Abstract
Introduction
High levels of plasmatic branched-chain amino acids (BCAA), commonly used as dietary supplements, are linked to metabolic risk factors for Alzheimer's disease (AD). BCAA directly influence amino acid transport to the brain and, therefore, neurotransmitter levels. We thus investigated the impact of BCAA on AD neuropathology in a mouse model.
Methods
3xTg-AD mice were fed either a control diet or a high-fat diet from 6 to 18 months of age. For the last 2 months, dietary BCAA content was adjusted to high (+50%), normal (+0%), or low (−50%).
Results
Mice fed a BCAA-supplemented high-fat diet displayed higher tau neuropathology and only four out of 13 survived. Mice on the low-BCAA diet showed higher threonine and tryptophan cortical levels while performing better on the novel object recognition task.
Discussion
These preclinical data underscore a potential risk of combining high-fat and high BCAA consumption, and possible benefits from BCAA restriction in AD.
Keywords: Alzheimer's disease, Branched-chain amino acids, 3xTg-AD mice, Dietary protein, Tau pathology
1. Introduction
Alzheimer's disease (AD) is the most prevalent neurodegenerative disease, and the number of demented patients in the world is predicted to double within the next 15 years because of the aging population [1]. Given the lack of disease-modifying treatments, controlling environmental risk factors remains an attractive opportunity to reduce the incidence of AD [2]. Changing dietary habits has been proposed as a means of delaying the onset of the disease either through direct effects on brain function or indirectly by improving peripheral metabolic determinants [3]. Indeed, common nutrients such as lipids, amino acids, and polyphenols may have significant impact on cognitive performance [4], [5], [6]. However, the optimal diet to be used in prevention trials remains to be determined.
Branched-chain amino acids (BCAA; leucine, isoleucine, and valine) are essential amino acids, comprising approximately 20% of protein intake. BCAA are commonly used as dietary supplements, by doubling the daily intake of those amino acids, to increase muscular mass and recovery after exercise [7], [8]. However, a BCAA-related signature is identifiable in a context of metabolic disorders: circulating BCAA are increased in people suffering from obesity and have been shown to predict insulin resistance and other complications related to metabolic diseases [9], [10]. Evidence from metabolomics studies indicate that high-fat diet (HFD) acts in synergy with BCAA to induce metabolic disorders, at least in animal models [11].
BCAA enter the central nervous system via the large neutral amino acids transporter (LAT1) at the blood-brain barrier, where they compete with tryptophan and tyrosine, which are direct precursors to serotonin and dopamine, respectively [12]. Thus, circulating BCAA can modulate levels of these key neurotransmitters in the brain, thereby altering brain function and behavior [13], [14], [15], [16]. Because cognitive impairments observed in AD have been associated with a neurotransmitter imbalance [17], we hypothesize that BCAA intake can modulate AD-like behavior. Indeed, the maple syrup urine disease is a neurometabolic disorder characterized by a toxic accumulation of BCAA and their metabolites, which leads to severe mental retardation and encephalopathy [18], [19].
It is only recently that studies have provided clues to a potential link between BCAA and AD pathogenesis. A metabolomic analysis from the ADNI cohort recently reported inverse correlations between plasmatic valine concentrations and cognitive deficits, as well as ventricle volume in symptomatic stages [20]. Another metabolomic study in large prospective cohorts relates higher levels of plasma BCAA with a lower risk of dementia [21]. Conversely, a genome-wide association study suggests that single-nucleotide polymorphisms (SNPs) associated with higher plasma isoleucine levels are positively associated with the risk for developing AD [22]. However, no studies have focused yet on the effect of BCAA consumption on AD pathology. Thus, we investigated the impact of both BCAA supplementation and restriction in old 3xTg-AD mice, an animal model of age-related behavioral impairments and Aβ/tau neuropathologies.
2. Methods
2.1. Animals and diets
The 3xTg-AD (APPswe, PS1M146V, tauP301L) mouse model of genetically induced AD-like neuropathology was used with an equal number of males and females in each group. Mice produced at our animal facility received either a control diet (CD; 12% kcal fat) or a high-fat diet (HFD; 60% kcal fat) for a 10-month period starting at the age of 6 months. At 16 months of age, groups of mice were exposed until 18 months to the same diets modified to include high (+50%), normal (+0%), or low (−50%) BCAA content (Fig. 1A). Table 1 provides a detailed description of the amino acids measured in the diets using gas chromatography with flame ionization detector (see methods below), whereas Supplementary Table 1 contains the list of amino acids added to the formulations. Supplementary Table 2 shows the description of fatty acids measured in the diets using gas chromatography. Diets were formulated and custom-made by Research Diets, Inc. (New Brunswick, NJ) to ensure uniformity between groups and consistency with our previous studies with the 3xTg-AD mouse [23], [24].
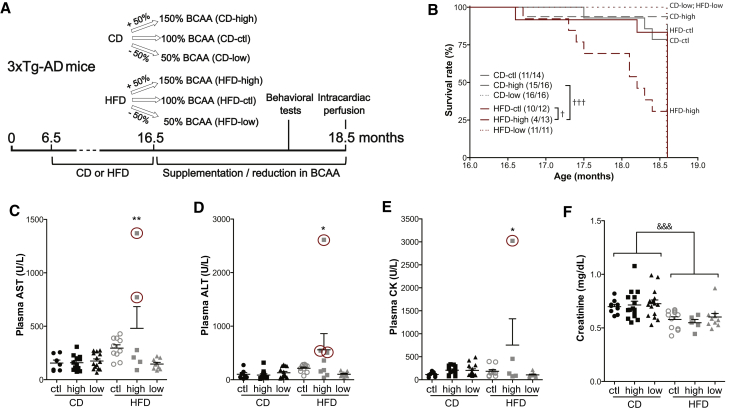
Fig. 1.
BCAA-supplemented HFD affects the survival of 3xTg-AD mice, increasing hepatic and muscular toxicity. Timeline of experimentation (A). Survival rate of mice fed the six different diets during a 2-month period (final vertical line indicates sacrifice) (B). Plasmatic concentrations of AST (C), ALT (D), CK (E), and creatinine (F) from intracardiac blood of 18-month-old 3xTg-AD mice sampled just before perfusion. Samples available and analyzed from moribund mice are identified with a red circle. Data are presented as mean ± SEM. Mantel-Cox test: †P < .05, †††P < .001. One-way ANOVA, Dunnett's (versus CD-ctl) post hoc analysis: *P < .05, **P < .01. Two-way ANOVA, followed by Tukey's HSD, effect of the diet: &&&P < .001. Abbreviations: CD, control diet; HFD, high-fat diet; ctl, normal levels of BCAA; high, BCAA-supplemented diet by 50%; low, BCAA-reduced diet by 50%; AST, aspartate aminotransferase; ALT, alanine aminotransferase, CK, creatine kinase; BCAA, branched-chain amino acids.
Table 1.
Description of dietary treatments
| Content | Control 5% (w/w) fat |
High fat 35% (w/w) fat |
||||
|---|---|---|---|---|---|---|
| Normal | High-BCAA | Low-BCAA | Normal | High-BCAA | Low-BCAA | |
| Protein (% w/w) | 20.3 | 23.3 | 18.4 | 27.4 | 31 | 25 |
| Carbohydrate (% w/w) | 66 | 63.5 | 67.5 | 25.3 | 24.1 | 26.1 |
| Fat (% w/w) | 5 | 4.8 | 5.1 | 35.1 | 33.4 | 36.3 |
| Calorie per diet weight (kcal/g) | 3.9 | 3.9 | 3.9 | 5.3 | 5.2 | 5.3 |
| Ingredient (g/kg) | ||||||
| Amino acids | ||||||
| L-cystine | 0.2 | 1.8 | 2.5 | 0.4 | 3.4 | 5.2 |
| Isoleucine | 6.4 | 16.2 | 2.9 | 8.9 | 22.2 | 5.3 |
| Leucine | 16.3 | 38.4 | 7.3 | 23.3 | 49.9 | 14.2 |
| Lysine | 15.2 | 13.2 | 13.7 | 22.9 | 20.2 | 24.9 |
| Methionine | 7.0 | 4.1 | 4.5 | 9.9 | 6.6 | 8.4 |
| Phenylalanine | 10.3 | 10.2 | 11.2 | 14.8 | 14.1 | 21.1 |
| Threonine | 5.2 | 5.0 | 5.3 | 7.5 | 6.8 | 9.0 |
| Tryptophan∗ | 0 | 1.1 | 1.3 | 0 | 1.1 | 1.3 |
| Valine | 7.4 | 20.6 | 3.2 | 11.6 | 26.8 | 6.4 |
| Histidine | 4.4 | 3.8 | 4.3 | 7.3 | 5.7 | 7.2 |
| Alanine | 4.9 | 5.3 | 5.2 | 6.6 | 5.6 | 6.7 |
| Arginine∗ | 0 | 3.2 | 3.9 | 0 | 3.2 | 3.9 |
| Aspartic acid | 9.9 | 9.0 | 9.9 | 14.5 | 13.3 | 15.9 |
| Glutamic acid | 40.9 | 37.1 | 39.0 | 55.9 | 58.8 | 61.8 |
| Glycine | 2.5 | 3.1 | 3.3 | 3.7 | 4.6 | 4.9 |
| Proline | 16.6 | 17.2 | 18.8 | 23.8 | 23.8 | 30.7 |
| Serine | 7.4 | 7.5 | 7.4 | 10.7 | 15.4 | 12.2 |
| Tyrosine | 10.9 | 10.3 | 10.3 | 13.2 | 12.8 | 18.3 |
| Total | 166 | 207 | 154 | 235 | 294 | 257 |
| Corn starch | 150 | 150 | 150 | 25 | 25 | 25 |
| Maltodextrin 10 | 0 | 0 | 0 | 100 | 100 | 100 |
| Sucrose | 500 | 500 | 500 | 52.5 | 52.5 | 52.5 |
| Cellulose, BW200 | 50 | 50 | 50 | 50 | 50 | 50 |
| Corn oil | 30 | 30 | 30 | 0 | 0 | 0 |
| Safflower oil | 0 | 0 | 0 | 125 | 125 | 125 |
| Lard | 0 | 0 | 0 | 135 | 135 | 135 |
| Soybean oil | 10 | 10 | 10 | 0 | 0 | 0 |
| Canola oil | 10 | 10 | 10 | 0 | 0 | 0 |
| Mineral (S19101) | 35 | 35 | 35 | 35 | 35 | 35 |
| Vitamins (V15908) | 10 | 10 | 10 | 10 | 10 | 10 |
| Choline bitartrate | 2 | 2 | 2 | 2 | 2 | 2 |
| Cholesterol, USP | 0.6 | 0.6 | 0.6 | 3 | 3 | 3 |
Abbreviation: BCAA, branched-chain amino acid.
NOTE. Amino acid content of the diets, accounting for casein and amino acid supplementation, was determined by GC-FID.
Arginine cannot be determined with the technique used and tryptophan degrades quickly during acid hydrolysis.
All mice were put under deep anesthesia with a ketamine/xylazine i.p. injection (100 mg/kg ketamine, 10 mg/kg xylazine) and sacrificed by intracardiac perfusion with 50 mL of ice-cold 0.1 M phosphate buffer saline (PBS) with a cocktail of inhibitors of phosphatases (sodium pyrophosphate 1 mM and sodium fluoride 50 mM) and proteases (SigmaFAST protease inhibitor tablets; Sigma-Aldrich, St. Louis, USA) as described elsewhere [23]. Rapidly, one hemisphere of the brain was dissected and frozen at −80°C until processing for Western blot, ELISA, or measurements of amino acids and neurotransmitters. All experiments were performed in accordance with the Canadian Council on Animal Care and were approved by the Institutional Committee of the Centre Hospitalier de l'Université Laval (CHUL).
2.2. Biochemical analyses
Aspartate aminotransferase, alanine aminotransferase, creatine kinase, triglycerides, and cholesterol were analyzed in plasma from intracardiac blood (centrifuged 5 min, 3000 rpm) sampled just before intracardiac perfusion at sacrifice with a modular analyzer (Roche). Only samples with more than 100 μL of plasma could be analyzed with that method. When the hemolysis level reached more than 50 g/dL, samples were eliminated from the analysis. Creatinine was determined with an ELISA kit (B-Bridge International Inc, Santa Clara, CA, USA) according to the manufacturer's instructions.
2.3. Behavioral assessment
Recognition memory and locomotor activity were evaluated with the novel object recognition test and the open field test, respectively, as previously described (see the Supplementary Material) [25].
2.4. Amino acid quantification in diet, plasma, and brain
Amino acids in the diets, plasma, and cortex homogenates were analyzed by gas chromatography with flame ionization detector as described in Supplementary Material [26].
2.5. Quantification of monoamines in the prefrontal cortex and hippocampus
Dopamine (DA), serotonin (5-HT), 5-hydroxyindolacetic acid (5-HIAA), homovanillic acid (HVA), and 3,4-dihydroxyphenylacetic acid (DOPAC) were quantified in the prefrontal cortex and hippocampus of mice by electrochemical high-performance liquid chromatography (HPLC) following the detailed method described in Supplementary Material [27].
2.6. Protein extraction and Western immunoblotting
For Western immunoblotting and β-Amyloid ELISA, proteins were extracted from the parieto-temporal cortex. The protein extraction method results in a TBS-soluble fraction (intracellular and extracellular fraction), a detergent-soluble fraction (membrane fraction) and a detergent-insoluble fraction (insoluble proteins resuspended in formic acid) as previously described [25]. The detailed method for Western immunoblotting is described elsewhere [24]. The list of primary antibodies used in our experiments is available in Supplementary Table 5. Homogenates were all run on the same gel for each experiment.
2.7. Aβ40 and Aβ42 quantification
Aβ40 and Aβ42 were measured in TBS-soluble and detergent-insoluble fractions from the parieto-temporal cortex using sensitive human β-Amyloid ELISA kits (Wako, Osaka, Japan) according to the manufacturer's instructions, as shown [24]. Plates were read at 450 nm using a Synergy HT multidetection microplate reader (Biotek, Winooski, VT, USA).
2.8. Statistics
Data are presented as the mean ± SEM. Statistical analysis used and the numbers of mice per group are specified in each figure or legend. Briefly, one- (one independent variable) or two-way (two independent variables) ANOVAs were used when more than two groups were compared and were followed by Tukey's post hoc (equal variance). In case of unequal variance, Dunnett's post hoc was used or a Kruskal-Wallis followed by Dunn's. The log-rank Mantel-Cox test was used for the analysis of the survival curves. Correlations between variables were investigated using linear regression analyses. All statistical analyses were performed with Prism 5 (GraphPad software, San Diego, CA, USA) or JMP (version 12.1.0; SAS Institute Inc., Cary, IL, USA) softwares, and statistical significance was set at P < .05.
3. Results
Experiments were performed in the 3xTg-AD mouse model of AD, which develops behavioral impairments as well as Aβ and tau neuropathologies with age. Because age-related factors are essential to the development of AD [28], [29], old 3xTg-AD mice were selected. To evaluate the impact of BCAA on AD pathogenesis, 3xTg-AD mice were fed a CD or HFD from 6 to 16 months of age and then both diets were either supplemented or restricted in BCAA by 50% for 2 months (Table 1; Fig. 1A). Animals were exposed to an HFD because it is more representative of prevalent dietary habits in Western societies [30], and also because HFD has been shown to accelerate AD-like pathology [23], [24], [31], [32] and to synergize with BCAA to induce metabolic disorders [11] in animal models.
3.1. BCAA supplementation combined with HFD led to the premature death of two-thirds of 3xTg-AD mice
At the end of the study, only four out of 13 mice (30.8%) in the group fed the HFD survived the 2-month supplementation with BCAA, which is significantly less than the group fed an HFD only, in which 10 mice out of 12 mice (83.3%) survived (Fig. 1B). Interestingly, no mice restricted in BCAA for the 2-month period died prematurely, regardless of the base diet. Although a total of 15 animals from all groups died before the end of the planned study, we were able to sacrifice a few (n = 7) in the high-BCAA HFD group while they were in critical conditions, allowing collection of intracardiac blood and brains as described in the Methods. These moribund mice fed the combination of HFD and BCAA (identified with red circles in Fig. 1) displayed higher aspartate aminotransferase, alanine aminotransferase, and creatine kinase plasmatic concentrations (Fig. 1C, D and E), suggesting major liver, muscular, or cardiac damage [33]. A two-way ANOVA revealed that mice fed the HFD, independently of BCAA intake, had lower plasmatic creatinine concentrations, possibly due to reduced muscle mass (Fig. 1F) [34]. No differences were found in plasma triglycerides and cholesterol levels between each group (Supplementary Table 4).
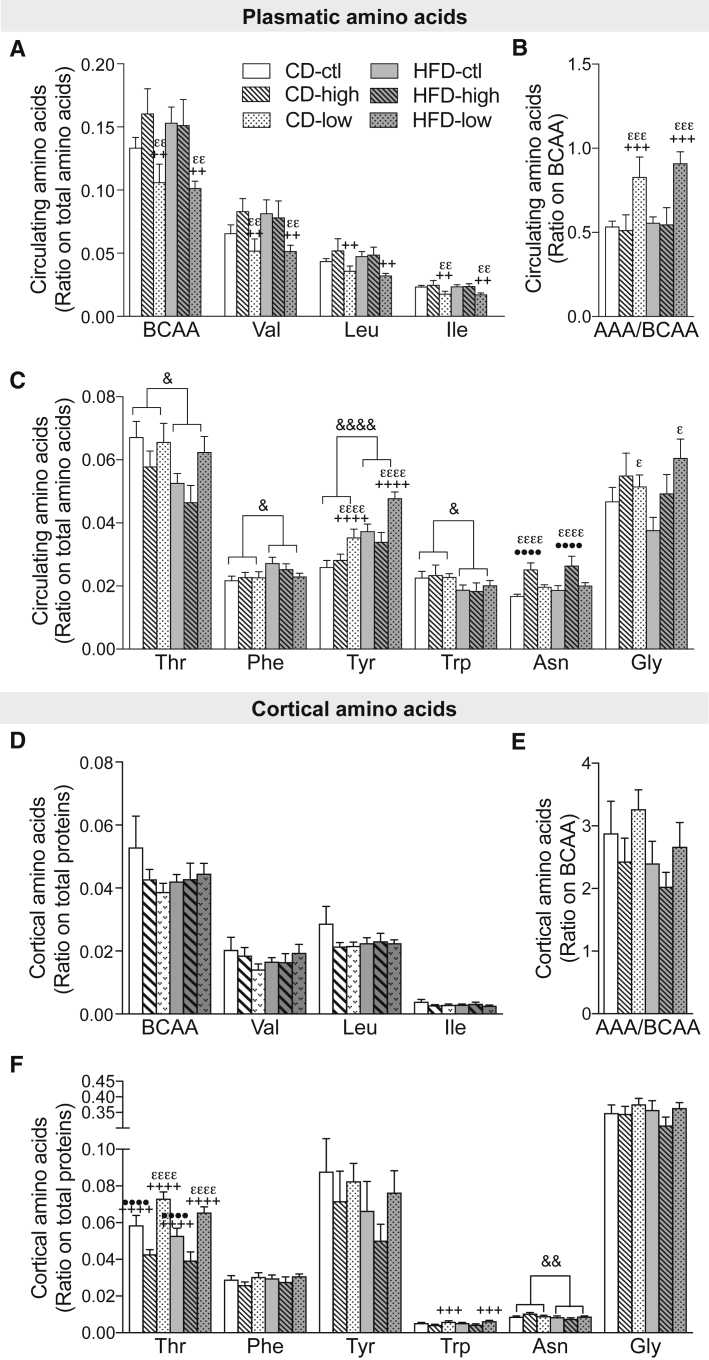
3.2. Titrating BCAA supply modifies plasma and brain amino acids and monoamines levels in 18-month-old 3xTg-AD mice
High BCAA consumption was reported to directly modify levels of plasmatic BCAA and aromatic amino acids (AAA) in humans [14]. Because tryptophan, threonine, tyrosine, valine, leucine, and isoleucine all compete for LAT1 for brain entry, specific changes in circulating amino acids may impact their balance in the brain, as well as downstream monoaminergic neurotransmitters [13], [14]. We thus measured levels of amino acids in the plasma and parieto-temporal cortex (Fig. 2). We first confirmed that BCAA restriction decreased valine, leucine, and isoleucine concentrations in the plasma in both CD and HFD groups, but not in the cerebral cortex (Fig. 2A, D). Lower BCAA intake also increased the ratio of circulating AAA to BCAA and levels of glycine and tyrosine under both types of diets (Fig. 2B, C). Although a 50% higher consumption of BCAA was not reflected in significantly higher plasmatic or cortical concentrations, increased asparagine levels were noted in the plasma of mice supplemented with BCAA in either the CD or HFD (Fig. 2C). Moreover, threonine levels were markedly modulated in the brain with higher and lower concentrations following low and high BCAA intake, respectively, whether in CD or HFD (Fig. 2F). Changes in cortical tyrosine levels were not significant, probably due to interindividual variations (Fig. 2F), whereas cortical concentrations of tryptophan, but not phenylalanine, were inversely associated with levels of BCAA in the plasma in both CD and HFD (Fig. 2F).
Fig. 2.
High or low BCAA dietary intake modifies plasmatic and cerebral amino acids levels in 18-month-old 3xTg-AD mice. BCAA (Val, Leu, Ile) and AAA (Phe, Trp, Tyr, His) in intracardiac blood sampled just before perfusion, expressed as a ratio on total plasma amino acids (n = 4–6 per group) (A–C). BCAA and AAA in TBS-soluble fraction of cortex homogenates, expressed as a ratio on total protein content (n = 10–16 per group) (D–F). Data are presented as mean ± SEM. Two-way ANOVA, followed by Tukey's HSD; effect of the diet: &P < .05, &&P < .01, &&&&P < .0001; effect of BCAA: ε different from ctl, + different from high, • different from low. Abbreviations: CD, control diet; HFD, high-fat diet; ctl, normal levels of BCAA; high, BCAA-supplemented diet by 50%; low, BCAA-reduced diet by 50%; AAA, aromatic amino acid; BCAA, branched-chain amino acid.
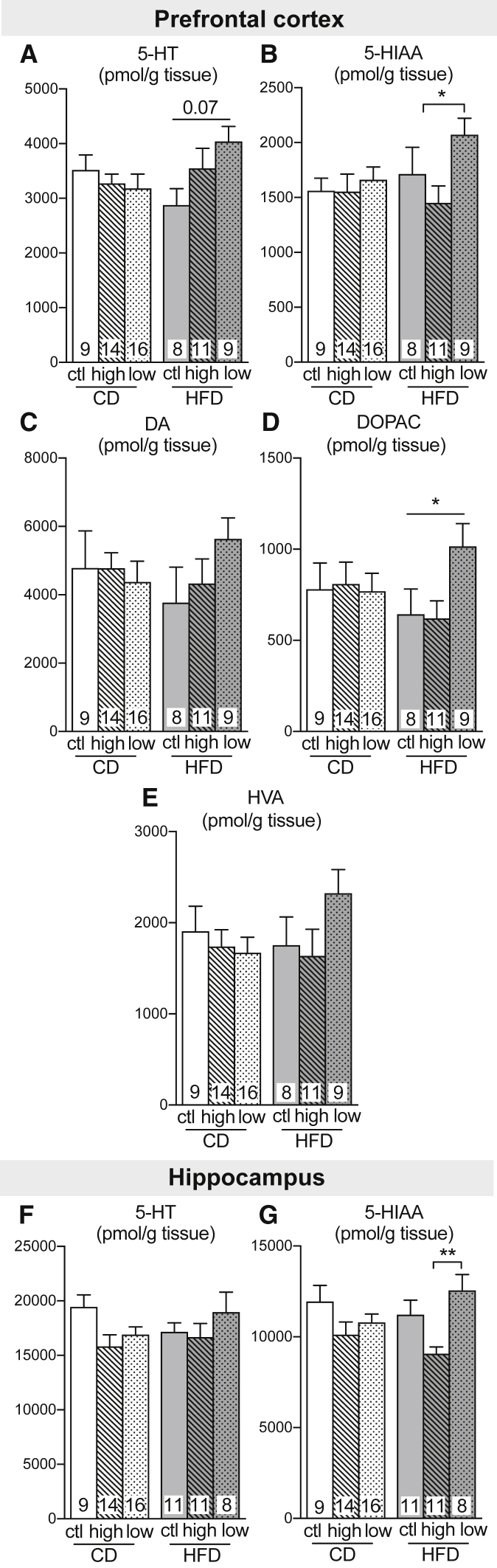
To confirm that modulations in plasmatic and brain amino acids impact neurotransmitter synthesis, we measured catecholamines (dopamine, DA) and indolamines (serotonin, 5-HT) and their metabolites in the prefrontal cortex and hippocampus of the 3xTg-AD mice (Fig. 3). Whereas BCAA intake did not affect DA concentrations in 3xTg-AD mice, a trend toward higher 5-HT content was observed in both the prefrontal cortex and hippocampus of mice fed an HFD reduced in BCAA compared with mice fed an HFD only (Fig. 3A, F). In addition, a low BCAA intake led to higher levels of metabolites 5-hydroxyindoleacetic acid (5-HIAA) and 3,4-dihydroxyphenylacetic acid (DOPAC) in both the prefrontal cortex and hippocampus, compared to the HFD supplemented with BCAA (Fig. 3B, D, G).
Fig. 3.
High or low BCAA supplies modify monoamines levels in 18-month-old 3xTg-AD mice. Levels of dopamine and serotonin and their metabolites measured in the prefrontal cortex (A–E) and hippocampus (F, G). Data are presented as mean ± SEM. One-way ANOVA, Kruskal-Wallis followed by Dunn's: *P < .05. Abbreviations: CD, control diet; HFD, high-fat diet; BCAA, branched-chain amino acid; ctl, normal levels of BCAA; high, BCAA-supplemented diet by 50%; low, BCAA-reduced diet by 50%; 5-HT, serotonin; 5-HIAA, 5-hydroxyindoleacetic acid; DA, dopamine; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid.
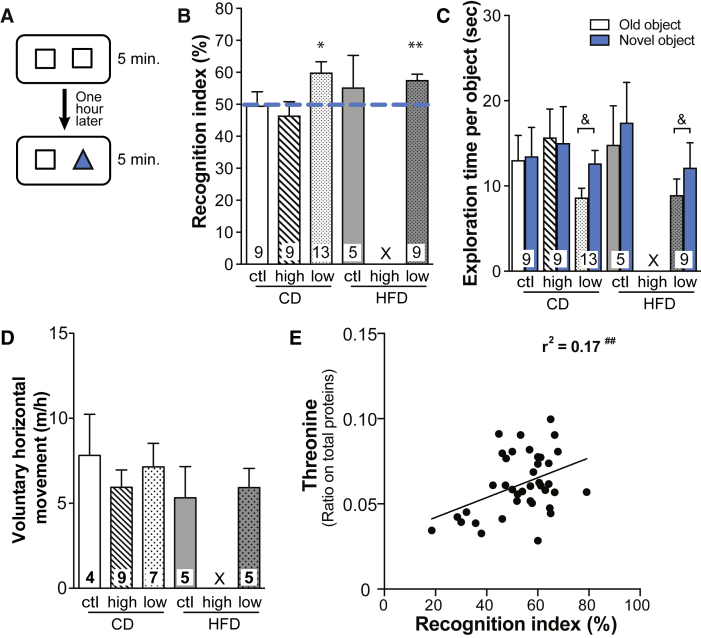
3.3. Low BCAA dietary intake improves object recognition memory in old 3xTg-AD mice
To assess the effect of BCAA dietary intake on memory function, all groups of mice underwent the novel object recognition task at 18 months (Fig. 4A). This test requires minimal movement and induces no anxiety, which makes it a suitable choice for old 3xTg-AD mice [24]. The test first confirmed that 18-month-old 3xTg-AD mice, fed either a CD or a HFD, did not discriminate the novel from the old object (Fig. 4B, C), as previously shown [25]. Similarly, 3xTg-AD mice fed the high BCAA diet failed to recognize the novel object. In contrast, 3xTg-AD mice fed the low BCAA diets, either CD-low or HFD-low, spent significantly more time exploring the novel than the familiar object, suggesting memory improvement after BCAA restriction. The open field test confirmed that no group of mice demonstrated impaired voluntary locomotor activity, which could have affected exploratory behavior (Fig. 4D). The time the mice spent on a wire confirms that muscular strength was not affected by dietary treatments (Supplementary Fig.1). Interestingly, cortical threonine levels were correlated with the recognition index (Fig. 4E).
Fig. 4.
BCAA-reduced diet improves object recognition memory in 18-month-old 3xTg-AD mice. The novel object recognition test paradigm (A). Performance at the novel object recognition task of 18-month-old 3xTg-AD mice determined with the recognition index (= time exploring the novel object/total time exploring both objects) (B) and the time spent exploring the old and the novel objects (C). Voluntary horizontal movement (D) measured during a 1-hour open field test. Correlation between cortical threonine levels and recognition index of the mice (E). Dotted line represents a recognition index of 50%, meaning the mouse does not discriminate between both objects. Almost all mice under the BCAA-supplemented HFD died before the test. Data are presented as mean ± SEM. One-sample t-test: *P < .05, **P < .01 versus random chance (recognition index of 50%). Wilcoxon signed-rank test (nonparametric paired t-test): &P < .05. Pearson's coefficient (r2) was determined using linear regression: ##P < .01. X: insufficient number of healthy mice. Abbreviations: CD, control diet; HFD, high-fat diet; ctl, normal levels of BCAA; high, BCAA-supplemented diet by 50%; low, BCAA-reduced diet by 50%; BCAA, branched-chain amino acid.
3.4. BCAA supplementation in an HFD context worsens tau hyperphosphorylation but not amyloid pathology in old 3xTg-AD mice
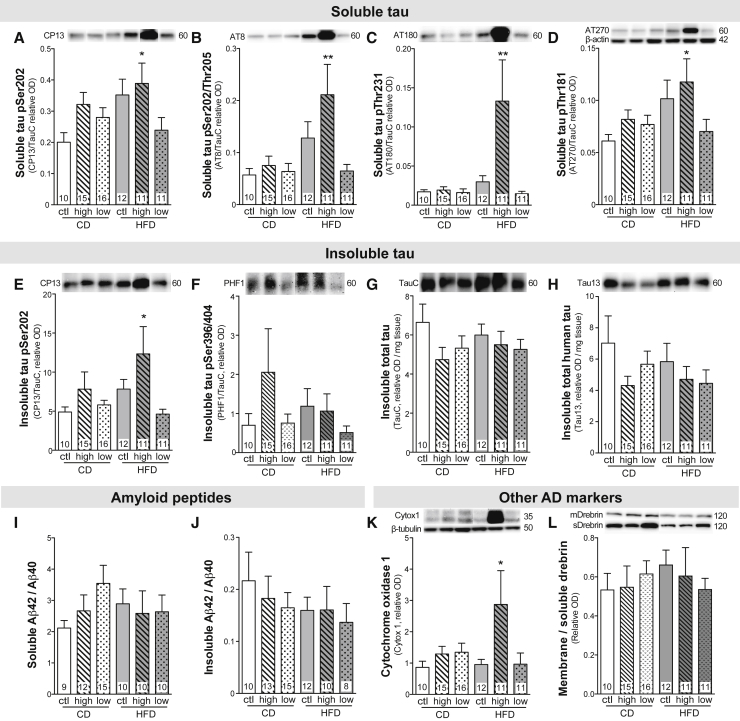
Tau hyperphosphorylation is one of the main neuropathological hallmarks of AD, correlating with cognitive deficits in patients with AD [28], [35]. Here, we found that a 2-month BCAA supplementation combined with HFD was sufficient to increase soluble tau pSer202 (+94%), pSer202/Thr205 (+271%), pThr231 (+680%), and pThr181 (+93%) compared with control mice, without changing the level of total tau (Fig. 5A–D). Strikingly, phosphorylation of detergent-insoluble tau at pSer202 was also increased in the HFD-high group (+151%), whereas tau pSer396/404 and both total tau and total human tau were unchanged (Fig. 5E–H). In contrast, reducing the BCAA resulted in a tau phosphorylation status similar to mice fed the CD. However, we did not find any changes in protein levels of the main kinases involved in tau phosphorylation (Supplementary Table 5).
Fig. 5.
BCAA-supplemented HFD worsens tau pathology but not amyloid pathology in 18-month-old 3xTg-AD mice. TBS-soluble tau phosphorylation at pSer202, pSer202/Thr205, pThr231, and pThr181 (A–D), detergent-insoluble tau phosphorylation at pSer202 and pSer396/404 (E, F), detergent-insoluble total tau and total human tau (G, H), TBS-soluble and detergent-insoluble Aβ42/Aβ40 (I, J), detergent-soluble cytochrome oxidase 1 (K), and ratio of detergent-soluble on TBS-soluble drebrin (L) measured by Western blot in cortex homogenates. Data for tau phosphorylation were calculated as the ratio of the phosphoepitope to total tau concentration. Data are presented as mean ± SEM. One-way ANOVA, Dunnett's (vs CD) post hoc analysis: *P < .05, ∗∗P < .01. Representative bands were selected from nonconsecutive samples run on the same gel. Abbreviations: BCAA, branched-chain amino acid; CD, control diet; HFD, high-fat diet; ctl, normal levels of BCAA; high, BCAA-supplemented diet by 50%; low, BCAA-reduced diet by 50%; OD, optical density; Aβ, amyloid β.
Accumulation of brain Aβ peptides and extracellular plaques are key markers of AD replicated in the 3xTg-AD mouse [25], [28]. However, we did not observe any effect of BCAA consumption on Aβ40 or Aβ42 peptides in soluble or detergent-insoluble fractions (Fig. 5I, J). Finally, we found that the cytochrome oxidase protein, a marker of cellular activity, was greatly increased in the cortex of mice fed the HFD supplemented with BCAA (Fig. 5K). No changes in drebrin (Fig. 5L) or other AD-relevant markers were noted (Supplementary Table 5).
4. Discussion
The consumption of high levels of BCAA is prevalent in our contemporary society, but no study to our knowledge has directly probed for the impact of high or low BCAA intake in a context of AD and old age [8], [36]. Correlative data linking BCAA and AD exist [20], [21], [22], but conclusions on causality require controlled intervention difficult to achieve in a clinical setting. In this study, 3xTg-AD mice were either supplemented or restricted in BCAA over a CD or HFD from 16 to 18 months of age. We found that BCAA supplementation combined with a HFD resulted in a significant mortality rate and strongly increased tau neuropathology. In contrast, reducing BCAA in both the CD and HFD did not result in any premature death, improved cognitive performance, and led to increased levels of threonine, tryptophan, 5-HT, 5-HIAA, and DOPAC in the brain.
4.1. BCAA toxicity
A first striking consequence of BCAA supplementation when combined with an HFD was a shortening of the lifespan of 3xTg-AD mice. Rises in plasmatic alanine aminotransferase, aspartate aminotransferase, and creatine kinase were observed in moribund animals and represent a possible signature of hepatic and muscular toxicity, corroborating previous studies reporting BCAA-induced liver injuries in HFD-fed obese mice, inflammation and oxidative stress [33], [37]. Although the cause of death for all the animals included in our study is not definitive, major brain damages (atrophy, edema, etc.) were not observed, suggesting that hepatic and muscular toxicity played a more direct role in their premature death. Although little is known about BCAA metabolism in the elderly, some studies have revealed that the response to BCAA supplementation is different in old age [38]. Thus, 18-month-old 3xTg-AD mice could display a failure in catabolizing BCAA, thus disturbing amino acids homeostasis in the brain and peripheral tissues.
4.2. Modulation of amino acids by BCAA intake: Plasma and brain levels
Our results further confirm that BCAA dietary supply modulates plasma and brain amino acids and catecholamines. Perhaps surprisingly, BCAA supplementation was not accompanied by a significant increase in plasmatic and cortical concentrations in the 3xTg-AD mouse, but rather by higher levels of asparagine in the plasma and lower cortical threonine. It should be kept in mind that the present measurement reflect an equilibrium reached following 2 months of altered BCAA intake, which is also affected by other factors such as distribution of amino acids in tissue where they integrate into proteins and elimination (metabolism and renal clearance). Still, BCAA restriction lowered BCAA and increased tyrosine concentrations in the plasma, whereas in the cortex, levels of threonine and tryptophan were upregulated, suggesting complex interactions in amino acid homeostasis.
Previous studies have shown that BCAA administration decreases cortical tyrosine and tryptophan in the hippocampus of rats [39]. The likely mechanism is competition for amino acid transporters at the blood-brain barrier [12]. However, despite obvious trends in the means, no significant change in brain tyrosine content was detected here due to interindividual variation. More clearly, increases and decreases in BCAA intake induced a strong modulation in cortical concentrations of threonine, possibly due to the fact that brain uptake of threonine is highly sensitive to competition from BCAA [40]. Finally, although we did not observe a decrease in serotonin following BCAA supplementation as previously reported [15], [39], a 2-month reduction in dietary BCAA combined to a HFD led to a trend toward increased serotonin and dopamine levels. This tendency was supported by significant increases in serotonin metabolite 5-HIAA and dopamine metabolite DOPAC, changes typically interpreted as signs of increased secretion and metabolism of these neurotransmitters [41]. Overall, our results confirm that BCAA dietary supply modulates plasmatic and cerebral amino acids and neurotransmitters, thus providing a means by which they can modify brain homeostasis and function.
4.3. BCAA and memory
The observed failure of 18-month-old 3xTg-AD mice to recognize the novel object is consistent with previous work in the same model at similar ages [5], [24], [25]. As old transgenic mice did not recognize the novel object, it was predictable that further deterioration of memory could not be detected at this age even after BCAA supplementation. However, a 2-month reduction in BCAA intake improved recognition memory in mice fed either the CD or HFD. These results were intriguing because HFD is known to worsen cognitive defects in 3xTg-AD mice [24]. The observed increase in cortical amino acids and monoamines may underlie these memory improvements, as suggested by the fact that the animals with the higher levels of brain threonine performed better at the novel object recognition task. In particular, threonine, tryptophan, and serotonin are known to be involved in behavioral changes [15], [42].
4.4. BCAA and AD neuropathology
BCAA have been only recently implicated in AD. Plasmatic valine has been linked to cognitive score and brain atrophy in an AD cohort [20], while a recent Mendelian randomization analysis indicates that genetic susceptibility to high plasmatic isoleucine is associated with a higher risk of developing the disease [22]. APP/PS1 mice, a mouse model of amyloid pathology, spontaneously display increased plasmatic BCAA, without obesity, suggesting that AD pathology potentiates defects in BCAA metabolism, putting patients with AD at a higher risk of BCAA-induced brain damages [43], [44]. Conversely, tau pathology is one of the major neuropathological markers of AD, strongly associated with antemortem cognitive deficits [35], [45]. We found that a 2-month BCAA supplementation combined with a HFD strongly increased both soluble and detergent-insoluble tau hyperphosphorylation in the cortex of old 3xTg-AD mice. These results suggest that the combination of BCAA supplementation and HFD has synergistic deleterious effects on AD pathology and neuronal function. To our knowledge, it is the first time that BCAA supplementation has been implicated in tau phosphorylation. Although synaptic and amyloid pathologies were unaffected by BCAA modulation, the rise in cytochrome oxidase 1 protein in cortex homogenates of mice under BCAA-supplemented HFD suggests an increase in mitochondrial oxidative activity in those mice, possibly reflecting a state of neuronal oxidative stress [46].
5. Conclusion
There is scientific evidence that dietary intervention can reduce the risk of developing age-related cognitive impairment or AD [3], [47]. Thus, nutritional strategies aimed at reducing AD pathology are of interest in the field. This study provides controlled preclinical data pinpointing the specific role of BCAA in an animal model of AD. For the first time, our results identify the potential risks of combining high BCAA consumption with a HFD, not only regarding peripheral toxicity but also with respect to the aggravation of AD-like tau neuropathology. On a more positive note, our results suggest that reducing the BCAA content in the diet supply, while maintaining protein intake, could have a beneficial effect on memory and could be considered in nutritional interventions aimed at improving cognitive performance and/or reducing the incidence of AD. Thus, it would be interesting to test whether protein supplementation with low BCAA concentration could prevent mood and memory impairment. Another easier intervention for the elderly would be to promote foods rich in protein but low in BCAA [36].
Research in Context.
-
1.
Systematic review: We performed an extensive literature review using PubMed on branched-chain amino acids (BCAA) and brain function. Although BCAA are now increasingly studied in metabolic disorders, very little is known on BCAA in the field of Alzheimer's disease (AD).
-
2.
Interpretation: Our findings in a mouse model of AD reveal that high and low BCAA consumption modulates amino acid and monoamines levels in the brain as well as AD neuropathology and memory-related behavior.
-
3.
Future directions: Intervention and observational studies are needed to confirm whether high or low BCAA consumption could increase or decrease, respectively, the risk of developing dementia.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) (FC–MOP 102532 and 125930), the Alzheimer Society of Canada (FC), the Institute of Nutrition and Functional Foods, and the Canada Foundation for Innovation (10307). M.T. is funded by a scholarship from the Alzheimer Society of Canada. F.C. is a Fonds de recherche du Québec-Santé (FRQ-S) senior research scholar.
Footnotes
Supplementary data to this article can be found online at doi.org/10.1016/j.trci.2018.10.005.
Supplementary data
References
- 1.Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C.P. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Norton S., Matthews F.E., Barnes D.E., Yaffe K., Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 3.Ngandu T., Lehtisalo J., Solomon A., Levälahti E., Ahtiluoto S., Antikainen R. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 4.Kerdiles O., Layé S., Calon F. Omega-3 polyunsaturated fatty acids and brain health: Preclinical evidence for the prevention of neurodegenerative diseases. Trends Food Sci Technol. 2017;69:203–213. [Google Scholar]
- 5.Dal-Pan A., Dudonné S., Bourassa P., Bourdoulous M., Tremblay C., Desjardins Y. Cognitive-Enhancing effects of a polyphenols-rich extract from fruits without changes in neuropathology in an animal model of Alzheimer's disease. J Alzheimers Dis. 2017;55:115–135. doi: 10.3233/JAD-160281. [DOI] [PubMed] [Google Scholar]
- 6.Van de Rest O., Van der Zwaluw N.L., de Groot L.C.P.G.M. Literature review on the role of dietary protein and amino acids in cognitive functioning and cognitive decline. Amino Acids. 2013;45:1035–1045. doi: 10.1007/s00726-013-1583-0. [DOI] [PubMed] [Google Scholar]
- 7.Ohtani M., Sugita M., Maruyama K. Amino acid mixture improves training efficiency in athletes. J Nutr. 2006;136:538S–543S. doi: 10.1093/jn/136.2.538S. [DOI] [PubMed] [Google Scholar]
- 8.Maughan R.J., Depiesse F., Geyer H., International Association of Athletics Federations The use of dietary supplements by athletes. J Sports Sci. 2007;25:S103–S113. doi: 10.1080/02640410701607395. [DOI] [PubMed] [Google Scholar]
- 9.Newgard C.B., An J., Bain J.R., Muehlbauer M.J., Stevens R.D., Lien L.F. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T.J., Larson M.G., Vasan R.S., Cheng S., Rhee E.P., McCabe E. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newgard C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pardridge W.M. Kinetics of competitive inhibition of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1977;28:103–108. doi: 10.1111/j.1471-4159.1977.tb07714.x. [DOI] [PubMed] [Google Scholar]
- 13.Fernstrom J.D. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids. 2013;45:419–430. doi: 10.1007/s00726-012-1330-y. [DOI] [PubMed] [Google Scholar]
- 14.Gijsman H.J., Scarnà A., Harmer C.J., McTavish S.B., Odontiadis J., Cowen P.J. A dose-finding study on the effects of branch chain amino acids on surrogate markers of brain dopamine function. Psychopharmacology (Berl) 2002;160:192–197. doi: 10.1007/s00213-001-0970-5. [DOI] [PubMed] [Google Scholar]
- 15.Coppola A., Wenner B.R., Ilkayeva O., Stevens R.D., Maggioni M., Slotkin T.A. Branched-chain amino acids alter neurobehavioral function in rats. Am J Physiol Endocrinol Metab. 2013;304:E405–E413. doi: 10.1152/ajpendo.00373.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scaini G., Jeremias G.C., Furlanetto C.B., Dominguini D., Comim C.M., Quevedo J. Behavioral responses in rats submitted to chronic administration of branched-chain amino acids. JIMD Rep. 2014;13:159–167. doi: 10.1007/8904_2013_274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y., Yan J., Zhou P., Li J., Gao H., Xia Y. Neurotransmitter receptors and cognitive dysfunction in Alzheime's disease and Parkinson' disease. Prog Neurobiol. 2012;97:1–13. doi: 10.1016/j.pneurobio.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zinnanti W.J., Lazovic J. Interrupting the mechanisms of brain injury in a model of maple syrup urine disease encephalopathy. J Inherit Metab Dis. 2012;35:71–79. doi: 10.1007/s10545-011-9333-5. [DOI] [PubMed] [Google Scholar]
- 19.Sitta A., Ribas G.S., Mescka C.P., Barschak A.G., Wajner M., Vargas C.R. Neurological damage in MSUD: the role of oxidative stress. Cell Mol Neurobiol. 2014;34:157–165. doi: 10.1007/s10571-013-0002-0. [DOI] [PubMed] [Google Scholar]
- 20.Toledo J.B., Arnold M., Kastenmüller G., Chang R., Baillie R.A., Han X. Metabolic network failures in Alzheimer's disease: A biochemical road map. Alzheimers Dement. 2017;13:965–984. doi: 10.1016/j.jalz.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tynkkynen J., Chouraki V., van der Lee S.J., Hernesniemi J., Yang Q., Li S. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer's disease: A prospective study in eight cohorts. Alzheimers Dement. 2018;14:723–733. doi: 10.1016/j.jalz.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson S.C., Markus H.S. Branched-chain amino acids and Alzheimer's disease: a Mendelian randomization analysis. Nat Publishing Group. 2017;7:13604. doi: 10.1038/s41598-017-12931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julien C., Tremblay C., Phivilay A., Berthiaume L., Emond V., Julien P. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol Aging. 2010;31:1516–1531. doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 24.Vandal M., White P.J., Tremblay C., St-Amour I., Chevrier G., Emond V. Insulin reverses the high-fat diet-induced increase in brain aβ and improves memory in an animal model of Alzheimer disease. Diabetes. 2014;63:4291–4301. doi: 10.2337/db14-0375. [DOI] [PubMed] [Google Scholar]
- 25.St-Amour I., Paré I., Tremblay C., Coulombe K., Bazin R., Calon F. IVIg protects the 3xTg-AD mouse model of Alzheimer's disease from memory deficit and Aβ pathology. J Neuroinflammation. 2014;11:54. doi: 10.1186/1742-2094-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbins R.J., Leonczak J., Johnson J.C., Li J., Kwik-Uribe C., Prior R.L. Method performance and multi-laboratory assessment of a normal phase high pressure liquid chromatography-fluorescence detection method for the quantitation of flavanols and procyanidins in cocoa and chocolate containing samples. J Chromatogr A. 2009;1216:4831–4840. doi: 10.1016/j.chroma.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Crumeyrolle-Arias M., Jaglin M., Bruneau A., Vancassel S., Cardona A., Daugé V. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–217. doi: 10.1016/j.psyneuen.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Querfurth H.W., LaFerla F.M. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 29.Rocca W.A., Petersen R.C., Knopman D.S., Hebert L.E., Evans D.A., Hall K.S. Trends in the incidence and prevalence of Alzheimer's disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7:80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D.D., Leung C.W., Li Y., Ding E.L., Chiuve S.E., Hu F.B. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174:1587–1595. doi: 10.1001/jamainternmed.2014.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reitz C., Tang M.-X., Schupf N., Manly J.J., Mayeux R., Luchsinger J.A. A summary risk score for the prediction of Alzheimer disease in elderly persons. Arch Neurol. 2010;67:835–841. doi: 10.1001/archneurol.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theriault P., ElAli A., Rivest S. High fat diet exacerbates Alzheimer's disease-related pathology in APPswe/PS1 mice. Oncotarget. 2016;5 doi: 10.18632/oncotarget.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang F., Zhao S., Yan W., Xia Y., Chen X., Wang W. Branched Chain Amino Acids Cause Liver Injury in Obese/Diabetic Mice by Promoting Adipocyte Lipolysis and Inhibiting Hepatic Autophagy. EBioMedicine. 2016;13:157–167. doi: 10.1016/j.ebiom.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harita N., Hayashi T., Sato K.K., Nakamura Y., Yoneda T., Endo G. Lower serum creatinine is a new risk factor of type 2 diabetes: the Kansai healthcare study. Diabetes Care. 2009;32:424–426. doi: 10.2337/dc08-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tremblay C., Francois A., Delay C., Freland L., Vandal M., Bennett Association of neuropathological markers in the parietal cortex with antemortem cognitive function in persons with mild cognitive impairment and Alzheimer Disease. J Neuropathol Exp Neurol. 2017;76:70–88. doi: 10.1093/jnen/nlw109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajendram R., Preedy V.R., Patel V.B. Humana Press; New-York, NY: 2015. Branched Chain Amino Acids in Clinical Nutrition. [Google Scholar]
- 37.Zhenyukh O., Civantos E., Ruiz-Ortega M., Sánchez M.S., Vázquez C., Peiró C. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic Biol Med. 2017;104:165–177. doi: 10.1016/j.freeradbiomed.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Tatpati L.L., Irving B.A., Tom A., Bigelow M.L., Klaus K., Short K.R. The effect of branched chain amino acids on skeletal muscle mitochondrial function in young and elderly adults. J Clin Endocrinol Metab. 2010;95:894–902. doi: 10.1210/jc.2009-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi S., Disilvio B., Fernstrom M.H., Fernstrom J.D. Oral branched-chain amino acid supplements that reduce brain s during exercise in rats also lower brain catecholamines. Amino Acids. 2013;45:1133–1142. doi: 10.1007/s00726-013-1566-1. [DOI] [PubMed] [Google Scholar]
- 40.Tovar A., Tews J.K., Torres N., Harper A.E. Some characteristics of threonine transport across the blood-brain barrier of the rat. J Neurochem. 1988;51:1285–1293. doi: 10.1111/j.1471-4159.1988.tb03098.x. [DOI] [PubMed] [Google Scholar]
- 41.Burchinsky S.G., Kuznetsova S.M. Brain monoamine oxidase and aging: a review. Arch Gerontol Geriatr. 1992;14:1–15. doi: 10.1016/0167-4943(92)90002-l. [DOI] [PubMed] [Google Scholar]
- 42.Markus C.R. Dietary amino acids and brain serotonin function; implications for stress-related affective changes. Neuromolecular Med. 2008;10:247–258. doi: 10.1007/s12017-008-8039-9. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz H.H., Chi T., Shin A.C., Lindtner C., Hsieh W., Ehrlich M. Increased susceptibility to metabolic dysregulation in a mouse model of Alzheimer's disease is associated with impaired hypothalamic insulin signaling and elevated BCAA levels. Alzheimers Dement. 2016;12:851–861. doi: 10.1016/j.jalz.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.González-Domínguez R., García-Barrera T., Vitorica J., Gómez-Ariza J.L. Metabolomic investigation of systemic manifestations associated with Alzheimer's disease in the APP/PS1 transgenic mouse model. Mol Biosyst. 2015;11:2429–2440. doi: 10.1039/c4mb00747f. [DOI] [PubMed] [Google Scholar]
- 45.Tremblay C., Pilote M., Phivilay A., Emond V., Bennett C.F. Biochemical characterization of Abeta and tau pathologies in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2007;12:377–390. doi: 10.3233/jad-2007-12411. [DOI] [PubMed] [Google Scholar]
- 46.Zhu X., Perry G., Moreira P.I., Aliev G., Cash A.D., Hirai K. Mitochondrial abnormalities and oxidative imbalance in Alzheimer disease. J Alzheimers Dis. 2006;9:147–153. doi: 10.3233/jad-2006-9207. [DOI] [PubMed] [Google Scholar]
- 47.Yurko-Mauro K., McCarthy D., Rom D., Nelson E.B., Ryan A.S., Blackwell A. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010;6:456–464. doi: 10.1016/j.jalz.2010.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.