Introduction
Dupilumab is a selectively immunosuppressive biologic therapy that treats chronic moderate-to-severe atopic dermatitis through inhibiting signaling transmission of interleukins (IL) 4 and 13. It is often prescribed when patients do not respond to or cannot tolerate conventional topical or systemic therapies. Most commonly reported side effects include injection site reactions, eye and eyelid inflammation, and nasopharyngitis.1, 2 This case report highlights a patient who experienced drug-induced alopecia at 18 weeks on dupilumab.
Case report
A 27 year-old Hispanic man was started on dupilumab after not responding to several topical steroids, tacrolimus ointment, oral steroids, and oral antihistamines. His baseline body surface area affected was 8% with an Investigator's Static Global Assessment of 3. He noted immediate improvements in his condition and quality of life after a few doses of dupilumab. The patient had an affected body surface area of 6% and Investigator's Static Global Assessment of 1 at 18 weeks on dupilumab when he noted increased hair loss on the scalp. The medication was otherwise well tolerated by the patient, and he reported no other significant side effects. Hair loss did not subside with daily clobetasol 0.05% foam and 3-times weekly ketoconazole 2% shampoo use. Diffuse pink background erythema with ill-defined areas of nonscarring alopecia on the crown and temporal scalp were noted on examination (Fig 1) Punch biopsy results showed alopecia areata-like hair miniaturization with peribulbar chronic inflammation and severe sebaceous gland atrophy (Figs 2 and 3).
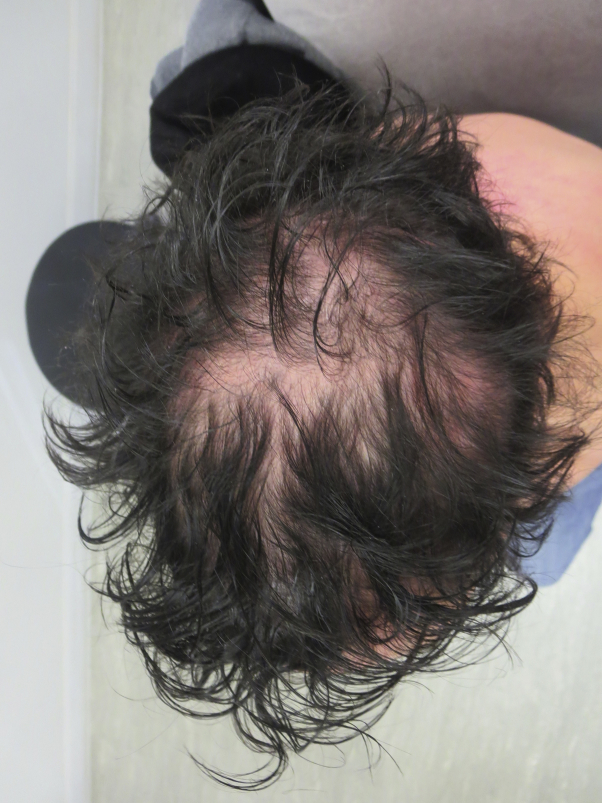
Fig 1.
Drug-induced alopecia areata–like reaction at 18 weeks on dupilumab. The reaction was noted throughout the entire scalp but was accentuated on the vertex and crown.
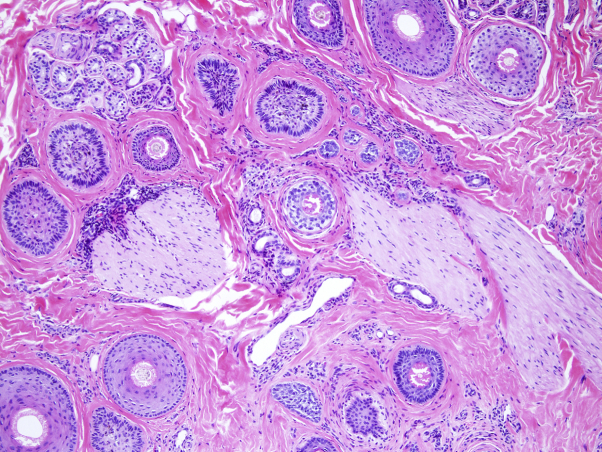
Fig 2.
Most hairs are miniaturized, and many of the hairs are in the catagen/telogen phase. Some mild chronic inflammation is evident.
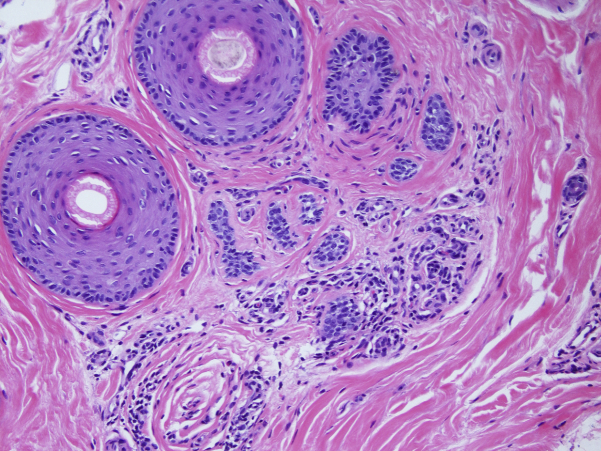
Fig 3.
In this image, 2 anagen phase and 1 telogen phase hairs are seen, but sebaceous glands are severely atrophic and can barely be recognized as sebaceous structures. Mild, chronic perivascular inflammation can also be appreciated at this level.
We speculated that if dupilumab was stopped and normal hair regrowth resumed, circumstantial evidence would point to a drug-induced alopecia rather than true alopecia areata. The patient discontinued dupilumab, continued topical triamcinolone acetonide 0.1% ointment to the scalp alternating weekly with tacrolimus 0.1% ointment, and received 1.3 mL of 10 mg/mL intralesional triamcinolone acetonide to approximately 30 sites. No remaining areas of alopecia, scale, or erythema were noted on examination 8 weeks later (Fig 4). Our patient's recovery was faster than anticipated given the extent of hair loss that he had compared with the minimal amount of intralesional steroid treatments administered.
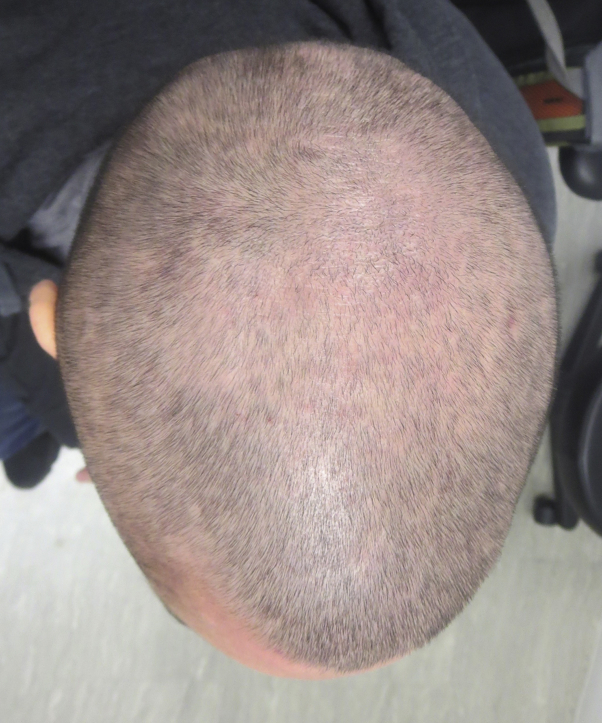
Fig 4.
Reversal of hair loss 8 weeks after discontinuing dupilumab and after 1 round of intralesional triamcinolone acetonide injections.
Discussion
The underlying autoimmune processes of alopecia areata are not easily illuminated, so the relationship between use of dupilumab and development of alopecia in this patient is strongly correlated but cannot be definitely declared as causative. Research has found that both atopic dermatitis and alopecia areata are involved in type 2 T-helper (Th) cytokine regulation.3, 4 Specifically, the expression of IL-4 and IL-13 has been positively correlated with the expression of sebaceous gland signatures.5 The mechanism of dupilumab in interrupting IL-4 and IL-13 processes in Th2-mediated inflammation and sebaceous gland development could therefore provoke an imbalance in interleukin milieu that contributes to sebaceous gland atrophy and nonscarring alopecia. Sebaceous gland atrophy is a feature of other drug-induced alopecia areata–like reactions and other inflammatory skin conditions such as psoriasis, but it is not seen in alopecia areata.6 More investigation is needed to establish how these pathologic conditions may further overlap; for example, the Th17/IL-23 pathway is one of many currently being investigated for its contribution to both atopic dermatitis and alopecia areata.4, 7, 8
One other reported case describes similar hair loss with dupilumab, and our case further lends support to this possible side effect.9 Conversely, one recently published case report describes effective treatment of both atopic dermatitis and pre-existing alopecia areata while on dupilumab.10 This difference in clinical outcomes, in conjunction with the observed difference in histologic features of drug-induced alopecia versus alopecia areata, suggests a phenotype for dupilumab-induced alopecia distinct from a true alopecia areata. Scalp biopsy of patients clinically suspicious for drug-induced alopecia is recommended to rule out this pathophysiology and guide treatment. It is important to make prescribers aware of newly discovered phase IV, postmarketing associations when they consider dupilumab with their patients in a clinical setting.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Beck L.A., Thaçi D., Hamilton J.D. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 2.Simpson E.L., Bieber T., Guttman-Yassky E. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 3.Werfel T., Allam J.P., Biedermann T. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138(2):336–349. doi: 10.1016/j.jaci.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Suárez-Fariñas M, Ungar B, Noda S et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136(5):1277-1287. [DOI] [PubMed]
- 5.Rittié L., Tejasvi T., Harms P.W. Sebaceous gland atrophy in psoriasis: an explanation for psoriatic alopecia? J Invest Dermatol. 2016;136:1792–1800. doi: 10.1016/j.jid.2016.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle L.A., Sperling L.C., Baksh S. Psoriatic alopecia/alopecia areata-like reactions secondary to anti-tumor necrosis factor-α therapy: a novel cause of noncicatricial alopecia. Am J Dermatopathol. 2011;33(2):161–166. doi: 10.1097/DAD.0b013e3181ef7403. [DOI] [PubMed] [Google Scholar]
- 7.Noda S., Shroff A., Mansouri Y. Alopecia areata and atopic dermatitis share common Th2 and IL-23 inflammatory pathways, with implications for targeted therapeutics. J Invest Dermatol. 2015;135:S119. [Google Scholar]
- 8.Koga C., Kabashima K., Shiraishi N. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128(11):2625–2630. doi: 10.1038/jid.2008.111. ISSN 0022-202X. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell K., Levitt J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4(2):143–144. doi: 10.1016/j.jdcr.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darrigade A.S., Legrand A., Andreu N. Dual efficacy of dupilumab in a patient with concomitant atopic dermatitis and alopecia areata. Br J Dermatol. 2018;179(2):534–536. doi: 10.1111/bjd.16711. [DOI] [PubMed] [Google Scholar]