Graphical abstract
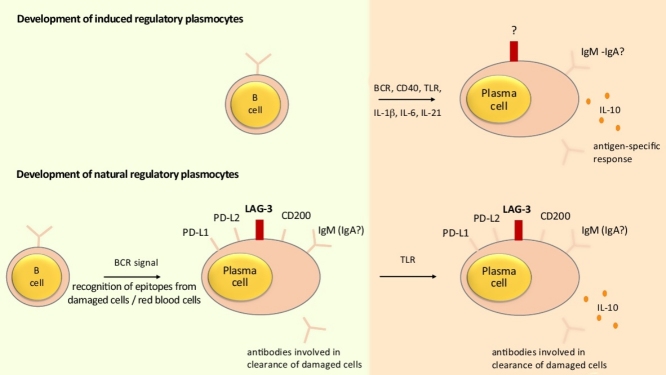
Some B cells have a particular capacity to upregulate IL-10 expression upon activation in vitro including those carrying high levels of surface CD1d including marginal zone and transitional T2-like B cells, as well as B cells that do not necessarily have this characteristic including B1a and Tim-1hi B cells. These B cells might give rise to induced regulatory plasma cells producing IL-10 upon appropriate activation in vivo (top part). These signals might involve BCR, CD40, TLR, and the cytokines IL-1β, IL-6, and IL-21, so that these cells would be specific for the antigen eliciting the response. Their phenotype is not defined. In addition, some B cells develop at steady state under the control of the BCR into LAG-3+CD138hi natural regulatory plasma cells (bottom part). Damaged cells (including red blood cells) might contribute to the generation of these cells. Upon challenge with agents that for instance provide abundant TLR agonists, these cells can upregulate IL-10 expression while remaining in a non-dividing state. The light green quadrant (left) indicates what is happening before any immune challenge. The orange quadrant (right) indicates what is happening after activation of an appropriate immune response.
Highlights
-
•
Some B cells differentiate into LAG-3+CD138hi plasma cells at steady state.
-
•
LAG-3+CD138hi plasma cells development is driven by BCR signals.
-
•
LAG-3+CD138hi plasma cells express several immune checkpoint receptors.
-
•
LAG-3+CD138hi plasma cells can recognize damaged cells.
-
•
LAG-3+CD138hi plasma cells have a unique capacity to produce IL-10 upon stimulation.
Abstract
B cells can generate several types of antibody-secreting cells, including plasmablasts that divide and are short lived, as well as plasma cells that do not proliferate and can persist for extended time periods. Here, we discuss the identification of a novel subset of non-dividing plasma cells specialized in the production of interleukin(IL)-10. These cells develop at steady state, including in germ-free mice, via a mechanism dependent on the B cell receptor for antigen and possibly involving the recognition of damaged cells. They are characterized by the expression of the inhibitory receptor LAG-3, and also express CD200, PD-L1, as well as PD-L2. Their specialized epigenome allows them to produce IL-10 within hours after stimulation, which altogether qualify these cells as natural regulatory plasma cells.
Current Opinion in Immunology 2018, 55:62–66
This review comes from a themed issue on Autoimmunity
Edited by Daniel Stetson
For a complete overview see the Issue and the Editorial
Available online 4th October 2018
https://doi.org/10.1016/j.coi.2018.09.012
0952-7915/© 2018 The Author. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Antibodies provide an essential line of defense against infectious diseases. The incidence and severity of infectious diseases are markedly increased in patients suffering from genetic deficiencies impairing antibody production [1]. The differentiation of antibody-secreting cells (ASC) is linked to B cell proliferation, epigenome remodeling, and the expression of transcription factors driving the functional plasma cell state [2]. Different ASC subtypes can be distinguished depending on their lifespan and degree of differentiation. Plasmablasts are less mature, proliferative, and short-lived, while plasma cells are more differentiated, do not divide, and can persist for a lifetime in dedicated niches [3]. In this review, we use the term plasmocyte as a general descriptor for ASC, including both plasmablasts and plasma cells. The durability of some non-dividing plasma cells might explain the remarkable persistence of serum antibody titers towards vaccine antigens, which for instance can reach half-lives up to 3014 years for measles [4]. It has long been considered that the unique function of plasmocytes (including plasmablasts and plasma cells) was to produce antibodies. However, recent studies demonstrated that some plasmocytes could produce cytokines with either pro- or anti-inflammatory functions including interleukin(IL)-10 [5,6]. The loss of suppressive plasmocytes might explain why some patients with immune-deficiencies also often present with an increased incidence of immune-mediated diseases. For instance, patients with common variable immune deficiencies (CVID) have an increased incidence of autoimmune manifestations including autoimmune cytopenia [7]. The exploration of this possibility requires the better characterization of anti-inflammatory plasmocytes. This review discusses the identification of a novel subset of regulatory plasma cells in mice.
The role of plasmocytes as mediators of immune suppression
B cells necessitate stimulatory signals to acquire immune suppressive activities, including activation via the B cell receptor for antigen (BCR), CD40, Toll-like receptors (TLR), and receptors for cytokines [8••,9, 10, 11,12•]. Their suppressive effect also depends on their expression of the transcription factors IRF4 and BLIMP-1 in vivo, which directly stimulate Il10 transcription. For instance, IRF4 binds to the conserved noncoding sequence (CNS) 9 located upstream of the Il10 transcription start [13••], and is important for the induction of IL-10 in B cells stimulated by the M2 protein of the murine gammaherpes virus 68 [14]. These transcription factors are well-known for their crucial role in ASC formation [2], and no B cell has been described so far that expresses elevated levels of BLIMP-1 and IRF4 that is not a plasmocyte. This establishes a molecular link between Il10 expression and plasmocyte differentiation. Accordingly, plasmocytes were identified as the major source of B cell-derived IL-10 in vivo in autoimmune, malignant, and infectious diseases [8••,13••,15, 16, 17]. The suppressive function of plasmocytes is further emphasized by the observation of a distinct subset of regulatory plasmocytes characterized by the expression of IL-35 [8••]. IL-10 and IL-35 from plasmocytes suppress immunity by acting on myeloid cells and T lymphocytes [6]. The identification of the anti-inflammatory activities of plasmocytes might explain why some antibody deficiencies are associated with defects in immune regulation, and immune-mediated pathologies.
Induced and natural regulatory plasmocytes
The finding that plasmocytes are a major source of B cell-derived IL-10 in vivo in immune mice asks for the identification of their precursor in the naïve immune system.
Several B cell subsets in naïve mice can give rise to IL-10-producing progeny in vitro, in particular those carrying high levels of surface CD1d including marginal zone and transitional T2-like B cells, as well as B cells that do not necessarily have this characteristic including B1a and Tim-1hi B cells [18, 19, 20]. These B cells can exert suppressive functions in an IL-10-dependent manner in recipient mice upon adoptive transfer, yet the phenotype of the B cells expressing IL-10 and actually achieving suppression in vivo in the recipient is not defined. In particular, it is not known whether they can produce IL-10 and suppress immunity in vivo without differentiating into ASC. These B cell subsets might therefore be a source of induced IL-10-expressing plasmocytes in vivo.
The existence of a bona fide IL-10-producing regulatory B cell subset, defined as a homogenous population exclusively having suppressive function has yet to be established. The B cell populations so far described as competent for IL-10 expression are in fact heterogenous since only a small fraction of these cells upregulate IL-10 after activation, even when strong pharmacological agents such as phorbol 12-myristate 13-acetate and ionomycin are used in addition to the B cell stimulus. To be defined as a natural regulatory population, a cell subset should display a rapid and homogenous upregulation of IL-10 expression upon stimulation in vivo, while maintaining its phenotypic identity. Immune responses associated with the rapid elicitation of IL-10 expression in B lineage cells appear well-suited to identify such natural regulatory B cell subset. IL-10-expressing B lineage cells appear within less than 24 hours post-infection (p.i.) in the spleen of mice challenged with the Gram-negative bacterial pathogen Salmonella typhimurium [15]. These IL-10-expressing B lineage cells were all found among CD138hi plasmocytes, yet only a fraction of the CD138hi cells in the spleen of infected mice expressed IL-10 [15]. The characterization of IL-10+CD138hi cells through genome-wide transcriptomics revealed that they distinctively expressed the inhibitory receptor lymphocyte-activation gene 3 (LAG-3) in comparison to IL-10−CD138hi cells [21••]. IL-10+LAG-3+CD138hi cells represented a homogenous population because LAG-3 was expressed at the surface of more than 80% of IL-10+CD138hi cells in the spleen of infected mice, and IL-10 displayed a homogenous expression within LAG-3+CD138hi cells [21••]. Thus, LAG-3 identifies IL-10+CD138hi cells. This is striking because LAG-3 was previously identified as a marker for IL-10-producing CD4+ Tr1 cells in mouse and human [22,23]. Tr1 cells are characterized by the expression of BLIMP-1 and c-Maf as well as Ahr [24]. Considering the role of BLIMP-1 in B cell-mediated immune regulation, it is plausible that a shared molecular mechanism controls both IL-10+ regulatory plasmocytes and CD4+ Tr1 cells. IL-10+LAG-3+CD138hi cells were in a non-proliferating state, displayed a plasmacytoid morphology, and constitutively produced antibodies, thus qualifying as plasma cells rather than plasmablasts [21••].
The presence of non-proliferating IL-10+LAG-3+CD138hi plasma cells at day 1 p.i. is intriguing because B cells normally require to proliferate over several days in order to differentiate into plasma cells. Accordingly, IL-10+LAG-3+CD138hi plasma cells did not develop from B cells, but instead from pre-existing LAG-3+CD138hi cells that were already present in the spleen of naïve mice, including germ-free mice, yet did not express IL-10 before immune challenge [21••]. The relation of ontogeny between IL-10−LAG-3+CD138hi cells from naïve mice and IL-10+LAG-3+CD138hi cells from infected mice was corroborated by the similitude of their BCR repertoire and DNA methylome, which differed from the one’s of LAG-3−CD138hi and IL-10−CD138hi ASC. Noteworthy, their epigenome was specialized for Il10 expression: LAG-3+CD138hi cells from naïve mice displayed the lowest degree of CpG DNA methylation at the Il10 locus compared to all the other subsets of B cells and plasmocytes found in naïve mice. Accordingly, the induction of IL-10 expression in splenic LAG-3+CD138hi cells was remarkably rapid p.i., being significant already at 3 hours p.i. and reaching a peak at 12 p.i. while these cells were not dividing. Based on their pre-existence in naïve mice, including germ-free mice, their distinctive epigenome, their rapid and homogenous upregulation of IL-10 expression, as well as the global maintenance of their phenotypic characteristics upon IL-10 expression, LAG-3+CD138hi cells fulfill the requirement to be defined as a natural regulatory plasma cell subset.
In sum, IL-10-producing plasmocytes can derive from natural regulatory plasma cells that pre-exist in the naïve repertoire, as well as from activated competent B cell subsets. These induced regulatory plasmocytes are likely to appear and act later during the course of the response compared to natural regulatory plasma cells. It is also likely that they have a different antigen-reactivity profile.
Signals implicated in the development of natural regulatory plasma cells
The presence of LAG-3+CD138hi plasma cells in the naïve mouse, including in those kept under germ-free condition, suggests an endogenous response directed towards self-antigens.
The BCR controls the differentiation of LAG-3+CD138hi cells. These cells are almost completely absent in mice deficient for the Bruton tyrosine kinase (Btk), which is essential for BCR signaling [25], and their numbers are profoundly reduced in mice lacking CD19, the major co-receptor for the phosphoinositide 3-kinase (PI3K) signaling of the BCR [26]. In contrast, they are more abundant in mice lacking the BCR inhibitory co-receptor CD72 [21••]. LAG-3+CD138hi cells have a distinct BCR repertoire compared to LAG-3−CD138hi cells. They contain the majority of the plasmocytes expressing the VH11+Vk14+ BCR otherwise found on B1a cells, and that recognizes phosphatidylcholine (PtC). However, LAG-3+CD138hi cells do not contain any VH12+ cells, while VH12+ BCR are found on B1a cells at frequency similar to VH11+ BCR. Different BCR therefore have different capacities to generate these cells, as also shown by the fact that mice in which all B cells carry a single BCR display an altered frequency of LAG-3+CD138hi cells compared to controls. Several B cell subsets (not only B1a cells) can generate LAG-3+CD138hi cell in vivo [21••]. The differentiation of B cells into LAG-3+CD138hi plasma cells is therefore an antigen-driven process controlled by the BCR at steady state.
In contrast to the necessity of BCR signals, CD40 and TLR signaling were dispensable for the formation of LAG-3+CD138hi cells at steady state. Considering that CD40 is necessary for efficient T:B cell cooperation as well as immunity towards T-cell dependent antigen, and that TLR signaling is mandatory for response to T-independent type 1 antigens, this suggests that T-independent type 2 (TI-2) antigens, which can trigger plasma cell differentiation independently of cognate T:B cell interaction and TLR signaling, might play an important role in the differentiation of LAG-3+CD138hi cells at steady state. TI-2 antigens are good candidates to trigger an endogenous plasma cell response at steady state, when the availability of activated antigen-specific CD4+ T helper cells or TLR agonists are likely to be low. As further support to this notion, some immunization protocols can induce long-lived TI-2-antigen specific plasma cells expressing membrane IgM, and capable of expressing IL-10 upon stimulation [27], alike LAG-3+CD138hi cells.
What could be the function of regulatory plasma cells targeting endogenous TI-2 antigens? The maintenance of immunological tolerance towards endogenous TI-2 antigens that would become available only sporadically, for instance during acute tissue damage, might represent a challenge, because such antigens might be present in too low amount under normal physiological condition to induce clonal deletion or anergy in specific B cell clones. Immunity towards such antigens would not be controlled by T cell-mediated mechanisms of dominant tolerance because TI-2 antigens are not visible to CD4+Foxp3+ T regulatory cells or other suppressive T cell subsets. The function of LAG-3+CD138hi cells might thus be to provide a mechanism of dominant immune regulation towards endogenous TI-2 antigens. These cells regulate humoral immunity because a lack of LAG-3 expression on these cells led to the development of stronger humoral immunity after immunization [21••]. The exploration of this notion will require the identification of endogenous TI-2 antigens and of the antigens recognized by LAG-3+CD138hi cells.
Natural regulatory plasma cells: an interface between the immune system and damaged cells?
The characterization of the BCR repertoire of LAG-3+CD138hi cells led to some first clues on the antigens they recognize. Indeed, LAG-3+CD138hi cells distinctively express the PtC-reactive VH11+Vk14+ antibody, which is present on about 1.5–2% of these cells and absent from LAG-3−CD138hi cells [21••]. This antibody is known to bind to apoptotic cells and damaged red blood cells [28], establishing a link between the development of LAG-3+CD138hi cells and the recognition damaged cells such as apoptotic and senescent cells as well as aged red blood cells. LAG-3+CD138hi cells also differentially express other molecules associated with the handling of apoptotic cells including Sirp1α and Nr4a1 (also called Nur77) [21••]. Sirp1α recognizes the broadly expressed surface molecule CD47 that prevents the phagocytosis of healthy cells including healthy red blood cells [29,30]. The nuclear receptor Nr4a1 is involved in the anti-inflammatory effect of apoptotic cells [31]. Collectively, this literature suggests that LAG-3+CD138hi cells are at the interface of the immune system and damaged cells, including apoptotic bodies that constitute an important source of autoantigens [32].
This observation extends previous findings that associated B cell-mediated regulation with the recognition and clearance of apoptotic cells. This was for instance documented in a model of ulcerative colitis that spontaneously developed in TCRα-deficient mice. Mice that additionally lacked B cells developed an exacerbated form of this disease associated with an increased accumulation of apoptotic cells in the spleen, mesenteric lymph node, and colon of these mice, compared to their B cell-sufficient counterpart [33]. B cells contributed to the clearance of these apoptotic cells through the production of antibodies, and could be functionally replaced by the transfer of antibodies. The administration of a mixture of five monoclonal autoantibodies reacting towards colonic cells was sufficient to improve the course of colitis in TCRα-deficient mice lacking B cells [33]. The link between B cell-mediated immune regulation and apoptotic cells is underlined by the observation that the expression of TIM-1, a cell surface receptor that binds phosphatidylserine on the surface of apoptotic cells [34], identifies a subset of B cells with an increased competence for IL-10 production [20]. Finally, B cells contribute to the suppressive effect of apoptotic cells administered in recipient mice [35].
Conclusions
The identification of natural regulatory plasma cells provides a new light on B cell-mediated regulatory activities. In particular, it shows that this form of immune regulation is part of the natural function of the immune system because LAG-3+CD138hi cells develop at steady state independently of any immune challenge. They accumulate in spleen, bone marrow, and mesenteric lymph nodes where they constitutively produce antibodies that might help to remove cell debris. They constitutively express IL-10 in bone marrow, where they display an enrichment in VH11+Vk14+ cells. This might be related to the role of the bone marrow in the removal of damaged red blood cells, and as a hematopoietic organ. Further studies shall aim at defining the function of these cells at steady state, at determining their immune reactivity profile, and at elucidating whether a human counterpart exists. To this end, we have developed mice enabling the constitutive or inducible ablation of LAG-3+ cells by introducing the sequences coding for the diphteria toxin A, or diphteria toxin receptor, respectively, in the Lag3 gene. These sequences are preceded by a STOP cassette flanked by two LoxP sites, so that their expression can be restricted to LAG-3-expressing cells of the B (or T) cell lineage using appropriate Cre-expressing mouse lines.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgments
SF lab is supported by ERC PREG-LAB 647696, AXA Chair Translational Immunology, Agence Nationale de la Recherche (ANR-16-CE18-0007-01), Chair of Excellence (Université Sorbonne Paris Cité).
References
- 1.Durandy A., Kracker S., Fischer A. Primary antibody deficiencies. Nat Rev Immunol. 2013;13:519–533. doi: 10.1038/nri3466. [DOI] [PubMed] [Google Scholar]
- 2.Nutt S.L., Hodgkin P.D., Tarlinton D.M., Corcoran L.M. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15:160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida T., Mei H., Dorner T., Hiepe F., Radbruch A., Fillatreau S., Hoyer B.F. Memory B and memory plasma cells. Immunol Rev. 2010;237:117–139. doi: 10.1111/j.1600-065X.2010.00938.x. [DOI] [PubMed] [Google Scholar]
- 4.Amanna I.J., Carlson N.E., Slifka M.K. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 5.Dang V.D., Hilgenberg E., Ries S., Shen P., Fillatreau S. From the regulatory functions of B cells to the identification of cytokine-producing plasma cell subsets. Curr Opin Immunol. 2014;28:77–83. doi: 10.1016/j.coi.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Shen P., Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15:441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 7.Patuzzo G., Barbieri A., Tinazzi E., Veneri D., Argentino G., Moretta F., Puccetti A., Lunardi C. Autoimmunity and infection in common variable immunodeficiency (CVID) Autoimmun Rev. 2016;15:877–882. doi: 10.1016/j.autrev.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 8••.Shen P., Roch T., Lampropoulou V., O’Connor R.A., Stervbo U., Hilgenberg E., Ries S., Dang V.D., Jaimes Y., Daridon C. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that surface IgM-expressing plasmocytes were the major source of B cell-derived IL-10 in autoimmune and infectious disease, and documented the existence of a distinct subset of IL-35-expressing plasmocyte.
- 9.Fillatreau S., Sweenie C.H., McGeachy M.J., Gray D., Anderton S.M. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 10.Lampropoulou V., Hoehlig K., Roch T., Neves P., Calderon Gomez E., Sweenie C.H., Hao Y., Freitas A.A., Steinhoff U., Anderton S.M. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 11.Yoshizaki A., Miyagaki T., DiLillo D.J., Matsushita T., Horikawa M., Kountikov E.I., Spolski R., Poe J.C., Leonard W.J., Tedder T.F. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Rosser E.C., Oleinika K., Tonon S., Doyle R., Bosma A., Carter N.A., Harris K.A., Jones S.A., Klein N., Mauri C. Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nat Med. 2014;20:1334–1339. doi: 10.1038/nm.3680. [DOI] [PubMed] [Google Scholar]; This study identified the role of two inflammatory cytokines that were partly controlled by the microbiota and regulated the suppressive function of B cells.
- 13••.Matsumoto M., Baba A., Yokota T., Nishikawa H., Ohkawa Y., Kayama H., Kallies A., Nutt S.L., Sakaguchi S., Takeda K. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41:1040–1051. doi: 10.1016/j.immuni.2014.10.016. [DOI] [PubMed] [Google Scholar]; This study established that the acquisition of IL-10 expression by human B cells is maximal during their acquisition of antibody-secreting function
- 14.Rangaswamy U.S., Speck S.H. Murine gammaherpesvirus M2 protein induction of IRF4 via the NFAT pathway leads to IL-10 expression in B cells. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neves P., Lampropoulou V., Calderon-Gomez E., Roch T., Stervbo U., Shen P., Kuhl A.A., Loddenkemper C., Haury M., Nedospasov S.A. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33:777–790. doi: 10.1016/j.immuni.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Shalapour S., Font-Burgada J., Di Caro G., Zhong Z., Sanchez-Lopez E., Dhar D., Willimsky G., Ammirante M., Strasner A., Hansel D.E. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94–98. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teichmann L.L., Kashgarian M., Weaver C.T., Roers A., Muller W., Shlomchik M.J. B cell-derived IL-10 does not regulate spontaneous systemic autoimmunity in MRL.Fas(lpr) mice. J Immunol. 2012;188:678–685. doi: 10.4049/jimmunol.1102456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanaba K., Bouaziz J.D., Haas K.M., Poe J.C., Fujimoto M., Tedder T.F. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Blair P.A., Chavez-Rueda K.A., Evans J.G., Shlomchik M.J., Eddaoudi A., Isenberg D.A., Ehrenstein M.R., Mauri C. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding Q., Yeung M., Camirand G., Zeng Q., Akiba H., Yagita H., Chalasani G., Sayegh M.H., Najafian N., Rothstein D.M. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Lino A.C., Dang V.D., Lampropoulou V., Welle A., Joedicke J., Pohar J., Simon Q., Thalmensi J., Baures A., Fluhler V. LAG-3 inhibitory receptor expression identifies immunosuppressive natural regulatory plasma cells. Immunity. 2018;49:120–133. doi: 10.1016/j.immuni.2018.06.007. e129. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies and characterizes a population of natural regulatory plasma cells.
- 22.Gagliani N., Magnani C.F., Huber S., Gianolini M.E., Pala M., Licona-Limon P., Guo B., Herbert D.R., Bulfone A., Trentini F. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 23.Okamura T., Fujio K., Shibuya M., Sumitomo S., Shoda H., Sakaguchi S., Yamamoto K. CD4+CD25-LAG3+ regulatory T cells controlled by the transcription factor Egr-2. Proc Natl Acad Sci U S A. 2009;106:13974–13979. doi: 10.1073/pnas.0906872106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao Y., Vent-Schmidt J., McGeough M.D., Wong M., Hoffman H.M., Steiner T.S., Levings M.K. Tr1 Cells, but not Foxp3+ regulatory T Cells, suppress NLRP3 inflammasome activation via an IL-10-dependent mechanism. J Immunol. 2015;195:488–497. doi: 10.4049/jimmunol.1403225. [DOI] [PubMed] [Google Scholar]
- 25.Corneth O.B.J., Klein Wolterink R.G.J., Hendriks R.W. BTK signaling in B cell differentiation and autoimmunity. Curr Top Microbiol Immunol. 2016;393:67–105. doi: 10.1007/82_2015_478. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y., Fairfax K., Light A., Huntington N.D., Tarlinton D.M. CD19 differentially regulates BCR signalling through the recruitment of PI3K. Autoimmunity. 2014;47:430–437. doi: 10.3109/08916934.2014.921810. [DOI] [PubMed] [Google Scholar]
- 27.Blanc P., Moro-Sibilot L., Barthly L., Jagot F., This S., de Bernard S., Buffat L., Dussurgey S., Colisson R., Hobeika E. Mature IgM-expressing plasma cells sense antigen and develop competence for cytokine production upon antigenic challenge. Nat Commun. 2016;7:13600. doi: 10.1038/ncomms13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw P.X., Horkko S., Chang M.K., Curtiss L.K., Palinski W., Silverman G.J., Witztum J.L. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bian Z., Shi L., Guo Y.L., Lv Z., Tang C., Niu S., Tremblay A., Venkataramani M., Culpepper C., Li L. Cd47-Sirpalpha interaction and IL-10 constrain inflammation-induced macrophage phagocytosis of healthy self-cells. Proc Natl Acad Sci U S A. 2016;113:E5434–5443. doi: 10.1073/pnas.1521069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardai S.J., McPhillips K.A., Frasch S.C., Janssen W.J., Starefeldt A., Murphy-Ullrich J.E., Bratton D.L., Oldenborg P.A., Michalak M., Henson P.M. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 31.Ipseiz N., Uderhardt S., Scholtysek C., Steffen M., Schabbauer G., Bozec A., Schett G., Kronke G. The nuclear receptor Nr4a1 mediates anti-inflammatory effects of apoptotic cells. J Immunol. 2014;192:4852–4858. doi: 10.4049/jimmunol.1303377. [DOI] [PubMed] [Google Scholar]
- 32.Casciola-Rosen L.A., Anhalt G., Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizoguchi A., Mizoguchi E., Smith R.N., Preffer F.I., Bhan A.K. Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. J Exp Med. 1997;186:1749–1756. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi N., Karisola P., Pena-Cruz V., Dorfman D.M., Jinushi M., Umetsu S.E., Butte M.J., Nagumo H., Chernova I., Zhu B. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray M., Miles K., Salter D., Gray D., Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci U S A. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]