Abstract
Impulsivity and the attentional orienting response to cocaine-associated cues (cue reactivity) promote relapse in cocaine-use disorder (CUD). A time-dependent escalation of cue reactivity (incubation) occurs during extended, forced abstinence from cocaine self-administration in rats. The investigational serotonin (5-HT) 5-HT2A receptor (5-HT2AR) antagonist/inverse agonist M100907 suppresses impulsive action, or the inability to withhold premature responses, and cocaine-seeking behaviors. The present preclinical study was designed to establish the potential for repurposing the Food and Drug Administration–approved selective 5-HT2AR antagonist/inverse agonist pimavanserin as a therapeutic agent to forestall relapse vulnerability in CUD. In male Sprague-Dawley rats, pimavanserin suppressed impulsive action (premature responses) measured in the 1-choice serial reaction time (1-CSRT) task, similarly to M100907. We also used the 1-CSRT task to establish baseline levels of impulsive action before cocaine self-administration and evaluation of cue reactivity (lever presses reinforced by the discrete cue complex previously paired with cocaine delivery). We observed an incubation of cocaine cue reactivity between day 1 and day 30 of forced abstinence from cocaine self-administration. Baseline levels of impulsive action predicted incubated levels of cocaine cue reactivity in late abstinence. We also found that baseline impulsive action predicted the effectiveness of pimavanserin to suppress incubated cue reactivity in late abstinence from cocaine self-administration at doses that were ineffective in early abstinence. These data suggest that integration of clinical measures of impulsive action may inform refined, personalized pharmacotherapeutic intervention for the treatment of relapse vulnerability in CUD.
Introduction
Cocaine use disorder (CUD) represents a significant public health challenge in the United States. The lack of effective pharmacotherapeutics to suppress relapse vulnerability is an unmet need in the treatment of CUD (Volkow and Skolnick, 2012). Impulsivity and cue reactivity are two key behavioral phenotypes that engender relapse vulnerability (O’Brien et al., 1998; Drummond, 2001; Moeller et al., 2001a; Koob and Volkow, 2010; Saunders et al., 2013). Impulsivity has been defined clinically as rapid, unplanned reactions to stimuli, reduced sensitivity to negative consequences, and a disregard for long-term consequences (Moeller et al., 2001a). Rapid-response impulsivity (impulsive action; difficulty withholding a prepotent response) and impulsive choice (decision-making; delayed reward measures) are dimensions of impulsivity that have been associated with CUD in humans (Moeller et al., 2001a,b; Patkar et al., 2004) and in rodents (Perry et al., 2005; Belin et al., 2008; Anastasio et al., 2014a). Cue reactivity refers to the attentional orienting response to drug-related stimuli that predict reward (Maas et al., 1998; Carter and Tiffany, 1999; Garavan et al., 2000; Field and Cox, 2008). We demonstrated that levels of impulsive action positively correlated with the attentional bias toward cocaine-associated cues in cocaine-dependent humans (Liu et al., 2011) and rodents (Anastasio et al., 2014b); two meta-analyses recently corroborated this link (Coskunpinar and Cyders, 2013; Leung et al., 2017). High baseline levels of self-reported impulsivity (Barrett Impulsivity Scale-11) (Moeller et al., 2001b, 2007) or attentional bias for cocaine-associated cues (Cocaine Word Stroop Task) (Carpenter et al., 2006) predicted poorer retention of cocaine-dependent participants in outpatient treatment trials. These findings suggest that pharmacotherapeutic strategies that effectively diminish impulsive action and cocaine cue reactivity may reduce relapse during abstinence in CUD patients.
Serotonin (5-hydroxytryptamine; 5-HT) neurotransmission regulates the limbic-corticostriatal circuitry associated with the development and maintenance of addiction, as well as vulnerability to relapse (for reviews, see Cunningham and Anastasio, 2014; Koob and Volkow, 2016). Serotonin actions are transduced by 14 receptor subtypes, and the G-protein–coupled 5-HT2A receptor (5-HT2AR) has been implicated in the neural mechanisms underlying impulsive action, defined as premature responses in the choice serial reaction time (CSRT) tasks (for review, see Cunningham and Anastasio, 2014). After systemic administration, the preferential 5-HT2AR agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) (Titeler et al., 1988; Wischhof et al., 2011) elevated impulsive action measured in the 5-CSRT task (Koskinen et al., 2000a,b; Blokland et al., 2005). The selective 5-HT2AR antagonist/inverse agonist M100907 (volinanserin) administered systemically suppressed premature responding assessed in the 1 or 5-CSRT task (Winstanley et al., 2004; Fletcher et al., 2007, 2011; Anastasio et al., 2011, 2015; Cunningham et al., 2013; Fink et al., 2015) and also suppressed cocaine- and cue-evoked reinstatement after extinction training from cocaine self-administration (Lacosta and Roberts, 1993; Fletcher et al., 2002; Filip, 2005; Nic Dhonnchadha et al., 2009). A time-sensitive increase in attentional bias toward cocaine-associated cues in rats (Neisewander et al., 2000; Grimm et al., 2001; Lu et al., 2004; Swinford-Jackson et al., 2016) has been observed during extended abstinence and is referred to as “incubation” (for review, see Pickens et al., 2011). In rodents, escalation of cue reactivity (lever presses that are reinforced by the discrete drug-paired cue complex) occurs up to 6 months after termination of cocaine self-administration (Neisewander et al., 2000; Grimm et al., 2001; Lu et al., 2004; Swinford-Jackson et al., 2016); however, the relationship between levels of impulsive action and incubated cue reactivity, as well as the effectiveness of a 5-HT2AR antagonist/inverse agonist to suppress cocaine cue reactivity during early versus late abstinence from cocaine self-administration, is unknown.
Preclinical and early clinical evaluations of selective 5-HT2AR antagonists support their potential efficacy as therapeutics in sleep disorders (Ancoli-Israel et al., 2011), psychosis (Weiner et al., 2001), psychosis in Parkinson’s disease (Meltzer et al., 2012), and other psychologic disorders (Roberts, 2006); however, the investigational compound M100907 (volinanserin), which exhibits >100-fold selectivity for the 5-HT2AR over the homologous 5-HT2BR and 5-HT2CR and other monoamine receptors (Kehne et al., 1996; Knight et al., 2004), never achieved approval for a clinical indication. The selective 5-HT2AR antagonist/inverse agonist pimavanserin (Nuplazid, Acadia Pharmaceuticals, San Diego, CA) is now clinically approved for the treatment of psychosis in Parkinson disease (Sahli and Tarazi, 2018) and exhibits >100-fold selectivity for the 5-HT2AR over 5-HT2BR, 5-HT2CR, and other monoamine receptors (Vanover et al., 2006; Hacksell et al., 2014). Notably, both M100907 and pimavanserin are potent 5-HT2AR antagonists in vivo; for example, both compounds suppressed head-twitch behaviors and prepulse inhibition deficits induced by DOI in rats (Sipes and Geyer, 1995; Fantegrossi et al., 2010; McFarland et al., 2011), whereas M100907 is well characterized to suppress the discriminative stimulus properties of DOI and other 5-HT2AR agonists (Smith et al., 2003; Winter et al., 2007).
This preclinical study was designed to establish the potential for repurposing pimavanserin as a therapeutic to forestall relapse vulnerability in CUD. Given that previous studies support the utility of the rodent CSRT tasks for screening pharmacologic interventions on impulsive action (for review, see Winstanley, 2011), we used the 1-CSRT task to test the hypothesis that pimavanserin would suppress impulsive action and cocaine cue reactivity during abstinence from cocaine self-administration, similar to M100907. Given that baseline levels of impulsive action positively correlated with cocaine cue reactivity in humans (Liu et al., 2011) and rodents (Anastasio et al., 2014b), we evaluated whether baseline levels of impulsive action are related to the effectiveness of pimavanserin to control cocaine cue reactivity in early (day 1) versus late abstinence (day 30).
Materials and Methods
General Methods
Animals.
Male Sprague-Dawley rats (n = 160; Harlan, Houston, TX) weighed 250–275 g at arrival and were housed in the colony room. Rats were housed two per cage under a 12-hour light/dark cycle with monitored and controlled temperature (21–23°C) and humidity levels (45%–50%). Rats were acclimated to the colony room for 7 days before handling and experimentation commenced. Rats were food-restricted to ∼90% free-feeding weight (confirmed by daily weights) during 1-CSRT task training and had ad libitum access to water except during daily operant sessions. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011) and with the University of Texas Medical Branch Institutional Animal Care and Use Committee approval.
Drugs.
(-)-Cocaine (National Institute on Drug Abuse Drug Supply Program, Bethesda, MD) was dissolved in 0.9% NaCl. M100907 [(R)-(2,3-dimethoxyphenyl)-[1-[2-(4-fluorophenyl)ethyl]piperidin-4-yl]methanol] (synthesized by Kenner Rice, National Institute on Drug Abuse, Bethesda, MD) was dissolved in 1% Tween-80 in 0.9% NaCl [vehicle (VEH) used for comparison with M100907]. Pimavanserin [1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)-1-(1-methylpiperidin-4-yl)urea] (Trylead Chemical Technology Co., Ltd., Hangzhou, China) was dissolved in 0.9% NaCl brought to a pH ∼6.0 using 1 M HCl (VEH used for comparison with pimavanserin). M100907 and pimavanserin were administered by the intraperitoneal or subcutaneous route, respectively.
1-Choice Serial Reaction Time Task.
All sessions conducted in the 1-CSRT task occurred in five-hole, nose-poke operant chambers containing a house light, food tray, and external pellet dispenser that delivered 45-mg dustless precision food pellets (Bio-Serv, Frenchtown, NJ) housed within ventilated, sound-attenuated cubicles (MedAssociates, St Albans, VT). The 1-CSRT task method was previously reported in detail (Anastasio et al., 2011, 2013, 2014b; Cunningham et al., 2013; Fink et al., 2015). To summarize, rats were initially exposed to a pretraining stage during which they were habituated to the test chamber. A nose-poke response into the illuminated-center stimulus hole (i.e., a target response) resulted in simultaneous illumination of the magazine light on the opposite chamber wall and delivery of a 45-mg food pellet. Rats were progressed through a series of training stages after completion of the pretraining stage. Each stage consisted of daily 100-trial sessions to be completed in a maximum of 30 minutes. The stimulus duration was incrementally shortened throughout each training stage until a final stage of 0.5 second was achieved with a limited hold of 5 seconds and an intertrial interval (ITI) of 5 seconds (ITI5).
A maximum of 100 target responses in a session resulted in a maximum of 100 reinforcers delivered. Incorrect nontarget responses, premature responses, or omissions resulted in a timeout period (5 seconds) that reduced the potential number of reinforcers delivered. Before progressing through each training stage, rats were required to achieve acquisition criteria: ≥50 reinforcers earned, >80% accuracy (target responses/(target + nontarget responses) ⋅ 100) and <20% omissions (omitted responses/trials completed ⋅ 100) (Anastasio et al., 2011, 2013, 2014b; Cunningham et al., 2013; Fink et al., 2015).
The number of premature responses, omissions, and reinforcers earned, percent accuracy, latency to first response, and time to finish the 1-CSRT task were recorded. Premature responses, the primary output measure to assess impulsive action, were categorized into three types: target, nontarget, and total (i.e., target + nontarget). The number of reinforcers earned assessed task competency and provides an additional measure of impulsive action. The percent accuracy was a general indication of attentional capacity. Percent omissions indicated motivation to perform the task, and latency to first response in the 1-CSRT task provided a secondary measure of motivation and an indication of general motor impairment.
Cocaine Self-Administration and Cue Reactivity.
Rats (n = 144) were anesthetized (8.6 mg/kg of xylazine, 1.5 mg/kg of acepromazine, 43 mg/kg of ketamine in bacteriostatic saline) before surgical implantation of indwelling jugular catheters with back mounts; rats were allotted 7 days to permit postoperative recovery (Cunningham et al., 2011, 2013; Anastasio et al., 2014a,b). Rats received a 0.1-ml infusion of a bacteriostatic saline solution that contained heparin sodium (10 U/ml; American Pharmaceutical Partners, East Schaumburg, IL), streptokinase (0.67 mg/ml; Sigma Chemical, St. Louis, MO), and ticarcillin disodium (66.67 mg/ml; Research Products International, Mt. Prospect, IL) into the catheter immediately after daily cocaine self-administration sessions to ensure catheter patency during experimentation.
The cocaine self-administration assay used standard operant conditioning chambers (Med Associates, Inc.) housed within sound-attenuated, ventilated cubicles equipped with fans (Med Associates, Inc.). Operant chambers were fitted with two retractable response levers, a stimulus light above each of the response levers, a houselight on the wall opposite the response levers, and an external pellet dispenser. Cocaine infusions were delivered through syringes that were loaded daily into infusion pumps (Med Associates, Inc.) located outside the cubicles. The infusion pumps were connected to liquid swivels (Instech, Plymouth Meeting, PA) fastened to catheters via polyethylene 20 tubing encased inside a metal spring leash (Plastics One, Roanoke, VA).
Cocaine self-administration training sessions were 180 minutes in duration and occurred daily. Rats were trained to perform a lever press response reinforced by a cocaine infusion (0.75 mg/kg/0.1 ml infusion) (Cunningham et al., 2011; Anastasio et al., 2014a,b; Swinford-Jackson et al., 2016). Schedule responses on the active lever resulted in delivery of a cocaine infusion over a 6-second period; each infusion was simultaneously paired with the illumination of the house and stimulus lights and activation of the infusion pump (i.e., discrete cue complex paired with cocaine delivery). Inactive lever presses were recorded but had no scheduled consequences. The stimulus light and infusion pump were inactivated following delivery of cocaine. The house light remained on to signal a timeout period (20 seconds); lever presses committed during the timeout period had no scheduled consequences. Rats were trained on a fixed ratio (FR)1 schedule of reinforcement and progressed to a FR5 schedule after achieving seven infusions/hour with less than 10% variability for 3 consecutive days. Once stable cocaine self-administration was acquired, rats were subjected to forced abstinence (FA) from cocaine self-administration for 1 (FA day 1) or 30 days (FA day 30). During the FA period, rats were returned to their home cages, weighed, and handled daily. After the assigned FA period, rats were evaluated in a cue reactivity test session (60 minutes) in which presses on the previously active lever were reinforced by the discrete cue complex (stimulus-light illuminated, infusion-pump activated) on an FR1 schedule. Inactive lever presses were recorded but had no scheduled consequences.
Research Design
Cohort 1: Pimavanserin and M100907 Suppress Impulsive Action Measured in the 1-CSRT Task.
Rats (n = 16) were required to meet acquisition criteria in the 1-CSRT task [≥50 target responses, >80% accuracy, and <20% omissions on the final training stage (0.5-second stimulus duration, 5-second limited hold, and ITI5)] for at least 3 consecutive days. Performance in the 1-CSRT task was assessed after systemic administration of M100907 or pimavanserin. Pretreatment with vehicle (1 ml/kg, i.p.) or M100907 (0.001, 0.01, 0.1 mg/kg, i.p) occurred 30 minutes before start of the 1-CSRT task session under an ITI5 schedule. Five rats failed to meet the acquisition criteria under an ITI5 schedule in the 1-CSRT task and were excluded from analysis of M100907 (n = 11 rats analyzed).
After pharmacologic evaluations with M100907, rats established stable 1-CSRT task training under an ITI5 schedule for a minimum of 30 days to permit drug washout. Pharmacological test sessions with pimavanserin commenced once rats met the 1-CSRT task acquisition criteria for at least 3 consecutive days. Pretreatment with VEH (1 ml/kg, s.c.) or pimavanserin (0.3, 1, 3 mg/kg, s.c.) occurred 30 minutes before start of 1-CSRT task sessions under an ITI5 schedule. Two rats failed to maintain stable performance under an ITI5 schedule in the 1-CSRT task and were excluded from analysis of pimavanserin (n = 9 rats analyzed). Rats were treated with VEH the day before drug pretreatments, which were separated by a minimum of 3 days. The order of M100907 and pimavanserin injections was randomized across rats in a within-subjects design.
Cohort 2: Baseline Levels of Impulsive Action Predict the Efficacy of Pimavanserin to Suppress Cue Reactivity.
Rats (n = 144) were trained on the 1-CSRT task. Baseline levels of impulsive action were established once rats met the acquisition criteria of ≥50 target responses, >80% accuracy, and <20% omissions on the final training stage (0.5-second stimulus duration, 5-second limited hold, ITI5) for 3 consecutive days. Three rats failed to maintain stable performance and were excluded from analysis. After identification of baseline levels of impulsive action, 1-CSRT task sessions ceased. Rats were returned to their home cages and were freely fed for at least 5 days before surgical implantation of indwelling jugular catheters. Cocaine self-administration began after at least 5 days postcatheterization. After acquisition of stable cocaine self-administration, rats were assigned to either FA day 1 or FA day 30 and returned to their home cages for the assigned FA duration. Fifteen rats were excluded from analysis for technical issues in the cocaine self-administration assay (n = 126 rats analyzed).
Pharmacologic test sessions were used to evaluate the efficacy of pimavanserin to suppress cocaine cue reactivity on FA day 1 or FA day 30. Pretreatment with VEH (1 ml/kg, s.c.) or pimavanserin (0.3, 1, 3, 10 mg/kg, s.c.) occurred 30 minutes before the cue reactivity session on FA day 1 (n = 68; n = 12–15/group) or FA day 30 (n = 58; n = 11 or 12/group) in a between-subjects design.
Statistical analyses.
A one-way repeated measures analysis of variance (ANOVA) was used to evaluate the effects of either M100907 (VEH, 0.001, 0.01, 0.1 mg/kg) or pimavanserin (VEH, 0.3, 1, 3 mg/kg) on 1-CSRT task measures (target, nontarget, or total premature responses, reinforcers earned, % omissions, % accuracy, latency to first response, time to finish the 1-CSRT task); Dunnett’s procedure was employed to analyze planned comparisons (Keppel, 1973). The dose of M100907 or pimavanserin estimated to decrease total premature responses by 50% of the maximum suppression (ID50) in the 1-CSRT task was quantified by a four-parameter logistic nonlinear regression (Ratkowsky and Reedy, 1986; Tallarida and Murray, 1987). Student’s t test was used to assess total cocaine intake throughout acquisition and maintenance of cocaine self-administration. A two-way ANOVA with the factors of FA day (FA day 1, FA day 30) and medication pretreatment (VEH, 0.3, 1, 3, 10 mg/kg of pimavanserin) was used to analyze 1-CSRT task performance to ensure equal distribution of rats across groups. A two-way ANOVA with the factors of FA day (FA day 1, FA day 30) and medication pretreatment (VEH, 0.3, 1, 3, 10 mg/kg of pimavanserin) was used to analyze previously active lever presses, inactive lever presses, and the latency to first response during the cue reactivity test session. A one-way ANOVA was used to analyze previously active lever presses at each FA day after medication pretreatment; planned comparisons were assessed using Dunnett’s procedure or Student’s t test, where appropriate (Keppel, 1973). A four-parameter logistic nonlinear regression was used to calculate the ID50 of pimavanserin to suppress previously active lever presses during the cue reactivity session on FA day 1 versus FA day 30 (Ratkowsky and Reedy, 1986; Tallarida and Murray, 1987). A one-way analysis of covariance (ANCOVA) was used to evaluate the relationship between target premature responses and previously active lever presses on FA day 1 versus FA day 30 after pretreatment with VEH. A one-way ANCOVA was used to evaluate the relationship between target premature responses and previously active lever presses at each FA day with five between-subject pretreatment conditions (VEH, 0.3, 1, 3, 10 mg/kg of pimavanserin) and the covariate (target premature responses in the 1-CSRT task).
Results
M100907 and Pimavanserin Suppress Impulsive Action.
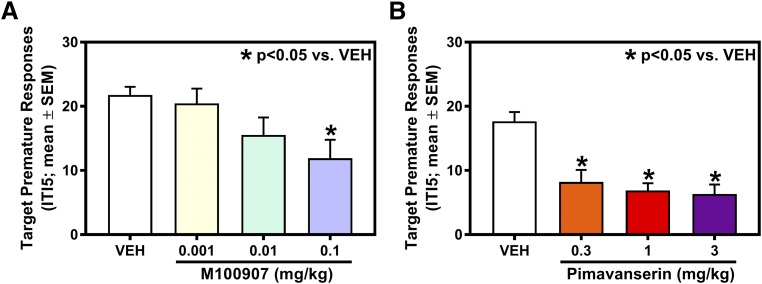
We tested the hypotheses that M100907 or pimavanserin would suppress impulsive action measured in the 1-CSRT task relative to VEH. Figure 1A displays the mean (±S.E.M.) number of target premature responses after pretreatment with VEH or M100907 (n = 11). There was a main effect of M100907 on target premature responses (F(3,30) = 4.17; P < 0.05); planned comparisons with Dunnett’s procedure showed that 0.1 mg/kg of M100907 decreased target premature responses versus VEH (P < 0.05; Fig. 1A). The ID50 of M100907 to suppress target premature responses was 0.007 mg/kg. In addition to target premature responses, there was a main effect of M100907 on total (target + nontarget) premature responses (F(3,30) = 4.88; P < 0.05), % omissions (F(3,30) = 8.69; P < 0.05), and time to finish the 1-CSRT task (F(3,30) = 7.21; P < 0.05); planned comparisons with Dunnett’s procedure showed that 0.1 mg/kg of M100907 decreased total premature responses, increased % omissions, and increased the time to finish the 1-CSRT task versus VEH (P < 0.05; Table 1). There was no main effect of M100907 on nontarget premature responses (F(3,30) = 1.55; n.s.], reinforcers earned [F(3,30) = 1.13; n.s.], % accuracy [F(3,30) = 0.422; n.s.], or latency to first response in the 1-CSRT task (F(3,30) = 1.04; n.s.) versus VEH (Table 1).
Fig. 1.
M100907 and pimavanserin suppress target premature responses in the 1-CSRT task. The effects of M100907 (0.001, 0.01, and 0.1 mg/kg; n = 11) or pimavanserin (0.3, 1, 3 mg/kg; n = 9) were each evaluated under an ITI5 schedule in the 1-CSRT task. (A) M100907 significantly decreased target premature responses at 0.1 mg/kg (*P < 0.05 vs. VEH). (B) Pimavanserin significantly decreased target premature responses at 0.3, 1, and 3 mg/kg (*P < 0.05 vs. VEH).
TABLE 1.
1-CSRT task descriptive statistics for M100907 and pimavanserin pretreatment (mean ± S.E.M.)
| Pretreatment | Dose | Premature Responses |
Reinforcers Earned | % Accuracy | % Omissions | Latency to First Response (s) | Time to Finish 1-CSRT Task (s) | ||
|---|---|---|---|---|---|---|---|---|---|
| Target | Nontarget | Total | |||||||
| M100907 | Vehicle | 21.8 ± 1.27 | 1.61 ± 0.265 | 23.4 ± 1.43 | 68.4 ± 1.33 | 97.0 ± 0.433 | 6.15 ± 0.817 | s 3.38 ± 1.85 | 872 ± 15.9 |
| 0.001 mg/kg | 20.5 ± 2.30 | 1.91 ± 0.719 | 22.4 ± 2.32 | 69.5 ± 1.06 | 97.1 ± 0.674 | 6.00 ± 1.95 | 1.00 ± 0.185 | 874 ± 23.7 | |
| 0.01 mg/kg | 15.5 ± 2.71 | 0.818 ± 0.325 | 16.4 ± 2.88 | 71.2 ± 2.60 | 97.5 ± 0.483 | 10.6 ± 2.83 | 2.88 ± 1.49 | 938 ± 40.6 | |
| 0.1 mg/kg | 11.9 ± 2.87* | 1.36 ± 0.338 | 13.3 ± 2.86* | 66.8 ± 2.12 | 97.7 ± 0.750 | 18.3 ± 2.70* | 2.17 ± 0.862 | 1022 ± 35.8* | |
| Pimavanserin | Vehicle | 17.7 ± 1.44 | 0.963 ± 0.286 | 18.6 ± 1.50 | 73.6 ± 1.40 | 97.5 ± 0.704 | 5.89 ± 0.957 | 1.44 ± 0.258 | 859 ± 11.5 |
| 0.3 mg/kg | 8.22 ± 1.88* | 1.00 ± 0.441 | 9.22 ± 2.09* | 79.6 ± 2.06 | 98.4 ± 0.631 | 9.89 ± 1.96 | 0.613 ± 0.161 | 898 ± 25.9 | |
| 1 mg/kg | 6.89 ± 1.11* | 0.333 ± 0.167 | 7.22 ± 1.16* | 77.2 ± 3.36 | 98.3 ± 0.674 | 14.1 ± 3.85* | 1.16 ± 0.378 | 948 ± 41.9* | |
| 3 mg/kg | 6.33 ± 1.47* | 0.667 ± 0.236 | 7.00 ± 1.53* | 74.4 ± 4.11 | 98.3 ± 0.657 | 17.1 ± 4.74* | 1.51 ± 0.236 | 984 ± 46.0* | |
P < 0.05 vs. Vehicle in each respective group.
Figure 1B displays the mean (±S.E.M.) number of target premature responses after pretreatment with pimavanserin (n = 9). There was a main effect of pimavanserin on target premature responses (F(3,24) = 17.3; P < 0.05); planned comparisons with Dunnett’s procedure showed that 0.3, 1, 3 mg/kg of pimavanserin decreased target premature responses versus VEH (P < 0.05; Fig. 1B). The ID50 of pimavanserin to suppress target premature responses was 0.05 mg/kg. In addition to target premature responses, there was a main effect of pimavanserin on total premature responses (F(3,24) = 15.8; P < 0.05), % omissions (F(3,24) = 5.29; P < 0.05), and time to finish the 1-CSRT task (F(3,24) = 7.79; P < 0.05); planned comparisons with Dunnett’s procedure showed that 0.3, 1, and 3 mg/kg of pimavanserin decreased total premature responses, whereas 1 and 3 mg/kg of pimavanserin increased % omissions, as well as the time to finish the 1-CSRT task versus VEH (P < 0.05; Table 1). There was no main effect of pimavanserin on nontarget premature responses (F(3,24) = 1.15; n.s.], reinforcers earned [F(3,24) = 1.25; n.s.], % accuracy [F(3,24) = 0.713; n.s.], or latency to first response in the 1-CSRT task [F(3,24) = 2.54; n.s.] versus VEH (Table 1).
Pimavanserin Suppresses Cue Reactivity on FA Day 30, but Not FA Day 1.
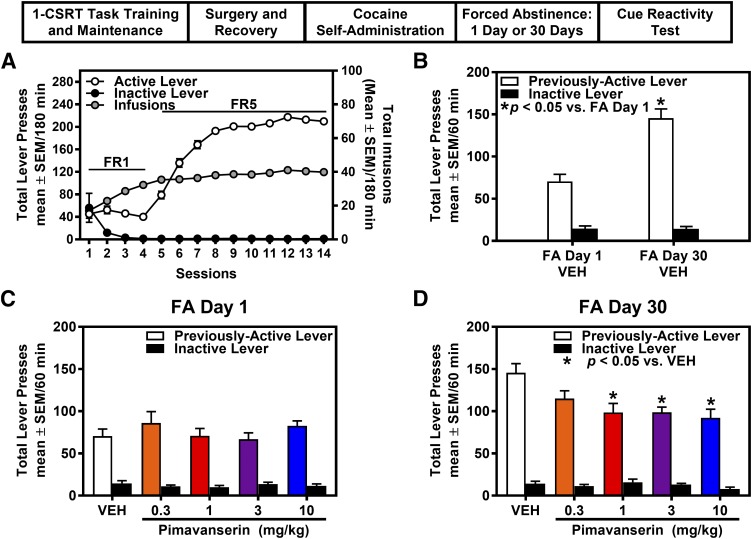
Rats (n = 126) were trained in the cocaine self-administration assay after screening on the 1-CSRT task. Rats stably acquired cocaine self-administration (i.e., FR5 schedule; seven infusions/hour for a minimum of three sessions) and displayed <10% variation in the number of cocaine infusions earned during maintenance (Fig. 2A). Rats were assigned to specific FA day and medication pretreatment group based on counterbalancing across target premature responses (Table 2), total cocaine intake across acquisition, and maintenance of self-administration. The results of a two-way ANOVA indicated no main effect of FA day, pretreatment, or FA day X pretreatment interaction for premature responses (target, nontarget, total), reinforcers earned, % accuracy, % omissions, latency to first response, and time to finish the 1-CSRT task between rats assigned to FA day 1 or FA day 30 and pimavanserin pretreatment groups (Table 2), indicating that rats were appropriately counterbalanced before training on cocaine self-administration. Total cocaine intake across self-administration sessions was not different in rats assigned to FA day 1 (361.8 ± 7.68 mg/kg; n = 68) or FA day 30 (364.3 ± 7.85 mg/kg; n = 58) [t(124) = 0.234; n.s.].
Fig. 2.
Pimavanserin suppresses cue reactivity on FA day 30, but not FA day 1, from cocaine self-administration. (A) Total presses (mean ± S.E.M.) on the active (white circles) or inactive lever (black circles; left y-axis), and total number of cocaine infusions (mean ± S.E.M.) obtained (gray circles; right y-axis) are presented for the acquisition and maintenance phase of cocaine self-administration. (B) Previously active and inactive lever presses (mean ± S.E.M.) are presented for the cue reactivity test session in VEH-treated rats on FA day 1 and FA day 30 from cocaine self-administration. Cue reactivity is significantly elevated on FA day 30 vs. FA day 1 from cocaine self-administration (*P < 0.05; n = 11 or 14/group). The effects of pimavanserin (0.3, 1, 3, 10 mg/kg) on previously active and inactive lever presses (mean ± S.E.M.) on (C) FA day 1 (n = 12–15/group) or (D) FA day 30 (n = 11–12/group) from the last cocaine self-administration session are presented. Pimavanserin suppressed previously active, but not inactive, lever presses on FA day 30 from cocaine self-administration (*P < 0.05 vs. VEH).
TABLE 2.
1-CSRT task descriptive statistics and results of two-way analysis of variance (ANOVA)
| Forced Abstinence (FA) Day |
Pimavanserin Pretreatment |
Premature Responses | Reinforcers Earned |
% Accuracy |
% Omissions |
Latency to First Response (s) |
Time to Finish 1-CSRT Task (s) |
||
|---|---|---|---|---|---|---|---|---|---|
| Target |
Nontarget |
Total |
|||||||
| Descriptive statistics FA day 1 | Vehicle | 25.7 ± 2.33 | 1.43 ± 0.327 | 27.1 ± 2.26 | 62.7 ± 1.91 | 98.6 ± 0.377 | 9.29 ± 1.98 | 1.16 ± 0.370 | 981 ± 49.4 |
| 0.3 mg/kg | 25.5 ± 2.00 | 2.00 ± 0.467 | 27.5 ± 2.21 | 61.6 ± 2.36 | 98.3 ± 0.530 | 9.77 ± 1.86 | 1.02 ± 0.221 | 953 ± 24.4 | |
| 1 mg/kg | 21.1 ± 2.25 | 1.40 ± 0.306 | 22.5 ± 2.19 | 65.2 ± 1.89 | 98.1 ± 0.371 | 11.1 ± 1.47 | 1.17 ± 0.347 | 948 ± 32.4 | |
| 3 mg/kg | 24.4 ± 2.05 | 1.21 ± 0.318 | 25.6 ± 2.11 | 65.9 ± 2.64 | 98.5 ± 0.597 | 7.57 ± 1.34 | 1.03 ± 0.171 | 912 ± 22.6 | |
| 10 mg/kg | 27.7 ± 1.76 | 2.00 ± 0.357 | 29.7 ± 1.76 | 61.9 ± 1.85 | 96.8 ± 0.895 | 6.58 ± 0.839 | 1.32 ± 0.241 | 948 ± 31.8 | |
| FA day 30 | Vehicle | 26.2 ± 2.28 | 2.27 ± 0.384 | 28.5 ± 2.33 | 60.4 ± 1.91 | 97.8 ± 0.702 | 9.91 ± 1.09 | 1.15 ± 0.299 | 984 ± 42.6 |
| 0.3 mg/kg | 26.9 ± 2.33 | 1.67 ± 0.541 | 28.6 ± 2.06 | 63.4 ± 2.09 | 98.7 ± 0.568 | 7.17 ± 1.24 | 1.57 ± 0.307 | 935 ± 26.5 | |
| 1 mg/kg | 28.5 ± 2.51 | 1.36 ± 0.432 | 29.9 ± 2.71 | 56.9 ± 2.09 | 97.5 ± 0.601 | 11.8 ± 2.42 | 1.37 ± 0.432 | 977 ± 44.2 | |
| 3 mg/kg | 20.5 ± 2.28 | 1.17 ± 0.322 | 21.7 ± 2.51 | 66.5 ± 3.29 | 98.4 ± 0.783 | 10.9 ± 2.25 | 1.55 ± 0.469 | 972 ± 37.2 | |
| 10 mg/kg | 28.3 ± 2.63 | 1.00 ± 0.275 | 29.3 ± 2.80 | 64.1 ± 1.96 | 99.2 ± 0.323 | 6.08 ± 1.52 | 14.6 ± 10.8 | 943 ± 42.2 | |
| Results of two-way ANOVA | |||||||||
| FA day | F(1,116) = 0.716 | F(1,116) = 0.208 | F(1,116) = 0.563 | F(1,116) = 0.671 | F(1,116) = 0.509 | F(1,116) = 0.087 | F(1,116) = 1.96 | F(1,116) = 0.358 | |
| Pretreatment | F(4,116) = 1.63 | F(4,116) = 1.06 | F(4,116) = 1.87 | F(4,116) = 1.53 | F(4,116) = 0.513 | F(4,116) = 2.26 | F(4,116) = 1.63 | F(4,116) = 0.451 | |
| FA day x pretreatment | F(4,116) = 1.66 | F(4,116) = 1.36 | F(4,116) = 1.63 | F(4,116) = 1.75 | F(4,116) = 2.18 | F(4,116) = 0.787 | F(4,116) = 1.52 | F(4,116) = 0.373 | |
We tested the hypothesis that pimavanserin would suppress cue reactivity (previously active lever presses for the discrete cue complex) during FA from cocaine self-administration. A main effect of FA day (F(1,116) = 34.1; P < 0.05), pretreatment (F(4,116) = 2.75; P < 0.05), and a FA day X pretreatment interaction (F(4,116) = 3.29; P < 0.05) was observed for previously active lever presses. Planned comparisons indicated that previously active lever presses were significantly elevated in rats treated with VEH on FA day 30 versus FA day 1 from cocaine self-administration [t(23) = 5.546; P < 0.05] (Fig. 2B). Further, planned comparisons revealed that pimavanserin (1, 3, 10 mg/kg) did not alter previously active lever presses on FA day 1 (Fig. 2C) but did suppress previously active lever presses on FA day 30 (Fig. 2D) relative to VEH (P < 0.05). The ID50 of pimavanserin to suppress previously active lever presses was 0.22 mg/kg on FA day 30; however, the four-parameter logistic nonlinear regression was unable to model the data for FA day 1, and an ID50 value was not determinable. There was no main effect of FA day (F(1,116) = 0.038; n.s.), pretreatment (F(4,116) = 1.154; n.s.), or a FA day X pretreatment interaction (F(4,116) = 1.003; n.s.) for inactive lever presses in the cue reactivity session. Further, there was no main effect of FA day (F(1,116) = 0.603; n.s.), pimavanserin pretreatment (F(4,116) = 0.465; n.s.), or a FA day X pretreatment interaction (F(4,116) = 1.227; n.s.) for latency to first response in the cue reactivity session.
Baseline Levels of Impulsive Action Predict the Efficacy of Pimavanserin in Suppressing Cocaine Cue Reactivity on FA Day 30.
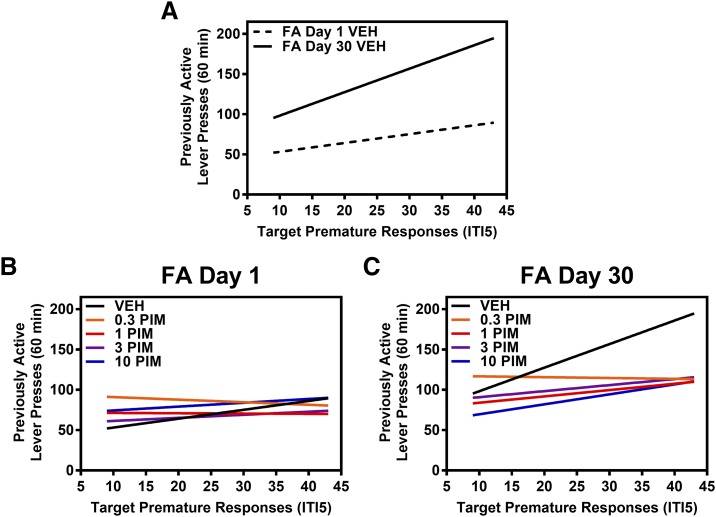
We tested the hypothesis that baseline levels of impulsive action would predict previously active lever presses during the cue reactivity test on FA day 1 or FA day 30 in VEH-treated rats (Fig. 3A). We found that the covariate target premature responses predicted the number of previously active lever presses exhibited on FA day 1 and on FA day 30 in VEH-treated rats (F(1,22) = 5.081, P < 0.05) (Fig. 3A). We also tested the hypothesis that baseline levels of impulsive action would predict the efficacy of pimavanserin to suppress previously active lever presses during the cue reactivity test on FA day 1 or FA day 30. No relationship between target premature responses and the number of previously active lever presses exhibited on FA day 1 after pretreatment with VEH or pimavanserin (F(4,62) = 0.405; n.s.) (Fig. 3B). Target premature responses predicted the number of previously active lever presses exhibited on FA day 30 after pretreatment with VEH or pimavanserin (F(4,52) = 4.04, P = 0.05) (Fig. 3C).
Fig. 3.
Baseline levels of impulsive action predict the efficacy of pimavanserin to suppress cocaine cue reactivity on FA day 30 from cocaine self-administration. Target premature responses under an ITI5 schedule in the 1-CSRT task (mean ± S.E.M.) are presented on the x-axis, and previously active lever presses (mean ± S.E.M.) on the cue reactivity test session are presented on the y-axis. The relationship between target premature responses and previously active lever presses after pretreatment with VEH or pimavanserin (PIM; 0.3, 1, 3, 10 mg/kg) is represented by a linear regression line for each pretreatment condition. (A) Target premature responses predicted the number of previously active lever presses exhibited on FA day 1 and on FA day 30 in VEH-treated rats (F(1,22) = 5.081, P < 0.05). (B) No relationship was found between target premature responses and the number of previously active lever presses exhibited on FA day 1 after pretreatment with vehicle or pimavanserin (F(4,62) = 0.405; n.s.). (C) Target premature responses predicted the number of previously active lever presses exhibited on FA day 30 after pretreatment with vehicle or pimavanserin (F(4,52) = 4.04, P = 0.05).
Discussion
We demonstrated that pimavanserin suppressed impulsive action measured in the 1-CSRT task, similarly to M100907 (Winstanley et al., 2004; Fletcher et al., 2007, 2011; Anastasio et al., 2011, 2015; Cunningham et al., 2013; Fink et al., 2015). Moreover, baseline levels of impulsive action predicted incubated levels of cocaine cue reactivity on FA day 30, replicating our previous observations that baseline impulsive action predicted cocaine cue reactivity on FA day 14 (Anastasio et al., 2014b). We also found that baseline impulsive action predicted the effectiveness of pimavanserin to suppress incubated cue reactivity in late abstinence from cocaine self-administration at doses that were ineffective in early abstinence. Taken together with the extinction-reinstatement studies with M100907 (Lacosta and Roberts, 1993; Fletcher et al., 2002; Filip, 2005; Nic Dhonnchadha et al., 2009), these data suggest that the length of abstinence and associated neuroadapations that drive incubated cocaine cue reactivity are interlocked with 5-HT2AR mechanisms that underlie rapid response impulsivity.
Our findings suggest that identification of baseline levels of impulsive action could be useful in guiding pharmacotherapeutic intervention in CUD. Baseline levels of impulsive action can be defined clinically through self-report questionnaire measures (e.g., Barrett Impulsiveness Scale; Eysenck Impulsiveness Questionnaire) or behavioral laboratory tasks (e.g., Go/No Go Task; Continuous Performance Test; Stop Signal Task; CSRT tasks) (for reviews, see Moeller et al., 2001a; Hamilton et al., 2015). The integration of clinical measures of impulsive action may inform refined, personalized pharmacotherapeutic intervention for the treatment of relapse vulnerability in CUD and improve patient care for afflicted populations.
Pimavanserin is marketed as a 5-HT2AR inverse agonist to treat Parkinson disease psychosis (Vanover et al., 2008; Meltzer et al., 2010; Sahli and Tarazi, 2018). Both pimavanserin and M100907 act as 5-HT2AR inverse agonists to attenuate basal constitutive 5-HT2AR signaling in cells designed with overexpression of the native 5-HT2AR or transfection with a 5-HT2AR mutation targeted to increase constitutive activity (Weiner et al., 2001; Vanover et al., 2004; Muntasir et al., 2006; Vanover et al., 2006). A definitive role for negative 5-HT2AR efficacy in the control of behavior is suggested by a limited literature in conditioned behaviors (Welsh et al., 1998; Romano et al., 2006). For example, reduced constitutive 5-HT2AR activity is proposed to account for the impairment of conditioned learning evoked by the 5-HT2AR antagonist/inverse agonist MDL11,939 (Welsh et al., 1998). Presently, we are unable to attribute definitively the effects of M100907 or pimavanserin to their 5-HT2AR antagonist versus inverse agonist properties.
Pimavanserin and M100907 promote the maintenance of sleep in humans (Rosenberg et al., 2008; Ancoli-Israel et al., 2011). Notably, sleep disorders (Morgan et al., 2010), as well as psychosis (Caton et al., 2000; Peer et al., 2009), commonly co-occur with CUD, and dopamine replacement therapy in Parkinson’s disease elicits side effects that include increased risk-taking and impulsive behaviors (Park and Stacy, 2011). Our present findings demonstrate that pimavanserin suppressed impulsive action, as well as cocaine cue reactivity. Thus, a compelling case can be made that pimavanserin or other 5-HT2AR antagonists/inverse agonists that may be ultimately available for clinical use will be efficacious in suppressing relapse vulnerability and potentially improve concomitant sleep and psychiatric disorders seen in CUD. The marketing of pimavanserin provides the opportunity to assess the efficacy of pimavanserin to extend abstinence and improve the health status of CUD patients.
Acknowledgments
We thank Amanda E. Price for helpful discussions and comments on the manuscript.
Abbreviations
- 5-HT
serotonin
- 5-HT2AR
5-HT2A receptor
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- CSRT
choice serial reaction time
- CUD
cocaine use disorder
- DOI
2,5-dimethoxy-4-iodoamphetamine
- FA
forced abstinence
- FR
fixed ratio
- ITI
intertrial interval
- VEH
vehicle
Authorship Contributions
Participated in research design: Sholler, Boone, Wang, Moeller, Anastasio, Cunningham.
Conducted experiments: Sholler, Stutz, Fox.
Contributed reagents or analytical tools: Rice.
Performed data analysis: Sholler, Anastasio.
Wrote or contributed to the writing of the manuscript: Sholler, Stutz, Fox, Boone, Wang, Moeller, Anastasio, Cunningham.
Footnotes
This work was supported by the NIH National Institute on Drug Abuse [Grants T32 DA07287 (D.J.S.), U54 DA038999 (F.G.M./K.A.C./N.C.A.), P50 DA033935 (K.A.C./F.G.M./N.C.A.)] and the Center for Addiction Research at the University of Texas Medical Branch. This research was supported in part by the Intramural Research Program of the National Institutes of Health [National Institute on Drug Abuse; National Institute of Alcohol Abuse and Alcoholism (K.C.R.)].
References
- Anastasio NC, Gilbertson SR, Bubar MJ, Agarkov A, Stutz SJ, Jeng Y, Bremer NM, Smith TD, Fox RG, Swinford SE, et al. (2013) Peptide inhibitors disrupt the serotonin 5-HT2C receptor interaction with phosphatase and tensin homolog to allosterically modulate cellular signaling and behavior. J Neurosci 33:1615–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Liu S, Maili L, Swinford SE, Lane SD, Fox RG, Hamon SC, Nielsen DA, Cunningham KA, Moeller FG. (2014a) Variation within the serotonin (5-HT) 5-HT2C receptor system aligns with vulnerability to cocaine cue reactivity. Transl Psychiatry 4:e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stoffel EC, Fox RG, Bubar MJ, Rice KC, Moeller FG, Cunningham KA. (2011) Serotonin (5-hydroxytryptamine) 5-HT(2A) receptor: association with inherent and cocaine-evoked behavioral disinhibition in rats. Behav Pharmacol 22:248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stutz SJ, Fink LH, Swinford-Jackson SE, Sears RM, DiLeone RJ, Rice KC, Moeller FG, Cunningham KA. (2015) Serotonin (5-HT) 5-HT2A receptor (5-HT2AR):5-HT2CR imbalance in medial prefrontal cortex associates with motor impulsivity. ACS Chem Neurosci 6:1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stutz SJ, Fox RG, Sears RM, Emeson RB, DiLeone RJ, O’Neil RT, Fink LH, Li D, Green TA, et al. (2014b) Functional status of the serotonin 5-HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology 39:370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Vanover KE, Weiner DM, Davis RE, van Kammen DP. (2011) Pimavanserin tartrate, a 5-HT(2A) receptor inverse agonist, increases slow wave sleep as measured by polysomnography in healthy adult volunteers. Sleep Med 12:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320:1352–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland A, Sik A, Lieben C. (2005) Evaluation of DOI, 8-OH-DPAT, eticlopride and amphetamine on impulsive responding in a reaction time task in rats. Behav Pharmacol 16:93–100. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Schreiber E, Church S, McDowell D. (2006) Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict Behav 31:174–181. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. (1999) Cue-reactivity and the future of addiction research. Addiction 94:349–351. [PubMed] [Google Scholar]
- Caton CL, Samet S, Hasin DS. (2000) When acute-stage psychosis and substance use co-occur: differentiating substance-induced and primary psychotic disorders. J Psychiatr Pract 6:256–266. [DOI] [PubMed] [Google Scholar]
- Coskunpinar A, Cyders MA. (2013) Impulsivity and substance-related attentional bias: a meta-analytic review. Drug Alcohol Depend 133:1–14. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC. (2014) Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology 76 Pt B:460–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, Watson CS, Gilbertson SR, Rice KC, Rosenzweig-Lipson S, et al. (2013) Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem Neurosci 4:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Fox RG, Anastasio NC, Bubar MJ, Stutz SJ, Moeller FG, Gilbertson SR, Rosenzweig-Lipson S. (2011) Selective serotonin 5-HT(2C) receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology 61:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC. (2001) Theories of drug craving, ancient and modern. Addiction 96:33–46. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH. (2010) Interaction of 5-HT2A and 5-HT2C receptors in R(-)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther 335:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Cox WM. (2008) Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend 97:1–20. [DOI] [PubMed] [Google Scholar]
- Filip M. (2005) Role of serotonin (5-HT)2 receptors in cocaine self-administration and seeking behavior in rats. Pharmacol Rep 57:35–46. [PubMed] [Google Scholar]
- Fink LH, Anastasio NC, Fox RG, Rice KC, Moeller FG, Cunningham KA. (2015) Individual differences in impulsive action reflect variation in the cortical serotonin 5-HT2A receptor system. Neuropsychopharmacology 40:1957–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. (2002) Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology 27:576–586. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Noble K, Higgins GA. (2011) Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT(2C) receptor stimulation and 5-HT(2A) receptor blockade. Neuropharmacology 61:468–477. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. (2007) Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 195:223–234. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. (2000) Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 157:1789–1798. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. (2001) Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412:141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacksell U, Burstein ES, McFarland K, Mills RG, Williams H. (2014) On the discovery and development of pimavanserin: a novel drug candidate for Parkinson’s psychosis. Neurochem Res 39:2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Littlefield AK, Anastasio NC, Cunningham KA, Fink LH, Wing VC, Mathias CW, Lane SD, Schütz CG, Swann AC, et al. (2015) Rapid-response impulsivity: definitions, measurement issues, and clinical implications. Pers Disord 6:168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PL, McCloskey TC, Johnson MP, et al. (1996) Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther 277:968–981. [PubMed] [Google Scholar]
- Keppel G. (1973) Design and Analysis: A Researcher’s Handbook, Prentice-Hall, Inc., Englewood Cliffs, NJ. [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. (2004) Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedebergs Arch Pharmacol 370:114–123. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen T, Ruotsalainen S, Puumala T, Lappalainen R, Koivisto E, Männistö PT, Sirviö J. (2000a) Activation of 5-HT2A receptors impairs response control of rats in a five-choice serial reaction time task. Neuropharmacology 39:471–481. [DOI] [PubMed] [Google Scholar]
- Koskinen T, Ruotsalainen S, Sirviö J. (2000b) The 5-HT(2) receptor activation enhances impulsive responding without increasing motor activity in rats. Pharmacol Biochem Behav 66:729–738. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Roberts DCS. (1993) MDL 72222, ketanserin, and methysergide pretreatments fail to alter breaking points on a progressive ratio schedule reinforced by intravenous cocaine. Pharmacol Biochem Behav 44:161–165. [DOI] [PubMed] [Google Scholar]
- Leung D, Staiger PK, Hayden M, Lum JA, Hall K, Manning V, Verdejo-Garcia A. (2017) Meta-analysis of the relationship between impulsivity and substance-related cognitive biases. Drug Alcohol Depend 172:21–33. [DOI] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Waters AJ, Cunningham KA, Moeller FG. (2011) Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am J Drug Alcohol Abuse 37:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. (2004) Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl) 176:101–108. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. (1998) Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry 155:124–126. [DOI] [PubMed] [Google Scholar]
- McFarland K, Price DL, Bonhaus DW. (2011) Pimavanserin, a 5-HT2A inverse agonist, reverses psychosis-like behaviors in a rodent model of Parkinson’s disease. Behav Pharmacol 22:681–692. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Mills R, Revell S, Williams H, Johnson A, Bahr D, Friedman JH. (2010) Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of parkinson’s disease psychosis. Neuropsychopharmacology 35:881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Elkis H, Vanover K, Weiner DM, Van Kammen DP, Peters P, Hacksell U. (2012) Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. SchizophrRes 141:144–152. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. (2001a) Psychiatric aspects of impulsivity. Am J Psychiatry 158:1783–1793. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. (2001b) The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat 21:193–198. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Steinberg JL, Green CM, Reist C, Lai LY, Swann AC, Grabowski J. (2007) Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse 33:367–378. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott E, Pittman B, Stickgold R, Malison RT. (2010) Normalizing effects of modafinil on sleep in chronic cocaine users. Am J Psychiatry 167:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntasir HA, Bhuiyan MA, Ishiguro M, Ozaki M, Nagatomo T. (2006) Inverse agonist activity of sarpogrelate, a selective 5-HT2A-receptor antagonist, at the constitutively active human 5-HT2A receptor. J Pharmacol Sci 102:189–195. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. (2000) Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA. (2009) Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci 123:382–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. (1998) Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 12:15–22. [DOI] [PubMed] [Google Scholar]
- Park A, Stacy M. (2011) Dopamine-induced nonmotor symptoms of Parkinson’s disease. Parkinsons Dis 2011:485063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar AA, Murray HW, Mannelli P, Gottheil E, Weinstein SP, Vergare MJ. (2004) Pre-treatment measures of impulsivity, aggression and sensation seeking are associated with treatment outcome for African-American cocaine-dependent patients. J Addict Dis 23:109–122. [DOI] [PubMed] [Google Scholar]
- Peer J, Bennett ME, Bellack AS. (2009) Neurocognitive characteristics of individuals with schizophrenia and cocaine dependence: comparison of currently dependent and remitted groups. J Nerv Ment Dis 197:631–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. (2005) Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 178:193–201. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. (2011) Neurobiology of the incubation of drug craving. Trends Neurosci 34:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratkowsky DA, Reedy TJ. (1986) Choosing near-linear parameters in the four-parameter logistic model for radioligand and related assays. Biometrics 42:575–582. [PubMed] [Google Scholar]
- Roberts C. (2006) ACP-103, a 5-HT2A receptor inverse agonist. CurrOpinInvestigDrugs 7:653–660. [PubMed] [Google Scholar]
- Romano AG, Quinn JL, Liu R, Dave KD, Schwab D, Alexander G, Aloyo VJ, Harvey JA. (2006) Effect of serotonin depletion on 5-HT2A-mediated learning in the rabbit: evidence for constitutive activity of the 5-HT2A receptor in vivo. Psychopharmacology (Berl) 184:173–181. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Seiden DJ, Hull SG, Erman M, Schwartz H, Anderson C, Prosser W, Shanahan W, Sanchez M, Chuang E, et al. (2008) APD125, a selective serotonin 5-HT(2A) receptor inverse agonist, significantly improves sleep maintenance in primary insomnia. Sleep 31:1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahli ZT, Tarazi FI. (2018) Pimavanserin: novel pharmacotherapy for Parkinson’s disease psychosis. Expert Opin Drug Discov 13:103–110. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. (2013) Preclinical studies shed light on individual variation in addiction vulnerability. Neuropsychopharmacology 38:249–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. (1995) DOI disruption of prepulse inhibition of startle in the rat is mediated by 5-HT(2A) and not by 5-HT(2C) receptors. Behav Pharmacol 6:839–842. [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. (2003) Discriminative stimulus properties of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(+/-)DOI] in C57BL/6J mice. Psychopharmacology (Berl) 166:61–68. [DOI] [PubMed] [Google Scholar]
- Swinford-Jackson SE, Anastasio NC, Fox RG, Stutz SJ, Cunningham KA. (2016) Incubation of cocaine cue reactivity associates with neuroadaptations in the cortical serotonin (5-HT) 5-HT2C receptor (5-HT2CR) system. Neuroscience 324:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. (1987) Manual of Pharmacologic Calculations with Computer Programs, Springer-Verlag, New York. [Google Scholar]
- Titeler M, Lyon RA, Glennon RA. (1988) Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology (Berl) 94:213–216. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Betz AJ, Weber SM, Bibbiani F, Kielaite A, Weiner DM, Davis RE, Chase TN, Salamone JD. (2008) A 5-HT2A receptor inverse agonist, ACP-103, reduces tremor in a rat model and levodopa-induced dyskinesias in a monkey model. Pharmacol Biochem Behav 90:540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover KE, Harvey SC, Son T, Bradley SR, Kold H, Makhay M, Veinbergs I, Spalding TA, Weiner DM, Andersson CM, et al. (2004) Pharmacological characterization of AC-90179 [2-(4-methoxyphenyl)-N-(4-methyl-benzyl)-N-(1-methyl-piperidin-4-yl)-acetamide hydrochloride]: a selective serotonin 2A receptor inverse agonist. J Pharmacol Exp Ther 310:943–951. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Weiner DM, Makhay M, Veinbergs I, Gardell LR, Lameh J, Del Tredici AL, Piu F, Schiffer HH, Ott TR, et al. (2006) Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther 317:910–918. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Skolnick P. (2012) New medications for substance use disorders: challenges and opportunities. Neuropsychopharmacology 37:290–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, Harvey SC, Donohue E, Hansen HC, Andersson CM, et al. (2001) 5-hydroxytryptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther 299:268–276. [PubMed] [Google Scholar]
- Welsh SE, Romano AG, Harvey JA. (1998) Effects of serotonin 5-HT(2A/2C) antagonists on associative learning in the rabbit. Psychopharmacology (Berl) 137:157–163. [DOI] [PubMed] [Google Scholar]
- Winstanley CA. (2011) The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol 164:1301–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. (2004) 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 176:376–385. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rice KC, Amorosi DJ, Rabin RA. (2007) Psilocybin-induced stimulus control in the rat. Pharmacol Biochem Behav 87:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischhof L, Hollensteiner KJ, Koch M. (2011) Impulsive behaviour in rats induced by intracortical DOI infusions is antagonized by co-administration of an mGlu2/3 receptor agonist. Behav Pharmacol 22:805–813. [DOI] [PubMed] [Google Scholar]