| CRP (mg/L) | 12.31 ± 5.70 | 3.21 ± 1.26a | 0.59 ± 0.31a | <0.001 for active vs control and active vs remission and 0.033 for remission vs control |
| ESR (mm/hour) | 60.30 ± 8.89 | 33.85 ± 5.90a | 8.53 ± 2.63ab | <0.001 for active vs control and active vs remission and 0.042 for remission vs control |
| Medications | ||||
| DMARDS% | 60 (74%) | 20 (51.2%) | <0.001 | |
| TNFI% | 17 (20.9%) | 6 (15.3%) | <0.001 | |
| Methotrexate% | 28 (34.5%) | 13 (33.3%) | <0.001 | |
| Corticosteroids% | 35 (43.2%) | 14 (35.8%) | <0.001 | |
All data are represented as mean ± SD and number (percent).
a: significant with active group, b: significant with Remission group.
RA, rheumatoid arthritis; SDAI, Simplified Disease Activity Index; DAS28, Disease Activity Score in 28 Joints; RF, rheumatoid factor; Anti-CCP, anti-cyclic citrullinated peptide; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; DMARDs, disease modifying antirheumatic drugs; TNFi, tumour necrosis factor inhibitor.
Patients in the active RA group have a significantly higher SDAI and DAS28 scores and more anti-CCP and RF positive patients compared with those in the RA in remission group (p = <0.001 for all). The inflammatory markers CRP and ESR were significantly higher in the active RA group compared to the RA in remission and control groups (p = <0.001 for both). Patients in the active RA group had a higher rate of use of disease modifying antirheumatic drugs (DMARD), tumor necrosis factor inhibitor (TNFI), methotrexate and corticosteroids compared with the RA in remission group (p = <0.001 for all).
3.2. Echocardiographic characteristics
3.2.1. LV function
The LV echocardiographic characteristics are shown in Table 2. The three groups did not differ with respect to LV septum thickness at end diastole, LV posterior wall thickness at end diastole, LV end diastolic volume, LV end-systolic volume, EF%, peak mitral E wave velocity, peak mitral A wave velocity, mitral E/e′ ratio and peak S′ velocity. The LV GLS value for active RA patients was significantly worse (less negative) compared to RA patients in remission and control group (p = <0.001 for both). Of note, the LV GLS value was comparable between patients with RA in remission and control group.
Table 2.
The LV echocardiographic characteristics of the studied groups.
| Active RA (n = 81) | Remission RA (n = 39) | Control | p-Value | |
|---|---|---|---|---|
| LV septum thickness at end diastole | 0.93 ± 0.09 | 0.94 ± 0.10 | 0.94 ± 0.10 | 0.766 |
| LV PW thickness at end diastole | 0.99 ± 0.79 | 0.90 ± 0.09 | 0.91 ± 0.08 | 0.614 |
| LVEDV | 121.59 ± 17.70 | 117.41 ± 15.58 | 118.30 ± 20.39 | 0.413 |
| LVESV | 46.58 ± 10.69 | 42.44 ± 9.52 | 42.50 ± 11.59 | 0.054 |
| EF% | 62.11 ± 5.06 | 63.33 ± 5.51 | 63.70 ± 5.36 | 0.229 |
| Mitral Peak E (m/s) | 0.77 ± 0.10 | 0.78 ± 0.10 | 0.80 ± 0.11 | 0.193 |
| Mitral Peak A (m/s) | 0.77 ± 0.10 | 0.73 ± 0.11 | 0.74 ± 0.11 | 0.077 |
| LV IVRT (ms) | 87.32 ± 11.17 | 85.77 ± 16.16 | 82.15 ± 9.73 | 0.096 |
| Mitral E/è | 11.04 ± 1.50 | 11.69 ± 1.62 | 11.20 ± 1.62 | 0.101 |
| Peak S′ (cm/s) | 10.99 ± 1.38 | 10.92 ± 1.40 | 11.43 ± 1.41 | 0.196 |
| LV GLS (%) | −18.56 ± 1.87 | −21.18 ± 1.27a | −20.78 ± 1.44a | <0.001 for active vs control and active vs remission and 0.027 for remission vs control |
All data are represented as mean ± SD and number (percent).
a: significant with active group, b: significant with Remission group.
RA, rheumatoid arthritis; LV, left ventricular; LVEDV, left ventricular end-diastolic volume index; LVESV = left ventricular end-systolic volume; EF%, Ejection fraction; E: peak flow velocity during the early rapid filling phase; A: peak flow velocity during atrial contraction; IVRT, Isovolumic relaxation time; E/ è, the ratio of early flow velocity to the early annular velocity; S′=systolic annulus velocity; GLS, global longitudinal strain.
3.2.2. RV function
The RV echocardiographic characteristics are reported in Table 3. There were no significant differences between the three groups regarding peak tricuspid E wave velocity, peak tricuspid A wave velocity, tricuspid E/e′ ratio, pulmonary artery systolic pressure, TAPSE, RVFAC, MPI-TDI. The RV GLS value for active RA patients was significantly worse (less negative) compared to RA patients in remission and control group (p = <0.001 for both). The RV GLS value did not differ between patients with RA in remission and control group.
Table 3.
The RV echocardiographic characteristics of the studied groups.
| Active | Remission | Control | p-Value | |
|---|---|---|---|---|
| Tricuspid E | 0.49 ± 0.09 | 0.50 ± 0.07 | 0.50 ± 0.07 | 0.672 |
| Tricuspid A | 0.40 ± 0.07 | 0.39 ± 0.08 | 0.39 ± 0.09 | 0.476 |
| Tricuspid E/è | 4.33 ± 0.84 | 4.13 ± 0.83 | 4.50 ± 0.72 | 0.126 |
| ESPAP | 30.75 ± 4.79 | 29.85 ± 4.68 | 30.75 ± 4.98 | 0.594 |
| TAPSE | 19.10 ± 2.19 | 19.03 ± 2.38 | 19.58 ± 2.30 | 0.476 |
| RVFAC | 37.27 ± 2.81 | 36.33 ± 1.78 | 37.23 ± 1.72 | 0.106 |
| MPI-TDI | 0.42 ± 0.07 | 0.39 ± 0.04 | 0.41 ± 0.07 | 0.106 |
| RV Global strain (%) | −18.99 ± 1.58 | −21.95 ± 1.69a | −23.03 ± 2.02a | <0.001 for active vs control and active vs remission and 0.031 for remission vs control |
All data are represented as mean ± SD and number (percent).
a: significant with active group, b: significant with Remission group.
E: peak flow velocity during the early rapid filling phase; A: peak flow velocity during atrial contraction; E/è, the ratio of early flow velocity to the early annular velocity; ESPASP, estimated systolic pulmonary artery pressure TAPSE, tricuspid annular plane systolic excursion; RVFAC, right ventricular fractional area change; MPI, myocardial performance index; TDI, tissue Doppler imaging; RV, right ventricular; GLS, global longitudinal strain.
3.2.3. Correlations
We assessed correlations between RA disease activity scores level (SDAI score and DAS28 score) and both LV GLS and RV GLS value. Calculation of Pearson’s correlation coefficient showed a statistically significant correlation between RA disease activity scores level (SDAI score and DAS28 score) and LV GLS value, increasing levels of disease activity was associated with worse LV GLS (r = −0.802, p value = <0.001) and r = −0.824, p value = <0.001) for SDAI and DAS28 scores respectively (Figure 3, Figure 4). Also, there were significant correlations between RA disease activity scores level (SDAI score and DAS28 score) and RV GLS value as the disease activity level increases the RV GLS value become worse (r = −0.682, p value = <0.001) and r = −0.731, p value = <0.001) for SDAI and DAS28 scores respectively (Fig. 5).
Figure 3.

Receiver operating curve characteristic (Roc) curve analysis for Simplified Disease Activity Index (SDAI) score as predictor reduced left ventricular global longitudinal strain (LV GLS).
Figure 4.
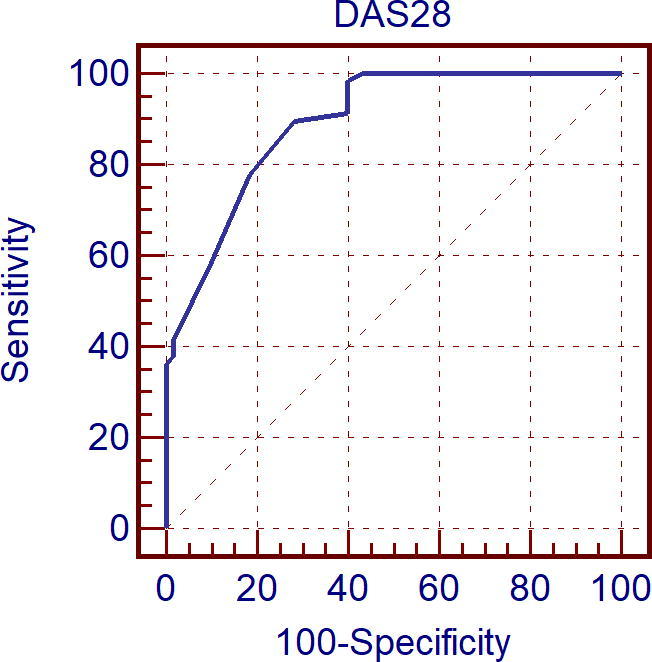
Receiver operating curve characteristic (Roc) curve analysis for Disease Activity Score in 28 Joints (DAS28) score as predictor reduced left ventricular global longitudinal strain (LV GLS).
Figure 5.
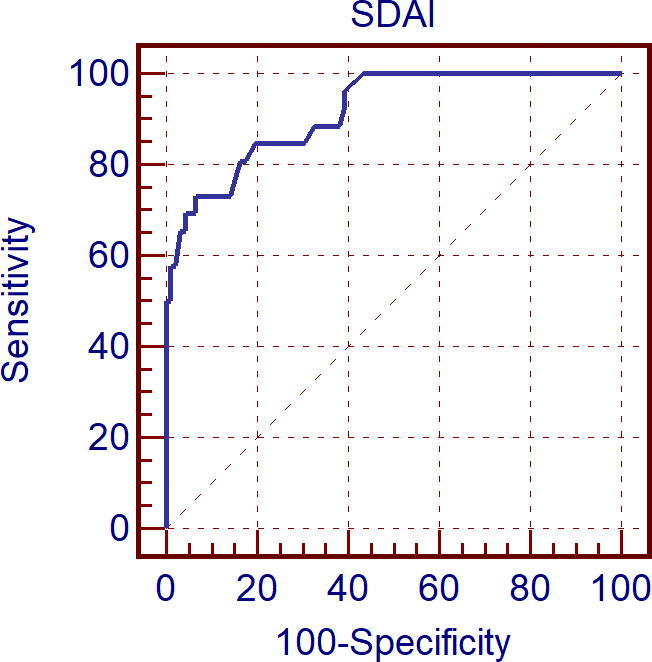
Receiver operating curve characteristic (Roc) curve analysis for Simplified Disease Activity Index (SDAI) score as predictor reduced right ventricular global longitudinal strain (RV GLS).
Receiver operating characteristic (ROC) curve analysis showed that SDAI score and DAS28 were predictive for reduced LV GLS with respect to the referral limit of −20% with a cut off value of >7 and >2.8 respectively with sensitivity of 77.6%, specificity of 85.0% and area under ROC curve = 90.4 for SDAI score and with sensitivity of 89.7%, specificity of 71.7% and area under ROC curve = 89.4 for DAS28 score (see Fig. 6).
Figure 6.
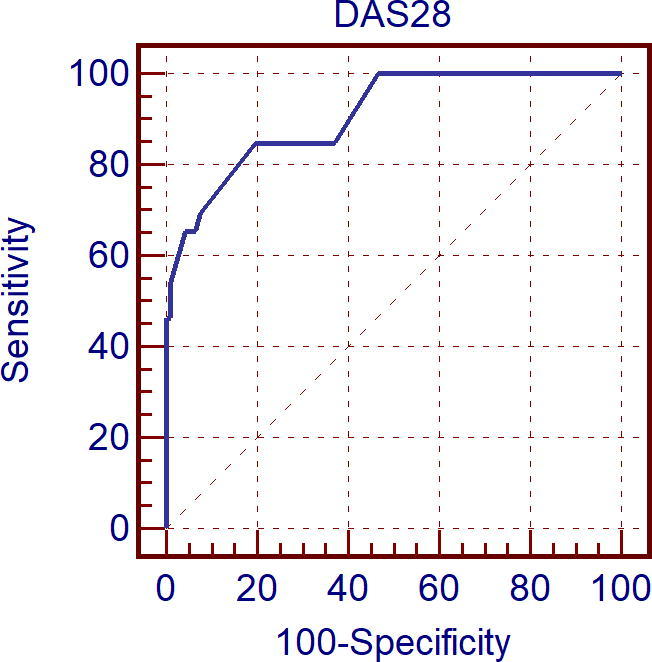
Receiver operating curve characteristic (Roc) curve analysis for Disease Activity Score in 28 Joints (DAS28) score as predictor reduced right ventricular global longitudinal strain (RV GLS).
Also, SDAI score and DAS28 were predictive for reduced RV GLS (less than the mean value for RV GLS in control group: −23%) with a cut off value of >11 and >3 respectively with sensitivity of 73.1%, specificity of 93.5% and area under ROC curve = 91.6 for SDAI score and with sensitivity of 84.6%, specificity of 80.4% and area under ROC curve = 90.8 for DAS28 score.
4. Discussion
The principle finding of this study was that GLS measured using STE of both left and right ventricle is reduced in patients with active RA without history of CVD and normal ejection fraction in comparison with RA Patients in remission phase and normal control groups. Moreover there was a positive correlation between the degree of GLS reduction of both left and right ventricles and the level of RA disease activity measured using SDAI score and DAS28 score.
Left ventricular diastolic function measured by echocardiography was nearly normal in the three studied groups and was not correlated with GLS reduction in RA active group, suggesting that GLS is sensitive tools in theses population in detecting the early stage of myocardial dysfunction. On the other hand, S′ measurement using TDI failed to predict those changes in myocardial function, and this could be explained by the fact that TDI is angle dependent and it measures only the function of the basal myocardium and cannot differentiate between active myocardial contraction and tethering effects of adjacent myocardium, while the GLS measure the global longitudinal function of the LV [17].
GLS is a well validated method in detecting reduction in ventricular systolic function even in the presence of normal ejection fraction [13]. Ejection fraction mainly measures radial left ventricular systolic function [18]. Ischemia due to coronary artery disease mainly affects the longitudinal fiber [19], and this reduction of GLS values in patients with active RA may point to the presence of subclinical ischemic heart disease and this is supported by the data from previous studies that patients with active RA have high prevalence of coronary artery disease [20]. In a recent study it was found that RA patient had more severe and prone to rupture coronary plaques in comparison to their counterparts control subjects [21]. The state of systemic inflammation in active RA is associated with release of inflammatory cytokines like IL-6 and TNF which lead to endothelial dysfunction, the first stage of atherosclerosis [22]. Moreover, it was found that there is a great similarity between atherosclerotic plaque and inflammatory processes in the rheumatoid synovium [23].
The high state of systemic inflammation in RA could lead to another possible mechanism of myocardial dysfunction through myocardial fibrosis in the absence of CAD [24]. In a recent study of RA patients using cardiac magnetic resonance the increased disease activity was associated with increased mid-wall fibrosis in the absence of ischemia [25]. In another recent study by Løgstrup et al. [26], they found that in RA patients, higher baseline anti-CCP was associated with worsening GLS over 2 years follow up in comparison to those with normal baseline anti-CCP (0.6 ± 1.8% vs −1 ± 2.8%; p = 0.04) despite there was no significant change in coronary calcium score over this period (23.8 ± 40.3 vs 22.6 ± 68.9; p = 0.96). Those findings support the result of our works that more the RA disease activity is, the more depression of left and right ventricular function.
The previous finding was supported by Midtbø et al. [27] who studied 78 patients with RA in different stages of disease activity and found that stress-corrected mid wall shortening (scMWS) and GLS, both reflect myocardial function, were reduced in patients with active RA compared with patients with RA in remission (95 ± 18% vs. 105 ± 17% and −18.9 ± 3.1% vs. −20.6 ± 3.5%, p < 0.01).
In another study of 87 patients with RA, GLS of left and right ventricle was reduced compared to normal population (p < 0.001) [28].
Ikonomidis et al. studied the effect of interleukin inhibitor on vascular function and myocardial deformation in 80 RA patients, and they found a significant improvement in GLS after treatment by interleukin inhibitors especially in CAD patients [29]. All these data support the finding of our study that higher disease activity is associated with worsening of myocardial function.
On contrast to our data, Logstrup et al. found that lower level of anti CCP antibodies is associated with reduced GLS [8].
Also Meune et al. who studied 27 patients with RA, found no significant difference in systolic strain between RA patients and control group [30]. A possible explanation of this finding is the method they used in measuring the strain. They used tissue Doppler rather than STE which is more sensitive and automated technique in measuring the strain.
There is scanty of data about assessment of RV function using STE in RA patients, in the current study we choose to assess the deformation of the RV using GLS only for two reasons. First, RV myocardial filaments are prevalently longitudinally arranged, and longitudinal strain is the rule procedure used to evaluate RV systolic function utilizing STE. Second Circumferential and spiral RV strain are profoundly actually difficult and not routinely performed in practice. Fine et al. [7] used the GLS to assess RV function in RA patients and compared them to normal subjects and they found a more negative GLS in RA patients (more worse function) (−17.9 ± 4.7% vs −20.7 ± 2.4%, p < 0.001).
Finally, the active RA group was significantly treated by corticosteroids, TNFI, and methotrexate. TNFI change the compliance of the ventricles and promote CHF [31], corticosteroids increase blood pressure, insulin resistant and augment hypercoagulability [32]. So, still these drugs could affect the results of GLS and not only the activity of RA.
5. Limitations
This was a single center study with relatively small number of patients, thus the current results need to be confirmed in larger multicenter study before routine clinical use of STE in RA patients in every day clinical practice. Moreover, the results of the current study cannot be applied on patients with established CVD, hypertension and diabetes who were excluded from the current study.
6. Conclusion
GLS measurement using STE is valuable and reproducible tool in detecting impairment of left and right ventricles systolic function in RA patients, even in the presence of normal ejection fraction. The degree of systolic function impairment is correlated to RA disease activity scores. This raises the concern that inappropriate management of RA activity could lead to development of heart failure. More prospective studies with larger number of patients and more long term follow up are needed to assess the long term clinical outcome of those patients with RA disease activity.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohamed Naseem, Email: mohamednasim2011@gmail.com.
Abeer Abd Elmonem Shahba, Email: abeer.ali2@med.tanta.edu.eg.
References
- 1.Curtis J.R., Yang S., Singh J.A. is rheumatoid arthritis a cardiovascular risk equivalent to diabetes? J Arthritis Care Res (Hoboken) 2018 doi: 10.1002/acr.23535. [DOI] [PubMed] [Google Scholar]
- 2.Nicola P.J., Maradit-Kremers H., Roger V.L. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52:412–420. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 3.Mantel Ä., Holmqvist M., Andersson D.C., Lund L.H., Askling J. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017;69(10):1275–1285. doi: 10.1016/j.jacc.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 4.Krum H., Abraham W.T. Heart failure. Lancet. 2009;373:941–955. doi: 10.1016/S0140-6736(09)60236-1. [DOI] [PubMed] [Google Scholar]
- 5.Mateen S., Zafar A., Moin S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin Chim Acta. 2016;1(455):161–171. doi: 10.1016/j.cca.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Crowson C.S., Myasoedova E., Davis J.M. Use of B-type natriuretic peptide as a screening tool for left ventricular diastolic dysfunction in rheumatoid arthritis patients without clinical cardiovascular disease. Arthritis Care Res. 2011;63:729–734. doi: 10.1002/acr.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fine N.M., Crowson C.S., Lin G. Evaluation of myocardial function in patients with rheumatoid arthritis using strain imaging by speckle-tracking echocardiography. Ann Rheum Dis. 2014;73(10):1833–1839. doi: 10.1136/annrheumdis-2013-203314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Løgstrup B.B., Deibjerg L.K., Hedemann-Andersen A. Left ventricular function in treatment-naive early rheumatoid arthritis. Am J Cardiovasc Dis. 2014;4:79–86. [PMC free article] [PubMed] [Google Scholar]
- 9.Arnett F.C., Edworthy S.M., Bloch D.A. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 10.Klarenbeek N.B., Koevoets R., van der Heijde D.M. Association with joint damage and physical functioning of nine composite indices and the 2011 ACR/ EULAR remission criteria in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1815–1821. doi: 10.1136/ard.2010.149260. [DOI] [PubMed] [Google Scholar]
- 11.Felson D.T., Smolen J.S., Wells G. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70:404–413. doi: 10.1136/ard.2011.149765. [DOI] [PubMed] [Google Scholar]
- 12.Prevoo M.L., 't Hof M.A., Kuper H.H. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 13.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28 doi: 10.1016/j.echo.2014.10.003. 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 14.Nagueh S.F., Appleton C.P., Gillebert T.C. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Rudski L.G., Lai W.W., Afilalo J. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Voigt J.U., Pedrizzetti G., Lysyansky P. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–193. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Voigt J.U., Nixdorff U., Bogdan R. Comparison of deformation imaging and velocity imaging for detecting regional inducible ischaemia during dobutamin. Eur Heart J. 2004;25(17):1517–1525. doi: 10.1016/j.ehj.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Marwick T.H. Methods used for the assessment of LV systolic function: common currency or tower of Babel? Heart. 2013;99:1078–1086. doi: 10.1136/heartjnl-2012-303433. [DOI] [PubMed] [Google Scholar]
- 19.Biering-Sørensen T., Hoffmann S., Mogelvang R. Myocardial strain analysis by2-dimensional speckle tracking echocardiography improves diagnostics of coronary artery stenosis in stable angina pectoris. Circ Cardiovasc Imaging. 2014;7:58–65. doi: 10.1161/CIRCIMAGING.113.000989. [DOI] [PubMed] [Google Scholar]
- 20.Solomon D.H., Reed G.W., Kremer J.M. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Ann Rheum Dis. 2015;67:1449–1455. doi: 10.1002/art.39098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karpouzas G., Malpeso J., Choi T., Li D., Munoz S., Budoff M. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis. 2014;73:1797–1804. doi: 10.1136/annrheumdis-2013-203617. [DOI] [PubMed] [Google Scholar]
- 22.Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2012;51:v3–v11. doi: 10.1093/rheumatology/kes113. [DOI] [PubMed] [Google Scholar]
- 23.Stevens R., Douglas K., Saratzis A., Kitas G. Inflammation and atherosclerosis inrheumatoid arthritis. Expert Rev Mol Med. 2005;7:1–24. doi: 10.1017/S1462399405009154. [DOI] [PubMed] [Google Scholar]
- 24.Mewton N., Liu C.Y., Croisille P. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ntusi N.A., Piechnik S.K., Francis J.M. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: insights from CMR T1 mapping. JACC Cardiovasc Imaging. 2015;8:526–536. doi: 10.1016/j.jcmg.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Løgstrup B.B., Masic D., Laurbjerg T.B. Left ventricular function at two-year follow-up in treatment-naive rheumatoid arthritis patients is associated with anti-cyclic citrullinated peptide antibody status: a cohort study. Scand J Rheumatol. 2017 Nov;46(6):432–440. doi: 10.1080/03009742.2016.1249941. [DOI] [PubMed] [Google Scholar]
- 27.Midtbø Helga, Semb Anne Grete, Matre Knut. Disease activity is associated with reduced left ventricular systolic myocardial function in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76:371–376. doi: 10.1136/annrheumdis-2016-209223. [DOI] [PubMed] [Google Scholar]
- 28.Fine Nowell M., Crowson Cynthia S. Evaluation of myocardial function in patients with rheumatoid arthritis using strain imaging by speckle-tracking echocardiography. Ann Rheum Dis. 2014;73(10):1833–1839. doi: 10.1136/annrheumdis-2013-203314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikonomidis I., Tzortzis S., Andreadou I. Increased benefit of interleukin-1inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ Cardiovasc Imaging. 2014;7:619–628. doi: 10.1161/CIRCIMAGING.113.001193. [DOI] [PubMed] [Google Scholar]
- 30.Meune C., Wahbi K., Assous N. Myocardial dysfunction in rheumatoid arthritis: a controlled tissue-Doppler echocardiography study. J Rheumatol. 2007;34:2005–2009. [PubMed] [Google Scholar]
- 31.Popa C., Netea M.G., Radstake T. Influence of anti-tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann Rheum Dis. 2005;64(2):303–305. doi: 10.1136/ard.2004.023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nashel D.J. Is atherosclerosis a complication of long-term corticosteroid treatment? Am J Med. 1986;80(5):925–929. doi: 10.1016/0002-9343(86)90639-x. [DOI] [PubMed] [Google Scholar]


