Abstract
Purpose
To determine whether there is a homogeneous reduction of sperm DNA fragmentation (SDF) in sperm samples recovered from the MACS procedure, compared to spermatozoa in the initial ejaculate (NEAT) and those retained in the column.
Methods
This study investigated the relative change in sperm DNA quality (SDF) of neat ejaculates (10 idiopathic infertile and 10 normozoospermic patients) to subpopulations of spermatozoa that had passed through the column (MACS−) and those retained (MACS+) by the annexin-V conjugated microbeads.
Results
While the MACS protocol was capable of reducing the mean proportion of SDF (59.2%; P = 0.000) and sperm with highly degraded DNA (SDD; 65.7%, P = 0.000) in all patients, the reduction was not homogeneous across the patient cohort. A significant positive correlation (r = 0.772, P = 0.000) was apparent between the level of SDF in the NEAT ejaculate and the efficacy of SDF reduction observed in the MACS− fraction.
Conclusion
MACS is capable of reducing the proportion of SDF, especially spermatozoa with a highly degraded DNA molecule. However, this reduction did not preclude the presence of a small subpopulation of spermatozoa with damaged DNA in the MACS− fraction. The MACS protocol was two- to threefold more efficient when the SDF in NEAT ejaculate was equal to or greater than 30%. In 4 of 20 individuals, the level of SDF after MACS resulted in semen for ICSI with a higher or non-significant reduction when compared to SDF observed in the NEAT ejaculate.
Keywords: Male factor, MACS, Sperm DNA fragmentation
Introduction
Sperm DNA damage in the ejaculate is a consequence of anomalies associated with sperm production (spermatocytogenesis, spermiogenesis, and epididymal maturation) and includes meiotic abnormalities, oxidative stress, and protamination failure and those resulting in adverse molecular changes to sperm proteins, lipids, and DNA [1–3]. When the sperm cell is stressed during production or experiences adverse molecular changes, it may experience cell death with or without apoptosis. If apoptosis occurs, one of the resulting processes is cell restructuration and externalization of phospholipid phosphatidylserine (PS) of the cell membrane. Randomization of the inner localization of phosphatidylserine in the sperm plasma membrane serves as a signal for phagocytosis, triggering pathways for apoptosis [4].
PS has high affinity for annexin V [5, 6], a molecule that can be conjugated with superparamagnetic microbeads for identification and selective sorting of apoptotic and non-apoptotic spermatozoa in a process commonly known as MACS (magnetic activated cell sorting). When a sperm sample is loaded into a column containing microbeads and a magnetic force applied, apoptotic spermatozoa expressing PS, attach to annexin V conjugated magnetized microbeads (annexin V positive fraction – MACS+), whereas non-apoptotic sperm (the annexin V negative fraction – MACS−) pass through the column to be collected for use in IVF procedures [7–9]. Given that it is widely acknowledged that altered or interference to normal apoptotic pathways are associated with sperm anomalies and male infertility [10, 11], it stands to reason, that selective removal of sperm cells exhibiting apoptotic markers, such as PS, should potentially improve reproductive outcomes [8].
Although an increasingly large number of infertile couples have been successfully fertilized using spermatozoa following MACS treatment, many of these studies have made the assumption that these outcomes are a consequence of the fact that sperm apoptosis is coincidently closely associated with sperm DNA fragmentation (SDF); the underlying premise is that there should be a lower proportion of DNA-damaged spermatozoa in the MACS− fraction and that this outcome should flow through to the generation of better quality embryos, improved rates of clinical pregnancy and reduced level of miscarriage [12].
Despite this assumption, there is yet to be a definitive study in which the level of SDF in the MACS− sperm sub-population has been directly compared to the MACS+ sperm sub-population retained in the column, nor the comparison of these findings with respect to the values of SDF observed in the original ejaculate. The underlying hypothesis of such a study would be that there is a homogeneous reduction of SDF in the MACS− subpopulation, with respect to whole ejaculate and the MACS+ subpopulation.
In order to explore this hypothesis, the current experiment compared the relative change in DNA quality of human spermatozoa in the freshly collected liquefied ejaculate (NEAT), to that of the DNA quality of spermatozoa that passed freely through the column (MACS−) and those that were retained (MACS+) by the annexin-V conjugated microbeads. To test for the efficacy of the MACS procedure in patients with different levels of SDF, the relative efficiency of the MACS procedure for improving sperm DNA quality in patients with high (≥ 30%) and low levels (< 30%) of SDF was also compared.
Material and methods
Patients and semen samples
As standard clinical practice, the Ginemed Clinic recommends the use of the MACS procedure for those infertile individuals presenting with SDF values in the neat ejaculate of greater than 30%. For the purpose of the current study, 10 idiopathic infertile individuals were selected from the 2015 assisted reproduction program at Ginemed Clinic; all these patients presented with SDF values higher than 30%. The mean ± standard deviation (SD) age of this cohort was 32 ± 6 years. In addition, a further 10 normozoospermic patients presenting with SDF values less than 30% were also included in the analysis; the mean (± SD) age of this cohort was 34 ± 5 years. All ejaculates collected, processed, and analyzed in this study were derived from individuals attending the clinic for their initial seminogram, so that no samples were used for the purposes of fertilization. As this was a prospective study, all patients signed an authorization to allow use of their semen sample for research purposes. This study was performed according to the Declaration of Helsinki and with the approval of the Ginemed Clinic Ethics Committee.
Sperm MACS sorting
Neat sperm samples were obtained by masturbation after the patients were requested to abstain from ejaculating for 4 days. Once the ejaculate had liquefied, semen was washed with Quinn’s Advantage™ Medium with HEPES (SAGE MEDIA™, Trumbull, Connecticut, USA). To prevent reactive saturation of the annexin V column, sperm concentration of each sample was adjusted to 25 × 106 mL−1 using an annexin-V binding buffer supplied within the assay kit (Miltenyi Biotec, Bergisch, Gladbach, Germany). For sperm sorting, 400 μL of the pre-washed semen sample was mixed with 100 μL of microbeads conjugated with annexin V found in the kit. This mixture was incubated at room temperature for 15 min before this solution was loaded on the separation column and placed in a magnetic field according to the manufacturer’s recommendations. The resulting first fraction, which shall be henceforth designated as MACS (−) fraction, was recovered in a 1.5-mL Eppendorf tube, centrifuged at 300g for 5 min and adjusted to a final sperm concentration of 10 × 106 mL−1 by re-suspending the pellet in Quinn’s Advantage™ Medium with HEPES (SAGE MEDIA™, Trumbull, Connecticut, USA). To isolate the MACS (+) fraction, the column was removed from the magnetic field and the retained fraction eluted using the annexin-V binding buffer included in the kit and adjusted to a final sperm concentration of 10 × 106 mL−1 using Quinn’s Advantage™ Medium with HEPES in preparation for the SDF assessment. The neat ejaculate was also diluted with Quinn’s Advantage™ Medium with HEPES to 10 × 106 mL−1.
Characterization of SDF using the Halosperm G2 assay
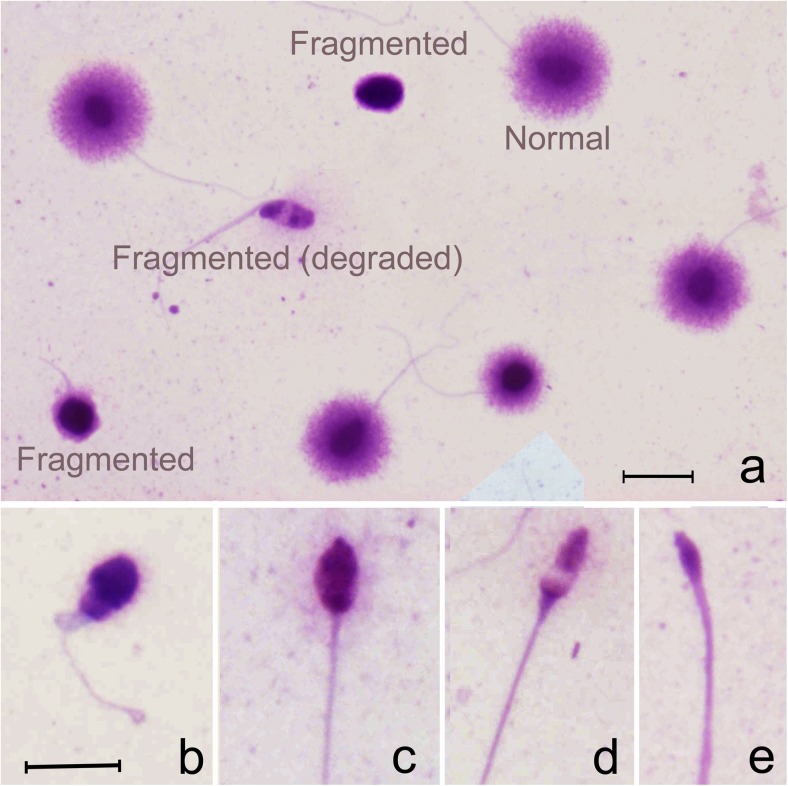
The neat ejaculate, MACS (−) and MACS (+) fractions were all assessed for SDF using the human sperm chromatin dispersion assay (Halosperm G2; Halotech DNA, Madrid, Spain) according to the manufacturer’s instructions. After processing the different semen samples, it was possible to identify three different categories of sperm nuclear morphotypes. We have previously shown that spermatozoa showing a large- or medium-sized halo of dispersed chromatin correspond to nuclei containing a non-fragmented DNA molecule (Fig. 1), whereas spermatozoa with a small halo around a compact core or with the absence of a halo altogether, correspond to sperm nuclei with fragmented DNA (Fig. 1; [13]). Additionally, with this technique, it was also possible to distinguish a third class of spermatozoa, which we have defined as “degraded” sperm (SDD: sperm DNA degradation; [13, 14]). This category was represented by a morphologically extreme variant of the fragmented sperm nuclei category defined by its reduced size as a result of massive chromatin dispersion following treatment (Fig. 1). These spermatozoa possessed only small remnants of chromatin associated with the scaffold of the sperm head. Variations in the size of these spermatozoa can be observed in Fig. 1b, c, d, e and have previously been identified as having the massive presence of double and single strand DNA breaks [14], a phenomenon which contributes to the dispersive loss of chromatin from the nucleoid in the microgel. Based on this characterization of sperm chromatin damage, sperm in the neat ejaculate and different sperm fractions obtained after MACS sorting (− and +) were simultaneously scored for the presence of sperm DNA fragmentation and degraded sperm. In this experiment, degraded spermatozoa were included in the overall SDF class, but to assess the effects of MACS sorting on this specific subpopulation, they were also analyzed separately.
Fig. 1.
a Morphology of Giemsa stained spermatozoa following the sperm chromatin dispersion test (Halosperm G2) resulting in the differentiation of three main sperm nuclear morphotypes: (1) normal sperm free of sperm DNA fragmentation and displaying large or moderate sized haloes around a compact core; (2) fragmented sperm displaying small or absence of haloes and (3) fragmented (degraded) sperm showing varying size of the faintly stained nuclear core (b–e). (Scale bar = 25 μm)
Statistical methods
Statistical analysis was conducted using the Statistical Package for Social Sciences (SPSS v.11, Chicago, IL, USA). Non-parametric statistics for group and paired samples using the Friedman and Wilcoxon signed-rank test were also conducted. Correlation analysis was performed using a Pearson correlation coefficient. Statistical significance was defined at P < 0.05.
Results
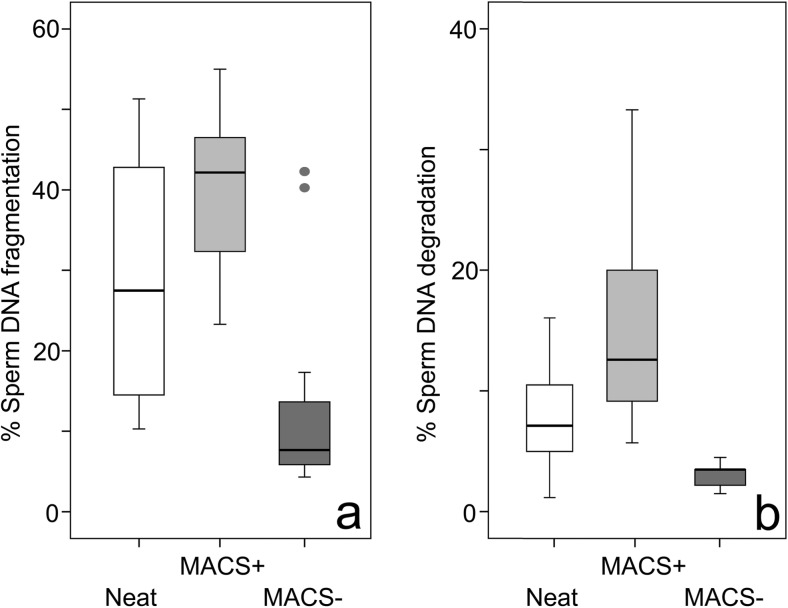
The proportion of SDF and SDD sperm prior to (NEAT ejaculate) and after sperm sorting (MACS+ and MACS−) fractions is reported in Fig. 2. Using the non-parametric Friedman test for paired samples, statistical differences were found for both SDF and SDD when all three groups (NEAT, MACS+, and MACS−) were compared (SDF: χ2 = 23. 57; df 2; P = 0.000; SDD: χ2=38.4; df 2; P = 0.000). To assess the statistical significance of SDF and SDD changes when NEAT samples were compared with MACS+/MACS− samples, the Wilcoxon signed-rank test was used. Significant differences were found for all cases and presented in Table 1. Following the MACS procedure and including all the individuals that were assessed in this experiment, there was a 59.2% reduction in SDF of the values observed in the MACS− sub-population compared with the NEAT ejaculate; this reduction reached 65.7% for SDD in the MACS− sub-population. In contrast, the proportion of SDF and SDD in the MACS+ sub-population increased by 34.2 and 109.2% respectively, when compared to the NEAT sample.
Fig. 2.
Box- and-whisker plots of percent a sperm DNA fragmentation (SDF) and b sperm DNA degradation (SDD) in neat seen samples and in MACS+ and MACS− sub-populations following the MACS procedure
Table 1.
Results of Wilcoxon signed-rank tests comparing the proportion of SDF (sperm DNA fragmentation) and SDD (Sperm DNA degradation) sperm cells found in neat semen samples and after separation in MACS+ and MACS− sperm sub-populations
| NEAT/MACS+ | NEAT/MACS− | |
|---|---|---|
| SDF | Z = −3.461; P = 0.001 | Z = −3.585; P = 0.000 |
| SDD | Z = −3.921; P = 0.000 | Z = −3.517; P = 0.000 |
The efficacy of MACS in reducing SDF and SDD when values of SDF were < 30 and ≥ 30 was also assessed and the results of this analysis presented in Table 2. Two individuals in the < 30 cohort (individuals 1 and 4 in Table 2) showed an increase in SDF with respect to those observed in the NEAT sample but there was no improvement in SDD. In the ≥ 30 cohort, two individuals (14 and 16 in Table 2) showed only a minor reduction in DNA damage removal after MACS but a higher efficacy in SDD removal was achieved.
Table 2.
Changes in SDF and SDD of individual patients following the MACS procedure (MACS− fraction)
| Cohort | Individual | SDF (NEAT) | SDF (MACS−) | SDD (NEAT) | SDD− (MACS−) |
|---|---|---|---|---|---|
| < 30% SDF | 1 | 10.3 | 13.3 | 4.0 | 4.0 |
| 2 | 11.0 | 7.6 | 1.0 | 1.0 | |
| 3 | 12 | 7 | 3 | 1.0 | |
| 4 | 12.3 | 17.3 | 4.0 | 4.0 | |
| 5 | 14.0 | 7.7 | 4.7 | 1.3 | |
| 6 | 15.0 | 6.0 | 3.0 | 0.3 | |
| 7 | 22.3 | 17.0 | 7.0 | 3.0 | |
| 8 | 22.7 | 4.3 | 7.0 | 3.0 | |
| 9 | 24.3 | 14.0 | 6.0 | 3.3 | |
| 10 | 25.0 | 5.2 | 8.0 | 3.0 | |
| ≥ 30% SDF | 11 | 30.0 | 6.7 | 9.7 | 1.7 |
| 12 | 33.0 | 5.6 | 10.6 | 1.0 | |
| 13 | 39.3 | 5.7 | 12.0 | 1.7 | |
| 14 | 41.0 | 42.3 | 6.0 | 2.6 | |
| 15 | 42.3 | 4.6 | 13.0 | 1.7 | |
| 16 | 43.3 | 40.3 | 5.0 | 3.3 | |
| 17 | 43.7 | 8.7 | 14.0 | 3.0 | |
| 18 | 45.3 | 8.7 | 9.3 | 3.0 | |
| 19 | 46 | 7.3 | 10.2 | 3.0 | |
| 20 | 51.3 | 9.6 | 16.0 | 2.3 |
Boldened data: individuals for which a clear decrease of SDF following the MACS procedure was not observed
SDF sperm DNA fragmentation, SDD sperm DNA degradation, NEAT neat ejaculate, MACS− sperm in the first fraction following the MACS procedure
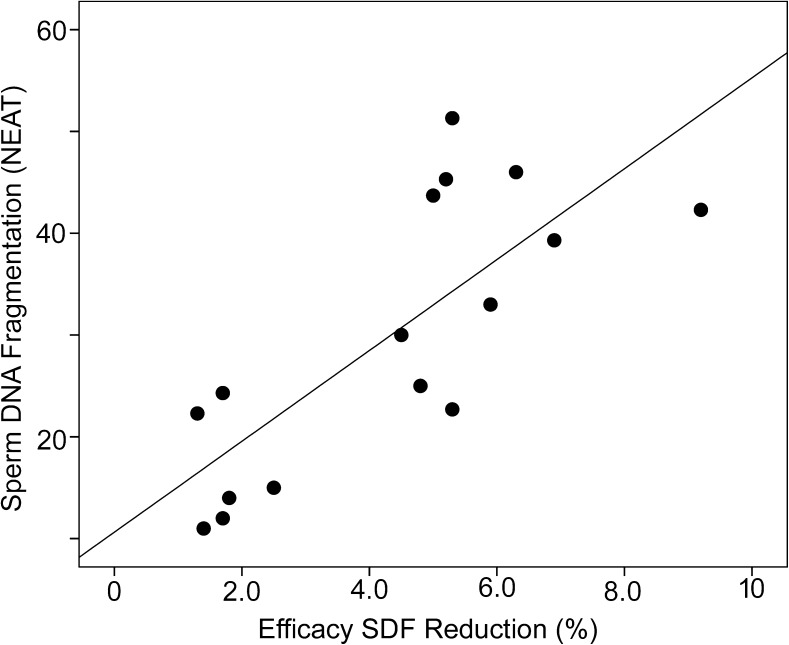
Table 3 reports the efficacy of SDF reduction following the MACS procedure in all patients and shows that there was a reduction in the level of SDF in the majority of the treated individuals (80% of the < 30 cohort and 80% of the ≥ 30 cohort). There was also a strong correlation when SDF values of the NEAT sample were plotted against the efficacy of SDF reduction (Fig. 3; Pearson r = 0.772, n = 16, P = 0.000).
Table 3.
Efficiency of the MACS procedure in reducing mean (± SD) SDF and SDD in patients with < 30 and ≥ 30 SDF in their neat ejaculate (NB individuals highlighted in bold type in Table 2 were excluded)
| SDF < 30% | SDF ≥ 30% | |
|---|---|---|
| SDF NEAT | 18.3 ± 5.8 | 41.4 ± 7.0 |
| SDF MACS− | 8.6 ± 4.5 | 7.1 ± 1.8 |
| % Efficiency | 2.1 X | 5.8 X |
| SDD NEAT | 5.0 ± 2.5 | 11.8 ± 2.3 |
| SDD MACS− | 2.2 ± 1.1 | 2.1 ± 0.8 |
| % Efficiency | 2.2 X | 5.6 X |
Fig. 3.
Correlation plot between the levels of sperm DNA fragmentation (SDF) observed in the NEAT sample and the percentage efficacy of sperm DNA fragmentation (SDF) reduction following the MACS procedure
Discussion
The results of this study have demonstrated that the MACS protocol is capable of reducing the proportion of SDF; especially those spermatozoa identified as presenting with a degraded DNA molecule. However, this reduction did not preclude the presence of a small subpopulation of spermatozoa with damaged DNA in the MACS− fraction. According to the results presented in Table 3, SDF was reduced following the MACS procedure but the protocol was two- to threefold more efficient when the SDF in NEAT ejaculate was equal to or greater than 30%. Additionally, this reduction was not homogeneous across all individuals; in fact, in 4 out of 20 individuals, the level of SDF after the MACS protocol resulted in semen samples for ICSI with a higher or non- significant reduction when compared with those values of SDF observed in the NEAT ejaculate. Nevertheless, in general, there was a strong correlation between the capacity to reduce SDF and the original level of SDF in the NEAT sample; i.e., SDF is more efficiently reduced when the level of SDF in the NEAT ejaculate is high.
While the MACS protocol appeared to provide a benefit for SDF reduction in most cases (80%), it is interesting that none of the sperm samples were completely free of spermatozoa with damaged DNA in the MACS− sperm fraction and that spermatozoa free of damaged DNA were also found in the MACS+ fraction. It is possible that these outliers might be attributed to subtle variations in apoptotic markers, how the PS marker is expressed in each cell or how the caspases are activated. Phosphatidylserine translocation in the plasma membrane, which is detected by annexin V, is an early marker of apoptosis [15, 16] but may not be the unique cell marker corresponding to sperm DNA fragmentation. Caspases are cell latent zymogens related with aspartate-specific cysteine proteinases. Once the caspase cascade is activated, there is a corresponding externalization of PS; this results in the stimulation of apoptosis by specific proteolysis of strategic cellular substrates [17]. With respect to the role of caspases and DNA fragmentation, there exists a caspase-regulated DNase complex known as DNA fragmentation factor [18]. This complex contains a caspase-activated DNase (CAD), but in parallel, there also exists a DNAse inhibitor for this activity (ICAD) that is complexed with CAD. In healthy male patients, this complex is functionally inactive if the cell is not undergoing apoptosis; however, when apoptosis is triggered, ICAD is cleaved by caspase-3 and -7, releasing CAD to induce nuclear DNA degradation [19]. The relationship between phenotypic changes in cell morphology and DNA fragmentation is not as clearly evident as it is for the externalization of PS [20]; for example, there are sperm cells that may overexpress ICAD. Although ICAD activity induces cellular arrest and these cells may exhibit topographies of apoptosis such PS externalization, DNA fragmentation, does not occur [19]. Rather, DNA fragmentation is a downstream effect of caspase activation and can be considered as dispensable for a primary triggering of future cell removal. In fact, it has been suggested that characteristic protein phosphorylation pathways occurring at different cell stages are involved in the triggering different apoptotic pathways [21].
Given this apoptotic mechanism, Marchetti and Marchetti [22] analyzed plasma membrane alterations associated with apoptosis in sperm ejaculated by flow cytometer after double labeling with annexin V antibody and propidium iodide (PI, membrane permeability marker). They determined three populations of sperm; (1) a sub-population of viable sperm (annexin V−, IP−); (2) a sub-population of necrotic sperm (annexin V+, IP+); and (3) a sub-population of viable sperm but with membrane alterations associated with apoptosis (annexin V±, IP+). Martí et al. [23] also analyzed PS externalization using simultaneous staining with CFDA (6-carboxy fluorescein-diacetate) and Annexin V and identified four different sperm subpopulations; (1) intact cells (CFDA+, annexin V), (2 and 3) sperm cells with two different levels of alteration (type I/II CFDA+, annexin V+), and (4) non-viable cells (CFDA−, annexin V−).
In addition to the highly variable nature of sub-cellular pathological expression, that is likely to be associated with apoptosis as indicated above, we further suggest that DNA fragmentation might also be considered as an additional defining parameter of sperm cell apoptosis, especially during the first stages of cell identification and caspase cascade triggering. We also propose that it is possible that apoptotic markers such as annexin V may or may not necessarily be expressed in association with DNA fragmented spermatozoa when the sperm pass through the column; this would help to explain the low but not complete absence of DNA-fragmented spermatozoa in the MACS− fraction and the presence of non-fragmented DNA sperm within the MACS+ sub-population. The presence of fragmented spermatozoa in the MACS− fraction could be the product of other DNA degradation processes not necessarily associated with apoptosis such as early necrotic DNA degradation, a phenomenon which requires further analysis with respect to spermatozoa [24] and a better understanding of the nature of the DNA breaks [25]. While spermatozoa carrying damaged DNA or chromosomal abnormalities are able to fertilize and produce negative effects on the progeny [26–28], direct evidence of an apoptotic sperm cell fertilizing an oocyte has yet to be described, so that the true clinical value of apoptotic sperm removal remains to be elucidated. Nevertheless, and as highlighted in the current study, the possibility of selecting an apoptotic sperm for ICSI cannot be not fully discarded.
What is interesting is the notable and homogeneous reduction of those spermatozoa containing a highly degraded DNA molecule (SDD). These spermatozoa were characterized by their retention of only the scaffold-like protein components of their original chromatin. After DNA denaturation and the selective removal of protein and linked DNA fragments, they stain up 60% less strongly than the rest of the sperm; similarly, after in situ nick translation procedure and the use of a two-dimensional comet assay under sequential neutral and alkaline conditions, these DDS sperm have also been shown to contain both massive double- and single-stranded DNA breaks [14]. For some reason, these spermatozoa appear to be efficiently removed by MACS and could potentially be interpreted as one of the fractions that are exhibiting apoptotic PS translocations of the cell membranes, presenting double-stranded DNA breaks as part of the apoptotic pathway linked to the action of caspases in the ejaculate. When DNA fragmentation occurs, it is generally considered a late apoptotic marker triggered by ICAD cleavage mediated by caspase-3 and -7 [16, 19, 20], however, given the massive loss of DNA as visualized with the two dimensional comet assay, the process of apoptosis in SDD sperm is probably linked to a more advanced pathological process than that affecting the rest of the cells.
Conclusions
This study has demonstrated that standard MACS processing of neat human semen reduces the proportion of sperm with DNA damage in the NEAT sample but that this reduction was not homogeneous across the patient cohort. In fact, the level of SDF after the MACS protocol in this study was actually higher than the SDF found in the NEAT ejaculate in 20% of the samples analyzed. A strong reduction the proportion of highly degraded spermatozoa was observed. For those ejaculates in which a SDF reduction was observed, there was a significant positive correlation between the level of SDF in the NEAT ejaculate and the efficacy of sperm DNA damage reduction observed in the MACS− fraction. Patients exhibiting an initial level of SDF ≥ 30% showed a two- to threefold more efficient reduction in SDF than those where the level of SDF was lower than 30%.
Acknowledgments
The authors want to acknowledge Francisca Arroyo for technical assistance.
Author’s contribution
PS-M, MD-S, JLF, SDJ and JG were involved in the experimental design. MD-S, JLF, and EG processed samples. EG, SDJ, and KG drafted the manuscript. SDJ revised the manuscript. JG conducted the statistical analysis.
Funding
This project was partially funded by the Spanish Ministry of Science and Innovation (BFU-2013- 44290-R). The funding body had no involvement in the study.
Compliance with ethical standards
This study was approved by the GINEMED Ethics Committee (Protocol Version v-4).
References
- 1.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;31:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38:955–1014. doi: 10.1590/S0100-879X2005000700003. [DOI] [PubMed] [Google Scholar]
- 4.Schlegel RA, Williamson P. Phosphatidylserine, a death knell. Cell Death Differ. 2001;8:551–563. doi: 10.1038/sj.cdd.4400817. [DOI] [PubMed] [Google Scholar]
- 5.Vermes I, Haanen C, Steffens-Nakken H, Reutellingsperger C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-I. [DOI] [PubMed] [Google Scholar]
- 6.van Genderen HO, Kenis H, Hofstra L, Narula J, Reutelingsperger CPM. Extracellular annexin A5: functions of phosphatidylserine-binding and two-dimensional crystallization. Biochim Biophys Acta Mol Cell Res. 2008;1783:953–963. doi: 10.1016/j.bbamcr.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Said T, Agarwal A, Grunewald S, Rasch M, Baumann T, Kriegel C, Li L, Glander HJ, Thomas AJ, Paasch U. Selection of nonapoptotic spermatozoa as a new tool for enhancing assisted reproduction outcomes: an in vitro model. Biol Reprod. 2006;74:530–537. doi: 10.1095/biolreprod.105.046607. [DOI] [PubMed] [Google Scholar]
- 8.Gil M, Sar-Shalom V, Melendez Sivira Y, Carreras R, Checa MA. Sperm selection using magnetic activated cell sorting (MACS) in assisted reproduction: a systematic review and meta-analysis. J Assist Reprod Genet. 2013;30:479–485. doi: 10.1007/s10815-013-9962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boomsma CM, Heineman MJ, Cohlen BJ, Farquhar C. Semen preparation techniques for intrauterine insemination. Cochrane Database Syst Rev. 2007;3:CD004507. doi: 10.1002/14651858.CD004507.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Bartke A. Apoptosis of male germ cells, a generalized or a cell type- specific phenomenon? Endocrinology. 1995;136:3–4. doi: 10.1210/endo.136.1.7828545. [DOI] [PubMed] [Google Scholar]
- 11.Sakkas D, Mariethoz E, St John JC. Abnormal sperm parameters in humans are indicative of an abortive apoptotic mechanism linked to the fas-mediated pathway. Exp Cell Res. 1999;251:350–355. doi: 10.1006/excr.1999.4586. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Martín P, Dorado-Silva M, Sánchez-Martín F, González-Martínez M, Johnston SD, Gosálvez J. Magnetic cell sorting of semen containing spermatozoa with high DNA fragmentation in ICSI cycles decreases miscarriage rate. Reprod BioMed Online. 2017;34:506–512. doi: 10.1016/j.rbmo.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Fernández JL, Muriel L, Goyanes V, Segrelles E, Gosálvez J, Enciso M, LaFromboise M, De Jonge C. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84:833–842. doi: 10.1016/j.fertnstert.2004.11.089. [DOI] [PubMed] [Google Scholar]
- 14.Gosálvez J, Rodríguez-Predreira M, Mosquera A, López-Fernández C, Esteves SC, Agarwal A, Fernández JL. Characterisation of a subpopulation of sperm with massive nuclear damage, as recognised with the sperm chromatin dispersion test. Andrologia. 2014;46:602–609. doi: 10.1111/and.12118. [DOI] [PubMed] [Google Scholar]
- 15.Almeida C, Sousa M, Barros A. Phosphatidylserine translocation in human spermatozoa from impaired spermatogenesis. Reprod BioMed Online. 2009;19:770–777. doi: 10.1016/j.rbmo.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Sakkas D, Moffatt O, Manicardi GC, Mariethoz E, Tarozzi N, Bizzaro D. Nature of DNA damage in ejaculated human spermatozoa and the possible involvement of apoptosis. Biol Reprod. 2002;66:1061–1067. doi: 10.1095/biolreprod66.4.1061. [DOI] [PubMed] [Google Scholar]
- 17.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 18.Slee EA, Adrain C, Martin SJ. Executioner Caspase-3, −6, and −7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–7326. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- 19.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 20.Collins JA, Schandl CA, Young KK, Vesely J, Willingham MC. Major DNA fragmentation is a late event in apoptosis. J Histochem Cytochem. 1997;45:923–934. doi: 10.1177/002215549704500702. [DOI] [PubMed] [Google Scholar]
- 21.Gjertsen BT, Cressey LI, Ruchaud S, Houge G, Lanotte M, Døskeland SO. Multiple apoptotic death types triggered through activation of separate pathways by cAMP and inhibitors of protein phosphatases in one (IPC leukemia) cell line. J Cell Sci. 1994;107:3363–3377. doi: 10.1242/jcs.107.12.3363. [DOI] [PubMed] [Google Scholar]
- 22.Marchetti C, Marchetti P. Detection of apoptotic markers in human ejaculated spermatozoa as new methods in human reproductive biology. Gynécol Obs Fertil. 2005;33:669–677. doi: 10.1016/j.gyobfe.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Martí E, Pérez-Pé R, Colás C, Muiño-Blanco T, Cebrián-Pérez JA. Study of apoptosis-related markers in ram spermatozoa. Anim Reprod Sci. 2008;106:113–132. doi: 10.1016/j.anireprosci.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Fraczek M, Hryhorowicz M, Gaczarzewicz D, Szumala-Kakol A, Kolanowski TJ, Beutin L, Kurpisz M. Can apoptosis and necrosis coexist in ejaculated human spermatozoa during in vitro semen bacterial infection? J Assist Reprod Genet. 2015;32:711–719. doi: 10.1007/s10815-015-0462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Didenko VV, Ngo H, Baskin DS. Early necrotic DNA degradation: presence of blunt-ended DNA breaks, 3′ and 5′ overhangs in apoptosis, but only 5′ overhangs in early necrosis. Am J Pathol. 2004;162:1571–1578. doi: 10.1016/S0002-9440(10)64291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernández-Gonzalez R, Moreira PN, Pérez-Crespo M, Sánchez-Martín M, Ramirez MA, Pericuesta E, Bilbao A, Bermejo-Alvarez P, de Dios Hourcade J, de Fonseca FR, Gutiérrez-Adán A. Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol Reprod. 2008;78:761–772. doi: 10.1095/biolreprod.107.065623. [DOI] [PubMed] [Google Scholar]
- 27.Marchetti F, Wyrobeck AJ. Mechanisms and consequences of paternally-transmitted chromosomal abnormalities. Birth Defects Res C. 2005;75:112–129. doi: 10.1002/bdrc.20040. [DOI] [PubMed] [Google Scholar]
- 28.Marchetti F, Essers J, Kanaar R, Wyrobeck AJ. Disruption of maternal DNA repair increases sperm-derived chromosomal aberrations. PNAS. 2007;104:17725–17729. doi: 10.1073/pnas.0705257104. [DOI] [PMC free article] [PubMed] [Google Scholar]