Abstract
Background
1,3-beta-D Glucan (BDG) assay has good accuracy for distinguishing patients with invasive fungal infections from patients without. Some procedures and medications affect BDG levels, resulting in false-positive BDG results. The extent of intestinal surgery on BDG kinetics is unknown. We evaluated the influence of laparoscopic and open intestinal surgery on peri- and postsurgical serum BDG values.
Methods
BDG was determined in 346 samples from 50 patients undergoing laparoscopic (24) or open (26) intestinal surgery at the following time points: after insertion of arterial but before skin incision, after skin incision but before dissection of the intestinal mucosa, after completion of anastomosis, after completion of skin sutures, in the evening after surgery, day 2 after surgery, 4–5 days after surgery.
Results
BDG was positive (ie, concentration ≥80 pg/mL) in 54% to 61% of patients during laparoscopic and open surgery (highest rates after completion of skin sutures). BDG was still positive in 12% (open) to 17% (laparoscopic) of patients without any suspected or proven fungal infection or anastomotic leakage 4–5 days after surgery. After completion of gut anastomosis, the BDG increase was higher in open compared with laparoscopic intestinal surgery.
Conclusions
The value of positive BDG tests in the perioperative setting up to 5 days postsurgery seems to be limited due to BDG elevations from intestinal surgical procedures.
Keywords: 1,3-ß-D-Glucan; surgery; Candida
Invasive candidiasis (IC) is the most common serious fungal infection in patients being cared for in the intensive care unit (ICU) [1]. Surgical ICU patients, mainly those undergoing procedures involving the gastrointestinal tract, have an increased risk of developing IC [2, 3]. Determination of 1,3-beta-D Glucan (BDG) is useful to differentiate Candida colonization from invasive candidiasis due to the high negative predictive value of this nearly “panfungal” antigen test [4–8]. Unfortunately, other glucans may interfere with BDG testing due to the close chemical structure of, for example, cellulose (1,4-beta-D Glucan) with fungal BDG. False-positive results have been linked to the use of cellulose-containing hemodialysis membranes, blood derivatives, broad-spectrum antibiotics, gram-positive and gram-negative bacteremia, severe mucositis, and surgery, especially with involvement of the intestinal tract [9–15]. The issue of BDG introduction into the bloodstream and its kinetics after intestinal mucosal damage (eg, in mucositis or gut surgery) is still poorly understood. Previously, 12%–57% of surgical ICU patients had high BDG values without any evidence of infection [11, 16]. It was speculated whether the following led to elevated BDG levels: iatrogenic causes such as translocation, leakage, or leaching; introduction of BDG into the bloodstream by using surgical gauze, performing transfusions, or hemodialysis; or administration of certain drugs [11]. Although leakage of the gut and subsequent translocation of fungal elements into the bloodstream have been shown to be a likely cause of increased blood BDG levels in patients with HIV infection [17], our study group has shown that mild to moderate chemotherapy-induced mucosal barrier damage is not associated with elevated blood BDG levels [18]. Recently, we also demonstrated that BDG testing is reliable in patients undergoing hemodialysis and that modern dialysis membranes do not release BDG [19].
As life-threatening intraabdominal candidiasis occurs in 30% to 40% of high-risk abdominal surgical ICU patients, it is of the utmost importance to obtain reliable BDG values for diagnosis or exclusion of invasive candidiasis in this patient cohort [16]. However, the potential introduction of BDG from surgical sponges or gauze and mucosal damage due to surgical impairment of mucosal integrity are key concerns when interpreting elevated blood BDG levels in the context of Candida infections after intestinal surgery. Compared with open intestinal surgery, sponges and gauze are less frequently used in laparoscopic intestinal surgery.
The aim of this study was to evaluate the influence of laparoscopic and open intestinal surgery on peri- and postsurgical serum BDG values.
METHODS
In this prospective study, all patients undergoing laparoscopic or open intestinal surgery involving the small bowel and/or colon and/or rectum at the Hospital of St. John of God (Barmherzige Brüder), Graz, Austria, between April and June 2018 were asked to participate in the study and included after they provided informed consent. Open and laparoscopic surgical procedures were performed as applied in clinical routine and published previously [20, 21]. Assignment to 1 of the groups was performed by the surgeons according to the underlying disease and technical aspects. Laparoscopic procedures were performed as reduced-port surgery using a multiport system (OCTOtmPORT) at the umbilical incision site and 1 additional trocart (12-mm diameter) in the right lower abdomen. An electric endoscopic stapling device (SIGNIA Stapling Device) was used for dividing/cutting the colon or rectum in the laparoscopic group. In open procedures, a mechanical stapling device (Contour Curved Cutter Stapler) was used to cut/divide the colon or rectum. In both groups, an electrothermal bipolar sealing device (LIGASUREtm) was used to dissect and seal tissue or vessels and to reduce blood loss [22]. Patients were not eligible for the study in case of (a) ongoing antifungal therapy for treatment of active fungal infection or antifungal therapy within 4 weeks before inclusion; (b) ongoing antibiotic therapy before surgery other than single-shot surgical prophylaxis; (c) treatment of bacteremia (eg, due to Enterococcus sp.) within 4 weeks before inclusion; (d) clinical, radiological, or laboratory evidence of current infectious disease as assessed by the treating physician; (e) administration of immunoglobulin, blood, or blood products (ie, thrombocytes, fresh frozen plasma) within 4 weeks before inclusion; (f) intestinal or other abdominal surgery (laparoscopic or open) or other major surgeries (eg, aortocoronary bypass) within 4 weeks before inclusion; (g) subsequent invasive candidiasis (defined according to proposed EORTC/MSG definitions of fungal infections in the ICU); or (h) other complicating infections occurring within 5 days after surgery. The complete observation period was 30 days after surgery for assessment of outcome and other parameters, for example, intrahospital or extrahospital death, necessity of antibiotic or antimycotic therapy due to complicating infectious disease, occurrence of anastomotic leakage, and subsequent surgical procedures.
Blood samples used for determination of BDG values were obtained at 7 scheduled time points as outlined below through arterial lines inserted just before sampling as a routine part of the anesthesia procedure: (1) 1 blood sample before intubation and before skin incision by the surgeon, (2) after skin incision but before dissection of intestinal mucosa (both samples were used for determination of BDG values prior to dissection of intestinal mucosa and possible influence of certain factors on BDG values, eg, insertion of vascular lines, single shot antibiotic prophylaxis, damaged mucosa in cancer or surgical preparation of intestine/colon for resection of cancer), (3) after completion of anastomosis, (4) after completion of skin sutures, (5) in the evening after surgery (ie, approximately 5–8 hours after surgery), (6) in the morning of day 2 after surgery (ie, approximately 18 hours after surgery), and (7) in the morning 4–5 days after surgery as part of a routine blood sampling procedure. The day of surgery was designated as day 1. Thus, 7 samples of 5 mL of whole blood were drawn in each patient using the S-Monovette collection system (Sarstedt, Nümbrecht, Germany). In case of signs and/or symptoms suggestive of candidemia and/or bacteremia and/or intraabdominal infection and/or other infectious complications, routine diagnostics were applied, for example, blood cultures and/or microbiological investigation of abdominal drainage fluids, as well as imaging studies as indicated by the treating physician. The serum BDG test was performed according to recently described methods using material from the Fungitell Assay (Associates of Cape Cod, Falmouth, MA) [4]. According to the manufacturer, a BDG concentration of ≥80 pg/mL was considered positive, whereas a concentration of <60 pg/mL was considered negative. Concentrations <80 pg/mL but ≥60 pg/mL were considered indeterminate, and <15.36 pg/mL was considered 0 pg/mL [19]. Statistical analysis was performed using R 3.1.1 (www.r-project.org) and SPSS Statistics 23. A P value of <.05 was considered statistically significant. The study protocol was approved by the ethics committee of the Medical University of Graz (nr 30–043 ex 17/18) and Hospital of St. John of God (Barmherzige Brüder), Graz, Austria. The study was registered at ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT03468803).
RESULTS
Baseline Demographics and BDG levels
Fifty patients were included in the study; 24 underwent laparoscopic and 26 open intestinal surgery. Demographic data, underlying diseases, and perisurgical interventions are shown in Table 1. Four of 350 samples could not be assigned to scheduled sampling dates and were not further analyzed. Thus, BDG tests were performed in 346 samples from 50 patients (2 samples from time point 5 and 2 samples from time point 7 were missing in 2 patients). There was no statistical difference between patients with elevated and nonelevated BDG with regard to observed demographic or clinical parameters (eg, sex, underlying diseases, type of antibiotic prophylaxis) except age (Table 1). None of the patients had renal replacement therapy before or after surgery; received immunoglobulins, red blood cells or other blood products; developed invasive candidiasis; or received systemic antimycotic treatment. All patients survived the observation period of 30 days.
Table 1.
Demographic Data of Both Patient Groups
| Laparoscopic surgery | Open surgery | P Value | |
|---|---|---|---|
| No. | 24 | 26 | |
| Female sex, No. (%) | 7 (29.3) | 13 (50) | .133 |
| Age, median (range), y | 68.32 (35.73–85.78) | 75.8 (56.08–91.56) | .008 |
| BMI, median (range), kg/m2 | 25.6 (19.26–32.39) | 26.87 (19.93–37.28) | .225 |
| Underlying disease, No. (%) | |||
| Colonic carcinoma | 12 (50.0) | 13 (52.0) | 1.000 |
| Rectal carcinoma | 4 (16.7) | 8 (30.8) | .243 |
| Diverticultis | 5 (20.8) | 3 (11.5) | .370 |
| Other | 3 (12.5) | 2 (7.7) | .571 |
| Endoscopic preoperative ink tattooing, No. (%) | 13 (54.2) | 7 (26.9) | .490 |
| Second intestinal surgery, No. (%) | 1 (4.2) | 3 (11.5) | .337 |
| Neoadjuvant chemotherapy, No. (%) | 1 (4.2) | 1 (3.8) | .954 |
| Leukocytes, median (range), G/L | 7.110 (4.900–7.940, n = 11) | 6.950 (5.215–9.975, n = 17) | .404 |
| CRP, median (range), mg/dL | 0.2 (0.1–0.3, n = 10) | 0.4 (0.13–2.47, n = 16) | .077 |
| Antibiotic prophylaxis, No. (%) | 24/24 (100) | 26/26 (100) | 1.000 |
| Antibiotic treatment, No. (%) | |||
| Day 2 (sample 6) | 4/24 (16.7) | 4/26 (15.4) | .902 |
| 4–5 d after surgery (sample 7) | 5/24 (20.8) | 6/26 (23.1) | .848 |
Abbreviations: BMI, body mass index; CRP, C-reactive protein.
Bold value indicates significant difference in age.
In the initial serum sample before skin incision, 6 of 50 patients (12%) had positive BDG values (3 patients were undergoing laparoscopic surgery and 3 open surgery). Five of these 6 patients had carcinoma of the colon or rectum, and 1 had nonmalignant tubulovilleous adenoma of the rectum. All 6 patients with initial BDG values ≥80 pg/mL had received single-shot antibiotic prophylaxis (5 received cefuroxime plus metronidazole, and 1 received ciprofloxacin plus metronidazole) before sampling for BDG determination. Eleven of 50 (22%) patients had postoperative antibiotic therapy as indicated by the treating physician. Two out of 7 patients with positive BDG values ≥80 pg/mL at sampling 4–5 days after surgery had antibiotic therapy, whereas 9 patients with antibiotic therapy at this time point had negative BDG levels. No patient developed fever within 5 days after surgery. Three patients underwent second surgery due to clinically suspected anastomotic leakage, peritonitis, and postsurgical intestinal bleeding. After the second surgery, BDG rose to values ≥80 pg/mL in 2 of 3 patients.
Dynamics of BDG Levels During and After Surgery
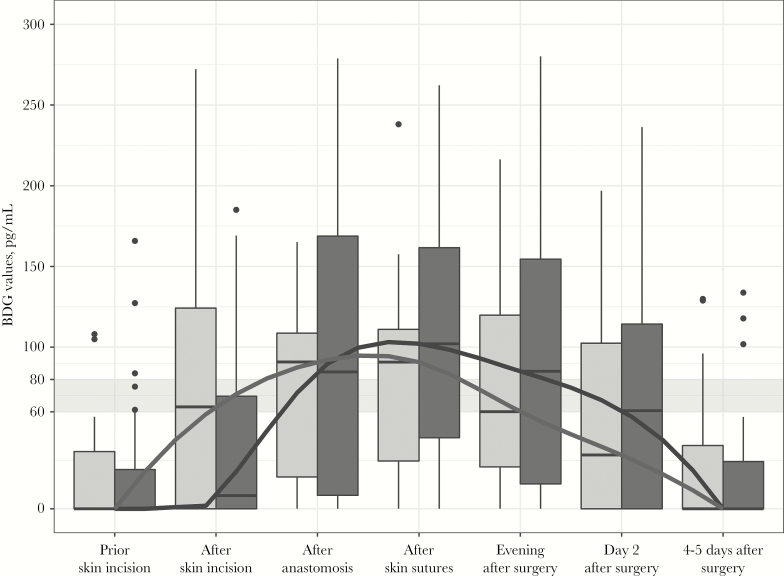
Boxplots and medians of BDG levels at all 7 sampling time points are displayed in Figure 1 and Table 2. BDG values of all patients are shown in Figure 2. The overall proportion of patients with BDG values ≥80 pg/mL and absolute serum BDG values increased significantly after skin incision, reaching its peak after completion of skin sutures (29/50 patients, 58%; median [interquartile range], 97 [33–136] pg/mL; P < .001). Thereafter, proportions of patients with BDG values ≥80 pg/mL decreased, but—when compared with baseline—remained elevated until (and including) the morning of day 2 after surgery.
Figure 1.
Boxplots and medians of 1,3-beta-D Glucan (BDG) levels at all 7 sampling time points in patients undergoing laparoscopic (light gray boxplots and lines) or open intestinal surgery (dark gray boxplots and lines). The gray band indicates the indeterminate zone of BDG values between 60 and 80 pg/mL. BDG values above 80 pg/mL are positive according to the instructions of the manufacturer. BDG values below 60 pg/mL are negative.
Table 2.
P Values for Differences in Proportions of All Patients With Elevated Serum BDG Values ≥80 pg/mL at Given Sampling Time Points Respective to Intestinal Surgery Time Points
| Proportions of Patients With BDG Values ≥80 pg/mLa | 1, Before Intubation, Before Skin Incision | 2, Just Before Dissection of Intestinal Mucosa | 3, After Completion of Anastomosis | 4, After Completion of Skin Sutures | 5, Evening After Surgeryb | 6, Morning of Day 2 After Surgery | 7, Morning 4–5 Days After Surgeryb | |
|---|---|---|---|---|---|---|---|---|
| Proportions of patients with BDG values ≥80 pg/mLa | - | 6/50 (12%) | 15/50 (30%) | 27/50 (54%) | 29/50 (58%) | 23/48 (48%) | 19/50 (38%) | 7/48 (15%) |
| 1, before intubation, before skin incision | 6/50 (12%) | - | .012 | <.001 | <.001 | <.001 | .001 | 1.000 |
| 2, just before dissection of intestinal mucosa | 15/50 (30%) | - | - | <.001 | .001 | .057 | .424 | .167 |
| 3, after completion of anastomosis | 27/50 (54%) | - | - | - | .687 | .581 | .057 | .001 |
| 4, after completion of skin sutures | 29/50 (58%) | - | - | - | - | .180 | .013 | <.001 |
| 5, evening after surgeryb | 23/48 (48%) | - | - | - | - | - | .219 | .003 |
| 6, morning of day 2 after surgery | 19/50 (38%) | - | - | - | - | - | - | .031 |
P values were calculated by McNemar test. Bold values are significant.
Abbreviation: BDG, 1,3-beta-D Glucan.
a≥80 pg/mL, ie, positive BDG values according to the manufacturer.
bTwo samples each are missing at sample time points 5 and 7.
Figure 2.
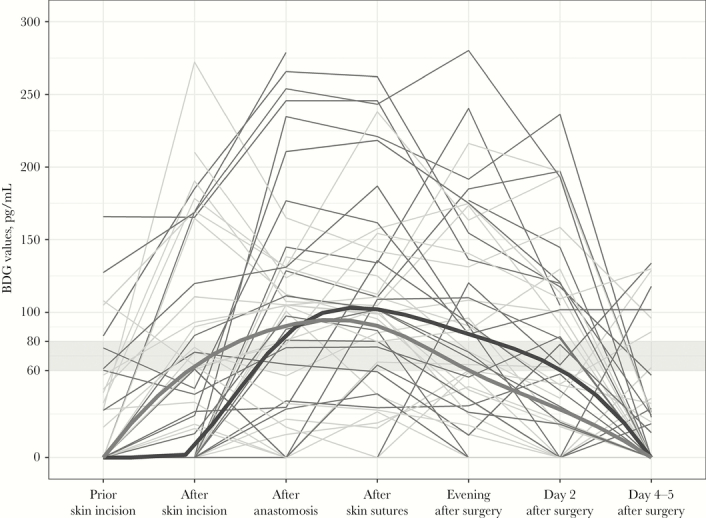
1,3-beta-D Glucan (BDG) values of all patients undergoing laparoscopic (light gray lines) or open intestinal surgery (dark gray lines). Medians are highlighted in bold. The gray band indicates the indeterminate zone of BDG values between 60 and 80 pg/mL. BDG values above 80 pg/mL are positive according to the instructions of the manufacturer. BDG values below 60 pg/mL are negative.
Differences in Dynamics of BDG Levels Between Laparoscopic and Open Surgery
Overall, the dynamics were similar in both groups. Significant differences for changes of BDG values were found for comparing the time point after completion of intestinal anastomosis with the time point after preparation of the intestine and just before dissection of the intestinal mucosa, with significantly higher increases in the open surgery group (P = .001). No differences between those groups were found when comparing other time points.
DISCUSSION
In our study, laparoscopic and open intestinal surgery led to elevated BDG values. In comparison with previous studies, we investigated BDG values in the perisurgical time frame to assess the immediate effect of intestinal surgery. Previously, BDG values were determined in patients after 48 hours of ICU treatment with an expected additional stay of at least 3 days [11]. No information could therefore be derived from the time frame during or immediately after surgery from that study. Nevertheless, the authors found a high frequency of positive BDG values early after ICU admission and a subsequent BDG decrease. Nine out of 35 patients (26%) without any evidence of invasive candidiasis had elevated BDG. In 8 of these patients, BDG decreased without antifungal therapy, raising the assumption that elevated BDG values might have been an effect of surgery. It was speculated whether elevated BDG was a result from the surgical intervention itself, medical treatment, or true postsurgical invasive candidiasis [11].
Our study found that intestinal surgery led to positive BDG values during surgery in 54% (laparoscopic surgery) to 62% (open surgery) of patients. Although we focused on the time frame of surgery starting with sampling after insertion of arterial lines for routine anesthesia until 5 days after surgery, we were not able to establish a direct causal relationship of certain underlying diseases, perisurgical interventions/procedures, and medications with elevated BDG values. Several procedures known to cause elevated BDG were applied during routine intestinal surgery, that is, antibiotic prophylaxis, use of gauze, and disintegration of intestinal mucosa. Different amounts of gauze are used in laparoscopic compared with open intestinal surgery. Considering that BDG levels tended to increase more during open surgery (ie, after completion of intestinal anastomosis), we assume that specific interventions in open surgery (eg, greater use of cellulose-based gauze, greater skin incision compared with laparoscopic surgery) might in part be responsible for elevation of BDG. However, BDG increased very early after preparation of the intestine and just before dissection of the intestinal mucosa both in laparoscopic and open surgery, indicating that cellulose-based gauze and skin trauma could not solely be responsible for elevation of BDG. As 12% of patients had BDG values ≥80 pg/mL before administration of perioperative antibiotic prophylaxis, intubation, abdominal skin disinfection, dressing, and skin incision, the underlying disease might have contributed to elevated BDG. However, as noted above, we found no causal relationship between underlying disease, stage of underlying disease, or demographic factors and elevated BDG. Whether placement of arterial lines (which was done before the first blood draw used for BDG testing) after skin disinfection using isopropanol- or octenidin-soaked gauze increased BDG remains unclear. As every patient received arterial lines but not every patient showed elevated BDG, it is unlikely that insertion of arterial lines led to positive BDGs. Other reasons previously suggested to cause elevated BDG were not used or were not present in our cohort, that is, administration blood products or immunoglobulins, bacterial infections (eg, bacteremia) before surgery, or invasive fungal infection. None of the patients received renal replacement therapy (RRT). In addition, we previously demonstrated that RRT using modern filters does not lead to elevated BDG [19]. Antibiotic prophylaxis, administered between sampling after skin incision but before dissection of intestinal mucosa, might be responsible for the BDG increase detected at the sampling time point before dissection of intestinal mucosa, together with skin incision and use of gauze in this procedure. However, although antibiotic prophylaxis was applied to all patients, BDG did not increase in all subjects. Overall, 56% (26/50) had negative BDG values (<60 pg/mL) at the time point after skin incision but before dissection of intestinal mucosa, and the rate was still 24% (12/50) at the time point after completion of anastomosis. Additionally, 9 of 11 patients with antibiotic therapy at time points 4–5 days after surgery had negative BDGs, indicating that administration of antibiotics is not associated with elevated BDG values in all patients. Overall, we were not able to associate the observed BDG increase with 1 particular standalone cause.
In summary, in patients undergoing intestinal surgery, positive BDG values have to be interpreted with great caution as surgical procedures increase BDG values. BDG reached concentrations ≥80 pg/mL in 54% to 61% of patients during laparoscopic and open surgery. BDG was still positive in 12% (open) to 17% (laparoscopic) of patients without any suspected or proven fungal infection or anastomotic leakage 4–5 days after surgery. Previously, BDG testing showed a high negative predictive value (99%) but a weak positive predictive value (72%) for detection of candidemia in the ICU setting, including patients undergoing surgery [23]. Our study adds data to the fact that BDG testing might be false positive due to surgical interventions, especially during and in the first days after intestinal surgery. We suggest that clinicians primarily rely on negative BDG tests in clinical workup of patients with a short history of up to 5 days after intestinal surgery and suspicion of fungal infection. The high negative predictive value of BDG testing will support subsequent therapeutic decisions [8]. The value of positive BDG tests in the perioperative setting up to 5 days after surgery seems to be limited due to BDG elevations by intestinal surgical procedures. Health care providers must have knowledge on the utility of the BDG testing and factors influencing test results to avoid unnecessary testing and unnecessary initiation of antifungal treatment in case of false-positive results [24].
Acknowledgments
Financial support. This work was supported by the Austrian Science Fund, grant number KLI 561-B31.
Potential conflicts of interest. R. Krause received research grants from Merck and served on the speakers’ bureau of Pfizer, Gilead, Astellas, Basilea, Merck, and Angelini. M. Hoenigl received research grants from Gilead and served on the speakers’ bureau of Gilead, Basilea, and Merck. J. Prattes received travel grants from Pfizer and Angelini and consulting fees from Gilead and served on the speakers’ bureau of Gilead. I. Zollner-Schwetz received research grants from Angelini and Astellas and served on the speakers’ bureau of Angelini and MSD. G. Gemes received consulting fees from Fresenius-Kabi. All other authors declare no conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20:133–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. León C, Ruiz-Santana S, Saavedra P, et al. ; Cava Study Group Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit Care Med 2009; 37:1624–33. [DOI] [PubMed] [Google Scholar]
- 3. Montravers P, Dupont H, Eggimann P. Intra-abdominal candidiasis: the guidelines-forgotten non-candidemic invasive candidiasis. Intensive Care Med 2013; 39:2226–30. [DOI] [PubMed] [Google Scholar]
- 4. Prüller F, Wagner J, Raggam RB, et al. . Automation of serum (1→3)-beta-D-Glucan testing allows reliable and rapid discrimination of patients with and without candidemia. Med Mycol 2014; 52:455–61. [DOI] [PubMed] [Google Scholar]
- 5. Cuenca-Estrella M, Verweij PE, Arendrup MC, et al. ; ESCMID Fungal Infection Study Group ESCMID* guideline for the diagnosis and management of Candida diseases 2012: diagnostic procedures. Clin Microbiol Infect 2012; 18(Suppl 7):9–18. [DOI] [PubMed] [Google Scholar]
- 6. Cornely OA, Bassetti M, Calandra T, et al. ; ESCMID Fungal Infection Study Group ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 2012; 18(Suppl 7):19–37. [DOI] [PubMed] [Google Scholar]
- 7. Marchetti O, Lamoth F, Mikulska M, et al. ; European Conference on Infections in Leukemia (ECIL) Laboratory Working Groups ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant 2012; 47:846–54. [DOI] [PubMed] [Google Scholar]
- 8. Prattes J, Hoenigl M, Rabensteiner J, et al. . Serum 1,3-beta-D-Glucan for antifungal treatment stratification at the intensive care unit and the influence of surgery. Mycoses 2014; 57:679–86. [DOI] [PubMed] [Google Scholar]
- 9. Ellis M, Al-Ramadi B, Finkelman M, et al. . Assessment of the clinical utility of serial beta-D-Glucan concentrations in patients with persistent neutropenic fever. J Med Microbiol 2008; 57:287–95. [DOI] [PubMed] [Google Scholar]
- 10. Yamanouchi K, Takatsuki M, Hidaka M, et al. . Significance of serum β-D-Glucan levels in recipients of living donor liver transplantation. J Hepatobiliary Pancreat Sci 2011; 18:432–5. [DOI] [PubMed] [Google Scholar]
- 11. Mohr JF, Sims C, Paetznick V, et al. . Prospective survey of (1→3)-beta-D-Glucan and its relationship to invasive candidiasis in the surgical intensive care unit setting. J Clin Microbiol 2011; 49:58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanamori H, Kanemitsu K, Miyasaka T, et al. . Measurement of (1-3)-beta-D-Glucan derived from different gauze types. Tohoku J Exp Med 2009; 217:117–21. [DOI] [PubMed] [Google Scholar]
- 13. Viswesh VV, Radosevich JJ, Green MR. Inclusion and recommendation of (1→3)-β-D-Glucan testing in the international guidelines for management of severe sepsis and septic shock. Crit Care Med 2013; 41:e487–8. [DOI] [PubMed] [Google Scholar]
- 14. Held J, Kohlberger I, Rappold E, et al. . Comparison of (1->3)-β-D-Glucan, mannan/anti-mannan antibodies, and Cand-Tec Candida antigen as serum biomarkers for candidemia. J Clin Microbiol 2013; 51:1158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pappas PG, Kauffman CA, Andes DR, et al. . Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tissot F, Lamoth F, Hauser PM, et al. ; Fungal Infection Network of Switzerland (FUNGINOS) β-Glucan antigenemia anticipates diagnosis of blood culture-negative intraabdominal candidiasis. Am J Respir Crit Care Med 2013; 188:1100–9. [DOI] [PubMed] [Google Scholar]
- 17. Hoenigl M, Pérez-Santiago J, Nakazawa M, et al. . (1→3)-β-D-Glucan: a biomarker for microbial translocation in individuals with acute or early HIV infection?Front Immunol 2016; 7:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prattes J, Raggam RB, Vanstraelen K, et al. . Chemotherapy-induced intestinal mucosal barrier damage: a cause of falsely elevated serum 1,3-beta-D-Glucan levels?J Clin Microbiol 2016; 54:798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prattes J, Schneditz D, Prüller F, et al. . 1,3-ß-D-Glucan testing is highly specific in patients undergoing dialysis treatment. J Infect 2017; 74:72–80. [DOI] [PubMed] [Google Scholar]
- 20. Kang JH, Lee SY, Kim CH, et al. . Comparison of the short-term outcomes of reduced-port laparoscopic surgery and conventional multiport surgery in colon cancer: a propensity score matching analysis. Ann Surg Treat Res 2018; 94:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakanishi M, Kuriu Y, Murayama Y, et al. . Usefulness of reduced port surgery for left colon cancer. Anticancer Res 2016; 36:4749–52. [DOI] [PubMed] [Google Scholar]
- 22. Gezen C, Kement M, Altuntas YE, et al. . Safety and effectiveness of 5-mm and 10-mm electrothermal bipolar vessel sealers (LigaSure) in laparoscopic resections for sigmoid colon and rectal cancers. J Laparoendosc Adv Surg Tech A 2012; 22:572–7. [DOI] [PubMed] [Google Scholar]
- 23. Posteraro B, De Pascale G, Tumbarello M, et al. . Early diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1→3)-β-D-Glucan assay, Candida score, and colonization index. Crit Care 2011; 15:R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fabre V, Markou T, DeMallie K, Forum SMO 2018 Single academic center experience of unrestricted β-D-Glucan implementation. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]