Abstract
BACKGROUND:
Neutropenia is a significant adverse event after heart transplantation (HT) and increases infection risk. Granulocyte colony-stimulating factor (G-CSF) is commonly used in patients with neutropenia. In this work, we assessed the adverse effects of G-CSF treatment in the setting of a university hospital.
METHODS:
Data on HT patients from January 2008 to July 2016 were reviewed. Patients who received G-CSF were identified and compared with patients without a history of therapy. Baseline characteristics, rejection episodes, and outcomes were collected. Data were analyzed by incidence rates, time to rejection and survival were analyzed using Kaplan–Meier curves, and odds ratios were generated using logistic regression analysis.
RESULTS:
Two hundred twenty-two HT patients were studied and 40 (18%) received G-CSF for a total of 85 total neutropenic events (0.79 event/patient year). There were no differences in baseline characteristics between the groups. In the 3 months after G-CSF, the incidence rate of rejection was 0.067 event/month. In all other time periods considered free of G-CSF effect, the incidence rate was 0.011 event/month. This rate was similar to the overall incidence rate in the non-GCSF group, which was 0.010 event/month. There was a significant difference between the incidence rates in the G-CSF group at 0 to 3 months after G-CSF administration and the non-GCSF group (p = 0.04), but not for the other time periods (p = 0.5). Freedom from rejection in the 3 months after G-CSF administration was 87.5% compared with 97.5% in the non-GCSF group (p = 0.006).
CONCLUSIONS:
G-CSF administration was found to be associated with significant short-term risk of rejection. This suggests the need for increased surveillance during this time period.
Keywords: neutropenia, G-CSF, acute rejection, heart transplantation, immunosuppression, infection prophylaxis
Neutropenia is known to occur frequently in heart transplantation (HT) patients, but published data are scarce regarding the true incidence of neutropenia and the consequences of its treatment after HT. The cause for neutropenia in this patient population is multifactorial, with contributory effects of bone marrow toxicity from immunosuppressive (anti-thymocyte globulin, mycophenolate mofetil) and anti-infective (ganciclovir, valganciclovir, sulfamethoxazole–trimethoprim) medications, and systemic infections, particularly cytomegalovirus (CMV). The goal of treatment of neutropenia in these patients is to prevent infection, but this is countered by a concern for an increase in rejection risk. In addition, in cases of severe infection or refractory neutropenia, there is sometimes a need to reduce immunosuppressive therapy, further increasing the risk of rejection.1 Therefore, a high threshold is usually maintained for treatment of neutropenia with a granulocyte-colony stimulating factor (G-CSF).
G-CSF is a cytokine that is made by many different tissues (fibroblasts, endothelial cells, stromal cells, macrophages, and lymphocytes) and stimulates the bone marrow to produce granulocytes and release them into the blood-stream.2 The pharmaceutical analogs of these naturally occurring cytokines stimulate production of neutrophil colonies, and shorten the time required for neutrophil precursors to mature and appear in the circulation. G-CSF administration for neutropenia has been studied most widely in bone marrow transplantation and oncology, and has been shown to reduce duration of neutropenia and increase nadir level.3–6 However, G-CSF may also lead to slight increases in circulating monocytes.4 The important role monocytes play in transplant rejection raises the question of whether G-CSF may be harmful to graft function.
Few reports on the use of G-CSF in the solid-organ transplantation population have been published, and the efficacy and safety of G-CSF in this group of patients remain unclear.7–17 Most of these studies have been done in the setting of kidney, liver, and pancreas transplantation, and, although the general consensus is that G-CSF is safe within the transplant population, most reports showed some instances of acute rejection after G-CSF administration. In addition, there is more ambiguity about the effect of G-CSF in the HT population as this is the one least studied. In this investigation, we evaluated the safety of G-CSF administration in HT patients and specifically evaluated its effect on the risk of rejection.
Methods
Patient population
Electronic medical records (EMRs) of all adult HT recipients at the University of Chicago Medicine from January 2008 to July 2016 were reviewed. Patients who received G-CSF for treatment of neutropenia were included in the G-CSF group, and those who did not receive G-CSF therapy were included in the non-GCSF group. Decisions to treat or not treat neutropenia with G-CSF were made by clinicians caring for the patients at the point of care. All patients included in this study received filgrastim. Other formulations of G-CSF were given for neutropenia, but these patients were excluded to avoid incongruent data. All patients received immunosuppressive and anti-infective agents according to standard protocol at the University of Chicago Medicine, with adjustments made based on the clinical status of the patient. Data collection was not started until approval was obtained from our institutional review board.
Acute rejection
Episodes of acute rejection in both groups were identified by EMR review. Acute rejection was defined as biopsy-proven cellular rejection that was International Society for Heart and Lung Transplantation (ISHLT) Grade 1B or greater, or non-cellular rejection. The rejection had to be treated with intravenous or oral immunosuppressive therapy to be considered a true episode of acute rejection.
Follow-up and end-points
Patients were followed from time of transplant to death or time of data analysis (October 10, 2016). In the G-CSF group, all episodes of treated neutropenia were identified. We defined the time effect of G-CSF to be 3 months after administration of the medication. All other time periods in which the patients were followed were considered to be free of G-CSF effects. This included time periods before G-CSF administration and the time periods after months 0 to 3 after G-CSF administration. We collected data on rejection episodes during these pre-specified time periods. The primary end-point consisted of the incidence rate per month of acute rejection during months 0 to 3 after administration of G-CSF, and the incidence rate per month of acute rejection during all other times in the patient’s follow-up. This was compared with the overall incidence rate of acute rejection in the non-GCSF group starting 4 months after transplantation. This time period was chosen because the median time to first neutropenic episode was 119.5 days in the G-CSF group, giving us the most matched comparator. An additional primary end-point compared freedom from rejection in the G-CSF group during months 0 to 3 after administration of G-CSF to freedom from rejection in the non-GCSF group. Secondary end-points consisted of left ventricular ejection fraction (LVEF) at end of follow-up and cumulative survival at 3 years. Finally, univariate and multivariate analyses were performed to identify significant predictors of rejection.
Statistical analysis
Continuous variables are expressed as median (interquartile range [IQR]) or as mean ± standard deviation. The independent t-test and Mann–Whitney U-test were used to analyze differences in continuous variables. Categorical variables are expressed as number (%) and compared using either the chi-square test or Fisher’s exact test, as appropriate. Multivariate analysis was conducted to assess whether there were significant associations between risk factors and rejection. Logistic regression analysis was performed to generate odds ratio (OR) and 95% confidence interval (95% CI). Kaplan–Meier curves were generated to describe time to rejection and survival, and tested using log rank test. Incidence rates were calculated utilizing STATA MP version 14 (StataCorp, College Station, TX) and its tables for epidemiologists, specifically incidence-rate ratios. All other statistical analyses were performed using SPSS version 24 (IBM SPSS, Armonk, NY). p < 0.05 was considered significant.
Results
Patients’ characteristics
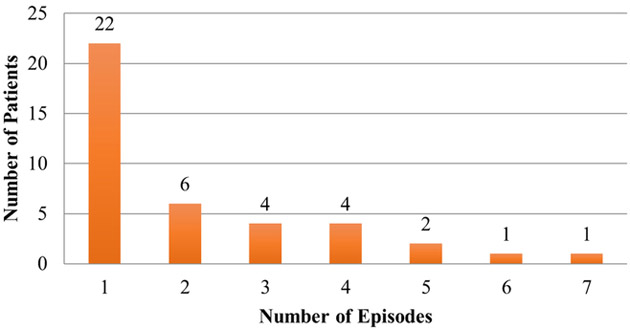
Patients’ characteristics are summarized in Table 1. Of the 222 HT patients studied, 40 (18.0%) received G-CSF for a total of 85 neutropenic events (0.79 event per patient-year). There were no significant differences in baseline characteristics between the 2 groups (Table 1). There was, however, a trend toward significance of older patients (60.5 years vs 56 years, p = 0.07) and high-risk CMV patients (35% vs 20.3%, p = 0.08) in the G-CSF group. Of the 40 patients treated with G-CSF, 22 patients had 1 episode of treated neutropenia, and 18 patients had 2 or more episodes of treated neutropenia (Figure 1). G-CSF doses separated by ≥7 days were considered different courses of therapy. The presumed etiologies of neutropenia were medication-induced in 59 (71%) patients (Table 2). Median time from transplant to first neutropenic event was 119.5 (57.3 to 236.5) days. Median duration of neutropenia was 3 (1 to 7) days. Absolute neutrophil count (ANC) before initiation of G-CSF was 775.0 ± 288.3 K/μL and the average cumulative dose of G-CSF given per neutropenic episode was ± 823.3 μg. The average peak ANC was 4,217.3 ± K/μL, the average nadir ANC was 703.3 ± 312.9 K/μL, and the average increase in ANC per neutropenic episode was 3,521.3 ± 4,371.6 K/μL (Table 3). Initial medication dose changes in response to neutropenia varied; however, in 43.4% of neutropenic episodes, no changes were made (Table 4).
Table 1.
Baseline Characteristics of Patients
| G-CSF group (N = 40) |
Non-G-CSF group (N =182) |
p-value | |
|---|---|---|---|
| Age at transplant, years | 60.50 (54.25 to 64.00) | 56.00 (46.00 to 63.00) | 0.08 |
| Sex | 0.41 | ||
| Male | 29 (72.5%) | 143 (78.6%) | |
| Female | 11 (27.5%) | 39 (21.4%) | |
| Race | |||
| Caucasian | 21 (52.5%) | 102 (56.4%) | 0.68 |
| African American | 16 (40.0%) | 69 (37.9%) | 0.81 |
| Other | 3 (7.5%) | 11 (6.1%) | 0.72 |
| HF etiology | 0.98 | ||
| NICM | 26 (65.0%) | 118 (64.8%) | |
| ICM | 14 (35.50%) | 64 (35.2%) | |
| CMV risk | |||
| Low | 3 (7.5%) | 29 (15.9%) | 0.22 |
| Intermediate | 23 (57.5%) | 116 (63.7%) | 0.46 |
| High | 14 (35%) | 37 (20.3%) | 0.07 |
| Induction agent | |||
| Basiliximab | 21 (52.5%) | 104 (57.1%) | 0.59 |
| Anti-thymocyte globulin | 15 (37.5%) | 69 (37.9%) | 0.96 |
| Basiliximab and anti-thymocyte globulin | 2 (5.0%) | 4 (2.2%) | 0.30 |
| None | 2 (5.0%) | 5 (2.7%) | 0.61 |
| Diabetes | 11 (27.5%) | 39 (21.4%) | 0.41 |
| Lab values before transplant | |||
| Creatinine (mg/dl) | 1.20 (1.00 to 1.95) | 1.4 (1.00 to 1.60) | 1.00 |
| Total bilirubin (mg/dl) | 0.55 (0.40 to 1.00) | 0.70 (0.40 to 1.08) | 0.21 |
| White blood cells (103/μl) | 7.35 (5.43 to 8.35) | 7.20 (5.70 to 8.45) | 0.76 |
| Hemoglobin (g/dl) | 11.43 ± 2.32 | 11.40 ± 1.91 | 0.94 |
| Platelets (103/μl) | 166 (145.00 to 229.75) | 202 (157.00 to 249.00) | 0.17 |
Data expressed as median (interquartile range) or number (%). CMV, cytomegalovirus; G-CSF, granulocyte colony-stimulating factor; HF, heart failure; ICM, ischemic cardiomyopathy; NICM, nonischemic cardiomyopathy.
Data expressed as mean ± standard deviation.
Figure 1.
Patient’s frequency of neutropenia.
Table 2.
Etioiogy of Neutropenia
| Percent (n) | |
|---|---|
| Medication-induced | 71.1% (59) |
| Infection-induced | 12% (10) |
| CMV-induced | 16.9% (14) |
CMV, cytomegalovirus.
Table 3.
Characteristics of Neutropenia
| Median or mean | |
|---|---|
| Time from transplant to first neutropenic episode | 119.5 days (57.3 to 236.5) |
| Duration of neutropenia | 3 (1 to 7) days |
| ANC before G-CSF | 779.2 ± 288.3 K/μl |
| Average dose of G-CSF given per episode | 1,017.2 ± 832.3 μg |
| Average nadir of ANC per episode | 703.3 ± 312.9 K/μl |
| Average peak of ANC per episode | 4,217.3 ± 4,241.3 K/μl |
| Average increase in ANC per episode | 3,521.3 ± 4,371.6 K/μl |
ANC, absolute neutrophil count; G-CSF, granulocyte colony-stimulating factor.
Data expressed as median (interquartile range) or mean +/− standard deviation.
Table 4.
Medication Changes in Response to Neutropenia
| Percent (n) | |
|---|---|
| MMF only | 20.5% (17) |
| TMP-SMX only | 1.2% (1) |
| Valganciclovir only | 7.2% (6) |
| Everolimus only | 1.2% (1) |
| MMF and TMP-SMX | 8.4% (7) |
| MMF and valganciclovir | 8.4% (7) |
| MMF, TMP-SMX, valganciclovir | 9.6% (8) |
| None | 43.4% (36) |
MMF, mycophenolate mofetil; TMP-SMX, trimethoprim–sulfamethoxazole.
Incidence of rejection and freedom from rejection
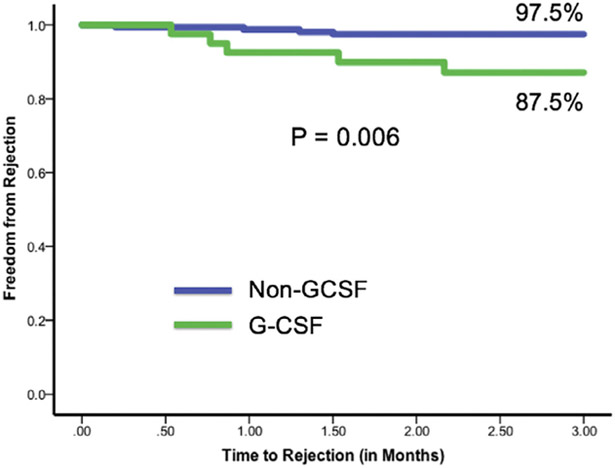
In the G-CSF group, the incidence rate per month of rejection was 0.067 during months 0 to 3 after administration of G-CSF for a total of 11 rejections out of 85 neutropenic episodes. During all other times considered free of G-CSF effect, the incidence rate per month was 0.011. In the non-GCSF group, the overall incidence rate of rejection was 0.010 event per month (Table 5). There was a significant difference between incidence rates in the G-CSF group during months 0 to 3 after G-CSF administration compared with the non-GCSF group (p = 0.04); however, there was no difference between the incidence rates in the G-CSF group during times considered free of G-CSF and the non-GCSF group (p = 0.5). The 3-month freedom from rejection in the G-CSF group from the time of G-CSF administration was 87.5%. In the non-GCSF group, the 3-month freedom from rejection from 4 months post-transplant was 97.5%. There was a statistically significant difference between these groups, with p = 0.006 (Figure 2).
Table 5.
Incidence Rates of Rejection
| Incidence rate (events per month) |
p-value | |
|---|---|---|
| Non-G-CSF group | 0.010 | |
| G-CSF group | ||
| Months 0 to 3 after G-CSF | 0.067 | 0.04 |
| All other time periods | 0.011 | 0.50 |
G-CSF, granulocyte colony-stimulating factor.
Figure 2.
Patients’ freedom from rejection.
Factors associated with rejection
On the basis of univariate analysis, the variables female gender, African American race, ischemic etiology of heart failure, high-risk CMV status, use of both basiliximab and anti-thymocyte globulin for induction, diabetes mellitus, and G-CSF use were chosen based on near statistical significance for the multivariate risk analysis for development of rejection. In the multivariate analysis, African American race (OR 6.1, 95% CI 2.1 to 18.3, p = 0.001), use of both basiliximab and anti-thymocyte globulin for induction (OR 7.8, 95% CI 1.2 to 49.6, p = 0.03), diabetes mellitus (OR 3.48, 95% CI 1.2 to 10.2, p = 0.02), and G-CSF use (OR 33.9, 95% CI 11.1 to 103.7, p < 0.001) were independent risk factors for developing rejection (Table 6).
Table 6.
Multivariable Risk Factor Analysis
| Univariate analyses |
Multivariate analyses |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Female gender | 0.65 | 0.24 to 1.81 | 0.41 | |||
| African American race | 3.30 | 1.48 to 7.34 | 0.03a | 6.10 | 2.04 to 18.3 | 0.001a |
| Ischemic etiology | 0.42 | 0.16 to 1.07 | 0.068 | |||
| High-risk CMV status | 1.26 | 0.52 to 3.03 | 0.61 | |||
| Basiliximab and anti-thymocyte globulin induction | 7.00 | 1.34 to 36.5 | 0.021a | 7.80 | 1.22 to 49.6 | 0.030a |
| Diabetes mellitus | 2.70 | 1.20 to 6.09 | 0.016a | 3.48 | 1.19 to 10.2 | 0.023a |
| G-CSF use | 21.2 | 8.52 to 53.0 | <0.01a | 33.9 | 11.09 to 103.7 | <0.001a |
CI, confidence interval; CMV, cytomegalovirus; G-CSF, granulocyte colony-stimulating factor; OR, odds ratio.
p < 0.05 by using logistic regression analysis.
Ejection fraction and survival
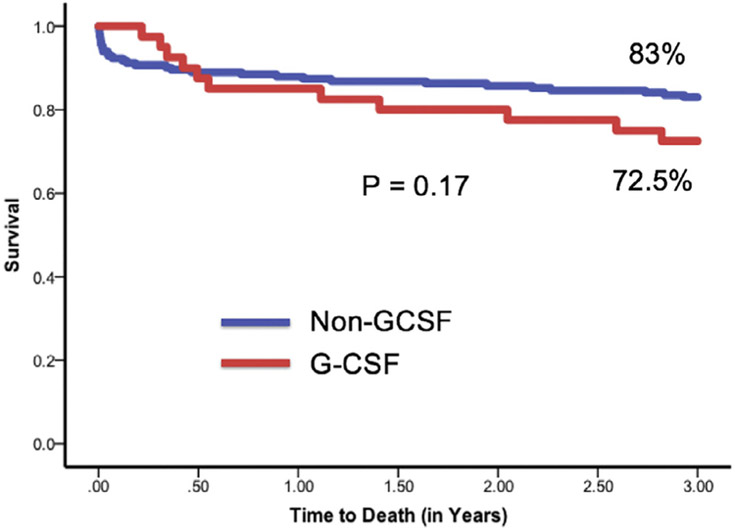
At time of death or end of follow-up, there was no significant difference in LVEF between the non-GCSF and G-CSF groups (59.8% vs 64.7%, p = 0.12). At 3 years, there was a trend toward reduced survival in patients who received G-CSF, but it did not reach statistical significance (83% vs 72.5%, p = 0.17; Figure 3).
Figure 3.
Patients’ survival at 3 years.
Discussion
In this study, we have assessed the safety of G-CSF for the treatment of neutropenia in HT patients and its association with rejection and survival. First, 20% of HT patients had neutropenia requiring G-CSF. Second, in the first 3 months after G-CSF therapy, there was a 6-fold increase in the rate of cellular rejection. Third, G-CSF therapy was found to be a significant and independent predictor of rejection. Last, there was a trend toward increased mortality among patients with neutropenia treated with G-CSF. To our knowledge, this is the first clinical report to suggest an increased risk of rejection with the use of G-CSF for neutropenia in HT patients. An earlier retrospective study suggested a decreased incidence of rejection in HT patients who received G-CSF for neutropenia.18 In that study, patients were evaluated between 2000 and 2007, whereas we evaluated patients between 2008 and 2016. Immunosuppressive regimens have changed over that time period, possibly explaining the differing findings in our study. In addition, the patient population studied by Vrotec et al differed greatly from ours, which may account for the differences in outcome. Our patient population had a higher percentage of African Americans, more with a non-ischemic etiology for heart failure, and more high-risk CMV patients. Other studies in solid-organ transplantation also suggested a decreased risk of rejection in patients treated with G-CSF. Proposed mechanisms to explain this include significant reductions in serum tumor necrosis factor levels, inhibition of T-cell proliferation, and mobilization of regulatory T cells associated with G-CSF treatment.11,19 These conflicting results from our study require further understanding of the proposed mechanisms by which G-CSF affects the risk of rejection.
Neutropenia after HT is mostly due to immunosuppressive medications used to prevent rejection, prophylactic medications used to prevent opportunistic infection, and CMV disease. The induction agents used currently include anti-thymocyte immune globulin and basiliximab. Anti-thymocyte immune globulin is a formulation of polyclonal anti-lymphocyte antibodies produced in rabbits that leads to substantial lymphocyte depletion with complement-dependent opsonization and cell lysis. Granulocytes and platelets are also bound by these antibodies, causing neutropenia and thrombocytopenia in the peripheral circulation.20 The anti-proliferative agents azathioprine (AZA) and mycophenolate mofetil (MMF) are also major contributors to the development of neutropenia. Both prevent the synthesis of DNA and thus proliferation of both T and B lymphocytes. In general, the side effect of myelosuppression is dose-dependent for both medications.21
The calcineurin inhibitors cyclosporine (CsA) and tacrolimus work by inhibiting transcription of interleukin-2 and other cytokines to suppress the immune system. This class of immunosuppressants mainly causes nephrotoxicity and, in general, is not usually implicated in the development of myelosuppression after HT.21 Notably, case reports have shown that neutropenia improves after discontinuation of tacrolimus and improvement with switching to CsA.22,23 Furthermore, a retrospective study examining post-HT patients grouped according to post-operative peripheral cytopenias showed a significant correlation between tacrolimus serum concentration and risk for leukopenia.24 The mechanism by which tacrolimus is thought to cause neutropenia is unknown. Finally, medications used to prevent opportunistic infection represent a commonly identified etiology of neutropenia after HT.
Ganciclovir and valganciclovir are widely used in the prevention of CMV disease after solid-organ transplantation, and commonly cause neutropenia. The mechanism of neutropenia appears to be dose-related inhibition of DNA polymerase in hematopoietic progenitor cells.25 Cessation of these medications can lead to CMV disease, which is also associated with neutropenia. A delicate balance is therefore necessary for the management of these patients. Finally, trimethoprim–sulfamethoxazole (TMP-SMX) is used in post-HT patients for prophylaxis against Toxo-plasma gondii and Pneumocystis jiroveci. Although the effects of TMP-SMX prophylaxis have not been described in post-HT patients, they have been described in the HIV population, who also require infection prophylaxis. In such a study, leukopenia from TMP-SMX was seen in up to 26% of patients.26
The risk of neutropenia as an adverse event after HT has not been formally described in large registries. However, small studies have evaluated the risk of post-HT neutropenia. One study looked at 22 HT patients divided into 2 groups: a cytopenic group and a non-cytopenic group. In comparing the 2 groups, basiliximab induction, higher average tacrolimus concentration, and longer duration of care in the intensive care unit were found to be risk factors for leukopenia after transplantation.24 Another small study sought to understand the role of CMV disease in leukopenia after HT. HT patients with no CMV disease were compared to those with CMV disease. The major findings of the study were that leukopenia developed mostly before the diagnosis of CMV disease, neutrophils and monocytes were most affected by CMV disease, and the reduction of leukocytes during CMV disease was independent of immunosuppression.27 Finally, a recent study evaluated the risk for development of leukopenia in relation to immunosuppressive medications. In the study, 2 groups were identified. Group 1 consisted of patients who were on an immunosuppressive regimen of tacrolimus or CsA, prednisone, and MMF, with the addition of valganciclovir and TMP-SMX for prophylaxis. Group 2 consisted of tacrolimus or CsA, prednisone, and MMF only (the older regimen). In comparing the 2 groups, there was a significant increase in leukopenia in Group 1, implicating valganciclovir and TMP-SMX as the key to development of leukopenia. In the study, decreasing the dose of MMF led to normalization of blood counts without an increase in risk for rejection.28 In our study, we identified a very high rate of neutropenia (18%) concurrent with the immunosuppressive regimen just described for tacrolimus or CsA, prednisone, and MMF, with the addition of valganciclovir and TMP-SMX for prophylaxis. However, we found that those patients had multiple episodes of rejection (0.79 event per patient-year), requiring adjustment of both the immunosuppressive regimen and prophylaxis therapy.
The use of G-CSF for treatment of neutropenia after solid-organ transplant has been evaluated in several small reports, predominantly in kidney and liver transplantation.7–17 Most reports did not show an increased risk of rejection, although the data are mixed. In general, cardiac allografts are viewed as more immunogenic than kidney and liver allografts, which may explain why our findings differ from those of earlier studies with other organs. In fact, several investigators have implied a protective role of the donor liver in inducing a degree of protection toward other tissues.29–31 The mechanism of this protective effect of the liver on other tissues and on preformed antibody states is not clear, although the relative resistance of the liver allograft to antibody damage is well demonstrated.
Our main finding is that the risk of rejection is increased in the early period (first 3 months) after G-CSF administration. This timing is congruent with previous reports in kidney and liver transplantation.7–17 It is important to highlight that, in our study, the risk of rejection in all other time periods was not significantly different from the baseline risk we identified in the rest of the patient population. Neutropenia may itself be protective against rejection; of 85 neutropenic episodes, there were 2 episodes of rejection in the month preceding G-CSF administration compared with 11 afterward. We also found that G-CSF use was an independent and significant predictor of rejection in the multivariate analysis. Our data thus suggest administration of G-CSF is associated with a significant short-term increase in rejection. The mechanism by which this occurs is possibly due to an increase in immune activity from mobilization of neutrophil precursors, although an increased risk of rejection due solely to an increased white blood cell count has not been described in the literature. Our data do show that the average increase in neutrophil count was 3,521.3 ± 4,371.6 K/μL, which represents a significant change and can explain the increased rejection rate in the G-CSF group.
In the first year post-transplant, rejection is a major cause of morbidity and mortality. A review of the Cardiac Transplant Research Database (CTRD) from 1990 to 2008 showed post-transplant death occurs in 2 phases: an early phase <1 year after transplant, and a late phase of low, gradually increasing risk. In the first early phase of acute risk, acute rejection was a cause of death in 12.5% of cases.32 Acute rejection is not a significant contributor to death beyond this acute phase. The implications of our results are thus important, and suggest that patients who receive G-CSF should undergo increased surveillance for rejection during the 3 months after its administration, especially in the first year post-transplant.
Study limitations
This is a retrospective analysis and we recognize the inherent limitations in such a review. In addition, our results are not widely applicable as they are based on chart review at a single institution. Furthermore, the management of neutropenic patients after HT is not standardized, and the doses of G-CSF given can vary widely by institution. Our study calls for a multicenter, prospective registry to assess the safety and efficacy of G-CSF therapy in HT patients.
In conclusion, G-CSF therapy appears to be associated with an increased short-term risk of rejection. This risk of rejection returns to the baseline risk of patients without a history of G-CSF therapy within 3 months after receiving G-CSF. G-CSF therapy has no long-term effects on survival or graft function. As such, enhanced surveillance of rejection in the 3 months after G-CSF use should be considered.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- 1.Rubin RH, Young LS. Clinical approach to infection in the compromised host. 3rd ed New York: Plenum; 1994. [Google Scholar]
- 2.Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature 1989;339:27–30. [DOI] [PubMed] [Google Scholar]
- 3.Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (2). N Engl J Med 1992;327:99–106. [DOI] [PubMed] [Google Scholar]
- 4.Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1). N Engl J Med 1992;327:28–35. [DOI] [PubMed] [Google Scholar]
- 5.Lindemann A, Herrmann F, Oster W, et al. Hematologic effects of recombinant human granulocyte colony-stimulating factor in patients with malignancy. Blood 1989;74:2644–51. [PubMed] [Google Scholar]
- 6.Hartmann LC, Tschetter LK, Habermann TM, et al. Granulocyte colony-stimulating factor in severe chemotherapy-induced afebrile neutropenia. N Engl J Med 1997;336:1776–80. [DOI] [PubMed] [Google Scholar]
- 7.Tajima A, Aso Y, Kawabe K, et al. Colony-stimulating factor for treatment of leukopenia after kidney allografting. Transplant Proc 1991;23:1369–70. [PubMed] [Google Scholar]
- 8.Colquhoun SD, Shaked A, Jurim O, et al. Reversal of neutropenia with granulocyte colony-stimulating factor without precipitating liver allograft rejection. Transplantation 1993;56:1593–5. [PubMed] [Google Scholar]
- 9.Page B, Morin MP, Mamzer MF, et al. Use of granulocyte-macrophage colony-stimulating factor in leukopenic renal transplant recipients. Transplant Proc 1994;26:283. [PubMed] [Google Scholar]
- 10.Squiers EC, Elkhammas EA, Henry ML. Use of granulocyte-macrophage colony-stimulating factor for reversal of neutropenia following combined kidney-pancreas transplantation. Transplant Proc 1995;27:3092–3. [PubMed] [Google Scholar]
- 11.Foster PF, Mital D, Sankary HN, et al. The use of granulocyte colony-stimulating factor after liver transplantation. Transplantation 1995;59:1557–63. [PubMed] [Google Scholar]
- 12.Peddi VR, Hariharan S, Schroeder TJ, et al. Role of granulocyte colony stimulating factor (G-CSF) in reversing neutropenia in renal allograft recipients. Clin Transplant 1996;10:20–3. [PubMed] [Google Scholar]
- 13.Minguez C, Mazuecos A, Ceballos M, et al. Worsening of renal function in a renal transplant patient treated with granulocyte colony-stimulating factor. Nephrol Dial Transplant 1995;10:2166–7. [PubMed] [Google Scholar]
- 14.Kutsogiannis DJ, Crowther MA, Lazarovits AI. Granulocyte macrophage colony-stimulating factor for the therapy of cytomegalovirus and ganciclovir-induced leukopenia in a renal transplant recipient. Transplantation 1992;53:930–2. [PubMed] [Google Scholar]
- 15.Patel HD, Anderson JR, Duncombe AS, et al. Granulocyte colony-stimulating factor. A new application for cytomegalovirus-induced neutropenia in cardiac allograft recipients. Transplantation 1994;58:863–7. [PubMed] [Google Scholar]
- 16.Gordon MS, O’Donnell JA, Mohler ER, et al. The use of granulocyte colony-stimulating factor in the treatment of fever and neutropenia in a heart transplant patient. J Heart Lung Transplant 1993;12:706–7. [PubMed] [Google Scholar]
- 17.Turgeon N, Hovingh GK, Fishman JA, et al. Safety and efficacy of granulocyte colony-stimulating factor in kidney and liver transplant recipients. Transpl Infect Dis 2000;2:15–21. [DOI] [PubMed] [Google Scholar]
- 18.Vrtovec B, Haddad F, Pham M, et al. Granulocyte colony-stimulating factor therapy is associated with a reduced incidence of acute rejection episodes or allograft vasculopathy in heart transplant recipients. Transplant Proc 2013;45:2406–9. [DOI] [PubMed] [Google Scholar]
- 19.Foster PF, Kociss K, Shen J, et al. Granulocyte colony-stimulating factor immunomodulation in the rat cardiac transplantation model. Transplantation 1996;61:1122–5. [DOI] [PubMed] [Google Scholar]
- 20.Lindenfeld J, Miller GG, Shakar SF, et al. Drug therapy in the heart transplant recipient: part I: cardiac rejection and immunosuppressive drugs. Circulation 2004;110:3734–40. [DOI] [PubMed] [Google Scholar]
- 21.Lindenfeld J, Miller GG, Shakar SF, et al. Drug therapy in the heart transplant recipient: part II: immunosuppressive drugs. Circulation 2004;110:3858–65. [DOI] [PubMed] [Google Scholar]
- 22.Amorim J, Costa E, Teixeira A, et al. Tacrolimus-induced neutropenia in a cardiac transplant patient. Pediatr Transplant 2014;18: 120–1. [DOI] [PubMed] [Google Scholar]
- 23.De Rycke A, Dierickx D, Kuypers DR. Tacrolimus-induced neutropenia in renal transplant recipients. Clin J Am Soc Nephrol 2011;6:690–4. [DOI] [PubMed] [Google Scholar]
- 24.Urbanowicz T, Straburzyńska-Migaj E, Klotzka A, et al. Induction therapy, tacrolimus plasma concentration, and duration if intensive care unit stay are risk factors for peripheral leucopenia following heart transplantation. Ann Transplant 2014;19:494–8. [DOI] [PubMed] [Google Scholar]
- 25.Fishman JA, Emery V, Freeman R, et al. Cytomegalovirus in transplantation—challenging the status quo. Clin Transplant 2007;21:149–58. [DOI] [PubMed] [Google Scholar]
- 26.Bozzette SA, Finkelstein DM, Spector SA, et al. A randomized trial of three antipneumocystis agents in patients with advanced human immunodeficiency virus infection. NIAID AIDS Clinical Trials Group. N Engl J Med 1995;332:693–9. [DOI] [PubMed] [Google Scholar]
- 27.Cooke ME, Potena L, Luikart H, et al. Peripheral blood leukocyte counts in cytomegalovirus infected heart transplant patients: impact of acute disease versus subclinical infection. Transplantation 2006;82:1419–24. [DOI] [PubMed] [Google Scholar]
- 28.Pazdernik M, Malek I, Koudelkova E, et al. Bone marrow suppression and associated consequences in patients after heart transplantation: a 6-year retrospective review. Biomed Pap Med Fac Univ Palacky Olo-mouc Czech Repub 2015;159:372–7. [DOI] [PubMed] [Google Scholar]
- 29.Kamada N, Davies HS, Roser B. Reversal of transplantation immunity by liver grafting. Nature 1981;292:840–2. [DOI] [PubMed] [Google Scholar]
- 30.Iwatsuki S, Iwaki Y, Kano T, et al. Successful liver transplantation from crossmatch-positive donors. Transplant Proc 1981;13:286–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Houssin D, Gigou M, Franco D, et al. Specific transplantation tolerance induced by spontaneously tolerated liver allograft in inbred strains of rats. Transplantation 1980;29:418–9. [DOI] [PubMed] [Google Scholar]
- 32.Tallaj JA, Pamboukian SV, George JF, et al. Have risk factors for mortality after heart transplantation changed over time? Insights from 19 years of Cardiac Transplant Research Database study. J Heart Lung Transplant 2014;33:1304–11. [DOI] [PubMed] [Google Scholar]