Graphical abstract
Keywords: Anti-insulin, Caspase 3, Imazamox, Immunohistochemistry, In situ hybridization
Highlights
-
•
Toxicity of an imazamox-based herbicide was evaluated in rats.
-
•
Blood samples were collected and serum ALP, AST, glucose, calcium and creatinine levels were determined.
-
•
Imazamox formulation induced an increase in serum glucose and calcium.
-
•
Liver and pancreatic tissue were studied by immunohistochemistry and in-situ hybridization.
-
•
Necrotic and degenerative changes were observed in insulin positive ß cells.
Abstract
We studied the acute toxicity of an imazamox-based herbicide at 12, 24 and 36 mg/kg body (bw) weight imazamox equivalent dose on the liver and pancreatic tissue in Sprague Dawley rats. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities, glucose, calcium as well as creatinine, were determined in blood samples, which were collected after 24, 48 and 72 h exposure. Caspase 3 and anti-insulin expression and immunopositivity were evaluated using in situ hybridization and immunohistochemistry, respectively. The imazamox-based herbicide evaluated in this study induced toxic effects even from the lowest dose tested (12 mg/kg bw). The two highest doses caused a statistically significant cytotoxicity on the Langerhans islet cells. Necrotic and degenerative changes were detected in hepatocytes at the two highest doses. Imazamox is considered to be poorly toxic to the liver. Nevertheless, the imazamox-based herbicide formulation tested here reduced the size of the β-islet cells, induced an elevation in serum glucose and calcium. Our data shows that commercial formulations of imazamox containing various co-formulants can have hepatic and pancreatic toxic effects.
1. Introduction
The use of herbicides is steadily increasing due to large-scale monoculture of crops and the rapid expansion of weed resistance to most pesticide active ingredients. This problem has been exacerbated in the last decade by the used of glyphosate-based herbicides on fields of glyphosate-tolerant genetically modified (GM) crops [1]. New crop varieties have been introduced to overcome glyphosate-resistant weeds by combining tolerances to several active ingredients such as 2,4-D, dicamba or imidazolinones. The exposure to these pesticides is thus anticipated to increase, which makes the study of their health effects a public health priority [2].
Imazamox is a member of the imidazolinone class of chemicals including imazapic, imazapyr, imazethapyr, imazamethabenz and imazaquinine [3], which are part of the Clearfield cropping system (https://agriculture.basf.com/us/en/Crop-Protection/Clearfield.html). It is an active ingredient in different herbicide formulations, as this class of compounds inhibits the enzyme acetohydroxyacid synthase, which catalyzes key reactions in the biosynthesis of branched-chain amino acids (valine, isoleucine, leucine) and regulates the end products of these pathways [[4], [5], [6]] (Fig. 1). Because these branched-chain amino acid biosynthesis pathways are not used in animals, imidazolinone herbicides are considered to be safe for non-target species, including humans [7]. However, the fact that imazamox does not have effects on amino acid biosynthesis in mammals does not mean that it cannot exert toxicity through interfering with other biochemical pathways.
Fig. 1.
Branched-chain amino acid (BCAA) biosynthetic pathway. Acetolactate synthase (ALS) catalyzes the first step of biosynthesis of BCAAs. Imazamox blocks the synthesis of valine, leucine, and isoleucine by inactivating the ALS enzyme [8].
Studies on imazamox toxicity are mostly available from studies submitted to regulatory agencies by companies seeking market approval. Imazamox is authorized in the EU with an acceptable daily intake of 3 mg/kg body weight/day (mg/kg bw/d), based on a rabbit developmental study [9,10]. The liver is the first organ reached by imazamox, as this compound is highly soluble in water and quickly accesses this organ following absorption via the portal vein. Liver intoxication can be reflected by the appearance of apoptosis and necrosis.
Apoptosis normally occurs during development and aging phases of life and helps to maintain homeostasis of cell populations in tissues. Apoptosis also occurs as a defense mechanism when toxic effects cause cell damage [11]. Many herbicides increase oxidative stress by causing excessive production of intracellular reactive oxygen species, which often cause apoptosis by altering mitochondrial function [12,13]. For instance, many studies show that synthetic pyrethroid and neonicotinoid exposure increased levels of oxidative stress markers and telomerase activity was found as well as increased organ inflammations, especially to the liver, and induced genotoxicity [[14], [15], [16]]. In addition to liver damage, agricultural chemicals and especially pesticides are suspected to be a significant contributor to the diabetes pandemic. It was recently shown that organophosphate compounds, which are mainly esters, amides or thiol derivatives, are widely used in agriculture to control insect vectors, in commercial buildings or domestic use in homes and gardens induce damage to pancreas (mitochondria damage in insulin-positive cells) and are positively associated with diabetes [17,18]. Evidence of the diabetic effects of herbicides was seen for the first time in Vietnam War veterans from the 1960s who had used Agent Orange, which is a herbicide containing dioxin contaminants [19]. Diabetes affects a large percentage of the human global population. Pancreatic islets of Langerhans play a key role in metabolism. The islets consist of five cell types and in rodents, the cell distribution is the following: β-cells (60%–75%), α-cells (20%), δ-cells (3–5%) and PP cells (F cells; 1–2%) [[20], [21], [22], [23]]. The β-cells constitute the major population in pancreas and regulate glucose homeostasis by insulin secretion. The pancreatic islets are a highly-vascularized micro-organ allowing them to efficiently exert their endocrine function [20] but also makes them readily accessible to water-soluble toxins [[24], [25], [26]].
Few studies have investigated the effects of imazamox on liver and pancreas function. Here we present the first study to investigate the effects of an imazamox-based herbicide on liver and pancreas function in a rat model system. Liver and pancreas function was assessed by measuring biochemical markers in serum (ALT and AST enzymatic activities, glucose, calcium as well as creatinine), organ histology and apoptosis status. We found clear evidence of pancreas structure and functional damage at two of the doses tested, suggesting that imazamox-based herbicides can exert toxicity in non-target mammalian species.
2. Material and methods
2.1. Chemicals and reagents
An Imazamox-based herbicide (Intervix® Pro) was purchased from BASF company (Turkey) and contained 40 g/L of imazamox (5-(methoxymethyl)-2-(4-methyl-5-oxo-4-propan-2-yl-1H-imidazol-2-yl)pyridine-3-carboxylic acid). This commercial formulation also contained sorbitan, monododecanoate, poly(oxy-1,2-ethanediyl) derives (<40%), ammonium hydroxide (<1.5%), 1,2-benzisothiazol-3(2 H)-one and 1,2-benzisothiazolin-3-one (<0.1%) and propane-1,2-diol (<20%) as co-formulants to increase the herbicidal activity of the formulation. Formaldehyde (37%) and phosphate buffer saline (PBS) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Ethics
This study was approved by the Ataturk University Local Board of Ethics Committee for Animal Experiments, Erzurum, Turkey (decision no: 42190979-01—02/2411). The study was in compliance with OECD principles of good laboratory practice, guidelines for testing of chemicals no. 407 and in accordance with standard operating procedures of the host institution.
2.3. Animals
A total of 50 male Sprague-Dawley rats (mean weight 300 ± 10 g SD) were used in this study. Animals were randomly assigned into 10 groups (n = 5/group) including control, three low dose groups (12 mg/kg bw; 24 h, 48 h, 72 h), three middle dose groups (24 mg/kg bw; 24 h, 48 h, 72 h) and three high dose groups (36 mg/kg bw; 24 h, 48 h, 72 h). All doses were calculated based on the LD50 and from the current/recent bibliography. Furthermore, we took into account the NOAEL doses from reports of risk assessment [9]. After a 5-day adaptation period, the imazamox-based herbicide was mixed with 0.9% isotonic sodium chloride to allow administration of a 12, 24 and 36 mg/kg bw imazamox equivalent dose. A volume of 1 mL was injected intraperitoneally. The animals were sacrificed at 24, 48 or 72 h following injection. Following imazmox administration, blood samples were collected by cardiac puncture into vacuum tubes with no anticoagulant (Vacutainer, BD-Plymouth, UK). Blood samples were centrifuged at 3000g for 10 min at room temperature to isolate serum and stored at −20 °C until analysis. Rats were decapitated rapidly under deep anesthesia (Sevoflurane, USA), livers and pancreases were excised and fixed in 10% neutral formaldehyde (Sigma, USA).
2.4. Biochemical assays
Serum enzyme activities for alanine aminotransferase (ALT) and aspartate aminotransferase (AST), glucose, calcium, and creatinine were determined using commercially available test kits (OSR6121 and OSR60117) on a biochemistry autoanalyzer (Cobas 6000/Roche Diagnostics, Germany).
2.5. Histopathological examination
Apoptosis was assessed by monitoring caspase activation. Procaspase-3 is a central player in apoptosis in different cell types [27]. Procaspase 3 is activated by induction of apoptosis in different forms and it is designated as cleaved or active caspase 3. We determined the presence of the cleaved or active forms of caspase 3 using in situ hybridization as a marker of apoptosis in cells / tissues [28,29]. The enzyme activation of caspase 3 has been studied by in situ hybridization, immunohistochemistry and Western blot [30]. Livers fixed in 10% neutral formaldehyde for 24–48 h were embedded in paraffin blocks. Paraffin-embedded tissues were processed to give 5 μm thick sections and were stained with hematoxylin–eosin, followed by microscopic examination. The histopathological findings in the sections were graded as 0 (none), 1 (mild), 2 (moderate), and 3 (severe) [31].
2.5.1. Immunohistochemical examination
After deparaffinization with graded alcohol and xylene for immunohistochemical staining, the slides were immersed in antigen retrieval solution (ab 96674, pH 6.0; Abcam, Cambridge, UK) and were heated in a microwave oven for 15 min to unmask antigens. The sections were then incubated in 3% H2O2 for 10 min. to block endogenous peroxidases. Liver sections were incubated at room temperature with polyclonal rabbit active/cleaved caspase 3 antibody (cat no. NB600-1235, dilution 1/200; Novus Biological, USA) for the detection of apoptosis. Pancreas sections were incubated at room temperature with monoclonal anti-insulin antibody clone K36AC10 (cat no. I2018-2ML, dilution 1/1000; Sigma Aldrich- USA). The antibody reacts specifically against insulin by RIA and immunocytochemistry (cytoplasmic expression). It exhibits cross-reactivity with human proinsulin [[32], [33], [34], [35]]. The EXPOSE Mouse and Rabbit Specific HRP/DAB Detection IHC Kit (ab80436) was used as follows. Sections were incubated with goat anti-mouse antibody, then with streptavidin peroxidase, and finally with 3,3′ diaminobenzidine chromogen. Slides were counter-stained with hematoxylin. Immunoreactivity was graded as 0 (none), 1 (mild), 2 (moderate), and 3 (severe) [36].
2.5.2. In situ hybridization
The paraffin sections were placed at 57 °C for 1 h and then passed through a series of xylol alcohols to perform deparaffinization. For the retrieval step, sections were incubated in pre-warmed Pepsin-HCl solution for 5 min and were washed with PBS. Caspase-3 mRNA was detected using the following biotynylated probe included oligonucleotide probe: AGATCATCACTGCTTCGTAATT / 3Bio (Exiqon, Product Name: Caspase 3 probe_1, Dilution rate: 1:50) and 50 μl solution was applied in each tissue sample and detection of hybridization employed the Hybridization Detection System for Biotinylated probes according to the manufacturer’s instructions (Dako, Cat.no: K0601). The sections were covered with coverslips and were incubated at 90 °C for 45 min. Nuclear fast red was used as a chromogen. The sections passed through alcohol and xylol baths were examined using a drop of entellan mounting medium (Merck 107961.0500) under light microscopy. Positivity for hybridisation was graded as 0 (none), 1 (mild), 2 (moderate), and 3 (severe).
2.6. Statistical analysis
All statistical analyses were carried out by using the SPSS statistical software (SPSS for windows, version 20.0). All data were presented in mean (±) standard deviation (S.D.). For biochemical analysis, differences were assessed using one-way analysis of variance (one-way ANOVA). For immunohistochemical analysis, differences in measured parameters between the groups were analyzed with a nonparametric test (Kruskal–Wallis). Dual comparisons between groups exhibiting significant values were evaluated with the Mann–Whitney U test (P <0.05).
3. Results
The aim of this study was to evaluate liver and pancreas toxicity of an imazamox-based herbicide. We assessed liver damage by evaluation of serum levels of ALT, AST, and creatinine (Table 1). Creatinine levels in some treatment groups (36 mg/kg for 24 h, 12 mg/kg for 48 h, 24 mg/kg for 48 h, 36 mg/kg for 48 h) were lower compared to the control group.
Table 1.
Measurement of creatinine and liver enzyme (ALT and AST) activity in serum of Sprague-Dawley rats intraperitoneally administred with imazamox-based herbicide formulation at the following doses: 12, 24, and 36 mg/kg bw compared to an untreated (control group).
| Time (hours) | Dose groups | Time (hours) | No. of animals | ALT (IU/L) | AST (IU/L) | CREATİNİNE (IU/L) |
|---|---|---|---|---|---|---|
| 24 h | Control | 24 | 5 | 52.2 ± 6.49 | 128.8 ± 13.88 | 0.53 ± 0.06 |
| 12 mg/kg | 24 | 5 | 57.4 ± 6.65 | 140.2 ± 15.02 | 0.47 ± 0.02 | |
| 24 mg/kg | 24 | 5 | 63 ± 7.31 | 107.2 ± 11.34* | 0.49 ± 0.03 | |
| 36 mg/kg | 24 | 5 | 55.6 ± 4.97 | 137 ± 14.17** | 0.45 ± 0.05* | |
| 48 h | Control | 48 | 5 | 52.2 ± 6.76 | 140.8 ± 17.79 | 0.53 ± 0.01 |
| 12 mg/kg | 48 | 5 | 70.4 ± 8.01* | 120.2 ± 15.61 | 0.46 ± 0.02* | |
| 24 mg/kg | 48 | 5 | 52.6 ± 4.15** | 124 ± 16.38 | 0.45 ± 0.03* | |
| 36 mg/kg | 48 | 5 | 51.4 ± 4.66** | 115.2 ± 17.68 | 0.44 ± 0.05* | |
| 72 h | Control | 72 | 5 | 50.2 ± 4.81 | 140.8 ± 15.18 | 0.53 ± 0.02 |
| 12 mg/kg | 72 | 5 | 58.8 ± 5.01* | 116.8 ± 10.25 | 0.52 ± 0.07 | |
| 24 mg/kg | 72 | 5 | 54 ± 2.91* | 101.2 ± 16.45 | 0.54 ± 0.02 | |
| 36 mg/kg | 72 | 5 | 52.4 ± 3.64* | 124.4 ± 13.61 | 0.46 ± 0.03 |
*,**
P < 0.05 as compared to control.
ALT was increased in the 12 mg/kg, 24 mg/kg, 36 mg/kg dose groups (following 48 h and 72 h post exposure) compared to the control group (P < 0.05, Table 1). The difference between the three doses investigated was not significant. AST serum levels did not show a correlation with ALT levels. AST levels decreased significantly following administration of 24 mg/kg for 24 h, but increased significantly following administration 36 mg/kg for 24 h (P < 0.05, Table 1). However, there was no significant difference in AST levels between the control group and some test groups (12 mg/kg for 24 h, 12 mg/kg for 48 h, 12 mg/kg for 72 h, 24 mg/kg for 48 h, 24 mg/kg for 72 h, 36 mg/kg for 48 h, and 36 mg/kg for 72 h).
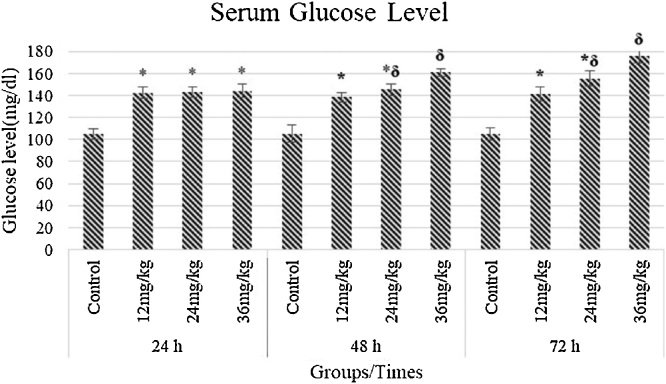
Following 24 h of exposure, serum glucose increased in all test groups, compared to the control (P < 0.05). At 48 h of exposure, serum glucose remained stable in the 12 mg/kg group and slightly increased in the 24 mg/kg group, but exhibited a further increase in the 36 mg/kg group, compared to the control (P < 0.05). Serum glucose levels following 72 h of exposure remained stable in the 12 mg/kg group, but further increased in the 24 mg/kg and 36 mg/kg groups, with a more pronounced increase in the latter (Fig. 2). When treatment groups compared with each other, following 48 h and 72 h of exposure the 12 mg/kg and 36 mg/kg groups were statistically significantly different between each other. However, there was no significance in the 24 mg/kg groups. Exposure for 24 h resulted in a slight but not significant increase in serum calcium levels in test groups compared to the control. However, following 48 h of exposure serum calcium was significantly increased in all test groups (P < 0.05). At 72 h of following exposure, serum calcium was slightly decreased in the 12 mg/kg group (P < 0.05) but remained stable in the 24 mg/kg and 36 mg/kg groups (P <0.05, Fig. 3). When treatment groups were compared with each other following 24 h,48 h and 72 h of exposure, there were no statistical difference between them.
Fig. 2.
Measurement of serum glucose of Sprague-Dawley rats intraperitoneally administred with imazamox-based herbicide formulation at the different doses of 12, 24, and 36 mg/kg bw compared to untreated animals (control group). * P < 0.05 as compared to control, δ P < 0.05 as compared to intergroups.
Fig. 3.
Measurement of serum calcium of Sprague-Dawley rats intraperitoneally administred by imazamox-based herbicide formulation at 12, 24, and 36 mg/kg bw, compared to untreated an control group. * P < 0.05 as compared to control.
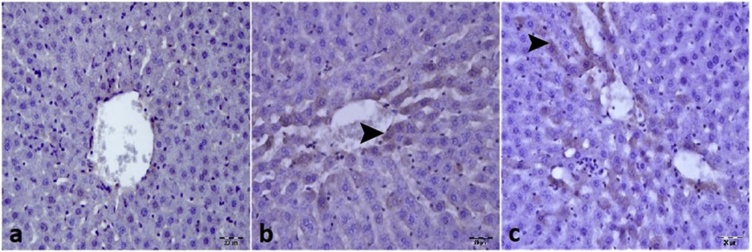
The signs of liver damage indicated by serum biochemical analysis (Table 1), were further investigated by immunohistochemistry and in situ hybridization. The liver of control rats had a normal histological appearance (Fig. 4a), whereas necrotic and degenerative lesions were detected in the 36 mg/kg in the 48 h (Fig. 4b) and 72 h (Fig. 4c) treatment groups. Immunohistochemistry analysis revealed a very modest level of cleaved caspase 3 positive apoptotic liver cells in the control group (Fig. 5a) and some treatment groups (12 mg/kg for 24 h, 12 mg/kg for 48 h, 12 mg/kg for 72 h, 24 mg/kg for 24 h, 24 mg/kg for 48 h, 24 mg/kg for 72 h and 36 mg/kg for 24 h) (Table 2). However, an approximately 6-fold increase in Cleaved Caspase 3 expression was observed in the 36 mg/kg for 48 h (Fig. 5b) and for 72 h (Fig. 5c) treatment groups and this increase was statistically significant (Table 2; P <0.05). Similarly, Cleaved Caspase 3 expression was very low in the liver of control rats (Fig. 6a) and the 12 mg/kg for 24 h, 12 mg/kg for 48 h, 12 mg/kg for 72 h, 24 mg/kg for 24 h, 24 mg/kg for 48 h, 24 mg/kg for 72 h and 36 mg/kg for 24 h treatment groups (Table 3). In contrast, cleaved caspase 3 expression as determined by in situ hybridization, was clearly evident in the 36 mg/kg for 48 h (Fig. 6b) and for 72 h (Fig. 6c) groups. There was no statistically significant difference in Cleaved Caspase 3 levels, with either of the methods used, between the control group and the 12 mg/kg 24 h, 12 mg/kg 48 h, 12 mg/kg 72 h, 24 mg/kg 24 h, 24 mg/kg 48 h, 24 mg/kg 72 h and 36 mg/kg 24 h treatment groups (Table 2). However, a significant difference was observed between the control group and the 36 mg/kg 48 h and 72 h groups (Table 2; P < 0.05).
Fig. 4.
Effect of imazamox-based herbicide formulation on rat liver tissue compared to control animals. a) Normal histologic appearance of liver in the control group b) Hydropic degeneration and necrosis of hepatocytes in the 36 mg/kg/day 48 h group (arrowhead) c) Necrosis of hepatocytes in the 36 mg/kg/day 72 h group (arrowhead). H&E x 20μ.
Fig. 5.
Immunohistochemical detection of cleaved caspase 3 in liver. a) Section from a control rat showing lack of immunostaining for cleaved caspase 3 staining. b) Section from rats treated by intraperitoneal injection with imazamox-based herbicide at an imazamox equivalent dose of 36 mg/kg bw for 48 h showing moderate positive immunoreactivity for cleaved caspase 3 (arrowhead). c) Section from rats intraperitoneally injected with imazamox at a dose of 36 mg/kg bw for 72 h showing intense positive immunoreactivity for cleaved caspase 3 (arrowhead). IHC x 20μ.
Table 2.
Differences between groups in terms of immunohistochemical staining and in situ hybridization for the detection of cleaved caspase 3 in liver.
| Groups | Caspase 3 |
|---|---|
| Control | 0.20 ± 0.002a |
| 12 mg/kg 24 h | 0.40 ± 0.024a |
| 12 mg/kg 48 h | 0.60 ± 0.04a |
| 12 mg/kg 72 h | 0.20 ± 0.008a |
| 24 mg/kg 24 h | 0.40 ± 0.014a |
| 24 mg/kg 48 h | 0.60 ± 0.029a |
| 24 mg/kg 72 h | 0.40 ± 0.011a |
| 36 mg/kg 24 h | 0.40 ± 0.024a |
| 36 mg/kg 48 h | 2.40 ± 0.13b |
| 36 mg/kg 72 h | 2.60 ± 0.25b |
Means with the same letter, per each column, are not significantly different according to Mann-Whitney U test, p ≤ 0.05, n = 5.
Fig. 6.
Detection of cleaved caspase 3 mRNA in liver tissue. Liver sections from untreated control and imazamox intraperitoneally injected rats were analysed for cleaved caspase 3 mRNA by in situ hybridization with a biotinylated oligonucleotide probe. a) Control group with negative staining for cleaved caspase 3 expression in hepatocytes. b) Moderate staining of cleaved caspase 3 expression in hepatocytes around the vena centralis in the 36 mg/kg bw imazamox treatment group at 48 h post-injection (arrowhead) c) Greater intensity of cleaved caspase 3 expression in the cytoplasm of hepatocytes around the vena centralis in the 36 mg/kg bw injected group at 72 h (arrowhead). ISH x 20μ.
Table 3.
Insulin positive islet size in different groups a) no significant difference compared to the control group, b) significant difference compared to the control group (P < 0.05).
| Groups | Size of islets |
|---|---|
| Control group | 36.40 ± 1.20a |
| 12 mg/kg for 24 h | 36.20 ± 0.87a |
| 12 mg/kg for 48 h | 36.80 ± 1.11a |
| 12 mg/kg for 72 h | 35.40 ± 0.67a |
| 24 mg/kg for 24 h | 37.20 ± 0.58a |
| 24 mg/kg for 48 h | 36.20 ± 0.58a |
| 24 mg/kg for 72 h | 35.60 ± 0.40a |
| 36 mg/kg for 24 h | 35.20 ± 1.39a |
| 36 mg/kg for 48 h | 27.00 ± 0.54b |
| 36 mg/kg for 72 h | 26.40 ± 0.50b |
Means with the same letter, per each column, are not significantly different according to One way ANOVA test, p ≤ 0.05, n = 5.
The results of the immunohistochemical examination of the pancreatic tissues are presented in Fig. 7 and Table 3. Our data show a statistically significant difference only between the control group and the groups that received the dose of 36 mg/kg for 48 and 72 h (Fig. 7i and j, Table 3; P < 0.05). In the other test groups, the pancreatic tissues had a normal histological appearance. Necrotic lesions and regions of degeneration were observed in insulin positive β-cells in the groups that received the 36 mg/kg dose for 48 and 72 h. According to our results, in those two groups the size of the insulin producing islets decreased by 36–38%, when compared to the control group. This finding is in accordance with the serum glucose levels and indicates a decreased insulin secretion (decrease in the β-islet cell population).
Fig. 7.
Effect of imazamox-based herbicide formulation on architecture of β-cells of pancreas tissue compared to the control group. a–h) Representative normal size of pancreatic cell islets immunostained with an anti-insulin antibody showing positive cells in control to 36 mg/kg bw dose groups, following 24 h of imazamox-based herbicide exposure. (i) Small size of pancreatic cell islets immunostained with anti-insulin antibody showing positive cells in the control to 36 mg/kg bw dose groups following 48 h of imazamox exposure and (j) 36 mg/kg bw dose group following 72 h of imazamox exposure IHC x 20μ.
4. Discussion
Xenobiotics such as pharmaceuticals, pesticides (herbicides, insecticides, fungicides, etc), and personal care products have caused harmful effects on the environment and public health [[37], [38], [39]]. Exposure of laboratory animals is used to predict these effects, which can include porphyria, liver damage, lipid mobilization, hypothyroidism, or testicular atrophy, among other adverse effects investigated in toxicity tests [[39], [40], [41], [42], [43]]. Imazamox is an imidazolinone herbicide ensuring control of numerous terrestrial and aquatic weeds. Despite adverse effects of Imazamox on plants, it does not have detectable signs of toxicity in mammals, even at very high doses, and its mechanism of action does not provide meaningful information for animal and human health risk assessment [3]. However, it is possible that exposure to imazamox at low doses, via contaminated water, over a prolonged period of time may cause a change in cellular structure and function, even if it is not toxic at high doses. It has been shown that there are no gross pathological or histopathological changes in short-term toxicity studies in rats, only a reduction in body weight, which is not considered toxicologically significant [44]. Furthermore, it has been reported that there are no macroscopic or microscopic events of toxicological significance in rats, at any treatment level with technical-grade imazamox, in long-term toxicity studies [45]. Although an increase in liver weight was observed, this was not considered treatment related. However, in these studies, imazamox was not tested as a pesticide formulation, but as a single technical-grade chemical. Our study reveals that co-formulants present in an imazamox-based herbicide formulation, can change the toxicity profile of this pesticide.
Other endpoints investigated in regulatory studies suggest that imazamox presents low toxicity. There was no evidence of neurotoxic effects related to imazamox observed in acute, sub-chronic, developmental, reproduction or chronic studies [46]. It has been reported that imazamox has no adverse effect on immune function [3]. The effects on organs associated with endocrine function are not reported in standard toxicity studies on this chemical, although it is known that the battery of tests currently applied can be inadequate to reveal endocrine disrupting effects. Furthermore, only a few studies have compared the toxicity of imazamox and its commercial formulations. Among these studies, one reported that both technical-grade imazamox [47] as well as an 11.83% formulation of this herbicide [48] caused a slight erythema on the skin.
In this study, we cannot definitely conclude if the toxic effects observed are attributed to imazamox itself or the different co-formulants present in the pesticide, or a combination of the two. The co-formulants 1,2-benzisothiazol-3(2 H)-one (BIT) and 1,2-benzisothiazolin-3-one have been shown to induce toxic effects [[49], [50], [51], [52]]. It is however crucial to test the commercial formulations of pesticides because they are the mixtures to which farmers and the general population are exposed [53].
It has been reported that imazamox and its major metabolites present low toxicity and no significant bioaccumulation. However, it has been observed that this herbicide can cause cell death, even though it does not bioaccumulate in tissues in sufficient amounts when taken at high doses, either acutely or chronically, by humans and animals [54].
Numerous pharmacological or chemical substances such as acetaminophen, CCl4, d-galactosamine, and dimethylnitrosamine have been shown to cause hepatic damage. When the liver is exposed to hepatotoxins at an excessive dose, these may induce acute liver injury characterized by necrosis, degeneration, or apoptosis of hepatocytes [[55], [56], [57], [58]]. In the present study, moderate necrotic and degenerative changes were detected in the liver of rats exposed to 24 and 36 mg/kg bw/d imazamox equivalent dose of a commercial formulation. Furthermore, the pancreas showed necrotic and degenerative changes in the groups that received 36 mg/kg bw/d for 48 and 72 h. The size of pancreatic islets in these groups was the smallest, when compared to other groups. Since degeneration and necrosis occur in the liver and insulin producing islets depending on the dose of imazamox formulation administered, our results suggest that this herbicide, commonly used in agriculture, may cause toxic effects in animals.
Caspase-3 (also known as CPP-32, Apoptain, Yama, SCA-1) has a key role in apoptosis, since it is either partially or totally responsible for the proteolytic cleavage of many key proteins, such as the nuclear enzyme poly (ADP-ribose) polymerase (PARP) [59]. Activation of caspase-3 requires proteolytic processing of its inactive zymogen into activated p17 and p12 fragments. Cleavage of caspase-3 requires aspartic acid at the P1 position [60,61]. Activated caspase-3 can induce a self-amplification cascade, which may directly roll-up cytoskeletal proteins in the cytosol, or directly activate nuclear DNAase and cause DNA breakage and apoptosis. Thus, caspase-3 plays an important role in the induction of apoptosis [62]. Activation of caspase-3 regulates inflammation and apoptosis signaling networks would eventually trigger hepatocyte apoptosis [63,64]. The results we present here showed that the immunopositivity of cleaved caspase-3 in liver tissue was higher in the group exposed to the highest dose of the imazamox-based herbicide. However, more studies are needed to determine the specific mechanisms of imazamox herbicide-induced apoptosis. In addition to apoptotic changes in the liver, we also observed necrotic and degenerative changes in pancreatic islets, which can play an important role in the pathogenesis of diabetes [65,66]. Many of the changes in pancreatic islet structure and function associated with diabetes are attributable to hyperglycemia [67]. Hyperglycemia may induce alterations in islet endothelium, potentially contributing to the progressive reduction of β-cell function [68]. It has been shown that the number of insulin positive cells is reduced in diabetes [69]. A report of pancreatic β-cell apoptosis in cases of diabetes type 1 also described that the CD8+ cytotoxic-T cells attack β-cells in this condition and consequently reduce the cell population [70]. Our findings showed that the size of pancreatic islets (with necrotic and degenerative changes) in the 36 mg/kg bw group was the smallest when compared to the other groups and that the insulin positive cell population decreased dramatically following exposure to the imazamox-based herbicide.
Blood serum changes are also important in the evaluation of toxicity and prognosis. Studies have demonstrated that paracetamol toxicity increases ALT and AST levels and that elevated ALT levels in the liver can be considered as a marker of fibrosis [71,72]. In our study, ALT levels increased in the 12 mg/kg treatment groups, at all time-points evaluated following administration. A decline in hepatic functional capacity results in decreased creatine production and lower serum levels of this compound. A study by Hu and colleagues showed that pesticide exposure adversely affects blood cells, liver and the peripheral nervous system, and has been shown to reduce creatinine levels, especially when leukocyte ratios are increased in participants [73].
Insulin-secreting pancreatic β-cells play a major role in glucose homeostasis [74]. Tizhe et al. reported that glyphosate-based herbicide Bushfire® exposure may change blood glucose homeostasis and effect insulin secretion in rats by damaging pancreatic islet and acinar cells [75]. In the present study, animals that received imazamox formulation for 24, 48 and 72 h in all treatment groups presented an increase of serum glucose, compared to the control group. Furthermore, serum calcium levels increased in 48 and 72 h in all treatment groups presented an increase of serum calcium, compared to the control group. Although little is known about how imazamox can induce hyperglycemia, it is suggested that the degeneration of β-cells in the pancreatic islet may lead to decrease insulin positive cells and it may cause increasing serum glucose levels. Decreased insulin levels may lead to increase serum calcium levels, because calcium is important for insulin mediated intracellular processes in insulin responsive tissues [76].
The current study evaluated the effect of imazamox alone in rats and its design presents the limitation that it does not simulate real-life human exposure (long-term, low-dose exposure to chemical mixtures). For this reason, these preliminary data should be reinforced with a study designed in a different way in order to mimic real-life conditions [[77], [78], [79]]. More studies are needed to determine the specific mechanisms of imazamox herbicide-induced toxicity in the vitals.
5. Conclusion
The current study presents new insights into the mechanism of imazamox-induced apoptosis and its toxicity in liver and pancreas. Furthermore, this is the first study on the apoptotic events related to imazamox and the cell changes induced in liver and pancreas. The present evidence shows an increase in hepatic caspase-3 activity, an apoptotic factor, and a decrease of the insulin islets in the pancreas. Thus, our study has demonstrated toxic effects of an imazamox herbicide formulation, which is widely used in agriculture and suggest that this pesticide should be used with caution.
Author contributions
ÇS participated in the rat experiment, performed biochemical and pathological analysis, and drafted the manuscript. SÇ, MÖ, AT and AK performed biochemical and pathological analysis. LK, TIB and AMT coordinated the investigation and assisted in the drafting of the manuscript. RM and MNA assisted in the drafting of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Transparency document
References
- 1.Owen M.D., Zelaya I.A. Herbicide-resistant crops and weed resistance to herbicides. Pest Manag. Sci. 2005;61:301–311. doi: 10.1002/ps.1015. [DOI] [PubMed] [Google Scholar]
- 2.Schütte G., Eckerstorfer M., Rastelli V., Reichenbecher W., Restrepo-Vassalli S., Ruohonen-Lehto M., Saucy A.W., Mertens M. Herbicide resistance and biodiversity: agronomic and environmental aspects of genetically modified herbicide-resistant plants. Environ. Sci. Eur. 2017;29:5. doi: 10.1186/s12302-016-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanek R., Research Everglades, Evaluation . In: Chapter 6: Everglades Research and Evaluation. Sklar Fred, Dreschel Thomas., editors. South Florida Environmental Report; 2013. [Google Scholar]
- 4.Gaston S., Zabalza A., Gonzalez E.M., Arrese-Igor C., Aparicio-Tejo P.M., Royuela M. Imazethapyr, an inhibitor of the branched-chain amino acid biosynthesis, induces aerobic fermentation in pea plants. Physiol. Plant. 2002;114:524–532. doi: 10.1034/j.1399-3054.2002.1140404.x. [DOI] [PubMed] [Google Scholar]
- 5.Elisakova V., Patek M., Holatko J., Nesvera J., Leyval D., Goergen J.L., Delaunay S. Feedback-resistant acetohydroxy acid synthase increases valine production in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2005;71:207–213. doi: 10.1128/AEM.71.1.207-213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCourt J.A., Pang S.S., King-Scott J., Guddat L.W., Duggleby R.G. Herbicide-binding sites revealed in the structure of plant acetohydroxyacid synthase. Proc. Natl. Acad. Sci. U. S. A. 2006;103:569–573. doi: 10.1073/pnas.0508701103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DEC N. New York State Department of Environmental Conservation; New York: 2003. Imazamox (Raptor) NYS DEC Letter – Active Ingredient Registration 3/03. [Google Scholar]
- 8.Shimizu T., Nakayama I., Nagayama K., Miyazawa T., Nezu Y. Springer; 2002. Acetolactate Synthase Inhibitors, Herbicide Classes in Development; pp. 1–41. [Google Scholar]
- 9.Authority E.F.S. Peer review of the pesticide risk assessment of the active substance abamectin. Efsa J. 2016;14(5) [Google Scholar]
- 10.Authority E.F.S. Peer review of the pesticide risk assessment of the active substance imazamox. Efsa J. 2016;14(4):4432. [Google Scholar]
- 11.Norbury C.J., Hickson I.D. Cellular responses to DNA damage. Annu. Rev. Pharmacol. Toxicol. 2001;41:367–401. doi: 10.1146/annurev.pharmtox.41.1.367. [DOI] [PubMed] [Google Scholar]
- 12.Li X., Sun A.Y. Paraquat induced activation of transcription factor AP-1 and apoptosis in PC12 cells. J. Neural Transm. 1999;106:1–21. doi: 10.1007/s007020050137. [DOI] [PubMed] [Google Scholar]
- 13.González-Polo R.A., Rodríguez-Martín A., Morán J.M., Niso M., Soler G., Fuentes J.M. Paraquat-induced apoptotic cell death in cerebellar granule cells. Brain Res. 2004;1011:170–176. doi: 10.1016/j.brainres.2004.02.078. [DOI] [PubMed] [Google Scholar]
- 14.Vardavas A.I., Fragkiadaki P., Alegakis A.K., Kouretas D., Goutzourelas N., Tsiaoussis J., Tsitsimpikou C., Stivaktakis P.D., Carvalho F., Tsatsakis A.M. Downgrading the systemic condition of rabbits after long term exposure to cypermethrin and piperonyl butoxide. Life Sci. 2016;145:114–120. doi: 10.1016/j.lfs.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Vardavas A.I., Stivaktakis P.D., Tzatzarakis M.N., Fragkiadaki P., Vasilaki F., Tzardi M., Datseri G., Tsiaoussis J., Alegakis A.K., Tsitsimpikou C. Long-term exposure to cypermethrin and piperonyl butoxide cause liver and kidney inflammation and induce genotoxicity in New Zealand white male rabbits. Food Chem. Toxicol. 2016;94:250–259. doi: 10.1016/j.fct.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Vardavas A.I., Ozcagli E., Fragkiadaki P., Stivaktakis P.D., Tzatzarakis M.N., Alegakis A.K., Vasilaki F., Kaloudis K., Tsiaoussis J., Kouretas D. The metabolism of imidacloprid by aldehyde oxidase contributes to its clastogenic effect in New Zealand rabbits. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018;829:26–32. doi: 10.1016/j.mrgentox.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Juntarawijit C., Juntarawijit Y. Association between diabetes and pesticides: a case-control study among Thai farmers. Environ. Health Prev. Med. 2018;23 doi: 10.1186/s12199-018-0692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ioanna K., Claudio C., Athanasios A., Manolis T., Elena V., Rizosa Apostolos K., Sarigiannisdef Dimosthenis A., Tsatsakis Aristides M. Estimation of daily intake and risk assessment of organophosphorus pesticides based on biomonitoring data – the internal exposure approach. Food Chem. Toxicol. 2018;123:57–71. doi: 10.1016/j.fct.2018.10.047. [DOI] [PubMed] [Google Scholar]
- 19.Kang H.K., Dalager N.A., Needham L.L., Patterson D.G., Lees P.S.J., Yates K., Matanoski G.M. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am. J. Ind. Med. 2006;49:875–884. doi: 10.1002/ajim.20385. [DOI] [PubMed] [Google Scholar]
- 20.Kim A., Miller K., Jo J., Kilimnik G., Wojcik P., Hara M. Islet architecture: a comparative study. Islets. 2009;1:129–136. doi: 10.4161/isl.1.2.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steiner D.J., Kim A., Miller K., Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2:135–145. doi: 10.4161/isl.2.3.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baskin D.G. A historical perspective on the identification of cell types in pancreatic islets of langerhans by staining and histochemical techniques. J. Histochem. Cytochem. 2015;63:543–558. doi: 10.1369/0022155415589119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodh S., O’Hare E.A., Zaghloul N.A. Primary cilia in pancreatic development and disease. Birth Def. Res. Part C Embryo today: Rev. 2014;102:139–158. doi: 10.1002/bdrc.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandler S., Jansson L. Vascular permeability of pancreatic islets after administration of streptozotocin. Virchows Arch.. A Pathol. Anatomy Histopathol. 1985;407:359–367. doi: 10.1007/BF00709983. [DOI] [PubMed] [Google Scholar]
- 25.Jansson L., Sandler S. The influence of cyclosporin A on the vascular permeability of the pancreatic islets and on diabetes induced by multiple low doses of streptozotocin in the mouse. Virchows Arch.. A Pathol. Anatomy Histopathol. 1988;412:225–230. doi: 10.1007/BF00737146. [DOI] [PubMed] [Google Scholar]
- 26.Moore P.G., James O.F. Acute pancreatitis induced by acute organophosphate poisoning. Postgrad. Med. J. 1981;57:660–662. doi: 10.1136/pgmj.57.672.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jänicke R.U., Sprengart M.L., Wati M.R., Porter A.G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 28.Arroyo J.A., Li C., Schlabritz-Loutsevitch N., McDonald T., Nathanielsz P., Galan H.L. Increased placental XIAP and caspase 3 is associated with increased placental apoptosis in a baboon model of maternal nutrient reduction. Am. J. Obstet. Gynecol. 2010;203(364):e313–364. doi: 10.1016/j.ajog.2010.05.021. e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng X.S., Li M.S., Du J., Jiang Q.Y., Wang L., Yan S.Y., Yu D.M., Deng J.B. Neuronal apoptosis in the developing cerebellum. Anatomia Histol. Embryologia. 2011;40:21–27. doi: 10.1111/j.1439-0264.2010.01033.x. [DOI] [PubMed] [Google Scholar]
- 30.Yang X., Yang S., Zhang J., Xue L., Hu Z. Role of Caspase 3 in neuronal apoptosis after acute brain injury. Chin. J. Traumatol. 2002;5:250–253. [PubMed] [Google Scholar]
- 31.Ozkaya A., Sahin Z., Dag U., Ozkaraca M. Effects of naringenin on oxidative stress and histopathological changes in the liver of lead acetate administered rats. J. Biochem. Mol. Toxicol. 2016;30:243–248. doi: 10.1002/jbt.21785. [DOI] [PubMed] [Google Scholar]
- 32.Keilacker H., Dietz H., Witt S., Woltanski K.P., Berling R., Ziegler M. Kinetic-properties of monoclonal insulin-antibodies. Biomed. Biochim. Acta. 1986;45:1093–1102. [PubMed] [Google Scholar]
- 33.Madsen O.D. Proinsulin-specific monoclonal-antibodies – immunocytochemical application as beta-cell markers and as probes for conversion. Diabetes. 1987;36:1203–1211. doi: 10.2337/diab.36.10.1203. [DOI] [PubMed] [Google Scholar]
- 34.Witt S., Dietz H., Ziegler B., Keilacker H., Ziegler M. Production and application of monoclonal-antibodies directed against glucagon and insulin – reduction of pancreatic insulin in rats after treatment with complete freund adjuvant. Acta Histochem. 1988:217–223. [PubMed] [Google Scholar]
- 35.Csaba G., Kovacs P., Pallinger E. Immunologically demonstrable hormones and hormone-like molecules in rat white blood cells and mast cells. Cell Biol. Int. 2004;28:487–490. doi: 10.1016/j.cellbi.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Aksu E.H., Kandemir F.M., Ozkaraca M., Omur A.D., Kucukler S., Comakli S. Rutin ameliorates cisplatin-induced reproductive damage via suppression of oxidative stress and apoptosis in adult male rats. Andrologia. 2017;49 doi: 10.1111/and.12593. [DOI] [PubMed] [Google Scholar]
- 37.Ikehata K., Gamal El-Din M., Snyder S.A. Ozonation and advanced oxidation treatment of emerging organic pollutants in water and wastewater. Ozone: Sci. Eng. 2008;30:21–26. [Google Scholar]
- 38.García-García C.R., Parrón T., Requena M., Alarcón R., Tsatsakis A.M., Hernández A.F. Occupational pesticide exposure and adverse health effects at the clinical, hematological and biochemical level. Life Sci. 2016;145:274–283. doi: 10.1016/j.lfs.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Georgiadis N., Tsarouhas K., Tsitsimpikou C., Vardavas A., Rezaee R., Germanakis I., Tsatsakis A., Stagos D., Kouretas D. Pesticides and cardiotoxicity. Where do we stand? Toxicol. Appl. Pharmacol. 2018 doi: 10.1016/j.taap.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Poland A., Knutson J.C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu. Rev. Pharmacol. Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 41.Bach D., Sela B.-A. Interaction of the chlorinated hydrocarbon insecticide lindane or DDT with lipids—a differential scanning calorimetry study. Biochem. Pharmacol. 1984;33:2227–2230. doi: 10.1016/0006-2952(84)90659-2. [DOI] [PubMed] [Google Scholar]
- 42.Bagchi D., Bagchi M., Hassoun E.A., Stohs S.J. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995;104:129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez A.F., Gil F., Lacasana M., Rodriguez-Barranco M., Tsatsakis A.M., Requena M., Parron T., Alarcon R. Pesticide exposure and genetic variation in xenobiotic-metabolizing enzymes interact to induce biochemical liver damage. Food Chem. Toxicol. 2013;61:144–151. doi: 10.1016/j.fct.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 44.Fischer J.E. 1996. A 28-day Dietary Toxicity Study in Albino Rats with AC 299, 263. Unpublished Report No. ID420-002 From American Cyanamid Co., Princeton, NJ, USA. Submitted to WHO by BASF Agro SAS, Levallois-perret, France. [Google Scholar]
- 45.Fischer J.E., Hess F.G. 1995. Chronic Dietary Toxicity and Oncogenicity Study with AC 299,263 in the Albino Rat (Volumes I-VI). Unpublished Report No. ID-427-002 From American Cyanamid Co., Princeton, NJ, USA. Submitted to WHO by BASF Agro SAS, Levallois-Perret, France. [Google Scholar]
- 46.U.S. EPA/OPP (U.S. Environmental Protection Agency/Office of Pesticide Programs). Imazamox – Report of the Hazard Identification Assessment Review Committee. Memorandum dated July 11, 2001 from P.V. Shah to William Donovan. OPP Official Record Health Effects Division Scientific Data Reviews EPA Series 361. U.S. EPA, Washington, DC. Copy courtesy of U.S. EPA/OPP. [FOIA01] 2001b.
- 47.J.E. Fischer, Skin Irritation Study in Albino Rabbits with AC 299,263 Technical: Lab Project Number: T-0519. Unpublished study prepared by American Cyanamid Co. MRID: 43193211. (MRID-DER01), 1992b 11.
- 48.Boczon, L., Skin Irritation Study in Albino Rabbits with AC 299,263 1AS Formulation: Lab Project Number: T-0645: A 94-10. Unpublished study prepared by American Cyanamid Co. MRID: 43193217. (MRID-DER01), 1994b 16.
- 49.Wieck S., Olsson O., Kümmerer K. Not only biocidal products: washing and cleaning agents and personal care products can act as further sources of biocidal active substances in wastewater. Environ. Int. 2018;115:247–256. doi: 10.1016/j.envint.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 50.Combrink K.D., Gulgeze H.B., Meanwell N.A., Pearce B.C., Zulan P., Bisacchi G.S., Roberts D.G., Stanley P., Seiler S.M. 1,2-Benzisothiazol-3-one 1,1-dioxide inhibitors of human mast cell tryptase. J. Med. Chem. 1998;41:4854–4860. doi: 10.1021/jm9804580. [DOI] [PubMed] [Google Scholar]
- 51.Chew A.L., Maibach H.I. 1,2-Benzisothiazolin-3-one (Proxel): irritant or allergen? A clinical study and literature review. Contact Derm. 1997;36:131–136. doi: 10.1111/j.1600-0536.1997.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 52.Vicini P., Manotti C., Caretta A., Amoretti L. Comparison of in vitro and ex vivo antiplatelet effects of 1,2-benzisothiazolin-3-one and its 2-amino derivative. ArzneimittelForschung. 1999;49:896–899. doi: 10.1055/s-0031-1300523. [DOI] [PubMed] [Google Scholar]
- 53.Mesnage R., Antoniou M.N. Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Front. Public Health. 2017;5:361. doi: 10.3389/fpubh.2017.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.BASF (BASF the Chemical Company) 2009. Clearcast® Herbicide. Research Triangle Park, North Carolina. [Accessed 9 September 2009] [Google Scholar]
- 55.Odukoya O., Sofidiya M., Ilori O., Gbededo M., Ajadotuigwe J., Olaleye O., Bouskela E., Cyrino F., Marcelon G., Brinkhaus B. Malondialdehyde determination as index of lipid peroxidation. Int. J. Biol. Chem. 1994;3:281–285. [Google Scholar]
- 56.Brattin W.J., Glende E.A., Jr, Recknagel R.O. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J. Free Radic. Biol. Med. 1985;1:27–38. doi: 10.1016/0748-5514(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 57.Nelson S.D. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- 58.Matsumaru K., Ji C., Kaplowitz N. Mechanisms for sensitization to TNF-induced apoptosis by acute glutathione depletion in murine hepatocytes. Hepatology. 2003;37:1425–1434. doi: 10.1053/jhep.2003.50230. [DOI] [PubMed] [Google Scholar]
- 59.Fernandes-Alnemri T., Litwack G., Alnemri E.S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J. Biol. Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- 60.Nicholson D.W., Ali A., Thornberry N.A., Vaillancourt J.P., Ding C.K., Gallant M., Gareau Y., Griffin P.R., Labelle M., Lazebnik Y.A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 61.Hashemzaei M., Barani A.K., Iranshahi M., Rezaee R., Tsarouhas K., Tsatsakis A.M., Wilks M.F., Tabrizian K. Effects of resveratrol on carbon monoxide-induced cardiotoxicity in rats. Environ. Toxicol. Pharmacol. 2016;46:110–115. doi: 10.1016/j.etap.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 62.Ren R., Sun D.J., Yan H., Wu Y.P., Zhang Y. Oral exposure to the herbicide simazine induces mouse spleen immunotoxicity and immune cell apoptosis. Toxicol. Pathol. 2013;41:63–72. doi: 10.1177/0192623312452488. [DOI] [PubMed] [Google Scholar]
- 63.Taylor R.C., Cullen S.P., Martin S.J. Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008;9:231. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 64.Wu Z.M., Wen T., Tan Y.F., Liu Y., Ren F., Wu H. Effects of salvianolic acid a on oxidative stress and liver injury induced by carbon tetrachloride in rats. Basic Clin. Pharmacol. Toxicol. 2007;100:115–120. doi: 10.1111/j.1742-7835.2007.00020.x. [DOI] [PubMed] [Google Scholar]
- 65.Baynes J.W., Thorpe S.R. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 66.Ihara Y., Toyokuni S., Uchida K., Odaka H., Tanaka T., Ikeda H., Hiai H., Seino Y., Yamada Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48:927–932. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- 67.Brereton M.F., Iberl M., Shimomura K., Zhang Q., Adriaenssens A.E., Proks P., Spiliotis I.I., Dace W., Mattis K.K., Ramracheya R. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat. Commun. 2014;5:4639. doi: 10.1038/ncomms5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Favaro E., Miceli I., Bussolati B., Schimitt-Ney M., Perin P.C., Camussi G., Zanone M.M. Hyperglycemia induces apoptosis of human pancreatic islet endothelial cells: effects of pravastatin on the Akt survival pathway. Am. J. Pathol. 2008;173:442–450. doi: 10.2353/ajpath.2008.080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greeley S.A.W., Zielinski M.C., Poudel A., Ye H., Berry S., Taxy J.B., Carmody D., Steiner D.F., Philipson L.H., Wood J.R. Preservation of reduced numbers of insulin-positive cells in sulfonylurea-unresponsive KCNJ11-related diabetes. J. Clin. Endocrinol. Metab. 2016;102:1–5. doi: 10.1210/jc.2016-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tomita T. Apoptosis of pancreatic beta-cells in Type 1 diabetes. Bosn. J. Basic Med. Sci. 2017;17:183–193. doi: 10.17305/bjbms.2017.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan L., Deng Y., Zhou J., Zhao H., Wang G., C.H.-R.F.A.R. Group Serum YKL-40 as a biomarker for liver fibrosis in chronic hepatitis B patients with normal and mildly elevated ALT. Infection. 2018;46:385–393. doi: 10.1007/s15010-018-1136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGovern A.J., Vitkovitsky I.V., Jones D.L., Mullins M.E. Can AST/ALT ratio indicate recovery after acute paracetamol poisoning? Clin. Toxicol. 2015;53:164–167. doi: 10.3109/15563650.2015.1006399. [DOI] [PubMed] [Google Scholar]
- 73.Hu R., Huang X., Huang J., Li Y., Zhang C., Yin Y., Chen Z., Jin Y., Cai J., Cui F. Long- and short-term health effects of pesticide exposure: a cohort study from China. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quesada I., Tudurí E., Ripoll C., Nadal Á. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. J. Endocrinol. 2008;199:5–19. doi: 10.1677/JOE-08-0290. [DOI] [PubMed] [Google Scholar]
- 75.Tizhe E., Ibrahim N., Fatihu M., Ambali S., Igbokwe I., Tizhe U. Pancreatic function and histoarchitecture in Wistar rats following chronic exposure to Bushfire®: the mitigating role of zinc. J. Int. Med. Res. 2018;46:3296–3305. doi: 10.1177/0300060518778640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.KanchanaN S.P. Serum calcium levels in type 2 Diabetes Mellitus. IOSR-JDMS. 2014;13:01–03. [Google Scholar]
- 77.Tsatsakis A.M., Docea A.O., Tsitsimpikou C. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi-chemicals’ low dose exposure. Food Chem. Toxicol. 2016;96:174–176. doi: 10.1016/j.fct.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 78.Tsatsakis A.M., Kouretas D., Tzatzarakis M.N., Stivaktakis P., Tsarouhas K., Golokhvast K.S., Rakitskii V.N., Tutelyan V.A., Hernandez A.F., Rezaee R., Chung G., Fenga C., Engin A.B., Neagu M., Arsene A.L., Docea A.O., Gofita E., Calina D., Taitzoglou I., Liesivuori J., Hayes A.W., Gutnikov S., Tsitsimpikou C. Simulating real-life exposures to uncover possible risks to human health: a proposed consensus for a novel methodological approach. Hum. Exp. Toxicol. 2017;36:554–564. doi: 10.1177/0960327116681652. [DOI] [PubMed] [Google Scholar]
- 79.Docea A.O., Gofita E., Goumenou M., Calina D., Rogoveanu O., Varut M., Olaru C., Kerasioti E., Fountoucidou P., Taitzoglou I., Zlatian O., Rakitskii V.N., Hernandez A.F., Kouretas D., Tsatsakis A. Six months exposure to a real life mixture of 13 chemicals’ below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats. Food Chem. Toxicol. 2018;115:470–481. doi: 10.1016/j.fct.2018.03.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.