Abstract
Purpose
Investigating whether pre-ovulatory follicular fluid (FF) levels of selected proteins differ between women who do or do not develop severe ovarian hyperstimulation syndrome (OHSS) and evaluate whether they potentially could guide a “freeze-all” strategy.
Methods
FF was collected during a randomized controlled trial comparing OHSS in antagonist versus agonist protocol including 1050 women in their first assisted reproductive technology (ART) cycle during year 2009–2013. The present sub-study is a matched case-control study comparing FF levels of soluble urokinase plasminogen activator receptor (suPAR), C-reactive protein, placental growth factor, vascular endothelial growth factor, and angiopoietins 1 and 2 in OHSS cases (n = 25, severe OHSS, and ≥ 15 oocytes), high-risk controls (n = 25, no OHSS, and ≥ 15 oocytes), and low-risk controls (n = 25, no OHSS, and 5–8 oocytes).
Results
FF level of suPAR differed significantly between the three groups (p = 0.018) with mean (SD) levels of 2.3 (0.4) μg/L, 2.6 (0.8) μg/L, and 2.8 (0.6) μg/L in OHSS cases, high-risk controls, and low-risk controls, respectively. Receiver operating characteristic curve analysis demonstrated that suPAR levels could predict severe OHSS (AUC 0.678; 95% CI 0.553–0.803) with a sensitivity of 64% and a specificity of 66%. None of the other investigated proteins differed between the three groups or between OHSS cases and combined controls.
Conclusion
The pre-ovulatory FF level of suPAR was significantly lower in women developing severe OHSS, indicating that the plasminogen activator system could be involved in the pathophysiology of OHSS. However, suPAR did not provide a satisfying predictive value for the prediction of OHSS.
Keywords: Ovarian hyperstimulation syndrome (OHSS), suPAR, Follicular fluid, CRP, VEGF, Angiopoietin 1, Angiopoietin 2
Introduction
Ovarian stimulation is essential for the success in assisted reproductive technology (ART), but as a side effect introduces a risk of ovarian hyperstimulation syndrome (OHSS), one of the most serious complications in ART. OHSS is characterized by ovarian enlargement and shifting of fluid to the third space, potentially causing ascites, pleural effusion, hemoconcentration, electrolyte disturbances, liver and kidney dysfunction, and thromboembolic events [1]. Based on the clinical symptoms and laboratory findings, OHSS has traditionally been classified as mild, moderate, and severe [2, 3]. Mild OHSS rarely causes clinical problems, whereas severe OHSS is a potentially life-threatening condition. The pathophysiological basis for OHSS is still not completely understood. However, it is believed that the hyper stimulated ovaries produce and secrete factors that cause OHSS, presumably proteins involved in inflammation [4, 5] and proteins involved in angiogenesis [6, 7].
As there are no specific treatment options for severe OHSS, effective prevention depends on reliable clinical and biochemical markers for OHSS. Several patient and stimulation characteristics are known to predict the risk of developing OHSS [8]. Especially the number of follicles ≥ 10–11 mm on the day of triggering final oocyte maturation correlates with the risk of OHSS [9–11]. However, some women with a high number of follicles do not develop OHSS. This could reflect that not only the quantity, but also the quality of each growing follicle, may contribute to the development of OHSS. It is possible that the intra-follicular milieu in women predisposed for the syndrome differs from that of women without this predisposition. Follicular fluid (FF) represents the intra-follicular milieu, and thus, proteins present in the FF may be involved in the development of OHSS and could be important biomarkers for severe OHSS. FF is easily accessible, as it is routinely aspirated during oocyte pick-up (OPU), and if potential biomarkers could be measured before embryo transfer, this could assist clinicians in identifying women that would benefit from postponing transfer of an embryo to a subsequent cycle (freeze-all strategy) to prevent the development or aggravation of OHSS.
Thus, the aim of this study was to determine whether FF concentrations of six selected candidate proteins involved in inflammation and angiogenesis differed between women that developed severe OHSS and women that did not develop OHSS with or without a high-risk profile.
Methods and materials
Participants
This study is a secondary sub-study of a large-phase IV, dual-center, randomized controlled trial on 1050 women, included from 2009 to 2013 in Denmark, with the objective to compare short GnRH antagonist protocol and long GnRH agonist protocol in an unselected population referred for their first ART treatment. Exclusion criteria were uterine abnormality, age > 40 years, use of testicular sperm extraction/aspiration, and severe co-morbidity [11]. The present sub-study is a case-control sub-study including three groups: (1) patients with ≥ 15 oocytes retrieved who developed severe OHSS (n = 25, OHSS cases), (2) patients with ≥ 15 oocytes who did not develop moderate-severe OHSS (n = 25, high-risk controls), and (3) patients with 5–8 oocytes who did not develop moderate-severe OHSS (n = 25, low-risk controls). For each OHSS case (n = 25), one high-risk control (n = 25) and one low-risk control (n = 25) were selected by matching patients by treatment protocol (antagonist versus agonist), pregnancy (±), age, BMI, and anovulation (±). We chose to use the number of oocytes retrieved to identify the high- and low-risk groups for the following reasons: (1) the exact number of follicles ≥ 10–11 mm on the day of triggering final oocyte maturation was not recorded in the original dataset; (2) the number of oocytes retrieved correlates with the number of growing follicles; (3) a large-scale report on 256,381 IVF cycles demonstrated a significantly increased OHSS risk when more than 15 oocytes were retrieved [12].
Treatment protocols
Participants were randomized to the standard GnRH agonist or antagonist protocol using a fixed rFSH (Puregon®) dose of 150 IU or 225 IU according to age ≤ 36 years or > 36 years, respectively. After 6 days of stimulation, the rFSH dose were adjusted according to ovarian response using transvaginal ultrasonography. In both groups, 6500 IU hCG (Ovitrelle®) was administered to induce final oocyte maturation. OPU was performed 36 h after hCG administration. Embryo transfer was performed 2 days after oocyte retrieval [11].
OHSS measurement
Transvaginal ultrasound scan and clinical examinations related to OHSS were performed at three occasions: day of OPU, OPU + 5 days, and day of pregnancy test (13–15 days after embryo transfer). Rates of no, mild, moderate, and severe OHSS were registered. OHSS was defined according to the Golan criteria with the addition of a few well-defined criteria from the Navot classification [11]. Severe OHSS was diagnosed in patients that in addition to abdominal distension and discomfort along with nausea, vomiting and/or diarrhea, enlarged ovaries, and ultrasonographic evidence of ascites, had clinical evidence of ascites and/or hydrothorax or breathing difficulties and could have hemoconcentration, liver or kidney dysfunction, and decreased renal perfusion. The diagnostic criteria are thoroughly described in the primary analysis of the RCT [11]. The degree of OHSS was evaluated at all three examination days, but when calculating the OHSS rates, only the most severe OHSS grade was counted for each patient.
Follicular fluid collection
FF from one large pre-ovulatory follicle with a diameter of > 17 mm was aspirated from each patient. The first clear follicular fluid aspirate with the presence of an oocyte and without contamination of blood or flushing medium was used for analysis. After removal of the oocyte, the fluid was centrifuged at 1200 rpm for 10 min to remove granulosa cells and debris. The supernatant was stored at − 20 °C until transfer to − 80 °C. They were once thawed to 0 °C, divided into aliquots, and refrozen to − 80 °C before final analysis.
Biochemical measurements
Soluble urokinase plasminogen activator receptor (suPAR) was determined in singlets using the suPARnostic AUTO Flex ELISA kit (ViroGates A/S, Denmark) on an automated Siemens BEP2000 platform. Intra- and inter-assay coefficients of variation (CVs) were 3.5% and 5.1%, respectively. Placental growth factor (PlGH) and vascular endothelial growth factor (VEGF) were determined by electrochemiluminescence immunoassays and C-reactive protein (CRP) by a high-sensitivity immunoturbidimetric assay on Cobas 6000 (Roche Diagnostics Ltd., Switzerland). The corresponding intra- and inter-assay CVs were as follows: 1.1% and 2.7% (placental growth factor (PlGF)); 1.9% and 2.5% (VEGF), and 1.6% and 3.9% (CRP). Angiopoietin 1 and angiopoietin 2 were measured by enzyme-linked immunosorbent assays (R&D Systems Inc., Minneapolis, USA). For angiopoietin 1, the intra- and inter-assay CVs were 2.4 and 6.4%, respectively. For angiopoietin 2, the intra- and inter-assay CVs were 6.9 and 10.4% respectively.
Statistical analysis
Data are presented as mean (SD) and number (%) as relevant. For continuous variables, differences between groups were evaluated using non-parametric tests (the Kruskal-Wallis or Mann-Whitney test). Categorical data were compared using χ2 test. Prediction of severe OHSS by FF suPAR levels was examined by receiver operator characteristics (ROC) curve analyses.
Results
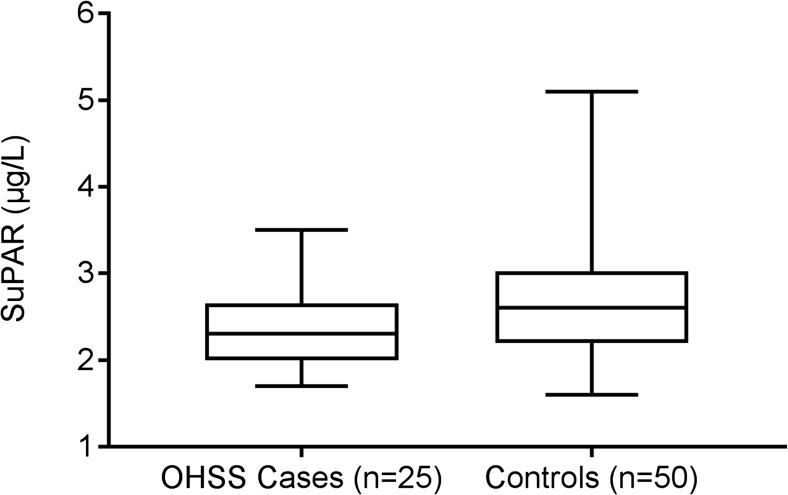
Table 1 presents patient characteristics and demonstrates the resemblance of the groups. The groups differed as expected regarding their risk profile for OHSS, with the low-risk control group having lower p-estradiol levels at OPU and fewer oocytes retrieved despite similar FSH stimulation dosage received (Table 1). The number of oocytes retrieved did not differ significantly between the two high-risk groups (p = 0.11). The high-risk control group did, however, have significantly lower p-estradiol levels as compared with the OHSS group (p = 0.01). The concentrations of the six selected candidate proteins measured in the FF are presented in Table 2. The FF levels of suPAR differed significantly between the three groups (p = 0.018) with mean (SD) levels of 2.3 (0.4) μg/L, 2.6 (0.8) μg/L, and 2.8 (0.6) μg/L in OHSS cases, high-risk controls, and low-risk controls, respectively. When OHSS cases (n = 25) where compared to the combined control groups (no OHSS, n = 50), the mean difference between groups was − 0.40 μg/L (95% confidence interval − 0.71 to − 0.09 μg/L), (p = 0.012) (Fig. 1). A total of 19 patients had ≥ 20 oocytes retrieved (11 in the OHSS group and 8 in the high-risk control group). There was no statistically significant difference in FF suPAR levels between OHSS cases with ≥ 20 oocytes retrieved and OHSS cases with 15–19 oocytes retrieved (2.2 (0.5) vs 2.4 (0.4) μg/L, p = 0.08), as well as no difference between high-risk controls with ≥ 20 oocytes retrieved and high-risk controls with 15–19 oocytes retrieved (2.6 (0.6) vs 2.7 (0.9) μg/L, p = 0.56). The OHSS group with ≥ 20 oocytes had near-significant lower mean FF suPAR levels compared with the non-OHSS patients with ≥ 20 oocytes retrieved (2.2 (0.5) μg/L vs 2.6 (0.6) μg/L, p = 0.05).
Table 1.
Group characteristics of matching criteria
| OHSS cases, n = 25 | High-risk controls, n = 25 | Low-risk controls, n = 25 | p values* | |
|---|---|---|---|---|
| Age in years, mean (SD) | 30.9 (4.1) | 30.4 (3.9) | 30.6 (4.2) | NS |
| BMI in kg/m2, mean (SD) | 23.3 (2.8) | 24.6 (3.9) | 23.6 (3.7) | NS |
| Anovulation, n (%) | 8 (32%) | 7 (28%) | 8 (32%) | NS |
| GnRH antagonist protocola, n (%) | 8 (32%) | 8 (32%) | 10 (40%) | NS |
| Total FSH dosage in IU, mean (SD) | 1755 (445) | 1677 (409) | 1818 (492) | NS |
| p-Estradiol OPU in nmol/L, mean (SD) | 8.8 (4.4) | 6.2 (3.4) | 2.7 (1.5) | < 0.0001 |
| No. of oocytes retrieved, mean (SD) | 20.2 (4.6) | 18.5 (4.2) | 6.5 (1.1) | < 0.0001 |
| Ongoing pregnancy, n (%) | 10 (40%) | 11 (44%) | 10 (40%) | NS |
BMI body mass index, OPU oocyte pick-up
*The Kruskal-Wallis
aPatients received either the GnRH antagonist protocol or the GnRH agonist protocol
Table 2.
Follicular fluid concentrations of selected biomarkers
| OHSS high risk, n = 25 | No OHSS high risk, n = 25 | No OHSS low risk, n = 25 | p values* | |
|---|---|---|---|---|
| SuPAR (μg/L) | 2.3 (0.4) | 2.6 (0.8) | 2.8 (0.6) | 0.018 |
| CRP (mg/L) | 1.7 (1.7) | 2.0 (2.0) | 1.7 (2.1) | 0.83 |
| VEGF (ng/L) | 1198 (263) | 1216 (200) | 1276 (328) | 0.64 |
| PlGF (ng/L) | 38.4 (19.9) | 37.0 (10.8) | 31.6 (9.1) | 0.26 |
| Angiopoietin 1 (ng/L) | 366 (277) | 439 (260) | 439 (364) | 0.53 |
| Angiopoietin 2 (ng/L) | 29,458 (19,162) | 34,521 (24,351) | 33,500 (22,758) | 0.67 |
All data presented as mean (SD)
*The Kruskal-Wallis test
Fig. 1.
Follicular fluid suPAR levels in OHSS cases (n = 25) compared with combined controls (n = 50), presented as median, interquartile range, and minimum and maximum value
The FF level of CRP as well as selected candidate proteins involved in angiogenesis (VEGF, PlGF, and angiopoietins 1 and 2) did not differ between the three groups (Table 2) or between cases and combined controls.
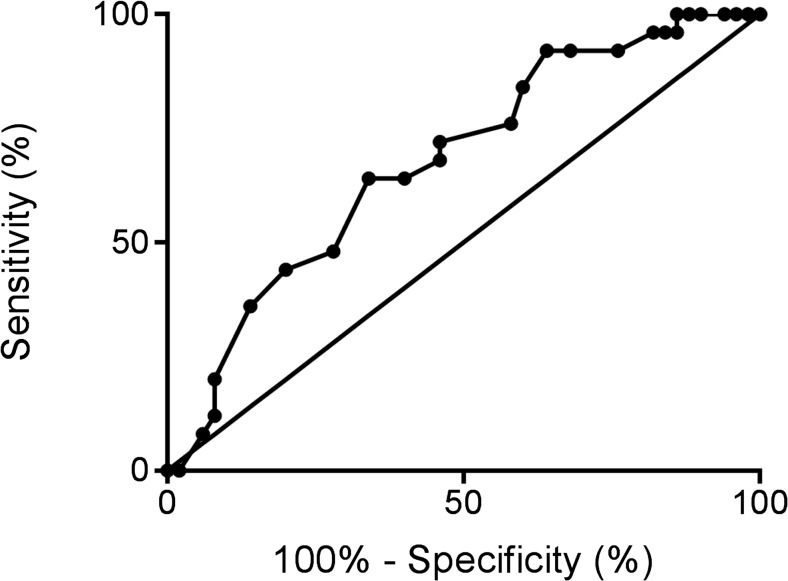
ROC curve analysis demonstrated that the level of suPAR in the FF could predict severe OHSS (AUC 0.678; 95% CI 0.553–0.803, p = 0.01, Fig. 2) with a sensitivity of 64% and a specificity of 66% using a cut-off value of 2.4 μg/L.
Fig. 2.
Receiver operator characteristics (ROC) curve for FF suPAR levels predicting severe OHSS
Patients in the three groups were matched for anovulation (±) with a total of 23 subjects presenting anovulation and 52 with ovulation. The FF level of CRP was significantly higher among patients with anovulation, with a mean (SD) of 2.58 (2.20) mg/L compared to 1.44 (1.44) mg/L in regular cycling women (p = 0.03). Furthermore, there was a trend toward higher FF suPAR levels among patients with anovulation compared to regular cycling women (2.6 (0.5) μg/L vs 2.5 (0.9) μg/L, p = 0.06). There was no association between anovulation and the angiogenetic factors VEGF, PlGF, and angiopoietins 1 and 2 in the FF.
Discussion
The present study evaluated the concentration of several proteins associated with inflammation and angiogenesis in pre-ovulatory FF from three groups of women: a group of women that developed severe OHSS, a high-risk control group that had a high number of oocytes retrieved at OPU but did not develop OHSS, and a third group that comprised the low-risk control group. Levels of suPAR were significantly reduced in FF from women who developed severe OHSS compared with the two control groups. In contrast, the concentrations of the other measured proteins including CRP, VEGF, PlGF, and angiopoietins 1 and 2 were similar between the three groups of women, indicating that the FF concentrations of these proteins were not associated with the development of OHSS. Despite the significantly reduced FF suPAR levels in the group that developed severe OHSS, the area under the ROC curve was too low to warrant a direct clinical use. Collectively, the present results suggest that the regulation of the plasminogen activator system within the follicle may be associated with development of OHSS, although the present study does not provide a causal explanation.
This is the first study to evaluate suPAR in FF from women undergoing ovarian stimulation and the first to place a central component in the plasminogen activator system, known to be intimately connected to ovulation, in the etiology of OHSS. SuPAR originates from cleavage and release of the cell membrane-bound urokinase plasminogen activator receptor (uPAR), which is the specific receptor for urokinase plasminogen activator (uPA) [13]. uPA converts inactive plasminogen to the robust protease plasmin. Among different substrates, plasmin degrades fibrin and several components of the extra cellular matrix. In this context, uPA and other plasminogen activators play a central role during ovulation in humans by securing the release of the cumulus oocyte complex to the oviduct [12]. It is believed that the primary role of uPAR is to localize uPA activity to specific extracellular sites rather than to modulate its proteolytic activity [13]. The molecular role of the soluble form of the receptor (suPAR) is not clearly understood, but several studies conclude that suPAR is a chemotactic agent stimulating the immune response and, furthermore, that suPAR is involved in the regulation of uPA/uPAR actions through competitive inhibition of uPAR as a scavenger of uPA [14].
Thus, it could be hypothesized that the reduced levels of suPAR within the follicle could result in a more diffuse action of plasmin rather than a localized proteolytic action at the site of follicular rupture. Decreased FF suPAR levels could leave more uPA to interact with the membrane-bound uPAR, resulting in a higher extracellular plasminogen activation and subsequently a more widespread extracellular proteolytic degradation leading to an increased dysregulation of angiogenesis and an increased risk of fluid extravasation and movement of fluid into the third space, thus promoting OHSS. Although this hypothesis requires further experimental work to be documented, the confined physical environment of the ovarian follicles may very well provide a suitable experimental environment.
CRP is a well-known marker of inflammation and has previously been reported to be elevated in patients with early OHSS [4, 15, 16], suggesting that inflammatory processes could be involved in the pathogenesis of OHSS. In this study, FF CRP levels were not increased among women with severe OHSS compared with controls without moderate-severe OHSS.
Several factors related to angiogenesis are believed to be involved in the pathogenesis of OHSS, with VEGF being the most important [17]. VEGF is one of the main regulators of angiogenesis [18] and directly stimulates increased vascular permeability [19]. It is the most extensively studied angiogenic factor in connection with OHSS, and it has been proposed that VEGF concentrations in serum and FF may predict occurrence of OHSS [20, 21]. However, other studies have found VEGF to be of limited predictive value [22, 23]. In the present study, FF VEGF levels did not differ between women that developed severe OHSS and women that did not develop moderate-severe OHSS.
PlGF is a member of the VEGF sub-family and displays 53% homology with VEGF [24]. Its association with OHSS has not previously been investigated, but the findings of several studies suggest that the ovary is the primary site of production during ovarian stimulation [25, 26], and PlGF may play a role in the PCOS pathogenesis and its angiogenic dysregulation [26]. However, as with VEGF, we did not demonstrate any association between OHSS and FF levels of PlGF.
In addition to VEGF and PlGF, we assessed FF angiopoietin 1 and 2 levels. The production of angiopoietins are believed to assist in mediating the triggering of OHSS by hCG [27], and it has been demonstrated that angiopoietin 2, in the presence of VEGF, mediates vascular leakage to the third space [28]. Still, in the present study, we did not find an association between angiopoietins and OHSS.
The diverging results in the literature may be related to variating classification of OHSS patients and selection of controls. The present study included two control groups that did not develop OHSS: a high-risk control group that were matched for important predictors of OHSS and a low-risk control group. We chose a cut-off value of 15 oocytes retrieved in order to identify high-risk patients of moderate-severe OHSS based on previously published results [12]. However, different cut-off values have been presented in the literature to predict severe OHSS, including cut-off values of 18–20 oocytes [29, 30], but also as high as 30 oocytes [31]. In order to assess the use of a higher cut-off value, we did a sub-analysis on the patients with more than 20 oocytes retrieved. The sub-analysis confirmed the association between suPAR and OHSS, but with fewer patients in each group. Furthermore, this study was designed as a matched case-control, which warrants cation when interpreting the sub-analysis. However, we are planning a retrospective study in order to investigate whether the number of oocytes retrieved affects levels of suPAR and whether a higher cut-off value for number of oocytes predicting OHSS provides a better association with suPAR.
Despite the groups being formed based on data from a large RCT, the number of women developing OHSS was still limited and it was not possible to match the high-risk groups completely regarding p-estradiol levels. However, a larger dataset would be difficult to obtain as current clinical practice employs the use of GnRH agonist trigger and a “freeze-all” strategy in high-risk patients to prevent severe OHSS.
Furthermore, FF concentrations from a single follicle may not truly reflect total granulosa cell production in the ovaries as substantial interfollicular variation in FF steroid and cytokine concentrations have been reported [32]. In this study, only FF from one mature pre-ovulatory follicle had been stored in the biobank from the original study, as it represents an easily accessible tool in daily clinical practice. Future studies on granulosa cell expression and pooled FF aspirates may provide additional knowledge on the role of suPAR and OHSS and elucidate whether suPAR in pooled FF has a higher predictive value for OHSS.
In conclusion, our findings indicate that the intra-follicular activation state of the immune system at OPU is not increased in women that develop severe OHSS measured by CRP and suPAR levels in the FF. On the contrary, suPAR levels were reduced in women with severe OHSS compared with controls, most likely reflecting an interesting unexplored role of the plasminogen activator system and OHSS. FF suPAR levels did not display convincing predictive value in a clinical setting with low sensitivity and specificity for predicting severe OHSS. However, this is the first study to evaluate suPAR levels in relation to ART; thus, further studies are required to determine the role of plasma or FF suPAR levels in OHSS and other outcomes of ART. Furthermore, we found that several proteins involved in angiogenesis, (VEGF, PlGF, and angiopoietins 1 and 2) were unaltered in the FF of patients with severe OHSS.
Funding
For the primary RCT, an unrestricted research grant was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD). The funders had no influence on the idea, data collection, analyses, or conclusions of the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
References
- 1.Kumar P, Sait SF, Sharma A, Kumar M. Ovarian hyperstimulation syndrome. J Hum Reprod Sci. 2011;4:70–75. doi: 10.4103/0974-1208.86080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv. 1989;44:430–440. doi: 10.1097/00006254-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Practice Committee of American Society for Reproductive Medicine Ovarian hyperstimulation syndrome. Fertil Steril. 2008;90:S188–S193. doi: 10.1016/j.fertnstert.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Korhonen KVM, Savolainen-Peltonen HM, Mikkola TS, Tiitinen AE, Unkila-Kallio LS. C-reactive protein response is higher in early than in late ovarian hyperstimulation syndrome. Eur J Obstet Gynecol Reprod Biol. 2016;207:162–168. doi: 10.1016/j.ejogrb.2016.10.051. [DOI] [PubMed] [Google Scholar]
- 5.Orvieto R. Controlled ovarian hyperstimulation--an inflammatory state. J Soc Gynecol Investig. 2004;11:424–426. doi: 10.1016/j.jsgi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Zhai J, Liu J, Zhao S, Zhao H, Chen Z-J, Du Y, et al. Kisspeptin-10 inhibits OHSS by suppressing VEGF secretion. Reproduction. 2017;154:355–362. doi: 10.1530/REP-17-0268. [DOI] [PubMed] [Google Scholar]
- 7.Miller I, Chuderland D, Grossman H, Ron-El R, Ben-Ami I, Shalgi R. The dual role of PEDF in the pathogenesis of OHSS: negating both angiogenic and inflammatory pathways. J Clin Endocrinol Metab. 2016;101:4699–4709. doi: 10.1210/jc.2016-1744. [DOI] [PubMed] [Google Scholar]
- 8.Papanikolaou EG, Humaidan P, Polyzos N, Kalantaridou S, Kol S, Benadiva C, Tournaye H, Tarlatzis B. New algorithm for OHSS prevention. Reprod Biol Endocrinol. 2011;9:147. doi: 10.1186/1477-7827-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarlatzi TB, Venetis CA, Devreker F, Englert Y, Delbaere A. What is the best predictor of severe ovarian hyperstimulation syndrome in IVF? A cohort study. J Assist Reprod Genet. 2017;34:1341–1351. doi: 10.1007/s10815-017-0990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griesinger G, Verweij PJM, Gates D, Devroey P, Gordon K, Stegmann BJ, Tarlatzis BC. Prediction of ovarian hyperstimulation syndrome in patients treated with corifollitropin alfa or rFSH in a GnRH antagonist protocol. PLoS One. 2016;11:e0149615. doi: 10.1371/journal.pone.0149615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papanikolaou EG, Pozzobon C, Kolibianakis EM, Camus M, Tournaye H, Fatemi HM, van Steirteghem A, Devroey P. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril. 2006;85:112–120. doi: 10.1016/j.fertnstert.2005.07.1292. [DOI] [PubMed] [Google Scholar]
- 12.Steward RG, Lan L, Shah AA, Yeh JS, Price TM, Goldfarb JM, Muasher SJ. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril. 2014;101:967–973. doi: 10.1016/j.fertnstert.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Toftager M, Bogstad J, Bryndorf T, Løssl K, Roskær J, Holland T, Prætorius L, Zedeler A, Nilas L, Pinborg A. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod. 2016;31:1253–1264. doi: 10.1093/humrep/dew051. [DOI] [PubMed] [Google Scholar]
- 14.Vassalli JD, Baccino D, Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985;100:86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y-X. Plasminogen activator/plasminogen activator inhibitors in ovarian physiology. Front Biosci. 2004;9:3356–3373. doi: 10.2741/1487. [DOI] [PubMed] [Google Scholar]
- 16.Thunø M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157–172. doi: 10.1155/2009/504294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin I, Gamzu R, Pauzner D, Rogowski O, Shapira I, Maslovitz S, Almog B. Elevated levels of CRP in ovarian hyperstimulation syndrome: an unrecognised potential hazard? BJOG. 2005;112:952–955. doi: 10.1111/j.1471-0528.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 18.Lamaita RM, Pontes A, Belo AV, Caetano JPJ, Andrade SP, Cãndido EB, Traiman P, Carneiro MM, Silva-Filho AL. Inflammatory response patterns in ICSI patients: a comparative study between chronic anovulating and normally ovulating women. Reprod Sci. 2012;19:704–711. doi: 10.1177/1933719111428518. [DOI] [PubMed] [Google Scholar]
- 19.Rizk B, Aboulghar M, Smitz J, Ron-El R. The role of vascular endothelial growth factor and interleukins in the pathogenesis of severe ovarian hyperstimulation syndrome. Hum Reprod Update. 1997;3:255–266. doi: 10.1093/humupd/3.3.255. [DOI] [PubMed] [Google Scholar]
- 20.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 21.Bates DO, Harper SJ. Regulation of vascular permeability by vascular endothelial growth factors. Vasc Pharmacol. 2002;39:225–237. doi: 10.1016/S1537-1891(03)00011-9. [DOI] [PubMed] [Google Scholar]
- 22.Gao M-Z, Zhao X-M, Sun Z-G, Hong Y, Zhao L-W, Zhang H-Q. Endocrine gland-derived vascular endothelial growth factor concentrations in follicular fluid and serum may predict ovarian hyperstimulation syndrome in women undergoing controlled ovarian hyperstimulation. Fertil Steril. 2011;95:673–678. doi: 10.1016/j.fertnstert.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 23.Jakimiuk AJ, Nowicka MA, Zagozda M, Koziol K, Lewandowski P, Issat T. High levels of soluble vascular endothelial growth factor receptor 1/sFlt1 and low levels of vascular endothelial growth factor in follicular fluid on the day of oocyte retrieval correlate with ovarian hyperstimulation syndrome regardless of the stimulation. J Physiol Pharmacol. 2017;68:477–484. [PubMed] [Google Scholar]
- 24.Mathur R, Hayman G, Bansal A, Jenkins J. Serum vascular endothelial growth factor levels are poorly predictive of subsequent ovarian hyperstimulation syndrome in highly responsive women undergoing assisted conception. Fertil Steril. 2002;78:1154–1158. doi: 10.1016/S0015-0282(02)04243-7. [DOI] [PubMed] [Google Scholar]
- 25.Geva E, Amit A, Lessing JB, Lerner-Geva L, Daniel Y, Yovel I, Azem F, Barak V. Follicular fluid levels of vascular endothelial growth factor. Are they predictive markers for ovarian hyperstimulation syndrome? J Reprod Med. 1999;44:91–96. [PubMed] [Google Scholar]
- 26.De Falco S. The discovery of placenta growth factor and its biological activity. Exp Mol Med. 2012;44:1–9. doi: 10.3858/emm.2012.44.1.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutman G, Barak V, Maslovitz S, Amit A, Lessing JB, Geva E. Regulation of vascular endothelial growth factor-A and its soluble receptor sFlt-1 by luteinizing hormone in vivo: implication for ovarian follicle angiogenesis. Fertil Steril. 2008;89:922–926. doi: 10.1016/j.fertnstert.2007.03.097. [DOI] [PubMed] [Google Scholar]
- 28.Tal R, Seifer DB, Grazi RV, Malter HE. Follicular fluid placental growth factor is increased in polycystic ovarian syndrome: correlation with ovarian stimulation. Reprod Biol Endocrinol. 2014;12:82. doi: 10.1186/1477-7827-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magnusson Å, Källen K, Thurin-Kjellberg A, Bergh C. The number of oocytes retrieved during IVF: a balance between efficacy and safety. Hum Reprod. 2018;33:58–64. doi: 10.1093/humrep/dex334. [DOI] [PubMed] [Google Scholar]
- 30.Chen CD, Wu MY, Chao KH, Chen SU, Ho HN, Yang YS. Serum estradiol level and oocyte number in predicting severe ovarian hyperstimulation syndrome. J Formos Med Assoc. 1997;96:829–834. [PubMed] [Google Scholar]
- 31.Asch RH, Li HP, Balmaceda JP, Weckstein LN, Stone SC. Severe ovarian hyperstimulation syndrome in assisted reproductive technology: definition of high risk groups. Hum Reprod. 1991;6:1395–1399. doi: 10.1093/oxfordjournals.humrep.a137276. [DOI] [PubMed] [Google Scholar]
- 32.Mendoza C, Cremades N, Ruiz-Requena E, Martinez F, Ortega E, Bernabeu S, Tesarik J. Relationship between fertilization results after intracytoplasmic sperm injection, and intrafollicular steroid, pituitary hormone and cytokine concentrations. Hum Reprod. 1999;14:628–635. doi: 10.1093/humrep/14.3.628. [DOI] [PubMed] [Google Scholar]