Abstract
Purpose
The aim of this study was undertaken to investigate the association of 78-kDa glucose-regulated protein (GRP78) gene promoter polymorphisms with risk of asthenozoospermia (AZS) men. In addition, we performed association analysis between GRP78 promoter mutations and serum GRP78 level in asthenozoospermia.
Methods
The study population comprised 400 subjects with AZS patients and 400 healthy controls. We assessed GRP78 rs3216733, rs17840761, and rs17840762 polymorphisms by using Snapshot SNP genotyping assays; serum GRP78 level was measured by enzyme-linked immunosorbent assay (ELISA). Semen quality was assessed by computer-assisted semen analysis.
Results
We found that rs3216733 was associated with increased risk of AZS (Gd vs. dd: adjusted OR = 1.42, 95% CI, 1.06–1.93, P = 0.020; Gd/GG vs. dd: adjusted OR = 1.43, 95% CI, 1.08–1.91, P = 0.013; G vs. d adjusted OR = 1.26, 95% CI, 1.03–1.56, P = 0.027). The haplotype analyses showed the frequency of G-C-C haplotype was significantly higher in AZS (P = 0.026). The percentage of progressive motility sperm was lower in the asthenozoospermic men with Gd and Gd/GG genotypes than dd genotype (P = 0.003). Moreover, the serum GRP78 levels were significantly lower in rs3216733 Gd/GG genotypes compared with the dd genotype (P < 0.001).
Conclusion
Our findings suggest that rs3216733 Gd/GG genotypes contribute to poor sperm motility, probably by decreasing the level of GRP78.
Keywords: GRP78/HSPA5 gene, Promoter polymorphisms, Asthenozoospermia, Single nucleotide polymorphism
Introduction
Human infertility is a universal and difficult problem which affects about 15% of couples in the world. Among the many aspects, the male factor is considered to play a critical role in infertility [1, 2]. Up to now, the etiology of infertile men is complex, exhibiting a high incidence of anatomic defects, molecular genetics disorders, urogenital tract infections, endocrine disturbances, genetic variations, or environmental toxins [3–9]. Semen quality analysis is an important reference data for infertile man’s evaluation as it provides information on sperm morphology, concentration, and motility [10]. Asthenozoospermia, characterized by poor sperm motility, is considered as an important factor of infertile men [11]. The exact pathogenesis of asthenozoospermia (AZS) is unclear. Relevant studies reported that the etiology of AZS is of functional defects (epididymitis, varicocele, orchitis) or an array of biochemical and genetic factors (genes variation, chromosomal abnormalities, Y microdeletions) [12–14]. Recently, the relationship between gene variation and AZS became a hot topic. Zhang (2016) analyzed 104 idiopathic asthenozoospermia and found a significant difference in the missense mutation frequency in the tektin-t gene and predicted that this mutation may be a possible risk for AZS [2]. Zhang (2016) reported that the incidence of the missense PATE1 gene mutation increases in AZS patients (72.22%) compared with the controls (52.83%) [15].
GRP78 (78 kDa glucose-regulated protein), also known as heat shock 70 kDa protein 5 (HSPA5) or immunoglobulin heavy chain-binding protein (BiP), is a member of the heat-shock protein 70 family and an important molecular chaperone of the endoplasmic reticulum (ER), involved in the folding and assembly of proteins [16]. Previous studies showed that in addition to being observed in human epididymal epithelium and testis spermatogenic cells, GRP78 protein persists in the neck region of ejaculated spermatozoa [17]. GRP78 protein modulates the binding interaction of sperm and zona pellucida though a calcium-dependent pathway, which can affect capacitation of human sperm [18, 19]. However, no studies have been reported on whether the GRP78 protein and its gene polymorphisms are involved in sperm motility. In this study, we investigated GRP78 promoter mutations and associated these with human sperm motility in asthenozoospermia.
Materials and methods
Subjects
In this case-control study, we recruited 400 subjects with AZS from the department of Reproduction and Genetics at the Affiliated Hospital of Youjiang Medical University for Nationalities (Guangxi, China). The patients with sperm morphology (> 4.0%), sperm concentration (≥ 15 × 106 spermatozoa/ml), leukocytospermia (< 1 × 106/ml), sperm viability (≥ 40%), forward progressive motility (< 32%), and negative for anti-sperm antibodies, who had been unable to accomplish pregnancy for at least 18 months of unprotected sexual intercourse, were participated in this study. Exclusion criteria included endocrine hypogonadism, Y chromosome microdeletions, karyotype anomalies, obstruction of the vas deferens, varicocele, cryptorchidism, orchitis, epididymitis and history of genital tract infection, and drug treatment. The 400 controls were recruited from the general population in the area of south China (Guangxi). The age-matched controls were fertility men who had fathered at least one child within the previous 2 years without using assisted reproduction. The routine sperm parameters in the control groups were normal following the World Health Organization (WHO) manuals (5th edition). Each subject was given a questionnaire to record information about his lifestyle, reproductive history, alcohol and cigarette smoking habits, medical history, and exposures. We could eliminate as much as possible the subjects of systemic diseases (hypertension, hypercholesterolemia, and diabetes mellitus), autoimmune diseases, and cancers.
All subjects carried out three complete semen analyses by a computer-assisted sperm analysis system (SCA5.0; Spain) after 2–7 days of sexual abstinence. Detailed characteristics are shown in Table 1. The study received the approval of the ethics committee of the Affiliated Hospital of Youjiang Medical University for Nationalities, and the informed consent was obtained from each subject. The study protocol followed the ethical principles of medical research involving human subjects in the Helsinki declaration.
Table 1.
Demographic characteristics and semen parameters of study subjects
| Sample parameters | Asthenozoospermic group (N = 400) | Control group (N = 400) | P value |
|---|---|---|---|
| Age (years) | 33.03 ± 6.8 | 32.29 ± 6.5 | 0.119 |
| pH value of semen | 7.35 ± 0.14 | 7.34 ± 0.15 | 0.831 |
| Ejaculate volume (mL) | 3.47 ± 1.39 | 3.25 ± 1.38 | 0.423 |
| Sperm concentration (× 106/mL) | 49.9 ± 20.7 | 50.3 ± 25.9 | 0.838 |
| Progressive motility (%) | 20.09 ± 8.18 | 57.16 ± 13.45 | < 0.001 |
| Curvilinear velocity (μm/s) | 32.03 ± 7.09 | 51.40 ± 8.88 | < 0.001 |
| Straight linear velocity (μm/s) | 12.71 ± 4.71 | 23.57 ± 5.66 | < 0.001 |
| Linearity (%) | 38.93 ± 9.03 | 45.86 ± 9.10 | < 0.001 |
| Smoking | |||
| Yes | 121 (30.3) | 137 (34.3) | 0.226 |
| No | 279 (69.7) | 263 (65.7) | |
| Drinking | |||
| Yes | 195 (48.8) | 181 (45.2) | 0.321 |
| No | 205 (51.2) | 219 (54.8) | |
| Sleeping status (day) | |||
| ≤ 8 h | 212 (53.0) | 193 (48.2) | 0.179 |
| > 8 h | 188 (47.0) | 207 (51.8) | |
SNP selection, DNA extraction and genotyping
The selection criteria of SNPs in GRP78 are as below: Firstly, we conducted a search for all SNPs of the promoter in the GRP78 gene from the international HapMap project databank (www.hapmap.org/) and the National Center for Biotechnology Information (NCBI). According to the above result, we selected SNPs that the frequency of the minor allele (MAF) outweighs 5% within Han Chinese. Secondly, Haploview software was used to pick tagging SNPs (r2 > 0.08) that can represent other SNPs in the same region of GPR78 gene. Thirdly, we examined previous papers that were executed to study the association between diseases and polymorphism in GRP78 gene promoter. Finally, we selected three eligible SNPs for genotyping (rs3216733, rs17840761, and rs17840762). Genomic DNA was extracted from blood leukocytes by using a whole-blood genome DNA isolation kit (Tianjin Inc., Beijing, China). Genotyping reactions were carried out using the multiple single nucleotide primer extension technique. The primers for PCR amplification were designed using online primer 3.0 (http://primer3.ut.ee/) (Table 2). All the resultant band lengths of the PCR products in this study have a length of 369 bp.
Table 2.
The primer sequences for the promoter region of the GRP78 gene
| SNP ID | Primer sequences |
|---|---|
| rs3216733 | F: 5′-CCCCTCCGCAATAAACGTCACTG-3′ |
| R: 5′-GCATCTAAGCTGCGACTGGTCTAC-3′ | |
| E: 5′-TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTCAATGAATCAGCTGGGGGGG-3′ | |
| rs17840761 | F: 5′-CCCCTCCGCAATAAACGTCACTG-3′ |
| R: 5′-GCATCTAAGCTGCGACTGGTCTAC-3′ | |
| E: 5′-CCCTAGGGGGTCGGAGTAG-3′ | |
| rs17840762 | F: 5′-CCCCTCCGCAATAAACGTCACTG-3′ |
| R: 5′-GCATCTAAGCTGCGACTGGTCTAC-3′ | |
| E: 5′-TTTTTTTTTTTTTTTTTTTTTTGAGTGACCCCCCGGGGCTG-′ |
F forward primer, R reverse primer, E extension primer
ELISA
Venous blood from the patients and healthy controls was clotted for 2 h at room temperature or overnight at 4 °C by serum separator tube before centrifugation for 20 min at approximately 1000×g and collected the supernatant at − 80 °C until use. The concentration of serum GRP78 levels was measured by enzyme-linked immunosorbent assay (ELISA) kits (LifeSpan BioSciences, Inc., USA; Catalog No. LS-F6280) following reagent manufacturer’s instructions. The optical density (OD) of the well is measured at a wavelength of 450 nm ± 2 nm (RT-6000, China). Each sample was analyzed in duplicate. The concentration of serum GRP78 levels was performed by a standard curve constructed with the kit’s standards.
Statistical analysis
The distribution of genotypes Hardy-Weinberg equilibrium was calculated by the chi-squared test between case and control subjects. The unconditional logistic regression was applied to estimate Odds ratios (ORs) and corresponding 95% confidence intervals (CIs). The online SHEsis software (http://analysis.bio-x.cn/myAnalysis. php) was used to analyze the haplotype analysis. Differences of variables were assessed by an unpaired t test. Statistical analysis of all data was performed using SPSS 17.0 software package (SPSS Inc., Chicago, USA). P < 0.05 was considered as statistically significant.
Results
Clinical characteristics of the study subjects
Table 1 compares the demographic and clinical characteristics of the study population. There was no significant difference between the AZS patients and controls regarding age, smoking status, alcohol consumption, sleeping status, and some semen parameters (pH, volume, concentration). However, the sperm motion kinetic parameters of the patients with AZS were significantly lower than those in the controls (the progressive motility, curvilinear velocity, straight linear velocity, and linearity, all P < 0.001).
Polymorphisms of GRP78 promoter and the risk of AZS
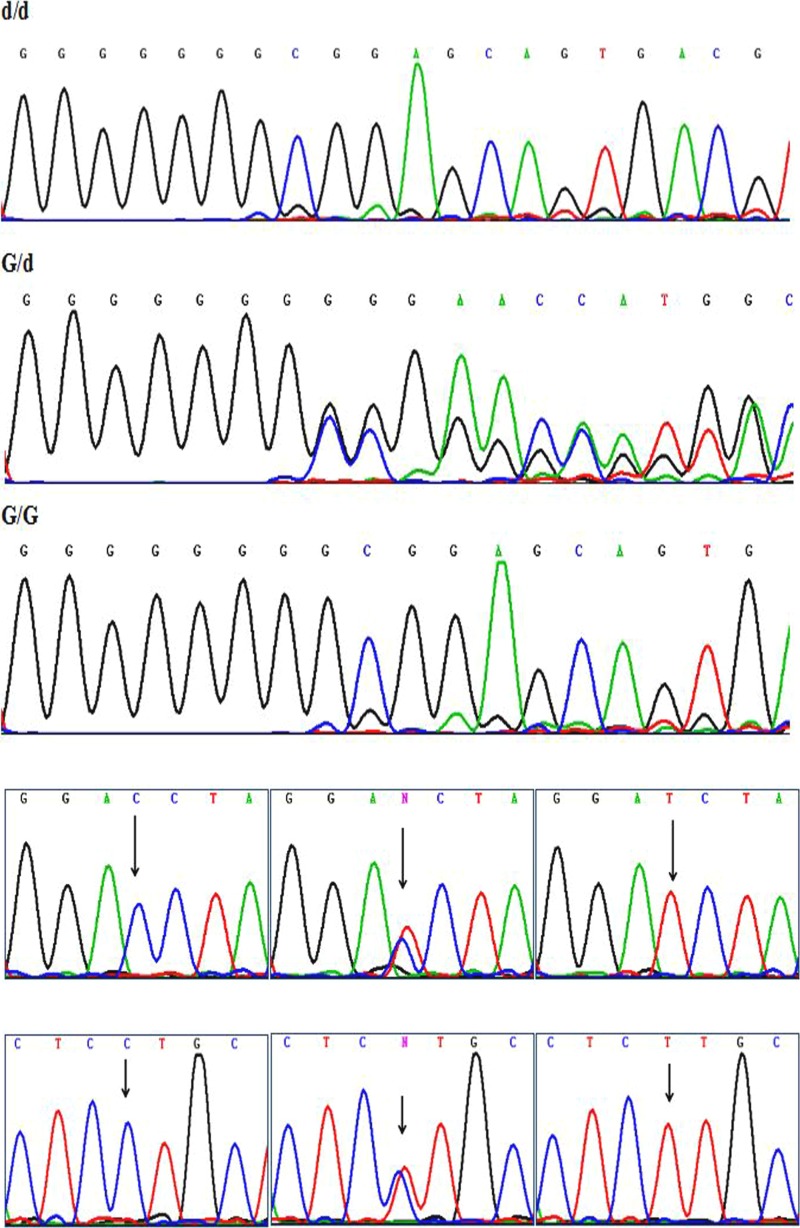
All of the SNPs have three genotypes respectively (Fig. 1). The genotype and allele frequencies for the GRP78 promoter polymorphism between asthenozoospermia and controls are shown in Table 3. The frequencies of the dd, Gd, GG genotypes and d, G alleles of rs3216733 polymorphism in the controls are 46.5%, 43.7%, 9.8% and 68.4%, 31.6% as against 38.0%, 49.8%, 12.2% and 62.9%, 37.1% in asthenozoospermia respectively. The frequencies of the CC, CT, TT genotypes and C, T alleles of rs17840761 polymorphism in the controls are 27.3%, 45.2%, 27.5% and 49.9%, 50.1% as against 28.0%, 47.5%, 24.5% and 51.8%, 48.2% in asthenozoospermia respectively. The frequencies of the CC, CT, TT genotypes and C, T alleles of rs17840762 polymorphism in the controls are 74.3%, 23.0%, 2.7% and 85.7%, 14.3% as against 75.0%, 23.5%, 1.5% and 86.8%, 13.2% in asthenozoospermia respectively. The Hardy-Weinberg equilibrium P value in two groups was > 0.05. In rs3216733, the Gd and Gd/GG genotypes were associated with increased risk of AZS (Gd vs. dd: adjusted OR = 1.42, 95% CI, 1.06–1.93, P = 0.020; Gd/GG vs. dd: adjusted OR = 1.43, 95% CI, 1.08–1.91, P = 0.013). Compared with the rs3216733 d allele, the rs3216733 G allele was significantly associated with an increased risk of AZS (OR = 1.26, 95% CI, 1.03–1.56, P = 0.027). The genotype and allele of rs17840761 and rs17840762 polymorphisms showed no significant differences in the two study groups (P > 0.05). By haplotype analyses, the G-C-C haplotype was 33.9 and 29.2% in both cases and controls, respectively. Compared with the controls, the G-C-C haplotype was significantly increased risk of AZS (P = 0.026) (Table 4).
Fig. 1.
Sequencing map of genotypes for GRP78 gene polymorphisms. Sequencing map showed dd, Gd, and GG genotypes for rs3216733; CC, CT, and TT genotypes for rs17840761; CC, CT, and TT genotypes for rs17840762, respectively. (From top to bottom)
Table 3.
Genotype and allele frequencies for the GRP78 promoter polymorphism between asthenozoospermia and controls
| Genotype/allele | Asthenozoospermic group (%) | Control group (%) | OR (95% CI)* | P value* |
|---|---|---|---|---|
| rs3216733 | ||||
| dd | 152 (38.0) | 186 (46.5) | 1.00 | |
| Gd | 199 (49.8) | 175 (43.7) | 1.42 (1.06–1.93) | 0.020 |
| GG | 49 (12.2) | 39 (9.8) | 1.45 (0.91–2.35) | 0.120 |
| Gd/GG | 248 (62.0) | 214 (53.5) | 1.43 (1.08–1.91) | 0.013 |
| d | 503 (62.9) | 547 (68.4) | 1.00 | |
| G | 297 (37.1) | 253 (31.6) | 1.26 (1.03–1.56) | 0.027 |
| rs17840761 | ||||
| CC | 112 (28.0) | 109 (27.3) | 1.00 | |
| CT | 190 (47.5) | 181 (45.2) | 1.05 (0.75–1.46) | 0.797 |
| TT | 98 (24.5) | 110 (27.5) | 0.86 (0.59–1.27) | 0.447 |
| CT/TT | 288 (72.0) | 291 (72.7) | 0.98 (0.71–1.34) | 0.880 |
| C | 414 (51.8) | 399 (49.9) | 1.00 | |
| T | 386 (48.2) | 401 (50.1) | 0.93 (0.76–1.13) | 0.443 |
| rs17840762 | ||||
| C/C | 300 (75.0) | 297 (74.3) | 1.00 | |
| C/T | 94 (23.5) | 92 (23.0) | 1.03 (0.74–1.44) | 0.864 |
| T/T | 6 (1.5) | 11 (2.7) | 0.51 (0.19–1.41) | 0.195 |
| CT/TT | 100 (25.0) | 103 (25.7) | 0.97 (0.70–1.34) | 0.866 |
| C | 394 (86.8) | 686 (85.7) | 1.00 | |
| T | 106 (13.2) | 114 (14.3) | 0.92 (0.69–1.23) | 0.583 |
SNPs single nucleotide polymorphisms, d deleted, OR odds ratio, CI confidence interval, dd deletion
*Adjusted for age, sex, smoking, drinking, and sleeping statuses by the logistic regression model
Table 4.
Haplotype distributions for the GRP78 promoter polymorphism in asthenozoospermia and controls
| Haplotype | Asthenozoospermic group (freq) 2n = 800 (%) | Control group (freq) 2n = 800 (%) | OR(95% CI) | P value |
|---|---|---|---|---|
| d-T-C* | 363 (45.4) | 384 (48.1) | 0.93 (0.76–1.13) | 0.446 |
| G-C-C* | 271 (33.9) | 234 (29.2) | 1.27 (1.03–1.57) | 0.026 |
| d-C-T* | 91 (11.4) | 111 (13.9) | 0.81 (0.60–1.09) | 0.158 |
| d-C-C* | 45 (5.6) | 51 (6.4) | 0.88 (0.58–1.33) | 0.543 |
| G-T-C | 15 (1.8) | 17 (2.1) | 0.90 (0.44–1.83) | 0.769 |
| G-C-T | 7 (0.9) | 3 (0.3) | – | – |
| G-T-T | 4 (0.5) | 0 (0.0) | – | – |
| d-T-T | 3 (0.4) | 0 (0.0) | – | – |
Major haplotypes—Haplotype frequencies less than 1% were not included in the statistical analysis
OR odds ratio, CI confidence interval
Sperm motion kinetics in asthenozoospermic and controls
We compared the association of sperm motion kinetic parameters (progressive motility, curvilinear velocity, straight linear velocity, and linearity) in the AZS patients and controls with different rs3216733 genotypes (Table 5). In the AZS patients, the sperm kinetic parameters were significantly lower in the Gd genotype vs. dd genotype with respect to progressive motility (P = 0.003), curvilinear velocity (P = 0.009), straight linear velocity (P = 0.004), and linearity (P = 0.010). Moreover, the patients with the Gd/GG genotypes had significantly lower sperm motion kinetics than the dd genotype with respect to progressive motility (P = 0.003), curvilinear velocity (P = 0.028), straight linear velocity (P = 0.019), and linearity (P = 0.014). Nevertheless, we failed to find the association of sperm motion kinetic parameters in controls with different rs3216733 genotypes (P > 0.05).
Table 5.
Comparison of sperm motion kinetics parameters in the polymorphism of rs3216733 among asthenozoospermic men and healthy controls
| Genotypes | Sperm motion kinetics parameters | |||
|---|---|---|---|---|
| Progressive motility (%) | Curvilinear Velocity, um/s | Straight Linear Velocity, um/s | Linearity, % | |
| Case subjects | ||||
| dd (n = 152) | 21.56 ± 6.56 | 33.03 ± 6.01 | 13.43 ± 4.13 | 40.34 ± 8.92 |
| Gd (n = 199) | 19.06 ± 9.31 | 31.14 ± 7.39 | 12.00 ± 4.85 | 37.82 ± 9.05 |
| t value | 2.948 | 2.633 | 2.898 | 2.595 |
| P value | 0.003 | 0.009 | 0.004 | 0.010 |
| dd (n = 152) | 21.56 ± 6.56 | 33.03 ± 6.01 | 13.43 ± 4.13 | 40.34 ± 8.92 |
| GG(n = 49) | 19.78 ± 7.05 | 32.55 ± 8.51 | 13.42 ± 5.43 | 39.01 ± 8.90 |
| t value | 1.618 | 0.431 | − 0.002 | 0.906 |
| P value | 0.107 | 0.667 | 0.998 | 0.336 |
| dd (n = 152) | 21.56 ± 6.56 | 33.03 ± 6.01 | 13.43 ± 4.13 | 40.34 ± 8.92 |
| Gd/GG (n = 248) | 19.21 ± 8.91 | 31.42 ± 7.64 | 12.28 ± 4.99 | 38.06 ± 9.01 |
| t value | 3.035 | 2.208 | 2.363 | 2.465 |
| P value | 0.003 | 0.028 | 0.019 | 0.014 |
| Control subjects | ||||
| dd (n = 186) | 57.72 ± 13.54 | 51.48 ± 9.61 | 23.41 ± 5.72 | 45.51 ± 8.66 |
| Gd (n = 175) | 56.45 ± 13.69 | 51.57 ± 8.16 | 23.85 ± 5.70 | 46.12 ± 9.42 |
| t value | 0.882 | − 0.099 | − 0.746 | − 0.645 |
| P value | 0.378 | 0.921 | 0.456 | 0.520 |
| dd (n = 186) | 57.72 ± 13.54 | 51.48 ± 9.61 | 23.41 ± 5.72 | 45.51 ± 8.66 |
| GG(n = 39) | 57.69 ± 12.04 | 50.28 ± 8.57 | 23.11 ± 5.30 | 46.42 ± 9.82 |
| t value | 0.011 | 0.718 | 0.298 | − 0.584 |
| P value | 0.991 | 0.473 | 0.766 | 0.560 |
| dd (n = 186) | 57.72 ± 13.54 | 51.48 ± 9.61 | 23.41 ± 5.72 | 45.51 ± 8.66 |
| Gd/GG (n = 214) | 56.68 ± 13.89 | 51.34 ± 8.23 | 23.71 ± 5.62 | 46.17 ± 9.47 |
| t value | 0.770 | 0.158 | − 0.550 | − 0.732 |
| P value | 0.442 | 0.874 | 0.582 | 0.465 |
Association of polymorphisms and serum GRP78 level
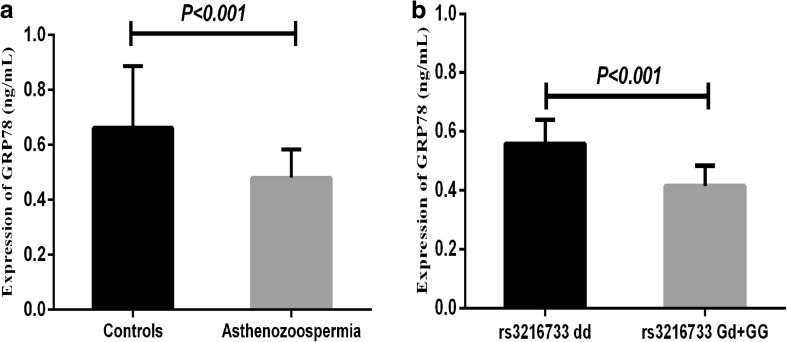
The concentration of GRP78 in the serum of AZS patients (0.479 ± 0.104 ng/mL) was significantly lower than that in controls (0.661 ± 0.225 ng/mL, P < 0.001; Fig. 2a). Besides, we also found that patients carrying rs3216733 Gd/GG genotypes (0.414 ± 0.069 ng/mL) had lower levels of GRP78 than carrying dd genotype (0.558 ± 0.082 ng/mL, P < 0.001; Fig. 2b). However, there was no relationship between serum GRP78 level and rs17840761, rs17840762 (P > 0.05, data not shown).
Fig. 2.
ELISA detection of GRP78 expression. a Serum level of GRP78 in patients with asthenozoospermia and controls. b The serum GRP78 level of rs3216733 Gd/GG genotype and dd genotype in asthenozoospermia patients
Discussion
In our study, we analyze the possible association of GRP78 gene polymorphisms (rs3216733, rs17840761 and rs17840762) with the risk of developing AZS. The results indicated that rs3216733 was associated with an increased risk of AZS in Gd genotype, Gd/GG genotype, G allele, and G-C-C haplotype. Moreover, we found that there was a significant correlation between the rs3216733 polymorphism and sperm motion kinetics in AZS patients. Patients with Gd and Gd/GG genotype had significantly lower values of progressive motility, curvilinear velocity, straight linear velocity, and linearity than patients with a dd genotype. Interestingly, the serum GRP78 levels were significantly lower in individuals with rs3216733 Gd/GG genotype than carrying dd genotype. These findings suggest that Gd/GG genotype in rs3216733 polymorphism may exert influences on the sperm motility and serum GRP78 expression in asthenozoospermia.
The GRP78 protein, located on chromosome 9q33-34 in humans, plays a key role in protein transport, folding, and assembly [20]. The core-promoter region of GRP78 has described in 2.1 kB upstream of exon 1 including rs3216733 (− 180 bp), rs17840762 (− 370 bp), and rs17840761 (− 378 bp), which involved in the binding of some transcription factors [21]. A functional study showed that rs391957/rs3216733 polymorphism alters basal promoter activity [22]. We found similar findings regarding the association of the GRP78 promoter polymorphism with the risk of several common diseases when we looked up the relevant reports of GRP78 polymorphism in recent years. Winder (2011) showed that gastric adenocarcinoma (GA) patients with rs391957 CT or TT genotypes were associated with an increased the risk of tumor recurrence and death than those with CC genotype [23]. Zhu (2013) reported that the rs391957 allele G was associated with higher levels of GRP78 mRNA and protein in hepatocellular carcinoma (HCC) and probably regulated the expression of GRP78 by providing an Ets-2 binding site [24]. Jia (2015) indicated that rs391957 polymorphism was an independent risk factor in the pathogenesis of type 2 diabetic peripheral neuropathy [25]. These reports support our hypothesis that rs3216733 is associated with the risk of AZS. In addition, Lobo (2015) found sperm GRP78 phosphorylation occurred spatial reorganization during the maturation of the epididymis, and compared with normozoosperm, phosphorylated GRP78 decreased over 2-fold in asthenozoosperm [26]. Shen (2013) showed that the GRP78 protein is differentially expressed between asthenozoosperm and normal individuals, suggesting that post-translational cutting and splicing play an important role in sperm motility [27]. Consistent with these results, we found that AZS patients with Gd/GG genotype have significantly lower values of sperm motion kinetics parameters, probably by the Gd/GG genotype that plays a key role in downregulating the expression of serum GRP78.
Previously, it was reported that the polymorphisms of rs17840761 and rs17840762 were not associated with gastric and cancer, chronic HBV infection, and Parkinson’s disease [23, 28, 29]. Obviously, these results are consistent with our results that the polymorphisms of rs17840761 and rs17840762 are not associated with the risk of AZS. However, we found that the G-C-C haplotype was significantly increased risk of AZS (P = 0.026) by haplotype analysis. Some possibilities should be considered to explain these issues. SNPs are the preferred marker for elucidating the relationship between genetic variation and disease susceptibility. However, a single SNP analysis usually provides limited information. Factors affecting the disease may exist within or near the haplotypes, so a better approach may be to analyze the haplotypes derived from SNPs [30–33].
The results of this study provide important information for GRP78 gene and the susceptibility of asthenozoospermia. rs3216733 may alter transcriptional activity and affect the differential expression of serum GRP78 protein resulting in an increased risk of asthenozoospermia. However, the limitations of this study should be pointed out. Firstly, this study only explored the polymorphism of GRP78 promoter region. Secondly, our study was limited to the subjects of Chinese population, and it was unclear whether the results were consistent with those in other populations. Therefore, further functional studies are necessary to clarify the exact role of GRP78 gene in asthenozoospermia pathogenesis.
Funding information
This work was supported by grants from the National Natural Science Foundation of China (No. 81560461), Research Key Laboratory of the Right River Valley Characteristics of Guangxi University (kfkt2017022).
Compliance with ethical standards
This study was approved by the ethics review board of the Affiliated Hospital of Youjiang Medical University for Nationalities, consistent with provisions of the Declaration of Helsinki. Voluntary written informed consent was obtained.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.de Kretser DM. Male infertility. Lancet. 1997;349(9054):787–790. doi: 10.1016/S0140-6736(96)08341-9. [DOI] [PubMed] [Google Scholar]
- 2.Zhang SH, Zhang JH, Ding XP, Zhang S, Chen HH, Jing YL. Association of polymorphisms in tektin-t gene with idiopathic asthenozoospermia in Sichuan, China. J Assist Reprod Genet. 2016;33(2):181–187. doi: 10.1007/s10815-015-0617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song P, Zou S, Chen T, Chen J, Wang Y, Yang J, Song Z, Jiang H, Shi H, Huang Y, Li Z, Shi Y, Hu H. Endothelial nitric oxide synthase (eNOS) T-786C, 4a4b, and G894T polymorphisms and male infertility: study for idiopathic asthenozoospermia and meta-analysis. Biol Reprod. 2015;92(2):38. doi: 10.1095/biolreprod.114.123240. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Sha YW, Mei LB, Ji ZY, Qiu PP, Ji H, et al. A familial study of twins with severe asthenozoospermia identified a homozygous SPAG17 mutation by whole-exome sequencing. Clin Genet. 2018;93(2):345–349. doi: 10.1111/cge.13059. [DOI] [PubMed] [Google Scholar]
- 5.Bracke A, Peeters K, Punjabi U, Hoogewijs D, Dewilde S. A search for molecular mechanisms underlying male idiopathic infertility. Reprod BioMed Online. 2018;36(3):327–339. doi: 10.1016/j.rbmo.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Yu B, Chen J, Liu D, Zhou H, Xiao W, Xia X, Huang Z. Cigarette smoking is associated with human semen quality in synergy with functional NRF2 polymorphisms. Biol Reprod. 2013;89(1):5. doi: 10.1095/biolreprod.113.109389. [DOI] [PubMed] [Google Scholar]
- 7.Gaskins AJ, Chavarro JE. Diet and fertility: a review. Am J Obstet Gynecol. 2018;218(4):379–389. doi: 10.1016/j.ajog.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vigano P, Chiaffarino F, Bonzi V, Salonia A, Ricci E, Papaleo E, et al. Sleep disturbances and semen quality in an Italian cross sectional study. Basic Clin Androl. 2017;27:16. doi: 10.1186/s12610-017-0060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahantigh D, Hosseinzadeh Colagar A, Salimi S. Genetic polymorphisms and haplotypes of the DJ-1 gene promoter associated with the susceptibility to male infertility. J Assist Reprod Genet. 2017;34(12):1673–1682. doi: 10.1007/s10815-017-1033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franken DR, Oehninger S. Semen analysis and sperm function testing. Asian J Androl. 2012;14(1):6–13. doi: 10.1038/aja.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu FJ, Liu X, Han JL, Wang YW, Jin SH, Liu XX, et al. Aged men share the sperm protein PATE1 defect with young asthenozoospermia patients. Hum Reprod. 2015;30(4):861–869. doi: 10.1093/humrep/dev003. [DOI] [PubMed] [Google Scholar]
- 12.Zuccarello D, Ferlin A, Garolla A, Pati MA, Moretti A, Cazzadore C, et al. A possible association of a human tektin-t gene mutation (A229V) with isolated non-syndromic asthenozoospermia: case report. Hum Reprod. 2008;23(4):996–1001. doi: 10.1093/humrep/dem400. [DOI] [PubMed] [Google Scholar]
- 13.Navarro-Costa P, Plancha CE, Goncalves J. Genetic dissection of the AZF regions of the human Y chromosome: thriller or filler for male (in)fertility? J Biomed Biotechnol. 2010;2010:936569. doi: 10.1155/2010/936569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAuliffe ME, Williams PL, Korrick SA, Dadd R, Perry MJ. The association between sperm sex chromosome disomy and semen concentration, motility and morphology. Hum Reprod. 2012;27(10):2918–2926. doi: 10.1093/humrep/des302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Wang QM, Ding XP, Wang T, Mu XM, Chen ZY. Association of polymorphisms in PATE1 gene with idiopathic asthenozoospermia in Sichuan, China. J Reprod Immunol. 2016;118:54–60. doi: 10.1016/j.jri.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35(4):373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Lachance C, Fortier M, Thimon V, Sullivan R, Bailey JL, Leclerc P. Localization of Hsp60 and Grp78 in the human testis, epididymis and mature spermatozoa. Int J Androl. 2010;33(1):33–44. doi: 10.1111/j.1365-2605.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 18.Lachance C, Bailey JL, Leclerc P. Expression of Hsp60 and Grp78 in the human endometrium and oviduct, and their effect on sperm functions. Hum Reprod. 2007;22(10):2606–2614. doi: 10.1093/humrep/dem242. [DOI] [PubMed] [Google Scholar]
- 19.Marin-Briggiler CI, Gonzalez-Echeverria MF, Munuce MJ, Ghersevich S, Caille AM, Hellman U, et al. Glucose-regulated protein 78 (Grp78/BiP) is secreted by human oviduct epithelial cells and the recombinant protein modulates sperm-zona pellucida binding. Fertil Steril. 2010;93(5):1574–1584. doi: 10.1016/j.fertnstert.2008.12.132. [DOI] [PubMed] [Google Scholar]
- 20.Endo S, Hiramatsu N, Hayakawa K, Okamura M, Kasai A, Tagawa Y, Sawada N, Yao J, Kitamura M. Geranylgeranylacetone, an inducer of the 70-kDa heat shock protein (HSP70), elicits unfolded protein response and coordinates cellular fate independently of HSP70. Mol Pharmacol. 2007;72(5):1337–1348. doi: 10.1124/mol.107.039164. [DOI] [PubMed] [Google Scholar]
- 21.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99(3):275–282. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 22.Hsu WC, Wang HK, Lee LC, Fung HC, Lin JC, Hsu HP, et al. Promoter polymorphisms modulating HSPA5 expression may increase susceptibility to Taiwanese Alzheimer’s disease. J Neural Transm (Vienna) 2008;115(11):1537–1543. doi: 10.1007/s00702-008-0117-5. [DOI] [PubMed] [Google Scholar]
- 23.Winder T, Bohanes P, Zhang W, Yang D, Power DG, Ning Y, Gerger A, Wilson PM, Tang LH, Shah M, Lee AS, Lenz HJ. GRP78 promoter polymorphism rs391957 as potential predictor for clinical outcome in gastric and colorectal cancer patients. Ann Oncol. 2011;22(11):2431–2439. doi: 10.1093/annonc/mdq771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu X, Zhang J, Fan W, Wang F, Yao H, Wang Z, Hou S, Tian Y, Fu W, Xie D, Zhu W, Long J, Wu L, Zheng X, Kung H, Zhou K, Lin MCM, Luo H, Li D. The rs391957 variant cis-regulating oncogene GRP78 expression contributes to the risk of hepatocellular carcinoma. Carcinogenesis. 2013;34(6):1273–1280. doi: 10.1093/carcin/bgt061. [DOI] [PubMed] [Google Scholar]
- 25.Jia Y, Tong Y, Min L. Significance of functional GRP78 polymorphisms in predicting the onset of type 2 diabetic peripheral neuropathy in Chinese population. Neurol Res. 2015;37(8):683–687. doi: 10.1179/1743132815Y.0000000054. [DOI] [PubMed] [Google Scholar]
- 26.Lobo V, Rao P, Gajbhiye R, Kulkarni V, Parte P. Glucose regulated protein 78 phosphorylation in sperm undergoes dynamic changes during maturation. PLoS One. 2015;10(11):e0141858. doi: 10.1371/journal.pone.0141858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen S, Wang J, Liang J, He D. Comparative proteomic study between human normal motility sperm and idiopathic asthenozoospermia. World J Urol. 2013;31(6):1395–1401. doi: 10.1007/s00345-013-1023-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X, Li DP, Fan WG, Lin MC, Wang JL, Lin SQ, et al. Lack of association between the GRP78 polymorphisms in the promoter and 3’ UTR and susceptibility to chronic HBV infection in a Chinese Han population. BMC Med Genet. 2010;11:83. doi: 10.1186/1471-2350-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CM, Wu YR, Hu FJ, Chen YC, Chuang TJ, Cheng YF, Lee-Chen GJ. HSPA5 promoter polymorphisms and risk of Parkinson’s disease in Taiwan. Neurosci Lett. 2008;435(3):219–222. doi: 10.1016/j.neulet.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 30.Daly MJ, Rioux JD, Schaffner SF, Hudson TJ, Lander ES. High-resolution haplotype structure in the human genome. Nat Genet. 2001;29(2):229–232. doi: 10.1038/ng1001-229. [DOI] [PubMed] [Google Scholar]
- 31.Meinderts SM, Sins JWR, Fijnvandraat K, Nagelkerke SQ, Geissler J, Tanck MW, Bruggeman C, Biemond BJ, Rijneveld AW, Kerkhoffs JLH, Pakdaman S, Habibi A, van Bruggen R, Kuijpers TW, Pirenne F, van den Berg TK. Nonclassical FCGR2C haplotype is associated with protection from red blood cell alloimmunization in sickle cell disease. Blood. 2017;130(19):2121–2130. doi: 10.1182/blood-2017-05-784876. [DOI] [PubMed] [Google Scholar]
- 32.Kakiuchi C, Ishiwata M, Nanko S, Kunugi H, Minabe Y, Nakamura K, Mori N, Fujii K, Umekage T, Tochigi M, Kohda K, Sasaki T, Yamada K, Yoshikawa T, Kato T. Functional polymorphisms of HSPA5: possible association with bipolar disorder. Biochem Biophys Res Commun. 2005;336(4):1136–1143. doi: 10.1016/j.bbrc.2005.08.248. [DOI] [PubMed] [Google Scholar]
- 33.Robertson D. Racially defined haplotype project debated. Nat Biotechnol. 2001;19(9):795–796. doi: 10.1038/nbt0901-795b. [DOI] [PubMed] [Google Scholar]