Abstract
Trichophyton benhamiae is an emerging zoonotic dermatophyte. We present a case of a small animal stock infected with two Trichophyton species. T. benhamiae was isolated from 15 out of 26 (58%) guinea pigs including two morphologically different phenotypes. Eight guinea pigs were infected with T. benhamiae and T. mentagrophytes simultaneously. The animals showed alopecia and crusts or no clinical signs at all. T. benhamiae was not detected in rats, rabbits and mice kept in the same stock.
Keywords: Trichophyton benhamiae, Trichophyton mentagrophytes, Zoonotic dermatophytes, Guinea pig, Mass spectrometry
1. Introduction
The dermatophyte T. benhamiae is an important zoonotic pathogen especially in small children in Germany [1]. The prevalence of zoonotic T. benhamiae infections has increased worldwide over the last 15 years [2]. T. benhamiae infections in humans often result in severe skin lesions like Tinea corporis, faciei and capitis [3] associated with extensive inflammation and secondary bacterial infections. Complications like kerion celsi have been described [3], [4]. Severe cases have mainly been recorded in children and immunosuppressed persons [5]. Treatment of this infection is complex and dermal scars are a common complication [6]. T. benhamiae was in the past often misdiagnosed as Microsporum canis [3], [7] or T. mentagrophytes var. porcellae [2] because of a similar colony color or as T. interdigitale because of microscopic similarities [3]. The histories of many zoonotic infections suggest that guinea pigs are the main source of transmission to humans [1]. Accordingly, Kupsch et al. found recently a high infection rate of 69% among German pet shop guinea pigs by culture [8]. Small rodents [3], rabbits [9], dogs [10] or even porcupines [11] have also been associated with T. benhamiae infection, but comparative investigations of different small animals have not been published to date. Infected companion animals commonly show no or only mild clinical signs such as alopecia and crusts [8]. Here we describe T. benhamiae and T. mentagrophytes infection in numerous guinea pigs of a mixed animal stock including other rodents and rabbits.
2. Case
2.1. Description of the small animal stock and the clinical signs
We report the screening results of 26 guinea pigs, 7 mice, 6 rats and 2 rabbits belonging to a single stock of animals showing partially alopecia. Guinea pigs and rabbits were kept together in a big open wooden box which was bedded with straw and hay and supplemented with hiding places made of wood. Mice and rats were held separately in plastic tubes with grid covers. All animals originated from three different breeders and were brought together in the respective stock. A distinction with regard to the original breeders was not possible. In the stock, the same animal care takers were responsible for all small animals. Eleven out of 26 (42%) guinea pigs presented clinical signs typical for dermatophytosis such as alopecia, itching, dull fur, crusts and scales found mainly on the abdomen and lower back (Fig. 1). All seven mice displayed mild clinical signs like dull fur and crusts mainly on the head area and lower back. Rats and rabbits showed no clinical signs.
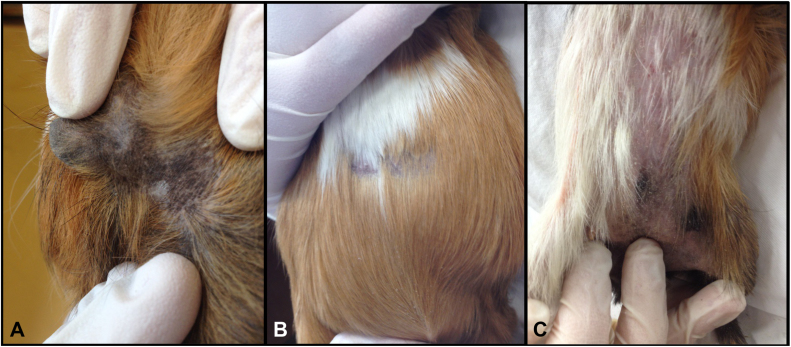
Fig. 1.
Guinea pigs infected with T. benhamiae displayed different clinical manifestations of dermatophyte infection. (A) Skin lesion localized behind the ear with alopecia and scantly crusts. (B) Mild clinical signs like hairless spots in the middle of the abdomen. (C) Severe clinical signs of T. benhamiae infection with extended hair loss, crusts, erythema associated with itching along the abdomen and inguinal area.
2.2. Sampling and mycological findings
All animals were sampled with toothbrushes to obtain hairs and skin scales. Particular attention was paid to the eye area and sites of alopecia. Soft toothbrushes were found to be good tools for sampling guinea pigs and rodents. Sample material was transferred from the toothbrush, used for the sampling of one animal only, to modified dermatophyte agar (Sifin Diagnostics GmbH, no. TN1054, Berlin Germany, containing additionally 0.4 mg/ml cycloheximide, 0.05 mg/ml gentamicin-sulfate, 0.05 mg/ml chlortetracycline, 0.1 mg/ml chloramphenicol, thiamin and inosite, [12]) and Sabouraud agar (Sifin). The samples were incubated at 28 °C for two weeks. After microscopic examination, the cultures were processed for identification by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) using a Microflex Biotyper (Bruker Daltonik GmbH, Bremen, Germany) as described [12]. Mass spectrometric identification of dermatophytes was conducted using an extended database as recently published [12]. Three isolates of T. benhamiae and 2 isolates of T. mentagrophytes were additionally identified by sequence analysis of the internal transcribed spacer region (ITS) of the ribosomal DNA. T. benhamiae was detected in 15 out of 26 (58%) guinea pigs by culture (Table 1). Two morphologically different biotypes of T. benhamiae, namely a brown-white and a yellow type were isolated (Fig. 2A). As described previously [8], [13], the brown-white type showed increased microconidia formation (Fig. 2B). Furthermore, T. mentagrophytes was also isolated from 8 of the 15 T. benhamiae infected guinea pigs. Three guinea pigs without clinical signs were infected with the brown-white and yellow type of T. benhamiae along with T. mentagrophytes. In three guinea pigs which presented with mild clinical signs, only T. mentagrophytes was isolated. Two out of 7 mice were positive for T. mentagrophytes, although all had mild clinical signs (Table 1). Rats and rabbits remained culturally negative for dermatophytes.
Table 1.
Results of cultural screening of 41 small animals of a single stock for infection with dermatophytes.
| species | Number of animals |
|||||
|---|---|---|---|---|---|---|
| Total | With clinical signs of dermatophytosisa | With confirmed dermato-phytosisb | Detection of T. ben-hamiaec | Detection of T. menta-grophytesc | Confirmed coinfection of T. benhamiaec and T. mentagrophytesc | |
| guinea pigs | 26 | 11 | 18 | 15 | 11 | 8 |
| rabbits | 2 | 0 | 0 | 0 | 0 | 0 |
| mice | 7 | 7 | 2 | 0 | 2 | 0 |
| rats | 6 | 0 | 0 | 0 | 0 | 0 |
| total | 41 | 18 | 20 | 15 | 13 | 8 |
clinical signs of dermatophytosis such as alopecia, itching, crusts, dull fur and scales.
based on cultural detection of Trichophyton species.
based on MALDI-TOF MS and ITS sequencing.
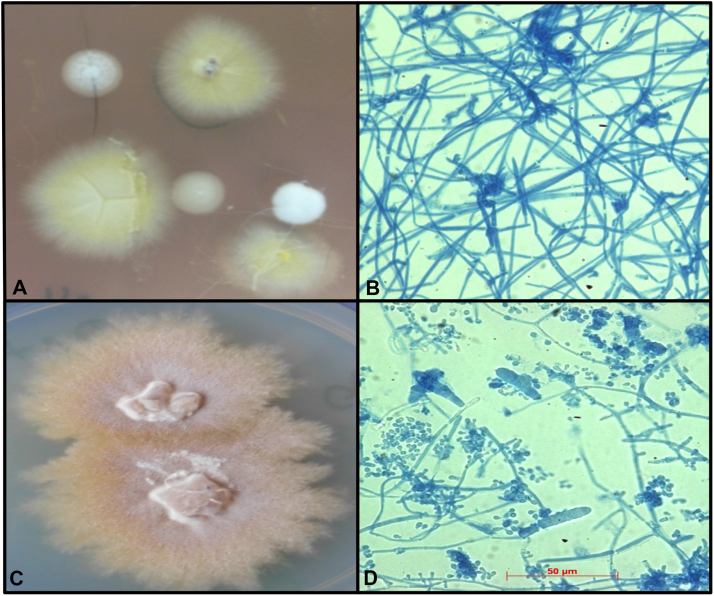
Fig. 2.
Macroscopic and microscopic characteristics of T. benhamiae isolates of this case belonging to a yellow or brown-white colony type (isolated from a guinea pig and cultured for 8 days at 28 °C aerobic on dermatophyte agar). (A) Yellow colored, flat and radial shaped colony morphology of T. benhamiae. (B) Microscopic structures of T. benhamiae yellow type like hyphae, macroconidia and few microconidia, lactophenolblue staining, 200×. (C) Brown to beige colored, flat and powdry colony morphology of T. benhamiae. (D) Microscopic structures of T. benhamiae brown-white type like hyphae, macroconidia and microconidia, lactophenolblue staining, 200×.
2.3. Mass spectrometric analysis of dermatophytes
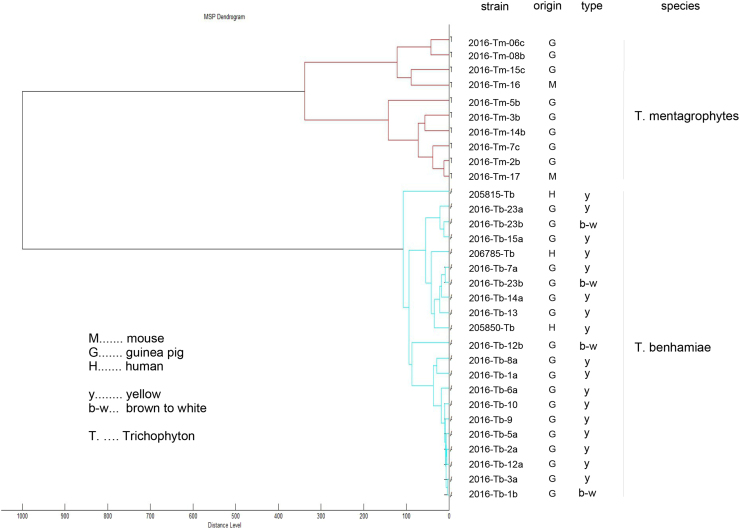
A distance dendrogram based on the mass spectrometric data of the dermatophytes (n = 31) isolated in this study and of three recent zoonotic T. benhamiae isolates was generated to investigate clustering of T. benhamiae and T. mentagrophytes isolates (Fig. 3). Mass spectrometric analysis revealed clear separation of T. mentagrophytes (n = 10) and T. benhamiae (n = 21) isolates into two clusters with a high distance level of 1000. Brown-white and yellow types of T. benhamiae were distributed equally among the T. benhamiae cluster. Visual inspection of the mass spectra confirmed no clear distinction between the yellow and brown-white T. benhamiae type (Fig. 4). Three human isolates of T. benhamiae, isolated from patients within the last three years in Saxony, Germany, within the diagnostic services of the Mycological Laboratory Mölbis, grouped together with guinea pig isolates in the same cluster with a high degree of similarity (distance level below 100 between 2 human and 7 guinea pig isolates, Fig. 3). This indicates a putative zoonotic potential of the T. benhamiae isolates of this study.
Fig. 3.
Distance dendrogram of MALDI-TOF MS analysis of T. benhamiae and T. mentagrophytes isolates. Ten T. mentagrophytes and 18 T. benhamiae isolated from the single small animal stock were included, as well as 3 human isolates of T. benhamiae. The cluster with the T. benhamiae isolates is clearly distinct from the cluster with the T. mentagrophytes isolates indicating a clear distinction between the two species. The human isolates showed high similarities in mass spectrometric analysis to the guinea pig isolates of this case.
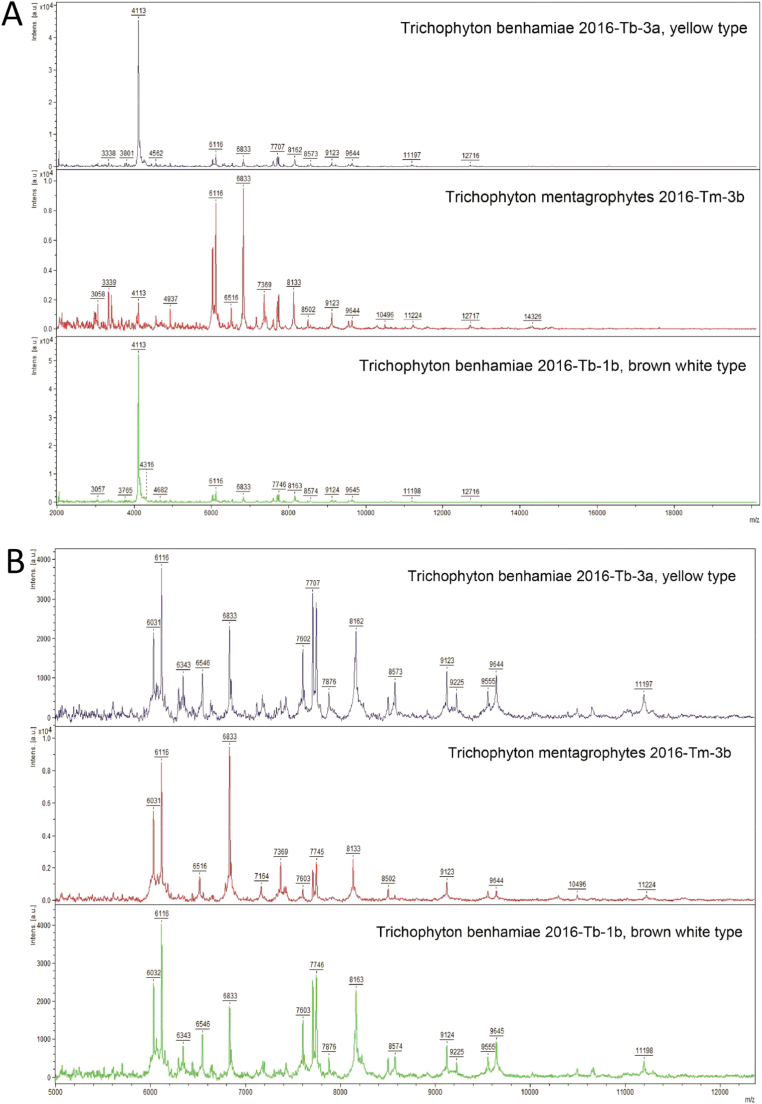
Fig. 4.
Examples of mass spectra of T. benhamiae and T. mentagrophytes isolates as indicated. The range from 2000 to 18.000 m/z is shown in (A) and from 5000 to 12.000 m/z in (B). The spectra of the brown-white and yellow phenotypes of T. benhamiae were very similar, while spectra of T. mentagrophytes were clearly distinct.
3. Discussion
In this study, we describe infestation of guinea pigs with T. benhamiae kept in a single stock together with other small animals. Interestingly, this pathogen was not detected in two rabbits staying in the same cage as the guinea pigs or in mice and rats housed in the same room but in adjacent cages despite all animals being taken care of by the same staff. The high infection rate of 58% confirms guinea pigs as a main reservoir for T. benhamiae in accordance with previous studies [1], [3], [6]. Furthermore, our findings suggest that rats, mice and rabbits are less susceptible to T. benhamiae infection than guinea pigs because these animals were treated and kept very similarly. Spread of T. benhamiae was possible due to the lack of biosecurity in this animal stock. The high infection rate of T. benhamiae might have been favored by the different origin of the small animals and the wooden boxes which are difficult to clean and disinfect. Because the animals were kept only as feed animals, no therapy of the skin infection was performed. Noteworthy, a yellow as well as a brown-white type of T. benhamiae was isolated from the same guinea pig in three cases. Different colony types have been described previously, but mainly as a yellow and white phenotype [2], [8], [14], [15].
We recently described a high similarity of T. benhamiae and T. verrucosum in mass spectrometric analysis [12]. Nevertheless, mass spectrometric differentiation of these two species was reliable [12]. Here we describe clear differentiation of T. benhamiae and T. mentagrophytes via MALDI-TOF MS, further supporting the power of this method to differentiate dermatophytes. However, isolates of the brown-white and yellow type of T. benhamiae did not form separate clusters, though ITS and 28 S rRNA gene sequencing indicated association of specific sequences with the two distinct colony types [8], [13].
Despite the high prevalence of T. benhamiae in the investigated animals, we are not aware of a zoonotic infection related to this stock. As these guinea pigs were kept as feed animals and only a few animal keepers had contact, transmission to humans might have been limited. However, as guinea pigs are popular companion animals, often in close contact to small children, a much higher risk of transmission might be common in various families [3]. Furthermore, in small children [6] and immunosuppressed humans [5], skin infections with T. benhamiae can lead to severe symptoms with secondary bacterial infections, dermal scars and kerion celsi [4]. Noteworthy, highly inflamed dermatophytosis caused by zoophilic dermatophytes such as T. benhamiae increases in humans [1]. Furthermore, Brasch et al. noted spread of T. benhamiae infections in Germany [15] which might be difficult to control as infected guinea pigs are often free of clinical signs of dermatophytosis. Accordingly, guinea pigs, at least in breeding stocks and pet shops, should be screened regularly for dermatophytes and respective biosecurity measures should be implemented to prevent this neglected zoonosis.
Acknowledgements
This study was financially supported by the German Federal Ministry of Education and Research (01K11713). We thank Viktoria Rungelrath for critically reviewing the manuscript.
Acknowledgments
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Nenoff P., Krüger C., Ginter-Hanselmayer G., Tietz H.J. Mycology – an update. Part 1: dermatomycoses: causative agents, epidemiology and pathogenesis. J. Dtsch Dermatol. Ges. 2014;12(3):188–209. doi: 10.1111/ddg.12245. (Epub 2014 Feb 17) [DOI] [PubMed] [Google Scholar]
- 2.Sabou M., Denis J., Boulanger N., Forouzanfar F., Glatz I., Lipsker D. Molecular identification of Trichophyton benhamiae in Strasbourg, France: a 9-year retrospective study. Med. Mycol. 2018;56(6):723–734. doi: 10.1093/mmy/myx100. [DOI] [PubMed] [Google Scholar]
- 3.Nenoff P., Uhrlaß S., Krüger C., Erhard M., Hipler U.C., Seyfarth F. Trichophyton species of Arthroderma benhamiae – a new infectious agent in dermatology. J. Dtsch Dermatol. Ges. 2014;12(7):571–581. doi: 10.1111/ddg.12390. [DOI] [PubMed] [Google Scholar]
- 4.Nenoff P., Schulze I., Uhrlaß S., Krüger C. Kerion caused by the zoophilic dermatophyte Trichophyton species of Arthroderma benhamiae in a child. A new emerging pathogen of dermatomycoses in Germany. Hautarzt. 2013;64(11):846–849. doi: 10.1007/s00105-013-2665-3. [DOI] [PubMed] [Google Scholar]
- 5.Budihardja D., Freund V., Mayser P. Widespread erosive tinea corporis by Arthroderma benhamiae in a renal transplant recipient: case report. Mycoses. 2010;53(6):530–532. doi: 10.1111/j.1439-0507.2009.01736.x. [DOI] [PubMed] [Google Scholar]
- 6.Hiernickel C., Wiegand C., Schliemann S., Seyfarth F., Jung K., Elsner P. [Trichophyton species of Arthroderma benhamiae: clinical therapeutic aspects of a new pathogen in dermatology] Hautarzt. 2016;67(9):706–711. doi: 10.1007/s00105-016-3837-8. [DOI] [PubMed] [Google Scholar]
- 7.Mayser P., Budihardja D. A simple and rapid method to differentiate Arthroderma benhamiae from Microsporum canis. J. Dtsch Dermatol. Ges. 2013;11(4):322–327. doi: 10.1111/j.1610-0387.2012.08057.x. (Epub 2012 Nov29) [DOI] [PubMed] [Google Scholar]
- 8.Kupsch C., Berlin M., Gräser Y. [Dermophytes and guinea pigs: an underestimated danger?] Hautarzt. 2017;68(10):827–830. doi: 10.1007/s00105-017-4009-1. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y., Kano R., Nakamura E., Saito K., Watanabe S., Hasegawa A. First report of human ringworm caused by Arthroderma benhamiae in Japan transmitted from a rabbit. Mycoses. 2002;45(3–4):129–131. doi: 10.1046/j.1439-0507.2002.00732.x. [DOI] [PubMed] [Google Scholar]
- 10.Sieklucki U., Oh S.H., Hoyer L.L. Frequent isolation of Arthroderma benhamiae from dogs with dermatophytosis. Vet. Dermatol. 2014;1:39–e14. doi: 10.1111/vde.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi H., Takahashi-Kyuhachi H., Takahashi Y., Yarita K., Takayama A., Inomata T. An intrafamilial transmission of Arthroderma benhamiae in Canadian porcupines (Erethizon dorsatum) in a Japanese zoo. Med. Mycol. 2008;46:465–473. doi: 10.1080/13693780801938996. [DOI] [PubMed] [Google Scholar]
- 12.Bartosch T., Heydel T., Uhrlaß S., Nenoff P., Müller H., Baums C.G. MALDI-TOF MS analysis of bovine and zoonotic Trichophyton verrucosum isolates reveals a distinct peak and cluster formation of a subgroup with Trichophyton benhamiae. Med. Mycol. 2018;56(5):602–609. doi: 10.1093/mmy/myx084. [DOI] [PubMed] [Google Scholar]
- 13.Symoens F., Jousson O., Packeu A., Fratti M., Staib P., Mignon B. The dermatophyte species Arthroderma benhamiae: intraspecies variability and mating behaviour. J. Med. Microbiol. 2013;62(3):377–385. doi: 10.1099/jmm.0.053223-0. (Epub 2012 Nov 22) [DOI] [PubMed] [Google Scholar]
- 14.Brasch J., Wodarg S. Morphological and physiological features of Arthroderma benhamiae anamorphs isolated in northern Germany. Mycoses. 2015;58(2):93–98. doi: 10.1111/myc.12280. (Epub 2014 Dec 22) [DOI] [PubMed] [Google Scholar]
- 15.Brasch J., Beck-Jendroschek V., Voss K., Uhrlaß S., Nenoff P. Arthroderma benhamiae strains in Germany: morphological and physiological characteristics of the anamorphs. Hautarzt. 2016;67(9):700–705. doi: 10.1007/s00105-016-3815-1. [DOI] [PubMed] [Google Scholar]