Anaplasma spp. are gram-negative obligate intracellular bacteria of remarkable importance in both human and veterinary health. Six Anaplasma species have currently been classified in the genus Anaplasma and are known as causative agents of tick-borne diseases1. The impacts of the diseases caused by Anaplasma on the productivity and health of animals have been recognized for over a century, and it is still a significant threat to livestock industries2. A zoonotic role has recently been uncovered for Anaplasma phagocytophilum, resulting in a rising interest in these organisms3. Anaplasma phagocytophilum has been considered the main cause of human anaplasmosis worldwide in recent years. Recently, a potential novel tick-transmitted Anaplasma species was identified in asymptomatic goats in China, and it has been recognized as a cause of human infection; the name “Anaplasma capra” was proposed provisionally4. Sequence and phylogenetic analyses of “Anaplasma capra” based on different gene loci revealed that this organism was distinct from all known Anaplasma species4. Although “A. capra” has not yet been formally recognized, human cases of “A. capra” infection have been recorded in northeastern China, and subsequent reports have also shown that “A. capra” seems to be widely distributed in China4–7, suggesting this agent may pose a substantial public health concern and may be responsible for anaplasmosis cases of unknown cause.
Wild animals are important reservoirs for tick-borne pathogens. The zoonotic A. phagocytophilum has been identified in a great number of wild animal species2. In previous reports, specific DNA of “A. capra” has been detected in domestic and some species of wild animals5,7–9, and “A. capra”-like bacteria were identified in ticks10, indicating “A. capra” might have a broad host range and genetic diversity.
This study was conducted in wild animals from Tangjiahe National Nature Reserve, which is a national protected area located in Sichuan Province, China (Supplementary Figure S1). This reserve is one of the biodiversity hotspots in the world and is an important natural habitat for a great number of endangered wildlife. From March 2016 to April 2017, 13 dead free-ranging wild animals were found in the field, including the first-grade nationally protected takin (Budorcas taxicolor) (five) and forest musk deer (Moschus berezovskii) (one); the second-grade nationally protected Himalayan goral (Naemorhedus goral) (three); and two nonendangered protected species, Reeves’s muntjac (Muntiacus reevesi) (three), and wild boar (one). Blood or liver samples were collected individually from animals, frozen at −20 °C, and transported in a freezer pack to a laboratory immediately. The total DNA was extracted using a QIAamp DNA Mini Kit according to the protocol. This study was approved by the Animal Ethics Committee of Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences.
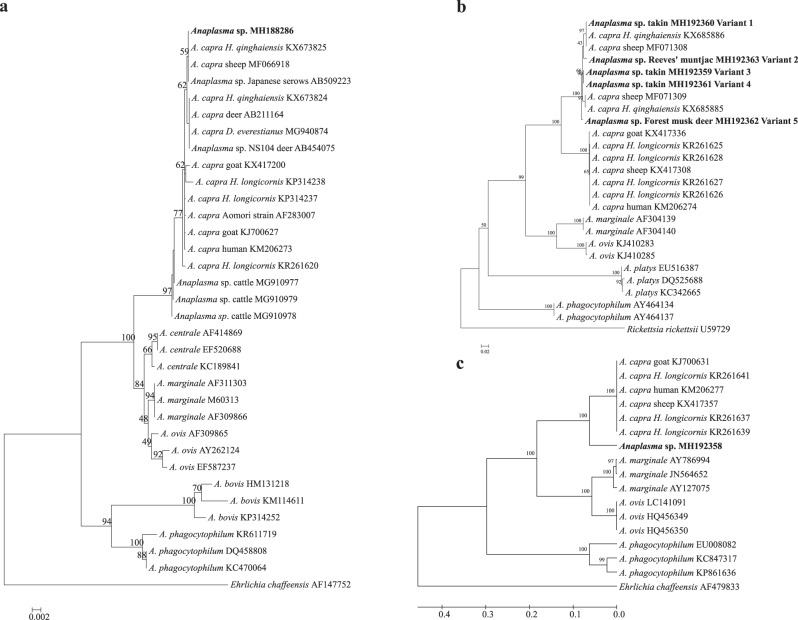
AllDNA samples were screened for the presence of “A. capra” by nested PCR targeting on gltA gene as previously described4,10. Positive (DNA extracted from a goat infected by “A. capra”, KX417336) and negative controls (double-distilled water) were included in each assay. “Anaplasma capra” was detected in six wild animals, including three takins, two Reeves’s muntjacs, and one forest musk deer (Supplementary Table S1). To further characterize the “A. capra” strains identified in this study, the 16S rRNA and msp4 genes were amplified from the gltA gene-positive samples5,10. The PCR products of the partial 16S rRNA (1261 bp), gltA(594 bp), and msp4(656 bp) gene were purified using the AxyPrep DNA gel extraction kit (Axygen, USA), cloned (pGEM-T Easy vector, Promega, USA) and sequenced (GenScript, Nanjing, China). Sequence analysis revealed that the 16S rRNA sequences (MH188286) obtained in this study were 100% identical to each other and to those of “A. capra” isolates identified from sheep (MF066918), H. qinghaiensis ticks (KX673825) and Japanese serows (AB509223); and they were 99.8% identical to those of the isolates identified from humans (HLJ-14, KM206273) and H. longicornis ticks (KP314237). Phylogenetic analysis based on the 16S rRNA gene demonstrated that the isolates were clustered within the clade inside the“A. capra” but distinct from other, well-defined Anaplasma species (Fig. 1a).
Fig. 1.
Phylogenetic analysis of “Anaplasmacapra” based on partial sequences of the 16S rRNA (1261 bp, a), gltA (594 bp, b), and msp4 genes (656 bp, c). Phylogenetic trees were constructed based on the sequence distance method using the neighbor-joining (NJ) algorithm with the Kimura two-parameter model in MEGA 4.0 software. Bootstrap analysis was performed with 1000 replicates. Ehrlichia chaffeensis or Rickettsia rickettsii was used as the outgroup. Boldface indicates the sequences obtained in this study
According to the gltA sequence alignment, five sequence variants (MH192359–MH192363) with 2–6 bp nucleotide substitutions were obtained and showed 98.4–100% identity to those of “A. capra” isolates from sheep (MF071308 and MF071309) and H. qinghaiensis ticks (KX685885 and KX685886), but they were 87.2–88.3% identical to the isolate HLJ-14 from human (KM206274). Moreover, the msp4sequences of these isolates showed 88.4% identity to “A. capra” isolate HLJ-14 (KM206277). Phylogenetic analysis of both gltA and msp4 gene sequences revealed that the “A. capra” strains were clustered independently from Anaplasma species identified previously and formed two distinct subclades with high bootstrap values. The isolates identified in this study were closely related to “A. capra” isolates from H. qinghaiensis and sheep but clearly distinct from human isolate HLJ-14 (Fig. 1b, c).
Anaplasma circulation in nature involves tick vectors and a great number of vertebrates that act as hosts and sources of infection for ticks, animals, and humans2. Since Anaplasma spp. are transmitted by ixodid ticks transstadially rather than transovarially, the reservoir hosts play a critical role in the maintenance and dispersal of these bacteria11. Numerous wild animal species have been considered competent reservoir hosts for Anaplasma, especially wild ruminants11, such as red deer (Cervus elaphus), roe deer (Capreolus capreolus), white-tailed deer(Odocoileus virginianus), mouflon (Ovis musimon), and chamois (Rupicapra rupicapra)11. In the present study, the novel tick-transmitted zoonotic “A. capra” was first identified in free-ranging wild animals (takin, Reeves’s muntjac, and forest musk deer) from a national nature reserve, where contact between wild and domestic animals rarely occurs. These results suggest that “A. capra” is endemic in this protected area and that those wild animals can act as competent sylvatic reservoirs for this organism.
The 16S rRNA gene sequence of “A. capra” was first described in cattle from Japan in 200112, and the agent was mistakenly assumed to be A. centrale (Aomori strain, AF283007) (Fig. 1a)13. More recently, this novel Anaplasma species was recorded in goats from northeastern China, and it was subsequently identified as a human pathogen in an active surveillance of patients who sought treatment after a tick bite4. “Anaplasma capra” has been detected in several tick species, including Ixodes persulcatus, Haemaphysalis longicornis, and Haemaphysalis qinghaiensis4,6,10; however, the vector competence of these ticks for transmission of “A. capra” is still unclear and needs to be further evaluated. The infection of “A. capra” has also been reported in sheep and goats from several provinces of China5, in deer (Anaplasma sp. NS104, AB454075) and free-living serows (Anaplasma sp. Kamoshika17, AB509223) from Japan8, and in cattle (MG910977–9) from Malaysia9. Those findings together with the results obtained in this study suggested that additional tick species and/or domestic and wild animals might be involved in the transmission and maintenance of “A. capra”, which warrants further investigation.
Sequence and phylogenetic analysis based on the 16S rRNA gene demonstrated that “A. capra” isolates obtained from ticks, domestic, and wild animals as well as humans exhibit high sequence similarities to each other (99.8–100%) and cluster in a separate clade within the genus Anaplasma, but they are clearly distinct from the other members of Anaplasma, indicating the novelty of this causative agent. Obviously, these isolates of “A. capra” were a single species according to the classification criteria of bacteria1,13 (>99% 16S rRNA gene sequence similarity). However, they were classified into two different groups with low sequence similarity and a divergent phylogenetic relationship based on the gltA and msp4 genes (87.2–88.3% for gltA and 88.4% for msp4), indicating two genotypes of “A. capra” in ticks and reservoir hosts. The “A. capra” genotype 1 identified in I. persulcatus, H. longicornis, sheep, goat, and human significantly differs from the genotype 2 identified in H. qinghaiensis, takin, Reeves’s muntjac and forest musk deer, suggesting a high degree of genetic diversity and host tropisms of “A. capra”, as has been documented in A. phagocytophilum2. However, it is unclear whether these two genotypes of “A. capra” have pathogenicity variation for animals and human, which should be clarified in the future.
In the past three decades, an increasing number of novel tick-associated microbes with zoonotic potential continue to be discovered, representing a considerable impact on public health worldwide. Since A. phagocytophilum was recognized as the causative agent of human granulocytic anaplasmosis, the cases have constantly increased in many countries14. However, numerous anaplasmosis cases of undetermined cause remain, indicating additional Anaplasma species are responsible for human anaplasmosis2,15. However, clinical cases of anaplasmosis might be generally neglected because the symptoms of the illness are notoriously nonspecific, making it likely to be confused with other illnesses14. The identification of the novel tick-transmitted zoonotic “A. capra” calls for clinicians to be aware of the possible infection from this causative agent in patients suspected of anaplasmosis or other tick-borne diseases, especially in areas where anaplasmosis can occur.
In summary, “A. capra” infection was first documented in three species of free-ranging wild ruminants from a national nature reserve of China, and a novel genotype of “A. capra” was identified that clearly differed from the genotype identified from human. Further studies should be conducted to fully elucidate the host range, vector ticks, pathogenicity, and geographic distribution of this organism.
Supplementary Information
Acknowledgements
This study was financially supported by the National Key Research and Development Program of China (2016YFC1202002); the NSFC (31502091); ASTIP, FRIP (2014ZL010), CAAS; NBCIS (CARS-37); and the Jiangsu Co-innovation Center Program for Prevention and Control of Important Animal Infectious Diseases and Zoonosis.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41426-018-0212-0).
References
- 1.Dumler JS, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 2.Battilani M, De Arcangeli S, Balboni A, Dondi F. Genetic diversity and molecular epidemiology of Anaplasma. Infect. Genet. Evol. 2017;49:195–211. doi: 10.1016/j.meegid.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, et al. Human infection with a novel tick-borne Anaplasma species in China: a surveillance study. Lancet Infect. Dis. 2015;15:663–670. doi: 10.1016/S1473-3099(15)70051-4. [DOI] [PubMed] [Google Scholar]
- 5.Yang, J. F. et al. A novel zoonotic Anaplasma species is prevalent in small ruminants: potential public health implications. Parasit. Vectors10, 264 (2017). [DOI] [PMC free article] [PubMed]
- 6.Sun XF, Zhao L, Wen HL, Luo LM, Yu XJ. Anaplasma species in China. Lancet Infect. Dis. 2015;15:1263–1264. doi: 10.1016/S1473-3099(15)00377-1. [DOI] [PubMed] [Google Scholar]
- 7.Peng Y, et al. Detection and phylogenetic characterization of Anaplasma capra: an emerging pathogen in sheep and goats in China. Front. Cell. Infect. Microbiol. 2018;8:283. doi: 10.3389/fcimb.2018.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato M, et al. Phylogenetic analysis of the 16S rRNA gene of Anaplasma species detected from Japanese serows (Capricornis crispus) J. Vet. Med. Sci. 2009;71:1677–1679. doi: 10.1292/jvms.001677. [DOI] [PubMed] [Google Scholar]
- 9.Koh, F. X., Panchadcharam, C., Sitam, F. T. & Sun, T. T. Molecular investigation of Anaplasma spp. in domestic and wildlife animals in Peninsular Malaysia. Vet. Parasitol. Regional Studies Rep.13, 141–147 (2018). [DOI] [PubMed]
- 10.Yang J, et al. Molecular survey and characterization of a novel Anaplasma species closely related to Anaplasma capra in ticks, northwestern China. Parasit. Vectors. 2016;9:603. doi: 10.1186/s13071-016-1886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rar V, Golovljova I. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 2011;11:1842–1861. doi: 10.1016/j.meegid.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Inokuma H, Terada Y, Kamio T, Raoult D, Brouqui P. Analysis of the 16S rRNA gene sequence of Anaplasma centrale and its phylogenetic relatedness to other ehrlichiae. Clin. Diagn. Lab. Immunol. 2001;8:241–244. doi: 10.1128/CDLI.8.2.241-244.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khumalo ZTH, et al. Evidence confirming the phylogenetic position of Anaplasma centrale (ex Theiler 1911) Ristic and Kreier 1984. Int. J. Syst. Evol. Microbiol. 2018;68:2682–2691. doi: 10.1099/ijsem.0.002832. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of lyme disease, human granulocytic Anaplasmosis, and Babesiosis: a review. JAMA. 2016;315:1767–1777. doi: 10.1001/jama.2016.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyer AR, Carlyon JA. Of goats and men: rethinking anaplasmoses as zoonotic infections. Lancet Infect. Dis. 2015;15:619–620. doi: 10.1016/S1473-3099(15)70097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.