Abstract
HIV-infected pregnant women face complex decisions about whether and how to disclose their serostatus. Previous studies have shown that HIV disclosure is associated with better care engagement, emotional adjustment to the disease, and reduced risk of HIV transmission, but women face both real and perceived barriers to disclosure. We examined patterns and predictors of HIV disclosure in a cohort of 200 women diagnosed or confirmed to have HIV during antenatal care in the Kilimanjaro region of Tanzania and followed participants to three months postpartum. Twenty women also completed qualitative in-depth interviews during pregnancy and three months postpartum. During the pregnancy period (at least 30 days post-diagnosis), 79.5% of women had disclosed to at least one other person, with disclosures generally restricted to the father of the child and/or a small number of close family members. By three months postpartum, 11.9% of women had still not disclosed to anyone. Women who presented to antenatal care with an established HIV diagnoses and married women were more likely to report disclosures. Social support was positively associated with disclosure. In qualitative interviews, women pointed to community gossip and stigma as barriers to disclosure. Those who had not disclosed to the father of the child noted fears of abandonment during the vulnerable pregnancy period. Despite expressed fears, participants reported overall positive experiences of disclosure that led to increased support. Taken together, these results point to the need for comprehensive, flexible, and culturally informed interventions that assist pregnant and postpartum women in deciding when and how to disclose. Such interventions should acknowledge and explore common barriers to disclosure, including fears of public stigma and personal consequences. Given the strong associations between disclosure, social support, and community stigma, interventions for disclosure should be nested in broader efforts of public education and HIV stigma reduction.
Keywords: Antiretroviral adherence, HIV disclosure, Option B+, Prevention of mother-to-child transmission of HIV (PMTCT), Stigma reduction, Tanzania, Test and treat
Background
For people living with HIV (PLWH), disclosure of one’s serostatus creates an opportunity to access social support, allows openness and exchange of information regarding HIV care-seeking, and reduces the risk of HIV transmission (Chaudoir et al., 2011, Evangeli and Wroe, 2017). While it is important to acknowledge the complexity of disclosure decisions and the potential for negative responses to disclosure, the benefits of making appropriate, well-informed disclosures are clear. In one mathematical modeling study, disclosing one’s serostatus was predicted to decrease the risk of HIV transmission by between 17.9% and 40.6% relative to no disclosure (Pinkerton & Galletly, 2007). Lack of disclosure, by contrast, is frequently associated with feelings of shame, anxiety, fear of mistreatment or abandonment by a partner or others, and poor engagement with HIV care (Knettel et al., 2018; Varni, Miller, McCuin, & Solomon, 2012).
Concerns about the potential negative consequences of HIV disclosure contribute to non-disclosure in many settings, including sub-Saharan Africa (Chaudoir et al., 2011). In a study in Uganda, 31% of HIV-infected adults had not disclosed to their most recent sexual partner (King et al., 2008). This and other regional studies have demonstrated that HIV disclosure leads to positive health outcomes for both the patient and partner, including increased condom use, partner testing, improved care-seeking behavior, social support, emotional well-being, and an improved outlook for the future (King et al., 2008, Lugalla et al., 2012). Despite these positive outcomes, anticipation or fear of stigma and violence are key barriers to disclosure (Lugalla et al., 2012, Chan and Tsai, 2016). Studies consistently report that HIV disclosure may lead to threats or actual violence, stigma, separation/alienation, and expressions of blame (King et al., 2008, Shamu et al., 2014). Such instances reinforce fears of the negative consequences of disclosure and the importance of supporting HIV-infected individuals to make sound, well-informed decisions related to serostatus disclosures (Shamu et al., 2014, Ezebuka et al., 2015).
Serostatus disclosure among pregnant women
The evidence is clear that effective antiretroviral treatment (ART) can virtually eliminate the vertical transmission of HIV from mothers to their children (World Health Organization, 2017). National HIV programs have implemented specific interventions focused on the prevention of mother-to-child transmission of HIV (PMTCT), which include HIV testing for all pregnant women during antenatal care, dedicated clinic space and providers, and targeted adherence counseling for those who test positive (UNAIDS, 2014). However, despite increased global emphasis on improving HIV care among pregnant women (World Health Organization, 2017, UNAIDS, 2014), a recent meta-analysis found lower rates of retention in care among women in prevention of mother-to-child transmission (PMTCT) programs, compared to the general population of people receiving HIV care (Knettel et al., 2018). In light of these findings, research is needed to better understand the reasons for nondisclosure among pregnant women, and particularly nondisclosure to the partner, as well as implications for women’s long-term health, well-being, and transmission risk (Clouse et al., 2014, Psaros et al., 2015).
The emerging literature has demonstrated that serostatus disclosure (or lack thereof) may play a key role in ART adherence and retention in care among pregnant women with HIV (Chinkonde et al., 2009, Ekama et al., 2012, McMahon et al., 2017). A study in Malawi found that pregnant women often disengaged from care out of fear that they would be spotted at the clinic and their HIV status would become known to others (Chinkonde et al., 2009). In a PMTCT study in Nigeria, researchers found that HIV-infected women who had not disclosed their status were at the highest risk of disengagement from care and these women were significantly less likely to give birth in a health facility (Spangler, Onono, Bukusi, Cohen, & Turan, 2014). Thus, increasing early and effective HIV disclosure is likely to improve long-term HIV care engagement among pregnant women (Naigino et al., 2017).
While HIV care engagement is vital during pregnancy to prevent mother-to-child transmission, barriers to disclosure may also be heightened during this period. A systematic review of disclosure studies in sub-Saharan Africa found that disclosure among pregnant and postpartum varied widely across studies, but was generally lower than the general population of people living with HIV (Tam, Amzel, & Phelps, 2015). Women infected with HIV frequently describe concerns about abandonment by their partner and families if their status were to be known, citing emotional, physical, and financial vulnerability during the antepartum period (Naigino et al., 2017, Kiula et al., 2013). A recent study in Tanzania found that only 41% of HIV-infected pregnant women had disclosed their serostatus to their partner (Kiula et al., 2013), while an earlier Tanzanian study found similar prevalence of disclosure (40%) up to four years after pregnancy (Antelman et al., 2001). In both studies, women who were more financially dependent on their partners were significantly less likely to disclose, an indicator that fear of losing support played a role in disclosure decision-making.
In light of concerns about non-disclosure, some clinics have begun to implement programs to encourage disclosure. In one study in the Kilimanjaro region of Tanzania, disclosure to the partner was common (74.9%) and more likely among women who had received disclosure counseling, knew their partner’s status, and were currently cohabitating with the partner (Oshosen, Sabasaba, Ngocho, & Mmbaga, 2016). Some of the clinics in the region had implemented a policy where partners were required to accompany pregnant women to the first antenatal clinic visit for simultaneous HIV counseling and testing, which was hypothesized to contribute to higher rates of disclosure. Similar regional studies have highlighted fears of stigma and experiences of stigma, discrimination, and violence as key barriers to disclosure, suggesting that joint patient-partner testing, improved counseling, and stigma-reduction interventions would be necessary steps for increasing disclosures and subsequently reducing HIV transmission (Naigino et al., 2017).
With the widespread implementation of Option B+ guidelines for PMTCT, whereby all HIV+ pregnant women are initiated on lifelong ART regardless of clinical stage (World Health Organization, 2016), it is crucial to understand the role HIV disclosure in PMTCT care engagement (Coutsoudis et al., 2013, Tenthani et al., 2014). In the current study, we report mixed-methods data on HIV disclosure among a cohort of 200 HIV-infected pregnant women enrolled in PMTCT programs under Option B+ guidelines in Moshi, Tanzania. The objectives of the study were to examine: 1) prevalence of serostatus disclosure to sexual partners, family, and others during pregnancy and three months postpartum; 2) predictors of disclosure, including relevant demographics, anxiety, depression, internalized shame/stigma, perceived social support, and perceived support from the father of the child; and 3) qualitative data on decision-making related to disclosure obtained from in-depth interviews with a subset of cohort participants during pregnancy and three months postpartum.
Methods
We enrolled 200 HIV-infected pregnant women initiating PMTCT care under Option B+ in a prospective cohort study between July 2016 and August 2017. Women were recruited from nine clinical sites in the Kilimanjaro Region of northern Tanzania, with six being in an urban area and three in rural areas. The clinics ranged in size, but together they see approximately 7000–9000 pregnant women per year, with observed prevalence of HIV infection of 4.8%. All of the study sites were part of the Tanzanian National AIDS Control Program, under which all patients receive HIV clinical services, including ART, free of charge, according to the national PMTCT protocol (The United Republic of Tanzania Ministry of Health and Social Welfare, 2013). Under Option B+ guidelines, all pregnant women who test positive for HIV receive standard counseling from a clinic nurse, are prescribed ART on the same day as diagnosis, receive the medication from a clinic-based pharmacy, and are encouraged to commence taking the daily pill on that day, and continue taking it for life (The United Republic of Tanzania Ministry of Health and Social Welfare, 2013). Ongoing clinic engagement typically consists of monthly appointments and medication refills, but some patients may eventually shift to 60- or 90-day intervals between appointments after demonstrating a track record of consistent clinic attendance and self-reported adherence to treatment.
Procedures
Women were eligible to enroll in the cohort if they were at least 18 years of age, mentally competent to provide consent, had been diagnosed with HIV at least one month prior, and were at least 16 weeks pregnant. Potential participants were identified and invited to participate by the PMTCT nurses responsible for scheduling antenatal care (ANC) appointments. As part of routine care, the nurses identify women who are attending their first ANC appointment. When these women presented to the clinic, the PMTCT nurses briefly explained the study and referred interested participants to a private office where they met with a study nurse who provided additional screening and information, then completed informed consent and study enrollment for eligible participants. A separate review of patient medical records found that 436 women attended antenatal appointments at the nine clinic sites during the study period (Watt et al., 2018). A total of 221 of these women were screened to participate in the study. Of these, 4 gave birth prior to enrollment, 9 were not eligible based on the above criteria, and 8 declined to participate.
At baseline and three months after the birth of their child, enrolled participants completed an interviewer-administered survey capturing demographic information, HIV and pregnancy history, HIV disclosure, and key psychosocial variables. Of the 200 women who completed the baseline survey, 168 (84.0%) returned to the clinic for a second survey at 3 months postpartum. A subset of 24 women were selected to complete two additional 60 to 90 minute in-person interviews at baseline and 18 women completed an additional interview at approximately three months postpartum. Interview participants were purposely selected to include both women who were newly diagnosed with HIV during the current pregnancy and those who had established diagnoses prior to the current pregnancy. The interviews explored participant experiences with HIV diagnosis, treatment, disclosure, social support, and coping. Participants received 5000 Tanzanian shillings (approximately $2.40) at each survey and each interview to compensate for their time and travel costs. All participants provided written informed consent to participate in the study.
Surveys and interviews were conducted in Kiswahili by Tanzanian research assistants trained in data collection and receiving ongoing supervision throughout the study. All data collection occurred in private offices at the study clinics. Surveys were orally administered and responses were recorded on a paper form, and then transferred to a secure online database with double data entry and reconciliation to ensure accuracy. Interviews were audio recorded and later transcribed and translated to English by a professional translator. Ten percent of the translations were checked for accuracy by a second translator and confirmed to be of high quality. Study procedures received ethical approval from the institutional review boards at Duke University, Kilimanjaro Christian Medical College, and the Tanzanian National Institute for Medical Research.
Measures
The structured survey took approximately one hour to complete. All measures were created in English and translated to Kiswahili, back-translated, and checked for consistency and cultural compatibility by bilingual researchers. All surveys were administered in Kiswahili.
Demographic and HIV information
The survey included demographic questions related to the participant’s age, education, methods of transportation used, religion, and relationship status. Participants were also asked to report: (a) whether they were diagnosed with HIV during the current pregnancy or whether they had a pre-existing, “established” diagnosis, and (b) whether their current partner or father of the child had been tested for HIV and shared the result with the participant (i.e., “knowledge of partner’s status”).
HIV status disclosure
Our primary outcomes, disclosure of one’s HIV status and change in disclosure status from baseline to postpartum, were measured through five yes/no questions. Participants were first asked, “Have you ever told another person about your HIV status?” Those who responded yes to this first question were then asked, “Have you told your partner/husband?”, “Have you told any other sexual partners?”, “Have you told any family members?”, and “Have you told any friends?” We also report responses to two additional disclosure-related items: length of time from diagnosis to first HIV disclosure and whether anyone had ever disclosed the participant’s status without her consent (yes/no).
Edinburgh postnatal depression scale
To assess symptoms of depression, we administered the Edinburgh Postnatal Depression Scale (EPDS) (Cox, Holden, & Sagovsky, 1987) using a previously validated Kiswahili version with slight adaptations to the local dialect (Kumar, Ongeri, Mathai, & Mbwayo, 2015). The measure has been used and validated extensively, including in Tanzania (Rwakarema, Premji, Nyanza, Riziki, & Palacios-Derflingher, 2015), and is made up of 10 items rating the severity of symptoms from 0 to 3, with a total scale range from 0 to 30. Because of significant positive skew (z=6.98), we dichotomized the variable using the suggested cutoff of 10 or higher for identifying “possible depression” (Cox et al., 1987). Items include: “I have felt sad or miserable” and “I have been so unhappy that I have been crying.” (Cronbach’s α=.875).
Brief symptom inventory – anxiety
To assess symptoms of anxiety, we used the six-item anxiety subscale of the Brief Symptom Inventory (Lang, Norman, Means-Christensen, & Stein, 2009). Participants rated the severity of six symptoms in the past seven days on a scale from 0 (Not at all) to 4 (Extremely), with a total scale range from 0 to 24. Because the responses showed significant positive skew (z=7.64), we dichotomized the variable using the suggested cutoff of 7 or higher for “evidence of signs or symptoms of anxiety” (Derogatis, 1993). Items on the measure include, “Nervousness or shakiness inside,” or “Feeling fearful.” (Cronbach’s α=.920).
HIV shame and internalized stigma
To measure internalized feelings of shame and self-stigma related to one’s HIV diagnosis, we used Scale A (HIV-Related Shame) of the HIV and Abuse Related Shame Inventory (HARSI-A) (Neufeld, Sikkema, Lee, Kochman, & Hansen, 2012). The HARSI-A contains 13 items rated from 0 (Not at all) to 4 (Very much), with a total scale range from 0 to 52. Items include, “I put myself down for becoming HIV+” and “When I tell others I have HIV, I expect them to think less of me.” The measure demonstrated strong psychometric properties in a previous validation study (Neufeld et al., 2012) and excellent internal consistency in the current sample (Cronbach’s α=.856).
Perceived social support - general
To measure the level of support one perceives from her social network, we used the Perceived Availability of Support Scale (PASS) (O’Brien, Wortman, Kessler, & Joseph, 1993). The measure contains 8 items rated 1 (Definitely not) to 5 (Definitely yes), with a total scale range from 8 to 40. Questions include, “Is there someone you could contact if you wanted to talk about an important personal problem you were having?”, and “Is there someone who would help take care of you if you had to stay in bed for several weeks?” (Cronbach’s α=.824).
Social support – father of child
Based on past literature highlighting the importance of partner disclosure for HIV care, we adapted the Norbeck Social Support Questionnaire (NSSQ) (Norbeck, Lindsey, & Carrieri, 1983) to focus specifically on support from the father of the child. The measure contains 7 items rating the level of support from 1 (Not at all) to 5 (A great deal), with a total scale range of 7 to 35. Items include “How much can you confide in this person?”, and “How much does this person make you feel respected or admired?” (Cronbach’s α=.959).
Qualitative interview guides
In-depth interviews were conducted using semi-structured guides that included broad questions about the participant’s pregnancy, HIV diagnosis, feelings about living with HIV, and social support system. The guide included specific probes to explore HIV disclosure, to whom disclosures had been made, when they occurred, and how the other person responded. We also explored the influence of disclosures or non-disclosure on HIV care.
Data analysis
Sum scores were calculated for all continuous scaled measures. Missing items were imputed with the individual mean of completed items when at least 75% of items were completed, a robust method when both the number of participants missing data and the total number of missing items are small, as was the case in our data (Downey and King, 1998, Shrive et al., 2006). Significant intercorrelations were ubiquitous among the continuous variables but none exceeded pre-identified thresholds for multicollinearity (r>.80 or VIF>5.0) (Berry and Feldman, 1985, Kutner et al., 2013), with correlations ranging from r=-.133 to .519, and a mean VIF of 1.266. Therefore, all variables were retained in the analyses.
To describe the sample, we calculated basic frequencies and descriptive statistics for our demographic variables and disclosure items to provide a description of the study sample and overall prevalence of disclosure. We included frequencies of disclosure across subgroups for established diagnosis, age, education, and relationship status. Summary statistics for the continuous variables are presented as medians with interquartile ranges.
Next, we used Poisson regression with robust variance (Zou & Donner, 2013) to examine factors associated with HIV disclosure (to anyone). In this model, we assessed the relative influence on disclosure of depression, anxiety, internalized shame/stigma, perceived social support, and perceived support from the father of the child, while also assessing differences associated with age, education, relationship status, and time of diagnosis. Factors in the regression model were chosen based on published literature showing these variables to be independently associated with HIV disclosure, as well as the qualitative data collected from this cohort. To examine longitudinal changes in disclosure, we completed a second Poisson regression to assess the same variables, measured at baseline, as predictors of any new disclosures between the baseline and 3 months postpartum time points. For both time points, factors with a p-value of .10 or less in preliminary univariable analyses were included in final multivariable regression models.
Qualitative analysis for the in-depth interview data was completed using an inductive, thematic approach based in grounded theory and the constant comparative method (Glaser and Strauss, 1967, Guest et al., 2012). First, each interview transcript was summarized by a single team member in a narrative memo organized around the domains of the interview guide. For the current paper, those domains were (a) disclosure to the partner, (b) disclosure to others, (c) reasons for disclosure, and (d) barriers to disclosure. For each domain, the memo writer coded participant responses into smaller core ideas. Direct quotes from the transcripts were used throughout the memo to support coding, maintain the integrity and context of the participants’ responses, and give examples to illuminate core ideas. After initial coding, all transcripts and memos were reviewed by a second team member to confirm accuracy and clarity, and to review coding. The two team members then engaged in a consensus-building discussion to finalize coding within each transcript. Finally, the qualitative memos were combined and cross-analyzed to explore core ideas across interviews, and representative quotes were selected to provide evidence for the synthesized results.
Results
Description of the sample
At baseline, participants ranged from 18 to 44 years old with a mean of 30 years (SD=6.22). Education levels were varied, although the majority of participants (n=117, 58.5%) reported having a primary school education or less. A majority of participants (n=115, 57.5%) reached the clinic by local minibuses, with a smaller number walking (n=40, 20.0%) or taking a motorcycle taxi (n=30, 15.0%), and the average travel time was 49 minutes each way. All participants self-identified as either Christian (n=139, 69.5%) or Muslim (n=61, 30.5%).
At the time of the pregnancy interview, nearly half of participants (n=98, 49.0%) were married and 72 (36.0%) were in a relationship but not married. A total of 30 (15.0%) were not in a relationship: 21 were single, 6 were separated or divorced, and 3 were widowed. Personal incomes varied widely, from an equivalent of $0 to $960 U.S. dollars per month (median=$48/month, SD=$143). Approximately two-thirds of women (n=138, 69.0%) lived in homes with electricity, and 41 (21%) lived in homes with piped water. A partner or husband was most commonly the primary source of financial support (n=98, 49.0%), although 46 (23.0%) participants were primarily self-supported and 32 (16.0%) received support from a family member.
On average, participants were 28 weeks gestational age at the time of enrollment in the study (range 17 to 41 weeks); 41 women (20.5%) were pregnant for the first time; others, on average, had 2 previous pregnancies (range 1 to 6). About half (n=106, 53.0%) had been diagnosed with HIV prior to the index pregnancy, while 94 (47.0%) received their HIV diagnosis during the index pregnancy. For women with established diagnoses prior to the current pregnancy, there was a mean time since diagnosis of 6 years (range 0 to 16 years). Among all cohort participants, just over half (n=105, 52.5%) responded that they knew their partner’s HIV status, while 64 (32.0%) did not, and 31 (15.5%) were unsure. Among the 105 who knew their partner’s status, 44 (41.9%) reported their partner’s most recent test was negative.
Prevalence of disclosure
At the time of the baseline survey, 159 (79.5%) participants had disclosed their status to any other person outside of the HIV clinic. Among 168 participants who completed surveys at both time points, disclosure increased from 81.5% at baseline to 88.1% at three months (see Table 1). Disclosure to the father of the child increased from 65.5% at baseline to 69.6% at three months. A smaller proportion had disclosed to any family member at baseline (n=79, 47.0%) and three months (n=97, 57.7%). Few participants had disclosed to a friend at baseline (n=16, 9.5%) or three months (n=24, 14.6%). Women who completed the 3 month survey did not significantly differ in their baseline disclosures from those who did not complete the follow up survey (81.5% vs. 68.8%, Χ2=2.70, p=.149).
Table 1.
Frequencies of disclosure at baseline (N=200) and 3 months postpartum (n=168).
| Baseline, full sample (N=200) | Baseline, participants with 3-month data (n=168) | 3-Month Postpartum (n=168) | |
|---|---|---|---|
| Disclosed to anyone | |||
| Yes | 159 (79.5%) | 137 (81.5%) | 148 (88.1%) |
| No | 41 | 31 | 20 |
| Disclosed to father of child | |||
| Yes | 128 (64.0%) | 110 (65.5%) | 117 (69.6%) |
| No | 72 | 58 | 51 |
| Disclosed to any other sexual partners | |||
| Yes | 5 (2.5%) | 4 (2.4%) | 4 (2.4%) |
| No/NA | 192 | 164 | 164 |
| Disclosed to any family | |||
| Yes | 93 (45.5%) | 79 (47.0%) | 97 (57.7%) |
| No | 107 | 89 | 71 |
| Disclosed to a friend | |||
| Yes | 19 (9.5%) | 16 (9.5%) | 24 (14.6%) |
| No | 179 | 152 | 140 |
Among the 159 participants who had disclosed their status to anyone at baseline, 121 (76.1%) disclosed on the same day they were diagnosed with HIV, while one participant reported waiting nearly 9 years before making her first disclosure. Among those who waited beyond the first day, the median time from diagnosis to first disclosure was 45 days. Twelve participants (6.0%) reported that at some point their HIV status had been disclosed by someone else without their consent.
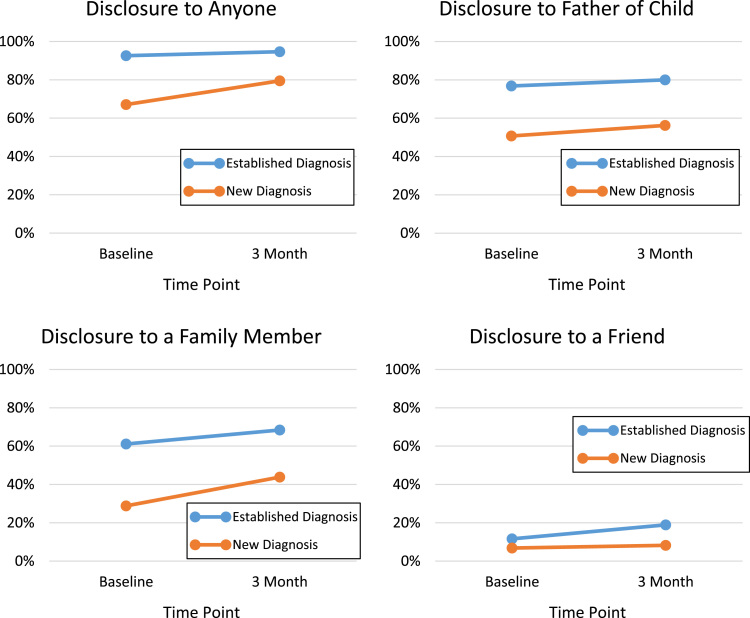
To explore potential challenges of early disclosure for newly diagnosed women, we compared prevalence of disclosure among participants who were diagnosed during the current pregnancy versus those with previously established diagnoses (see Fig. 1). Differences in disclosures to the father of the child were wide between groups at both baseline (76.8% for established diagnoses versus 50.7% for new) and three months postpartum (80.0% and 56.2%, respectively), with little change over time. Disclosures to family members were less common than disclosures to a partner for both groups at baseline (61.1% and 28.8%); however, there was a substantial increase in these disclosures by three months, particularly among women with new diagnoses (68.4% and 43.8%). Disclosure to a friend was rare in both groups at both baseline (11.6% and 6.8%) and three months (18.9% and 8.2%), and the gap between the new and established diagnosis group actually grew across time points, indicating these disclosures may be more likely to occur months or years after one’s initial diagnosis.
Fig. 1.
Disclosures among participants with new (n=73) versus established (n=95) diagnoses, for those with data at both time points.
Factors associated with HIV disclosure at pregnancy
Univariable analyses of predictors of disclosure at baseline (see Table 2) confirmed that participants with an established HIV diagnosis before the current pregnancy were significantly more likely to have disclosed their HIV status than those with a new diagnosis (PrR=1.46, 95% CI [1.25, 1.71], p<.001). Participants who were married (PrR=1.50, 95% CI [1.11, 2.02], p=.008) were also significantly more likely to disclose than those who were not in a relationship. Additionally, both general social support (PrR=1.02, 95% CI [1.00, 1.03], p=.003) and support from the father of the child (PrR=1.02, 95% CI [1.00, 1.02], p<.001) were significantly associated with increased likelihood of disclosure.
Table 2.
Factors associated with disclosure of HIV status at baseline (N=200).
| Disclosedn(%) | No Disclosuren | Univariable Analysis PrR (95% CI) | Multivariable Analysis PrR (95% CI) | |
|---|---|---|---|---|
| HIV diagnosis | ||||
| Newly Diagnosed | 60 (63.8) | 34 | REF | |
| Previously Established | 99 (93.4) | 7 | 1.46 (1.25 – 1.71)*** | 1.43 (1.22 – 1.66)*** |
| Age | ||||
| <25 years old | 34 (73.9) | 12 | REF | |
| 25–35 years old | 88 (81.5) | 20 | 1.10 (0.91 – 1.33) | |
| >35 years old | 37 (80.4) | 9 | 1.09 (0.87 – 1.36) | |
| Education | ||||
| Primary education or less | 96 (82.5) | 21 | 1.08 (0.93 – 1.25) | |
| Secondary or more | 63 (75.9) | 20 | REF | |
| Relationship status | ||||
| Married | 88 (89.8) | 10 | 1.50 (1.11 – 2.02)** | 1.17 (0.84 – 1.63) |
| In a relationship | 53 (73.6) | 19 | 1.23 (0.89 – 1.69) | 1.09 (0.81 – 1.48) |
| Single/Separated/ Divorced/Widow | 18 (60.0) | 12 | REF | REF |
| Depression | ||||
| No depression | 116 (77.3) | 34 | 0.90 (0.78 – 1.04) | |
| Symptoms of depression | 43 (86.0) | 7 | REF | |
| Anxiety | ||||
| No anxiety | 118 (78.7) | 32 | 0.97 (0.82 – 1.14) | |
| Symptoms of anxiety | 38 (80.9) | 9 | REF | |
| Disclosed median (IQR) | No Disclosure median (IQR) | Univariable Analysis PrR (95% CI) | Multivariable Analysis PrR (95% CI) | |
| HIV Shame/Stigma | 16 (12.0 – 25.0) | 19 (13.0 – 26.5) | 1.00 (0.99 – 1.00) | |
| Social Support – General | 29 (24.0 – 35.0) | 26 (21.0 – 31.0) | 1.02 (1.00 – 1.03)** | 1.01 (1.00 – 1.03)* |
| Social Support – Father of Child | 25 (18.0 – 31.0) | 20.5 (12.3 – 25.0) | 1.02 (1.00 – 1.02)*** | 1.01 (0.99 – 1.02) |
Note. *p < .05. **p < .01. ***p < .001. PrR, prevalence ratio. CI, confidence interval. REF, reference category. IQR, interquartile range.
In multivariable follow-up, an established HIV diagnosis (PrR=1.43, 95% CI [1.22, 1.66], p<.001) and greater social support (PrR=1.01, 95% CI [1.00, 1.03], p=.037) remained associated with increased prevalence of HIV disclosure. Relationship status and support from the father of the child were not significantly associated with disclosure in the final model.
Factors associated with new disclosures at three months postpartum
We examined the same baseline variables as potential predictors of new disclosures at 3 months postpartum using a subset of 164 participants who had completed the 3-month follow up survey (n=168) and had not already endorsed disclosures to every category at baseline (n=4). Twenty-nine of these participants (17.3%) had at least one new disclosure at 3 months postpartum. Univariable analysis yielded only one significant predictor of new disclosures, with married participants significantly less likely than non-married women to have new disclosures at 3 months (RR=.42, 95% CI [.19, .93], p=.032). This finding likely reflects that a majority of married women had already disclosed to their husband at baseline, thus limiting opportunities for new disclosures. No other variables were significantly associated with new disclosures at 3 months postpartum.
Qualitative findings
HIV disclosures
The 24 participants who completed a qualitative in-depth interview (IDI) ranged in age from 18–43 with a mean age of 29, similar to the overall cohort. Nineteen of twenty-four IDI participants had disclosed their HIV-positive status at baseline, while five had not disclosed their status to anyone. Four of the five women who had not disclosed were unmarried, and these women consistently expressed fears of being abandoned by a partner if their status became known. They also reported fears of stigma by family members or others in the community. For example, one woman shared, “To be open about this condition, some people do not accept it with understanding. Some receive the information but they begin to panic. I am also scared because in my condition I might lack assistance, and that is why I cannot tell anyone else.” Those in less stable relationships feared disclosure during pregnancy or soon after the birth due to fears of abandonment. For example, several participants reported being in relationships where the partner traveled for extended periods for work, but provided financial assistance from afar. These women expressed concern that disclosure of an HIV diagnosis would lead the partner to end the relationship or withdraw his support during the crucial months around the baby’s birth.
Among women who had disclosed their status, a majority had disclosed to their partner/husband, while disclosures to a sibling, mother, friend, or pastor were present but less common. Notably, none of the participants had disclosed to her father, and disclosures to family members were almost exclusively limited to female relatives. At baseline, few women expressed intent to disclose to additional people in the near future and most expressed either outright opposition or ambivalence to further disclosures. This was confirmed at the follow-up interviews, when only one participant reported a new disclosure, to the priest of her congregation.
Themes surrounding non-disclosure included fear of gossip and resulting stigma. When considering disclosure to a partner or husband, women frequently reported fear of abandonment or withdrawal of support, conflict, or violence. We discuss these reasons for non-disclosure, as well as experiences of disclosure among women who chose to disclose their status.
Fear of gossip and stigma
The most frequently cited reason for non-disclosure of one’s HIV status was fear of gossip and stigma among family and community members. As one participant shared, “Disclosure is not a simple thing because this disease is perceived as shameful and very dangerous.” Another stated, “I don’t wish to make myself dirty because (disclosing your HIV status) is the same as making yourself dirty. It is not a good label to have.” Some shared that a lack of understanding or misinformation surrounding the transmission of HIV led women with HIV to be shunned. For example, women with HIV may be excluded from communal food preparation or clothes-washing and do not receive visitors to their homes. Fears of stigma were particularly salient in the context of motherhood, as gossip and resulting stigma could extend to their children and others in the family. One participant noted her daughter no longer wanted to visit their home village because other children in the family ridiculed her about her mother’s HIV. Similarly, several other women indicated that they had not disclosed their own status in an effort to protect their children from stigma.
In addition to concrete examples of exclusion or mistreatment due to one’s HIV status, women frequently described HIV as a common topic of gossip in community circles. Even in the absence of enacted stigma, being labeled as a person living with HIV was a source of discomfort and could lower one’s standing in the eyes of others. This experience of community stigma greatly influenced quality of life among women with HIV. As one participant described it, “I don’t want to give people a reason to point fingers at me saying, ‘See that one, see that one!’ It’s like dying before your time is due.”
Fear of abandonment, conflict, or violence
Another common theme related to non-disclosure was the fear of abandonment or withdrawal of support. Among participants who had not disclosed their status to their partner/husband, there was frequently fear that the partner might leave the relationship or withdraw financial support. As one participant stated, “The first thing that came to my mind (when I tested positive) was that my husband would leave me or stigmatize me.” Women reported that disclosures were easier when the husband was also known to be positive. However, seven women reported they were in serodiscordant relationships (husband was known to be negative), which led to fears that the husband may accuse them of infidelity and leave the relationship.
Among the nine participants who did not know their partner’s status, six specifically shared that they suspected their partner to be HIV positive but that he either refused to test or had not disclosed the result of prior tests. In these instances, they reported disclosing their own status was easier as they felt they had leverage to argue that their husband had been infected first and therefore “brought the infection into the relationship.” By contrast, in situations where there was more ambiguity surrounding who had been infected first, women felt less confident to disclose. Participants also expressed concern that disclosure could lead to violence in the relationship. “The way I see it, if I tell (my husband), we could quarrel and fight a lot and in this condition that I am in now…I need to be careful with my pregnancy.”
Faith-based acceptance and belief in religious cures
When asked why they had chosen not to disclose their HIV status, women frequently described ‘handing over’ such decisions to the will of God. One participant shared she wished to “just let it be our life secret and let the almighty God do as he plans, and we will continue to live like that.” Similarly, when considering the challenges of disclosing to someone close to them, women regularly reverted to faith-based acceptance of the situation. For example, as one woman shared, “So, I think this is a matter for me to just keep to myself and God, that’s all.” In rare instances, women reported seeking religious cures for their HIV, with one woman waiting to disclose to others until “God has done a miracle” by curing her.
Experiences of disclosure
Despite nearly universal reports of hesitancy and fear to disclose one’s HIV status, the experiences of those who had disclosed their status were generally positive, accepting, and supportive. One participant reported that after she disclosed to her mother, “She became my pillar of support and hope. She was the one reminding me about the clinic days. She would come to my house and tell me, ‘let’s go to the clinic my daughter’“.
Women who had disclosed to their partners also described positive reactions, including a sense of relief that the relationship was open and built on a foundation of trust. This was true even among women who disclosed to their partners in serodiscordant relationships. As one woman shared, “Frankly, he accepted me and told me that I am his wife and he couldn’t leave me.”
As noted previously, women who expressed uncertainty about the source of infection in their relationships were frequently hesitant to disclose their HIV status to their partners. However, women who were confident that their husband’s behavior had been the source of infection, particularly through infidelity, often reported disclosing and confronting their partner. “What motivated me to disclose is I have never cheated on our marriage, so how did I contract HIV while I was with my husband? That was why I couldn’t remain silent.”
Partner testing, disclosure and engagement in HIV care
Some women described testing with their partners at their first antenatal care appointment; in some instances, the clinic had insisted on partner testing. One participant described that prior to testing with her partner, the clinic nurse asked the couple to commit to staying together, no matter the results. Because of this commitment, when the participant tested positive and the partner tested negative, she felt more confident that her partner would not abandon her.
During the baseline interview, women who had not disclosed their status reported that their pregnancy gave them a convenient reason to attend the clinic regularly for HIV care and to take a daily medication, which they could tell others was a vitamin or supplement. At the postpartum interviews, participants described needing to be more secretive about their HIV care, including making excuses to leave the house for appointments and hiding their medication from others. Participants did acknowledge their concerns about being spotted at the HIV clinic by someone they knew, which led some to seek care at clinics further from their homes. While no participants directly reported that they had discontinued care due to fear of disclosure, it was clear that secrecy surrounding their diagnosis led to added stress related to attending appointments and adhering to daily antiretroviral treatment.
Discussion
In the Option B+ era, antenatal care has proven to be an important catch point for HIV testing, diagnosis, and initiation of antiretroviral treatment. However, new mothers also consider themselves to be particularly vulnerable to the consequences of HIV stigma in the months before and after childbirth. As a result, there are substantial barriers to HIV disclosure during this period. Frameworks have been developed to understand the factors involved in disclosure decision-making among the general population (Chaudoir et al., 2011), but few have examined the unique challenges of disclosure for pregnant and postpartum women.
In the current study, we observed rates of disclosure slightly higher than past regional studies with HIV-infected adults and HIV-infected pregnant women, which may have been due in part to improved awareness of the importance of disclosure and clinic-level practices encouraging women to disclose to their partners (Tam et al., 2015, Kiula et al., 2013). However, one in eight participants had still not disclosed to another person at three months postpartum. Expectedly, women with established HIV diagnoses before the current pregnancy were more likely to have made previous disclosures given the longer time since diagnosis, with the gap between these two groups narrowing by approximately half by three months postpartum. Disclosures beyond the partner and immediate family were rare at both time points.
Regression analyses confirmed women with established HIV diagnoses before the current pregnancy were significantly more likely to have disclosed, while married participants were also more likely to have disclosed their HIV status than non-married participants. Additionally, greater social support, both generally and specifically from the father of the child, were significantly associated with disclosure. Three variables associated with emotional coping – anxiety, depression, and HIV shame – were not significantly associated with disclosure at baseline or follow-up. One possibility for this finding is that emotional distress may promote disclosure for some (i.e., lead them to reach out for help or support) and hinder disclosure for others (i.e., lead them to hide their problem).
In qualitative interviews, participants confirmed the cohort findings in describing their caution and selectivity surrounding disclosure. Frequently, disclosures were restricted to sexual partners and trusted female relatives or peers. None of the participants had shared their status widely, largely due to anticipated stigmas in the broader community, including unkind gossip and social exclusion. Among those who had not disclosed to their partner, the perceived stakes were higher, including fears of violence, abandonment, or withdrawal of financial support. Another common rationale for nondisclosure was a form of passive religious acceptance, where women felt the appropriate circumstances for disclosure would materialize if it was “God’s will.” Despite widespread concerns about disclosures, women who had disclosed said that others typically responded to disclosures very positively, providing encouragement, and in many cases became supporters and advocates for their engagement in HIV care.
When the results of the study are compared to common frameworks for HIV disclosure, such as the Disclosure Processes Model (Chaudoir et al., 2011), both similarities and key differences are present. For example, participants made careful appraisals of their goals prior to making disclosures and ultimately disclosed only to a select number of trusted individuals. Those who did disclose typically reported positive responses that resulted in improved HIV-related social support. A key difference in our sample was the focus on fear of abuse, abandonment, and withdrawal of practical support. It appears that pregnant mothers focus less on the emotional consequences of disclosure and more on the practical consequences, especially as they relate to fears for the long-term wellbeing of the baby. Future research may seek to examine this finding and its implications for interventions during the prenatal and postpartum periods.
These findings should be interpreted in light of the study’s limitations. Eligible participants were identified by the study nurses and may reflect women who were more engaged with their care at the clinic. Participants who could be reached for follow up surveys could similarly have been more likely to be engaged in care. Since participants were recruited at the antenatal clinic, we missed women who did not attend antenatal care. Although Tanzanian national PMTCT guidelines recommend disclosure counseling as part of the standard of care (The United Republic of Tanzania Ministry of Health and Social Welfare, 2013), our study did not specifically explore the extent to which counseling was provided or how it was perceived by patients. Disclosure counseling in PMTCT will be an important area for future research and intervention, in order to better integrate disclosure decision-making support into routine care. All measurements were completed by self-report and the quantitative analysis did not examine some potential confounding variables (e.g., financial status, intimate partner violence, food security) and potential sources of measurement bias such as social desirability were not examined. Finally, while this was intended to be a purely descriptive study, several participants noted that it was helpful for them to engage with the study nurses and discuss their experiences with HIV. Therefore, the presence of a compassionate listener who expressed concern about the participant may have influenced participants’ sense of well-being and perhaps even their decision-making surrounding HIV care and disclosure. Strengths of the study include a high rate of follow-up at 3 months postpartum (84.0%), triangulation of data through a mixed methods design, and the examination of unique aspects of the disclosure experiences during the pregnancy and postpartum periods.
We did not encounter any experiences where a disclosure led to violence or abandonment in the current study. However, it should be noted that disclosure may not always be the right decision, particularly in circumstances where one’s physical safety or personal well-being is at stake (Shamu et al., 2014). We agree with the astute assessment of Hutchinson and Dhairyawan that a key, universal goal should be to reduce or remove stigma as a factor in disclosure decision-making (Hutchinson & Dhairyawan, 2017). To date, research on combined HIV disclosure/stigma reduction interventions is scarce, particularly in the pregnancy and postpartum periods. Future studies should examine standard HIV counseling provided in antenatal care, which will inform improvements and ensure that patients receive adequate support and education. Our results point to the need for comprehensive, flexible, and culturally informed stigma interventions that assist patients in deciding when and how to disclose.
Interventions exploring disclosure decision-making should also acknowledge and explore common barriers to disclosure, including fears of public stigma and the potential for personal consequences such as violence or abandonment (Tam et al., 2015). Acknowledging these possibilities may open the door to education regarding their likelihood and build the foundation for accurate cost-benefit assessments to ensure disclosure decisions are well-informed. Some disclosure intervention models have successfully integrated peer mentors, women previously diagnosed with HIV, to share their experiences of diagnosis and disclosure and assist those who are newly diagnosed in navigating these steps (Bateganya et al., 2015, Karwa et al., 2017).
Additionally, given the strong associations between disclosure, social support, and community stigma, interventions for disclosure should be nested in broader efforts toward public education and stigma reduction related to HIV. By fostering a more accepting social environment for women living with HIV, women will be more likely to make disclosure decisions based on their practical and personal circumstances and needs, and not based on fear of mistreatment from others.
Ethics statement
Study procedures received ethical approval from our institutions.and conform to ethical standards for research with human participants. No financial conflicts of interest exist in relation to this research that should be disclosed. Each of the authors contributed meaningfully to the preparation of this manuscript.
Funding
This study was funded by a grant from the NIH National Institute of Allergies and Infectious Diseases (NIAID), Grant R21 AI124344. We also acknowledge support received from the Duke Center for AIDS Research (P30 AI064518).
References
- Antelman G., Smith Fawzi M.C., Kaaya S., Mbwambo J., Msamanga G.I., Hunter D.J., Fawzi W.W. Predictors of HIV-1 serostatus disclosure: A prospective study among HIV-infected pregnant women in Dar es Salaam, Tanzania. AIDS. 2001;15(14):1865–1874. doi: 10.1097/00002030-200109280-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateganya M.H., Amanyeiwe U., Roxo U., Dong M. Impact of support groups for people living with HIV on clinical outcomes: A systematic review of the literature. Journal of Acquired Immune Deficiency Syndromes. 2015;68:S368–S374. doi: 10.1097/QAI.0000000000000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry W.D., Feldman S. Multiple regression in practice. 1st ed. SAGE; Thousand Oaks, CA: 1985. [Google Scholar]
- Chan B.T., Tsai A.C. Trends in HIV-related stigma in the general population during the era of antiretroviral treatment expansion: An analysis of 31 Sub-Saharan African Countries. Journal of Acquired Immune Deficiency Syndromes (1999) 2016;72(5):558–564. doi: 10.1097/QAI.0000000000001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudoir S.R., Fisher J.D., Simoni J.M. Understanding HIV disclosure: A review and application of the Disclosure Processes Model. Social Science & Medicine (1982) 2011;72(10):1618–1629. doi: 10.1016/j.socscimed.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkonde J.R., Sundby J., Martinson F. The prevention of mother-to-child HIV transmission programme in Lilongwe, Malawi: Why do so many women drop out. Reproductive Health Matters. 2009;17(33):143–151. doi: 10.1016/S0968-8080(09)33440-0. [DOI] [PubMed] [Google Scholar]
- Clouse K., Schwartz S., Van Rie A., Bassett J., Yende N., Pettifor A. “What they wanted was to give birth, nothing else”: Barriers to retention in Option B+ HIV care among postpartum women in South Africa. Journal of Acquired Immune Deficiency Syndromes. 2014;67(1):e12–8. doi: 10.1097/QAI.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutsoudis A., Goga A., Desmond C., Barron P., Black V., Coovadia H. Is Option B+ the best choice? Lancet. 2013;381(9863):269–271. doi: 10.1016/S0140-6736(12)61807-8. [DOI] [PubMed] [Google Scholar]
- Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Derogatis L.R. Brief symptom inventory: Administration, scoring, and procedures manual. 4th ed. National Computer Systems; Minneapolis, MN: 1993. [Google Scholar]
- Downey R.G., King C. Missing data in Likert ratings: A comparison of replacement methods. The Journal of General Psychology. 1998;125(2):175–191. doi: 10.1080/00221309809595542. [DOI] [PubMed] [Google Scholar]
- Ekama S.O., Herbertson E.C., Addeh E.J., Gab-Okafor C.V., Onwujekwe D.I., Tayo F., Ezechi O.C. Pattern and determinants of antiretroviral drug adherence among Nigerian Pregnant Women. Journal of Pregnancy. 2012:2012. doi: 10.1155/2012/851810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangeli M., Wroe A.L. HIV disclosure anxiety: A systematic review and theoretical synthesis. AIDS and Behavior. 2017;21(1):1–11. doi: 10.1007/s10461-016-1453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezebuka O., Sam-Agudu N., Erekaha S., Dairo M. Correlates of intimate partner violence among HIV-positive women in southwest Nigeria. The Lancet Global Health. 2015;3:S23. [Google Scholar]
- Glaser B.G., Strauss A.L. Aldine; Chicago, IL: 1967. The discovery of grounded theory: Strategies for qualitative research. [Google Scholar]
- Guest G., MacQueen K.M., Namey E.E. SAGE; Thousand Oaks, CA: 2012. Applied thematic analysis. [Google Scholar]
- Hutchinson P., Dhairyawan R. Shame and HIV: Strategies for addressing the negative impact shame has on public health and diagnosis and treatment of HIV. Bioethics. 2017 doi: 10.1111/bioe.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwa R., Maina M., Mercer T., Njuguna B., Wachira J., Ngetich C., Pastakia S. Leveraging peer-based support to facilitate HIV care in Kenya. PLOS Medicine. 2017;14(7) doi: 10.1371/journal.pmed.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R., Katuntu D., Lifshay J., Packel L., Batamwita R., Nakayiwa S., Bunnell R. Processes and outcomes of HIV serostatus disclosure to sexual partners among people living with HIV in Uganda. AIDS and Behavior. 2008;12(2):232–243. doi: 10.1007/s10461-007-9307-7. [DOI] [PubMed] [Google Scholar]
- Kiula E.S., Damian D.J., Msuya S.E. Predictors of HIV serostatus disclosure to partners among HIV-positive pregnant women in Morogoro, Tanzania. BMC Public Health. 2013;13:433. doi: 10.1186/1471-2458-13-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knettel B.A., Cichowitz C., Ngocho J.S., Knippler E.T., Chumba L.N., Mmbaga B.T., Watt M.H. Retention in HIV Care during pregnancy and the postpartum period in the option B+ Era: A systematic review and meta-analysis of studies in Africa. Journal of Acquired Immune Deficiency Syndromes. 2018;77(5):427–438. doi: 10.1097/QAI.0000000000001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Ongeri L., Mathai M., Mbwayo A. Translation of EPDS questionnaire into Kiswahili: Understanding the cross-cultural and translation issues in Mental Health Research. Journal of Pregnancy and Child Health. 2015;2(1) doi: 10.4172/2376-127X.1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner M., Nachtsheim C., Neter J. Applied linear statistical models. 5th ed. McGraw-Hill; New York, NY: 2013. [Google Scholar]
- Lang A.J., Norman S.B., Means-Christensen A., Stein M.B. Abbreviated brief symptom inventory for use as an anxiety and depression screening instrument in primary care. Depression and Anxiety. 2009;26(6):537–543. doi: 10.1002/da.20471. [DOI] [PubMed] [Google Scholar]
- Lugalla J., Yoder S., Sigalla H., Madihi C. Social context of disclosing HIV test results in Tanzania. Culture, Health & Sexuality. 2012;14(Suppl. 1):S53–S66. doi: 10.1080/13691058.2011.615413. [DOI] [PubMed] [Google Scholar]
- McMahon S.A., Kennedy C.E., Winch P.J., Kombe M., Killewo J., Kilewo C. Stigma, facility constraints, and personal disbelief: Why women disengage from HIV care during and after pregnancy in Morogoro Region, Tanzania. AIDS and Behavior. 2017;21(1):317–329. doi: 10.1007/s10461-016-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naigino R., Makumbi F., Mukose A., Buregyeya E., Arinaitwe J., Musinguzi J., Wanyenze R.K. HIV status disclosure and associated outcomes among pregnant women enrolled in antiretroviral therapy in Uganda: A mixed methods study. Reproductive Health. 2017:14. doi: 10.1186/s12978-017-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld S.A.S., Sikkema K.J., Lee R.S., Kochman A., Hansen N.B. The development and psychometric properties of the HIV and Abuse Related Shame Inventory (HARSI) AIDS and Behavior. 2012;16(4):1063–1074. doi: 10.1007/s10461-011-0086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbeck J.S., Lindsey A.M., Carrieri V.L. Further development of the Norbeck Social Support Questionnaire: Normative data and validity testing. Nursing Research. 1983;32(1):4–9. [PubMed] [Google Scholar]
- O’Brien K., Wortman C.B., Kessler R.C., Joseph J.G. Social relationships of men at risk for AIDS. Social Science & Medicine (1982) 1993;36(9):1161–1167. doi: 10.1016/0277-9536(93)90236-w. [DOI] [PubMed] [Google Scholar]
- Oshosen M., Sabasaba A., Ngocho J.S., Mmbaga B.T. Prevalence and factors associated with HIV status disclosure among pregnant and lactating women on antiretroviral treatment after rollout of option B Plus in Urban Moshi Tanzania. Global Journal of Epidemiology and Public Health. 2016;3:8–15. [Google Scholar]
- Pinkerton S.D., Galletly C.L. Reducing HIV transmission risk by increasing serostatus disclosure: A mathematical modeling analysis. AIDS and Behavior. 2007;11(5):698–705. doi: 10.1007/s10461-006-9187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaros C., Remmert J.E., Bangsberg D.R., Safren S.A., Smit J.A. Adherence to HIV care after pregnancy among women in sub-Saharan Africa: Falling off the cliff of the treatment cascade. Current HIV/AIDS Reports. 2015;12(1):1–5. doi: 10.1007/s11904-014-0252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rwakarema M., Premji S.S., Nyanza E.C., Riziki P., Palacios-Derflingher L. Antenatal depression is associated with pregnancy-related anxiety, partner relations, and wealth in women in Northern Tanzania: A cross-sectional study. BMC Women’s Health. 2015;15 doi: 10.1186/s12905-015-0225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamu S., Zarowsky C., Shefer T., Temmerman M., Abrahams N. Intimate partner violence after disclosure of HIV test results among pregnant women in Harare, Zimbabwe. PLOS ONE. 2014;9(10):e109447. doi: 10.1371/journal.pone.0109447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrive F.M., Stuart H., Quan H., Ghali W.A. Dealing with missing data in a multi-question depression scale: A comparison of imputation methods. BMC Medical Research Methodology. 2006;6(1):57. doi: 10.1186/1471-2288-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler S.A., Onono M., Bukusi E.A., Cohen C.R., Turan J.M. HIV-positive status disclosure and use of essential PMTCT and maternal health services in rural Kenya. Journal of Acquired Immune Deficiency Syndromes (1999) 2014;67(Suppl. 4):S235–S242. doi: 10.1097/QAI.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M., Amzel A., Phelps B.R. Disclosure of HIV serostatus among pregnant and postpartum women in sub-Saharan Africa: a systematic review. AIDS Care. 2015;27(4):436–450. doi: 10.1080/09540121.2014.997662. [DOI] [PubMed] [Google Scholar]
- Tenthani L., Haas A.D., Tweya H., Jahn A., van Oosterhout J.J., Chimbwandira F., Ie D.E.A.S.A. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women ('Option B+’) in Malawi. AIDS. 2014;28(4):589–598. doi: 10.1097/QAD.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The United Republic of Tanzania Ministry of Health and Social Welfare. (2013). National guidelines for comprehensive care services for prevention of mother-to-child transmission of HIV and keeping mothers alive.
- UNAIDS. (2014). 2014 progress report on the global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva, Switzerland. Retrieved from 〈http://www.unaids.org/en/resources/documents/2014/JC2681_2014-Global-Plan-progress〉.
- Varni S.E., Miller C.T., McCuin T., Solomon S.E. Disengagement and engagement coping with HIV/AIDS stigma and psychological well-being of people with HIV/AIDS. Journal of Social and Clinical Psychology. 2012;31(2):123–150. doi: 10.1521/jscp.2012.31.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M.H., Cichowitz C., Kisigo G., Minja L., Knettel B.A., Knippler E., Mmbaga B.T. Predictors of postpartum HIV care engagement for women enrolled in prevention of mother-to-child transmission (PMTCT) programs in Tanzania. AIDS Care. 2018 doi: 10.1080/09540121.2018.1550248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2016). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Retrieved from 〈http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf〉. [PubMed]
- World Health Organization. (2017). Global guidance on criteria and processes for validation: Elimination of mother-to-child transmission of HIV and syphilis. Retrieved from 〈http://www.who.int/reproductivehealth/publications/emtct-hiv-syphilis/en/〉.
- Zou G., Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Statistical Methods in Medical Research. 2013;22(6):661–670. doi: 10.1177/0962280211427759. [DOI] [PubMed] [Google Scholar]