Abstract
Polyamines (PAs) are indispensable polycations ubiquitous to all living cells. Among their many critical functions, PAs contribute to the oxidative balance of the cell. Beginning with studies by the Tabor laboratory in bacteria and yeast, the requirement for PAs as protectors against oxygen radical–mediated damage has been well established in many organisms, including mammals. However, PAs also serve as substrates for oxidation reactions that produce hydrogen peroxide (H2O2) both intra- and extracellularly. As intracellular concentrations of PAs can reach millimolar concentrations, the H2O2 amounts produced through their catabolism, coupled with a reduction in protective PAs, are sufficient to cause the oxidative damage associated with many pathologies, including cancer. Thus, the maintenance of intracellular polyamine homeostasis may ultimately contribute to the maintenance of oxidative homeostasis. Again, pioneering studies by Tabor and colleagues led the way in first identifying spermine oxidase in Saccharomyces cerevisiae. They also first purified the extracellular bovine serum amine oxidase and elucidated the products of its oxidation of primary amine groups of PAs when included in culture medium. These investigations formed the foundation for many polyamine-related studies and experimental procedures still performed today. This Minireview will summarize key innovative studies regarding PAs and oxidative damage, starting with those from the Tabor laboratory and including the most recent advances, with a focus on mammalian systems.
Keywords: polyamine, spermidine, oxidative stress, redox regulation, reactive oxygen species (ROS), cancer biology, antioxidant, oxidase, homeostasis, free radicals, polyamine catabolism, spermine
Introduction
Polyamines (PAs)2 are naturally occurring polycationic alkylamines that are essential for growth and survival in all mammalian cells (1, 2). This absolute requirement is based on the multitude of roles PAs play, many of which relate to their positive charge at physiological pH. PAs, including putrescine (Put), spermidine (Spd), and spermine (Spm) (Fig. 1), contribute to critical cellular processes such as ion channel regulation, chromatin structure maintenance, DNA replication, transcription, and translation (3–6). They also act as free radical scavengers, and their catabolism can be a source of toxic reactive oxygen species (ROS), therefore implying their potential to affect oxidative status. The main purpose of this Minireview will be to cover the salient features of PAs and their catabolism in association with oxidative stress in both normal and disease processes.
Figure 1.
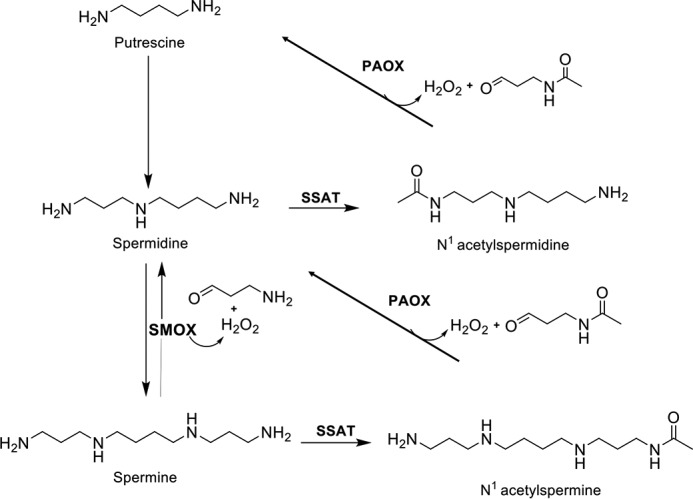
Polyamine catabolic pathway in mammals. SSAT catalyzes the acetyl-group transfer from acetyl-CoA to the aminopropyl end of spermidine or spermine, producing N1-acetylspermidine or N1-acetylspermine, respectively. These acetylated PAs are either excreted from the cell or used as substrates for PAOX, producing H2O2, 3-acetoamidopropanal, and either putrescine or spermidine, depending on the starting substrate. Alternatively, spermine can be directly oxidized back to spermidine by SMOX while generating H2O2 and 3-aminopropanal.
Contributions of polyamines to cellular redox balance
Oxidative stress occurs when ROS, such as those derived from hydrogen peroxide (H2O2), exceed the physiological levels required for normal redox reactions and cell signaling. The resulting oxidative damage to macromolecules is associated with aging and a variety of related pathologies, including cancer (7, 8). PAs play dual roles in maintaining cellular oxidative homeostasis by both protecting against free radical–mediated damage and acting as substrates for enzymes that produce ROS.
Polyamines as protection from oxidative damage
Polyamines protect against oxidative damage in microorganisms
The natural PAs are themselves capable of acting as free radical scavengers (9–12) and protect against oxidative damage of DNA and phospholipids in cell-free systems (13–15). These properties extend to bacteria and yeast, where Tabor and colleagues first demonstrated a protective role for PAs against oxidative damage in vivo. Spd-deficient Escherichia coli cells were hypersensitive to paraquat in the presence of oxygen, suggesting that Spd reduced superoxide-associated cell death (16). In subsequent studies, E. coli mutants lacking PAs were extremely susceptible to ROS toxicity when grown in 95% oxygen or exposed to H2O2 (17). These data were among the first to demonstrate polyamine-mediated protection against oxygen radicals.
The polyamine modulon of E. coli comprises a growing collection of genes that are stimulated by PAs at the level of translation (18). Recently, this modulon was expanded to include polyamine-inducible genes specific to oxidative stress conditions, including those governing the synthesis of superoxide dismutases (SODs), glutathione (GSH), and catalases (19, 20). Thus, in addition to the direct effects of PAs on ROS in E. coli, PAs can stimulate the expression of proteins essential to an effective antioxidant response.
Saccharomyces cerevisiae mutants lacking Spd and Spm also require exogenous PAs for protection against ROS, even in the presence of SOD overexpression (21, 22). Microarray studies comparing Spm-deficient yeast mutants containing low levels of Spd with those supplemented with Spd revealed that Spd altered the expression of at least 500 genes greater than 2-fold, including several oxidative stress-response genes (23). Recently, the S. cerevisiae polyamine exporter Tpo1 was discovered to participate in the oxidative stress response by modulating intracellular Spd and Spm levels in response to H2O2, thereby invoking the production of proteins necessary for oxidant tolerance, including SODs and heat shock proteins, governing the duration of cell cycle arrest, and allowing adaptation to elevated H2O2 levels (24).
In addition to the unmodified PAs, polyamine conjugates have been implicated in protection from oxidative stress. Glutathionylspermidine (Gsp) was first identified in E. coli by the Tabors in 1974 (25) and was later found to be a source of Spd for the bacteria upon the induction of growth from stationary phase (26). Glutathionylspermidine synthetase/amidase (GspSA) catalyzes both the formation and removal of an amide bond between GSH and Spd to govern Gsp abundance (27). The amidase domain of GspSA is sensitive to inactivation by oxidation, resulting in Gsp-modified proteins, including Gsp disulfides and protein thiols (28). These data are consistent with the hypothesis that the formation/hydrolysis of Gsp represents an oxidative stress mechanism that contributes to the maintenance of redox homeostasis in E. coli.
Confirmation of a role for GSH–Spd conjugates in redox reactions came with the discovery of trypanothione (N1,N8-bis(glutathionyl)spermidine), a cofactor for trypanosomatid GSH reductase (29). As an important mediator of redox balance in pathogenic trypanosomes, including those responsible for human African trypanosomiasis, leishmaniasis, and Chagas' disease, the generation, use, and redox recycling of trypanothione have become targets for antitrypanosomal drug development (30). Additionally, Spd and Spm protect against free radical–mediated lipid peroxidation in Trypanosoma cruzi (31).
Protective effects of polyamines against oxidative stress in mammalian cells
Spm and Spd also protect mammalian cells against ROS-mediated damage, and depletion of these PAs is known to arrest cellular growth. The Gy11 embryonic fibroblast cell line is deficient in Spm due to a mutation in the Spm synthase gene (32). These cells are more sensitive to the cytotoxic effects of H2O2 than their normal counterparts, and further depleting their PA levels induces DNA damage and apoptosis even in the absence of H2O2. Using combinations of enzyme inhibitors to adjust the intracellular Spd and Spm concentrations of these cells to within normal physiological ranges protected fibroblasts from H2O2 exposure. Additionally, depleting cellular GSH further sensitized PA-depleted cells to H2O2, indicating the involvement of PAs in a protective mechanism against ROS independent of GSH (33).
Oxidative stress activates translocation of the transcription factor NRF2 (nuclear factor (erythroid-derived 2)-like 2), which then stimulates expression of genes involved in the antioxidant response. Evidence suggests that NRF2 also regulates PA biosynthesis by inducing ornithine decarboxylase activity in response to oxidative stress, thereby elevating PA levels to potentially aid in the antioxidant response (34). However, activated PA catabolism through spermidine/spermine N1-acetyltransferase (SSAT), another transcriptional target of NRF2, also occurs in response to ROS, perhaps to limit tumor-promoting PA accumulation (34–36).
Polyamine catabolism as a source of ROS
Intracellular mammalian polyamine catabolism
Mammalian PA catabolism consists of highly regulated, inducible pathways that facilitate cellular PA homeostasis. It serves to balance PA transport and biosynthesis to maintain intracellular PAs within a cell type-specific range that is optimal for cellular function and proliferation. Mammalian PA catabolism has two distinct but interconnected pathways (Fig. 1), both of which contain oxidases that generate ROS in the form of H2O2.
The originally discovered catabolic mechanism is a two-step process, where Spd and Spm are acetylated in their N1 positions by the highly-inducible SSAT (37–39). N1-Acetylated PAs are either excreted from the cell or oxidized by peroxisomal N1-acetylpolyamine oxidase (PAOX), resulting in H2O2, 3-acetoamidopropanal, and Put or Spd, depending on the starting substrate (40–43). Pharmacological superinduction of SSAT as a chemotherapeutic strategy has antitumor effects through depletion of the natural PAs needed for basic cell functions (44, 45).
Spm can also be directly oxidized by Spm oxidase (SMOX; PAOh1) to produce H2O2, 3-aminopropanal (3-AP), and Spd (46, 47). Localized in the cytoplasm and nucleus of mammalian cells (48, 49), SMOX is highly inducible by many of the same stimuli that induce SSAT (46, 50). Thus, the H2O2 produced by SMOX has greater potential for producing genetic damage than that produced by PAOX, which, when produced in a normally functioning peroxisome, is in the presence of catalase. As Spm can exist in millimolar concentrations within the cell (1), the release of H2O2 via this pathway in circumstances of up-regulated SMOX activity is sufficient to evoke oxidative stress, particularly in the form of oxidative DNA damage. Furthermore, the 3-AP generated by SMOX spontaneously converts into the highly reactive and toxic unsaturated aldehyde acrolein (51, 52). Notably, the S. cerevisiae amine oxidase Fms1 also directly oxidizes Spm to Spd and was first described by the Tabor lab (53).
Recent studies have suggested a role for SSAT in the p53-mediated ferroptotic response to ROS stress, an iron-dependent, nonapoptotic mode of cell death characterized by accumulation of lipid ROS at the cell membrane. Activation of p53 by DNA-damaging agents induces SAT1, a direct target of p53, resulting in the induction of arachidonate 15-lipoxygenase, lipid peroxidation, and cell death. This SAT1 induction ultimately sensitizes the cells to ferroptosis in the presence of ROS, manifesting as tumor suppression in xenograft models. Similarly, in embryonic fibroblasts from p53 WT or p53 acetylation-deficient mutant mice, which retain the ability to stimulate ferroptosis, Sat1 expression is induced by p53 activation, and Sat1 knockdown partially prevents ferroptotic cell death. The results of these studies propose a tumor-suppressive role for p53-mediated SSAT by promoting ferroptosis (54).
Extracellular polyamine oxidases
In the early 1950s, it was reported that the addition of Spm or Spd to certain culture conditions was growth inhibitory to mycobacteria (55, 56). These studies led to the discovery of the first soluble amine oxidases (57). Crude preparations of sheep serum amine oxidase allowed kinetic studies indicating oxidative deamination of Spm and Spd at rates greater than 10-fold that of other amines and concluded that this oxidative “activation” of the PAs was responsible for their antibacterial effects (58). Tabor and colleagues purified a soluble amine oxidase from bovine plasma, bovine serum amine oxidase (BSAO), that had substrate specificity for Spm and Spd and catalyzed the stoichiometric formation of their corresponding aldehydes, ammonia, and H2O2 (Fig. 2) (59, 60). Subsequent studies revealed that the reaction by-products of Spm or Spd with BSAO were highly toxic to E. coli, Staphylococcus aureus, bacteriophages, and mammalian spermatozoa and caused immotility in Trypanosoma equiperdum (61, 62).
Figure 2.
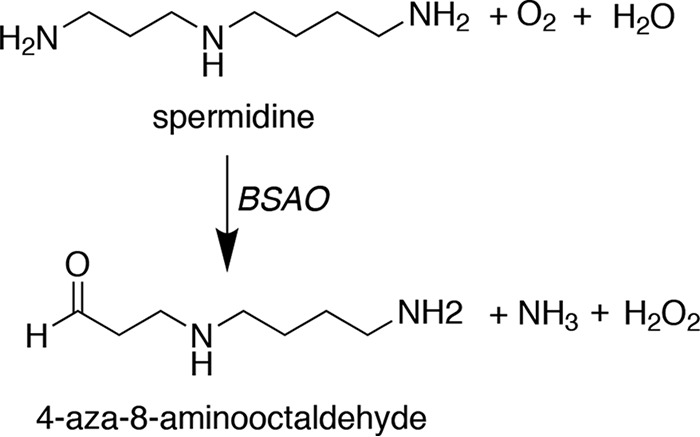
Extracellular polyamine oxidation. BSAO oxidizes the terminal aminopropyl nitrogens of spermine or spermidine (shown) to produce H2O2, ammonia, and the corresponding amino aldehydes.
Many studies have since concluded that adding Spd or Spm to mammalian cells in the presence of bovine serum results in extracellular oxidation of the PA and growth inhibition due to the oxidation products, not the exogenous PA (63–66). Studies testing the inhibitory effects of Spm, Spd, their aldehyde reaction products (which can convert to acrolein), and H2O2 in mammalian cell lines have indicated major roles for acrolein and H2O2 in the cytotoxic responses (51, 67–69), and in most systems, treatment with aldehyde dehydrogenase inhibitors and catalase together yielded protection from cytotoxicity (70, 71). Therefore, caution must be used when interpreting results involving PA treatment of cells in culture, particularly with regard to cellular processes involving ROS, such as autophagy. As virtually all of the mechanistic studies reported in mammalian cells regarding the role of PAs in autophagy were performed with high concentrations of PAs in medium containing bovine serum (72), the published interpretation of these studies is likely in error.
Early in vivo pharmacological studies in the Tabor lab provided evidence of Spm degradation to Spd following intraperitoneal injections of Spm in rabbits, mice, and rats, suggesting the presence of a BSAO-like enzyme in laboratory animals (61). In humans, extracellular oxidation of PAs and acetylpolyamines has been measured in plasma from patients suffering from cerebral stroke or chronic kidney disease (73, 74). Plasma amine oxidase activity in renal failure patients correlated with the severity of disease, reduction of Spm and Spd levels, and acrolein accumulation. Oxidation of Spm was inhibited in plasma of all patients examined using a common polyamine oxidase inhibitor; however, a copper-containing oxidase inhibitor, semicarbazide, inhibited Spm degradation in half of the patients. These data suggest the presence of a human extracellular, soluble, and semicarbazide-sensitive amine oxidase (SSAO) capable of oxidizing Spm (74).
Recent ocular research implicates vascular adhesion protein (VAP1/SSAO/AOC3)–mediated oxidation of Spm in the pathology of proliferative diabetic retinopathy (PDR) (75). Chronic inflammation and oxidative stress contribute to this pathology, and like BSAO, VAP1 oxidizes primary amines to generate H2O2, ammonia, and aldehydes capable of forming acrolein (76). Soluble VAP1 protein levels and acrolein adducts are increased in the vitreous fluid of patients with PDR, where PA levels are also elevated (77). This potential of VAP1 as an extracellular PA oxidase has implications for pathologies and/or treatment opportunities beyond the eye that warrant further evaluation and underscore the need for cautious experimental design and interpretation when considering the administration of natural PAs.
Physiological effects of polyamine-associated oxidative stress
Conditions that cause the release of free PAs, such as changes in the macromolecules to which PAs are bound, can stimulate PA catabolism through oxidation, resulting in the generation of ROS while lowering the abundance of free PAs available to serve in an antioxidant capacity (15). Consequently, elevated levels of free PAs are associated with a number of pathologies, including cancer, neurological disorders, stroke, and kidney dysfunction.
Infection and chronic inflammation-induced spermine oxidation
SMOX is induced by a variety of stimuli, including the inflammatory cytokines tumor necrosis factor-α, interleukin-1β, and interleukin-6 (78). As chronic inflammation contributes to the carcinogenic process through the generation of ROS, evidence from multiple models suggests that increased Spm oxidation serves as a molecular mechanism linking inflammatory stimuli to cancer initiation and/or progression through increased H2O2 generation and reduced Spm levels (Table 1) (79–82). The accumulation of genetic and epigenetic changes is a hallmark of cancer, and unrepaired DNA damage resulting from ROS exposure can cause mutations in driver genes that contribute to carcinogenesis. H2O2-induced DNA damage that occurs during chronic inflammation also contributes to epigenetic changes involving DNA methylation and histone modification patterns, which reduce or silence the expression of tumor suppressor genes (83). Thus, sub-lethal, chronically elevated SMOX increases the likelihood of mutagenic and epigenetic changes associated with cancer.
Table 1.
Cancers associated with induction of SMOX activity during chronic infection and/or inflammation
| Pathogen | SMOX-associated inflammatory condition | Precursor lesion | Associated carcinoma | Refs. |
|---|---|---|---|---|
| H. pylori | Gastritis/peptic ulcer | Intestinal metaplasia | Gastric | 84 |
| Enterotoxigenic B. fragilis | Colitis, inflammatory bowel disease | Left-sided tubular adenomas and low-grade dysplasias | Colorectal | 80, 92 |
| Undetermined | Prostatitis | Prostatic intraepithelial neoplasia | Prostate | 81 |
| Hepatitis C virus | Chronic hepatitis | Undetermined | Hepatocellular | 79, 93 |
Helicobacter pylori infection
H. pylori colonization of the stomach mucosa often persists for decades and causes chronic inflammation in the form of gastritis and peptic ulcers. In infected gastric epithelial cells, SMOX induction causes a chronic, low level of oxidative stress that has been directly linked to H2O2-dependent DNA damage without the induction of apoptosis or cell death (82, 84). SMOX expression is increased in gastric tissues from all stages of gastritis through carcinoma, relative to normal gastric mucosa, but is most highly expressed in the high-grade precursor lesion intestinal metaplasia (84). In H. pylori-positive gastritis patients living in geographically isolated high-risk versus low-risk regions of Colombia, SMOX was identified as the key factor influencing the progression to gastric cancer in high-risk regions (85). Further studies of these populations revealed differential expression of microRNA-124 that targets the 3′-UTR of SMOX and limits its translation. Analysis of DNA from the gastric mucosae of the Colombian patients revealed significantly higher levels of miR-124 gene methylation in those patients considered at high risk for progression to gastric cancer, consistent with the low expression levels of the mature miR-124 and unregulated production of SMOX activity in response to H. pylori infection (86). This uncontrolled production of ROS from SMOX combined with decreased levels of Spm for protection increases the likelihood of additional genetic and epigenetic changes in a potential feed-forward loop.
Enterotoxigenic Bacteroides fragilis infection
The induction of SMOX has also been observed following infection with Enterotoxigenic B. fragilis (ETBF), a colitis-inducing bacterium that is positively correlated with the development of colorectal cancer (CRC). Detection of the secreted virulence factor of ETBF, B. fragilis toxin, is an early marker for colon carcinogenesis that has been positively associated with early colonic neoplasms, particularly tubular adenomas and low-grade dysplasias biopsied from the left side of the colon (87). ETBF has been referred to as an “α-bug” or “driver” bacteria in CRC. While the toxin itself induces DNA damage, the host responds to infection with the production of ROS, cytokines, and chemokines, thereby producing an environment with an altered mucosal immune response and bacterial community that further potentiates oncogenesis (88–90). Using the multiple intestinal neoplasia mouse model of ETBF-induced colitis, a role for SMOX was identified in the accumulation of ROS-mediated DNA damage and subsequent development of colon carcinogenesis (80). Pharmacological inhibition of PA oxidation in ETBF-infected mice decreased intestinal inflammation, aberrant proliferation, and tumor number. In this same model, ROS resulting from ETBF infection caused recruitment of DNA-modifying enzymes to regions of DNA damage, resulting in epigenetic changes associated with aberrant tumor suppressor gene silencing (83).
More recently, levels of ETBF in paired biopsies of human primary CRCs and adjacent normal tissues were correlated with expression levels of PA metabolism genes. Both c-MYC and SMOX expression levels were increased in 80% of CRC tissues, with the greatest expression of SMOX observed in stage I and II cancers. Although the majority of patients were colonized with ETBF, the level of colonization was generally low, with the highest levels in earlier disease stages (92). These results are in line with those suggesting important roles for SMOX and ETBF in early stages of neoplasia and indicate that SMOX may remain elevated in the absence of ETBF.
Other inflammation-associated cancers
Elevated SMOX expression has also been documented in precancerous inflammatory conditions in the absence of infection. A tissue microarray of human prostate biopsies revealed the highest SMOX immunostaining in precursor prostatic intraepithelial neoplasia (PIN) lesions. This study also concluded that men who have developed PIN or prostate cancer have higher SMOX expression levels in benign prostate epithelium than men who have not had these lesions, suggesting increased SMOX as a risk factor for prostate carcinogenesis (81). Similarly, a recent study demonstrated increased SMOX expression in hepatic tissue from patients with chronic hepatitis. SMOX staining was further increased in hepatocellular carcinoma tissues and was positively correlated with poorer overall survival and relapse-free survival (93). Hepatitis is typically associated with hepatitis B or C virus infection and fibrosis, and recent in vitro studies have indicated that hepatitis C virus induces SMOX activity in hepatoma cells (79), suggesting a role for SMOX in hepatocellular carcinogenesis.
Immunomodulation through polyamine oxidation
In vitro studies have suggested that one way H. pylori infection alters the immune response is by highly inducing SMOX in macrophages, leading to their dysfunction and death and creating a permissive environment that allows for chronic infection (94). Recently, the effect of SMOX activity on immunomodulation was studied in mouse models of colitis due to pathogenic infection or epithelial injury-associated inflammation. SMOX is up-regulated in patients with ulcerative colitis and inflammatory bowel disease due to its high expression in infiltrating mononuclear cells, rather than colonic epithelial cells (95). In WT mice, infection with Citrobacter rodentium increased cytokine and chemokine levels and inflammatory cell infiltration in association with histological injury and mucosal hyperplasia, and these changes were diminished in SMOX knockout mice. Conversely, when colon inflammation resulted from administration of dextran sulfate sodium (DSS), SMOX knockout mice displayed increased histological damage and cytokine expression beyond that of DSS-treated WT mice, with more frequent colitis-associated mortality. These data suggest that in the context of infection, the regulation of intracellular polyamine levels by SMOX serves an immunomodulatory function, while in the setting of colitis associated with epithelial injury, SMOX-generated Spd may serve a protective role (96).
Ischemia/reperfusion injury
Ischemia reperfusion injury (IRI), physical trauma, and toxins induce PA catabolism through SSAT and SMOX in multiple organs, leading to tissue damage (73, 97–101). SSAT, in particular, plays a significant role in promoting kidney and liver damage in IRI (102), and conditional knockout of SSAT in proximal tubule epithelial cells, where the primary effects of IRI manifest, decreases renal damage severity via reductions in both PAOX and SMOX activities. Increasing SSAT expression in kidney cells caused increased mitochondrial damage, stimulating apoptosis and suggesting PA oxidation as a source of oxidative stress (103). Knockout of SSAT in mice or pharmacological inhibition of PA oxidation during IRI demonstrated that PA catabolism contributes to activation of the innate immune response, increasing inflammation and apoptosis at the site of injury. It therefore appears that PA catabolism functions in the initial injury as well as furthering damage via immunomodulation (103).
Snyder-Robinson syndrome
Snyder-Robinson syndrome (SRS), an X-linked mental retardation syndrome resulting from loss-of-function mutations in the Spm synthase gene, biochemically results in accumulation of high intracellular Spd levels and a near-complete lack of Spm (104). A recently developed Drosophila model of SRS suggests a role for increased Spd catabolism and the toxic metabolites it produces in establishing oxidative stress and lysosomal defects in the Drosophila nervous system, resulting in altered mitochondrial function and impaired autophagy that are also observed in affected SRS patient cells. ROS were highly elevated in the brains of mutant flies, and antioxidant therapies partially restored mitochondrial function, suggesting that increasing antioxidant capacity may be beneficial for SRS patients (105).
Therapeutic opportunities
Inhibition of polyamine oxidation
SMOX shares significant homology with PAOX as well as the histone-modifying enzyme lysine-specific demethylase-1 (106). As the crystallization of SMOX has eluded many attempts, the development of a specific inhibitor for SMOX alone has been challenging. However, studies with existing inhibitors and genetic manipulation of SMOX indicate it is an attractive therapeutic target for multiple pathologies. Most notably, the apparent role for SMOX induction in the development of epithelial cancers suggests the inhibition of SMOX as a potential target for prevention in individuals at risk for carcinogenesis, particularly in association with infection and chronic inflammation.
Of relevance to chemotherapy, a limitation of the common chemotherapeutic agent cisplatin is acute kidney injury (AKI). In mice with cisplatin-induced AKI, SSAT and SMOX levels increase, stimulating endoplasmic reticulum stress response genes that culminate in apoptosis and kidney damage (107). Knockout of SSAT or SMOX or neutralization of the by-products of PA oxidation reduces the severity of damage, implicating a role for PA catabolism in AKI. Thus, incorporation of an inhibitor of PA oxidation into a cisplatin treatment regimen could prevent kidney injury that might otherwise limit treatment. Furthermore, as organ damage due to insults such as ischemia/reperfusion, toxins, and trauma appears to have a PA catabolic regulatory component, suppression of these components is a promising strategy for the protection of tissues in a variety of contexts.
During stroke, PA catabolism is induced due to the release of free PAs from damaged RNA (73, 108), and the extent of this induction was recently correlated with aging (109). Acrolein, spontaneously formed from SMOX-generated 3-AP, is the metabolite most responsible for neuronal damage (110). Masuko et al. (111) designed inhibitors of the PA oxidases and investigated their effects on brain infarct sizes in a photochemically-induced thrombosis model. Inhibitor C9-4 (N1-nonyl-1,4-diaminobutane) most potently decreased brain infarct volume with a therapeutic window longer than 12 h and is thus a potential drug candidate for the treatment of brain ischemia.
Therapeutic induction of polyamine oxidation
In certain circumstances, the production of potentially harmful oxidative stress may have therapeutic potential. A major intracellular source of ROS is metabolic activity, and rapidly proliferating cells require a compensatory increase in metabolism. Therefore, populations such as cancer cells or pathogenic microorganisms may have increased sensitivity to treatments that produce additional oxidants.
BSAO as a mediator of polyamines and oxidative stress
The ability of BSAO to convert PAs into toxic aldehydes and H2O2, thus producing oxidative stress, has suggested its potential use in a therapeutic setting due to the abundance of PAs in proliferating cells, including tumor cells. Immobilized BSAO injected directly into B16 melanoma tumor xenografts in mice decreased tumor growth by ∼70% through the induction of apoptosis (112). Experiments in multidrug-resistant tumor cell lines have indicated their increased sensitivity to the oxidation products of BSAO and Spm (113–116). Recent advances in drug delivery technology have led to the incorporation of BSAO into nanoparticle formulations, thereby enhancing the stability of its catalytic activity and improving its potential as an in situ treatment strategy to reduce intratumoral PA levels while generating tumor-toxic oxidative stress (117–119).
Induction of SMOX by polyamine analogues
The use of PA analogues to exploit the self-regulatory nature of PA metabolism is a well-studied strategy for inhibiting growth of cancer cells. Members of the bis(ethyl) group of analogues strongly induce PA catabolism through large inductions of both SSAT and SMOX. In certain cancer cell types, cytotoxicity from these analogues is attributed to the production of H2O2 via SMOX simultaneously with depletion of the natural PAs (50, 121, 122). Curcumin, a natural polyphenol and popular dietary supplement, was recently shown to increase uptake of these PA analogues, resulting in enhanced polyamine depletion and growth inhibition and allowing a substantial reduction in the required effective analogue dose (123). As curcumin possesses multiple antitumor properties, this combination with a PA analogue would target multiple anticancer pathways (124). Finally, two PA analogues that have been studied clinically as single agents, bis(ethyl)norspermine and PG-11047 (45), have been incorporated into self-immolative nanoparticles capable of packaging and delivering therapeutic nucleic acids in addition to the PA analogue (91, 125). These prodrugs may allow for controlled drug release as well as the ability to simultaneously target additional antitumor pathways via specific cargo. These parent compounds and their prodrug derivatives were recently also shown to have an antiviral effect on Zika virus replication through the induction of SSAT and SMOX (120).
Conclusion
The multiple mechanisms through which PAs and their catabolism can affect oxidative homeostasis in organisms, including humans, allow for a wide array of possible outcomes in both normal and pathological states. Although a prime function of the PA catabolic pathways may be to maintain PA homeostasis at a set point, the potential to exploit the pathway for therapeutic benefits is great. This is especially true for the oxidation of Spm by SMOX in response to infection and inflammation. All data currently point to this enzyme as a rational target for chemoprevention strategies. Additionally, the targeted, tumor-specific super-induction of PA catabolism by specific PA analogues continues to hold promise for future anticancer therapies. Finally, in addition to PA catabolism being a homeostatic mechanism, there are other possibilities that should be explored. For instance, PA catabolism might provide important signaling molecules, like H2O2, at levels that are not injurious. Similarly, the recent linkage between PA catabolism and immune modulation opens new avenues for investigation and possible treatments. Although the catabolism of PAs has long been studied, with the Tabor laboratory leading the way in many aspects, many avenues remain to be explored.
This work was supported by National Institutes of Health Grant 1 R01 CA204345, the Maryland Cigarette Restitution Fund, the Samuel Waxman Cancer Research Foundation, and the Million Dollar Bike Ride (to the Casero laboratory) and by National Institutes of Health Grant 1 R01 CA204345 and the Doris Duke Charitable Foundation (to the Woster laboratory). This article is part of a series on “Polyamines,” written in honor of Dr. Herbert Tabor's 100th birthday. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- PA
- polyamine
- Put
- putrescine
- Spd
- spermidine
- Spm
- spermine
- ROS
- reactive oxygen species
- H2O2
- hydrogen peroxide
- SOD
- superoxide dismutase
- Gsp
- glutathionylspermidine
- GspSA
- glutathionylspermidine synthetase/amidase
- SSAT
- spermidine/spermine N1-acetyltransferase
- PAOX
- peroxisomal N1-acetylpolyamine oxidase
- SMOX
- spermine oxidase
- 3-AP
- 3-aminopropanal
- BSAO
- bovine serum amine oxidase
- SSAO
- semicarbazide-sensitive amine oxidase
- PDR
- progressive diabetic retinopathy
- ETBF
- enterotoxigenic Bacteroides fragilis
- CRC
- colorectal carcinoma
- PIN
- prostatic intraepithelial neoplasia
- DSS
- dextran sulfate sodium
- IRI
- ischemia/reperfusion injury
- SRS
- Snyder-Robinson syndrome
- AKI
- acute kidney injury.
References
- 1. Pegg A. E., and McCann P. P. (1982) Polyamine metabolism and function. Am. J. Physiol. 243, C212–C221 10.1152/ajpcell.1982.243.5.C212 [DOI] [PubMed] [Google Scholar]
- 2. Tabor C. W., and Tabor H. (1985) Polyamines in microorganisms. Microbiol. Rev. 49, 81–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pegg A. E. (2009) Mammalian polyamine metabolism and function. IUBMB Life 61, 880–894 10.1002/iub.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park M. H., Nishimura K., Zanelli C. F., and Valentini S. R. (2010) Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 38, 491–500 10.1007/s00726-009-0408-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Igarashi K., and Kashiwagi K. (2010) Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 42, 39–51 10.1016/j.biocel.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 6. Pasini A., Caldarera C. M., and Giordano E. (2014) Chromatin remodeling by polyamines and polyamine analogs. Amino Acids 46, 595–603 10.1007/s00726-013-1550-9 [DOI] [PubMed] [Google Scholar]
- 7. Hanahan D., and Weinberg R. A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 8. López-Otín C., Blasco M. A., Partridge L., Serrano M., and Kroemer G. (2013) The hallmarks of aging. Cell 153, 1194–1217 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan A. U., Mei Y. H., and Wilson T. (1992) A proposed function for spermine and spermidine: protection of replicating DNA against damage by singlet oxygen. Proc. Natl. Acad. Sci. U.S.A. 89, 11426–11427 10.1073/pnas.89.23.11426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ha H. C., Sirisoma N. S., Kuppusamy P., Zweier J. L., Woster P. M., and Casero R. A. Jr. (1998) The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. U.S.A. 95, 11140–11145 10.1073/pnas.95.19.11140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Das K. C., and Misra H. P. (2004) Hydroxyl radical scavenging and singlet oxygen quenching properties of polyamines. Mol. Cell. Biochem. 262, 127–133 10.1023/B:MCBI.0000038227.91813.79 [DOI] [PubMed] [Google Scholar]
- 12. Fujisawa S., and Kadoma Y. (2005) Kinetic evaluation of polyamines as radical scavengers. Anticancer Res. 25, 965–969 [PubMed] [Google Scholar]
- 13. Ha H. C., Yager J. D., Woster P. A., and Casero R. A. Jr. (1998) Structural specificity of polyamines and polyamine analogues in the protection of DNA from strand breaks induced by reactive oxygen species. Biochem. Biophys. Res. Commun. 244, 298–303 10.1006/bbrc.1998.8258 [DOI] [PubMed] [Google Scholar]
- 14. Khan A. U., Di Mascio P., Medeiros M. H., and Wilson T. (1992) Spermine and spermidine protection of plasmid DNA against single-strand breaks induced by singlet oxygen. Proc. Natl. Acad. Sci. U.S.A. 89, 11428–11430 10.1073/pnas.89.23.11428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pegg A. E. (2013) Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 26, 1782–1800 10.1021/tx400316s [DOI] [PubMed] [Google Scholar]
- 16. Minton K. W., Tabor H., and Tabor C. W. (1990) Paraquat toxicity is increased in Escherichia coli defective in the synthesis of polyamines. Proc. Natl. Acad. Sci. U.S.A. 87, 2851–2855 10.1073/pnas.87.7.2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chattopadhyay M. K., Tabor C. W., and Tabor H. (2003) Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc. Natl. Acad. Sci. U.S.A. 100, 2261–2265 10.1073/pnas.2627990100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Igarashi K., and Kashiwagi K. (2006) Polyamine modulon in Escherichia coli: genes involved in the stimulation of cell growth by polyamines. J. Biochem. 139, 11–16 10.1093/jb/mvj020 [DOI] [PubMed] [Google Scholar]
- 19. Sakamoto A., Terui Y., Yoshida T., Yamamoto T., Suzuki H., Yamamoto K., Ishihama A., Igarashi K., and Kashiwagi K. (2015) Three members of polyamine modulon under oxidative stress conditions: two transcription factors (SoxR and EmrR) and a glutathione synthetic enzyme (GshA). PLoS ONE 10, e0124883 10.1371/journal.pone.0124883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jung I. L., and Kim I. G. (2003) Transcription of ahpC, katG, and katE genes in Escherichia coli is regulated by polyamines: polyamine-deficient mutant sensitive to H2O2-induced oxidative damage. Biochem. Biophys. Res. Commun. 301, 915–922 10.1016/S0006-291X(03)00064-0 [DOI] [PubMed] [Google Scholar]
- 21. Balasundaram D., Tabor C. W., and Tabor H. (1993) Oxygen toxicity in a polyamine-depleted spe2 delta mutant of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 90, 4693–4697 10.1073/pnas.90.10.4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chattopadhyay M. K., Tabor C. W., and Tabor H. (2006) Polyamine deficiency leads to accumulation of reactive oxygen species in a spe2Δ mutant of Saccharomyces cerevisiae. Yeast 23, 751–761 10.1002/yea.1393 [DOI] [PubMed] [Google Scholar]
- 23. Chattopadhyay M. K., Chen W., Poy G., Cam M., Stiles D., and Tabor H. (2009) Microarray studies on the genes responsive to the addition of spermidine or spermine to a Saccharomyces cerevisiae spermidine synthase mutant. Yeast 26, 531–544 10.1002/yea.1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krüger A., Vowinckel J., Mülleder M., Grote P., Capuano F., Bluemlein K., and Ralser M. (2013) Tpo1-mediated spermine and spermidine export controls cell cycle delay and times antioxidant protein expression during the oxidative stress response. EMBO Rep. 14, 1113–1119 10.1038/embor.2013.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tabor C. W., and Tabor H. (1974) Glutathionylspermidine in Escherichia coli. Fed. Proc. 33, 1567–1567 [Google Scholar]
- 26. Tabor H., and Tabor C. W. (1975) Isolation, characterization, and turnover of glutathionylspermidine from Escherichia coli. J. Biol. Chem. 250, 2648–2654 [PubMed] [Google Scholar]
- 27. Bollinger J. M. Jr., Kwon D. S., Huisman G. W., Kolter R., and Walsh C. T. (1995) Glutathionylspermidine metabolism in Escherichia coli. Purification, cloning, overproduction, and characterization of a bifunctional glutathionylspermidine synthetase/amidase. J. Biol. Chem. 270, 14031–14041 10.1074/jbc.270.23.14031 [DOI] [PubMed] [Google Scholar]
- 28. Chiang B. Y., Chen T. C., Pai C. H., Chou C. C., Chen H. H., Ko T. P., Hsu W. H., Chang C. Y., Wu W. F., Wang A. H., and Lin C. H. (2010) Protein S-thiolation by glutathionylspermidine (Gsp): the role of Escherichia coli Gsp synthetASE/amidase in redox regulation. J. Biol. Chem. 285, 25345–25353 10.1074/jbc.M110.133363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fairlamb A. H., Blackburn P., Ulrich P., Chait B. T., and Cerami A. (1985) Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science 227, 1485–1487 10.1126/science.3883489 [DOI] [PubMed] [Google Scholar]
- 30. Schmidt A., and Krauth-Siegel R. L. (2002) Enzymes of the trypanothione metabolism as targets for antitrypanosomal drug development. Curr. Top. Med. Chem. 2, 1239–1259 10.2174/1568026023393048 [DOI] [PubMed] [Google Scholar]
- 31. Hernández S. M., Sánchez M. S., and de Tarlovsky M. N. (2006) Polyamines as a defense mechanism against lipoperoxidation in Trypanosoma cruzi. Acta Trop. 98, 94–102 10.1016/j.actatropica.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 32. Mackintosh C. A., and Pegg A. E. (2000) Effect of spermine synthase deficiency on polyamine biosynthesis and content in mice and embryonic fibroblasts, and the sensitivity of fibroblasts to 1,3-bis-(2-chloroethyl)-N-nitrosourea. Biochem. J. 351, 439–447 10.1042/bj3510439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rider J. E., Hacker A., Mackintosh C. A., Pegg A. E., Woster P. M., and Casero R. A. Jr. (2007) Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 33, 231–240 10.1007/s00726-007-0513-4 [DOI] [PubMed] [Google Scholar]
- 34. Smirnova O. A., Isaguliants M. G., Hyvonen M. T., Keinanen T. A., Tunitskaya V. L., Vepsalainen J., Alhonen L., Kochetkov S. N., and Ivanov A. V. (2012) Chemically induced oxidative stress increases polyamine levels by activating the transcription of ornithine decarboxylase and spermidine/spermine-N1-acetyltransferase in human hepatoma HUH7 cells. Biochimie 94, 1876–1883 10.1016/j.biochi.2012.04.023 [DOI] [PubMed] [Google Scholar]
- 35. Chopra S., and Wallace H. M. (1998) Induction of spermidine/spermine N1-acetyltransferase in human cancer cells in response to increased production of reactive oxygen species. Biochem. Pharmacol. 55, 1119–1123 10.1016/S0006-2952(97)00601-1 [DOI] [PubMed] [Google Scholar]
- 36. Wang Y., Xiao L., Thiagalingam A., Nelkin B. D., and Casero R. A. Jr. (1998) The identification of a cis-element and a trans-acting factor involved in the response to polyamines and polyamine analogues in the regulation of the human spermidine/spermine N1-acetyltransferase gene transcription. J. Biol. Chem. 273, 34623–34630 10.1074/jbc.273.51.34623 [DOI] [PubMed] [Google Scholar]
- 37. Casero R. A. Jr., and Pegg A. E. (1993) Spermidine/spermine N1-acetyltransferase–the turning point in polyamine metabolism. FASEB J. 7, 653–661 10.1096/fasebj.7.8.8500690 [DOI] [PubMed] [Google Scholar]
- 38. Pegg A. E. (2008) Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 294, E995–E1010 10.1152/ajpendo.90217.2008 [DOI] [PubMed] [Google Scholar]
- 39. Matsui I., Wiegand L., and Pegg A. E. (1981) Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J. Biol. Chem. 256, 2454–2459 [PubMed] [Google Scholar]
- 40. Vujcic S., Liang P., Diegelman P., Kramer D. L., and Porter C. W. (2003) Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem. J. 370, 19–28 10.1042/bj20021779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hölttä E. (1977) Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry 16, 91–100 10.1021/bi00620a015 [DOI] [PubMed] [Google Scholar]
- 42. Wu T., Yankovskaya V., and McIntire W. S. (2003) Cloning, sequencing, and heterologous expression of the murine peroxisomal flavoprotein, N1-acetylated polyamine oxidase. J. Biol. Chem. 278, 20514–20525 10.1074/jbc.M302149200 [DOI] [PubMed] [Google Scholar]
- 43. Wang Y., Hacker A., Murray-Stewart T., Frydman B., Valasinas A., Fraser A. V., Woster P. M., and Casero R. A. Jr. (2005) Properties of recombinant human N1-acetylpolyamine oxidase (hPAO): potential role in determining drug sensitivity. Cancer Chemother. Pharmacol. 56, 83–90 10.1007/s00280-004-0936-5 [DOI] [PubMed] [Google Scholar]
- 44. Casero R. A. Jr., Celano P., Ervin S. J., Wiest L., and Pegg A. E. (1990) High specific induction of spermidine/spermine N1-acetyltransferase in a human large cell lung carcinoma. Biochem. J. 270, 615–620 10.1042/bj2700615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murray-Stewart T. R., Woster P. M., and Casero R. A. Jr. (2016) Targeting polyamine metabolism for cancer therapy and prevention. Biochem. J. 473, 2937–2953 10.1042/BCJ20160383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y., Devereux W., Woster P. M., Stewart T. M., Hacker A., and Casero R. A. Jr. (2001) Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 61, 5370–5373 [PubMed] [Google Scholar]
- 47. Vujcic S., Diegelman P., Bacchi C. J., Kramer D. L., and Porter C. W. (2002) Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem. J. 367, 665–675 10.1042/bj20020720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murray-Stewart T., Wang Y., Goodwin A., Hacker A., Meeker A., and Casero R. A. Jr. (2008) Nuclear localization of human spermine oxidase isoforms–possible implications in drug response and disease etiology. FEBS J. 275, 2795–2806 10.1111/j.1742-4658.2008.06419.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cervelli M., Bellini A., Bianchi M., Marcocci L., Nocera S., Polticelli F., Federico R., Amendola R., and Mariottini P. (2004) Mouse spermine oxidase gene splice variants. Nuclear subcellular localization of a novel active isoform. Eur. J. Biochem. 271, 760–770 10.1111/j.1432-1033.2004.03979.x [DOI] [PubMed] [Google Scholar]
- 50. Devereux W., Wang Y., Stewart T. M., Hacker A., Smith R., Frydman B., Valasinas A. L., Reddy V. K., Marton L. J., Ward T. D., Woster P. M., and Casero R. A. (2003) Induction of the PAOh1/SMO polyamine oxidase by polyamine analogues in human lung carcinoma cells. Cancer Chemother. Pharmacol. 52, 383–390 10.1007/s00280-003-0662-4 [DOI] [PubMed] [Google Scholar]
- 51. Sharmin S., Sakata K., Kashiwagi K., Ueda S., Iwasaki S., Shirahata A., and Igarashi K. (2001) Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem. Biophys. Res. Commun. 282, 228–235 10.1006/bbrc.2001.4569 [DOI] [PubMed] [Google Scholar]
- 52. Houen G., Bock K., and Jensen A. L. (1994) HPLC and NMR investigation of the serum amine oxidase catalyzed oxidation of polyamines. Acta Chem. Scand. 48, 52–60 10.3891/acta.chem.scand.48-0052 [DOI] [PubMed] [Google Scholar]
- 53. Chattopadhyay M. K., Tabor C. W., and Tabor H. (2003) Spermidine but not spermine is essential for hypusine biosynthesis and growth in Saccharomyces cerevisiae: spermine is converted to spermidine in vivo by the FMS1-amine oxidase. Proc. Natl. Acad. Sci. U.S.A. 100, 13869–13874 10.1073/pnas.1835918100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ou Y., Wang S. J., Li D., Chu B., and Gu W. (2016) Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. U.S.A. 113, E6806–E6812 10.1073/pnas.1607152113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hirsch J. G. (1953) The antimycobacterial activity of various amines related to spermine in chemical structure. J. Exp. Med. 97, 323–325 10.1084/jem.97.3.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hirsch J. G., and Dubos R. J. (1952) The effect of spermine on tubercle bacilli. J. Exp. Med. 95, 191–208 10.1084/jem.95.2.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hirsch J. G. (1953) The essential participation of an enzyme in the inhibition of growth of tubercle bacilli by spermine. J. Exp. Med. 97, 327–343 10.1084/jem.97.3.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hirsch J. G. (1953) Spermine oxidase: an amine oxidase with specificity for spermine and spermidine. J. Exp. Med. 97, 345–355 10.1084/jem.97.3.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tabor C. W., Tabor H., and Rosenthal S. M. (1954) Purification of amine oxidase from beef plasma. J. Biol. Chem. 208, 645–661 [PubMed] [Google Scholar]
- 60. Tabor C. W., Tabor H., and Bachrach U. (1964) Identification of the aminoaldehydes produced by the oxidation of spermine and spermidine with purified plasma amine oxidase. J. Biol. Chem. 239, 2194–2203 [PubMed] [Google Scholar]
- 61. Tabor C. W., and Rosenthal S. M. (1956) Pharmacology of spermine and spermidine; some effects on animals and bacteria. J. Pharmacol. Exp. Ther. 116, 139–155 [PubMed] [Google Scholar]
- 62. Bachrach U., Tabor C. W., and Tabor H. (1963) Inactivation of bacteriophages by oxidized spermine. Biochim. Biophys. Acta 78, 768–770 10.1016/0006-3002(63)91055-2 [DOI] [PubMed] [Google Scholar]
- 63. Alarcon R. A., Foley G. E., and Modest E. J. (1961) Effects of spermine on mammalian cells. Arch. Biochem. Biophys. 94, 540–541 10.1016/0003-9861(61)90083-2 [DOI] [PubMed] [Google Scholar]
- 64. Higgins M. L., Tillman M. C., Rupp J. P., and Leach F. R. (1969) The effect of polyamines on cell culture cells. J. Cell. Physiol. 74, 149–154 10.1002/jcp.1040740206 [DOI] [PubMed] [Google Scholar]
- 65. Bachrach U. (1970) Oxidized polyamines. Ann. N.Y. Acad. Sci. 171, 939 10.1111/j.1749-6632.1970.tb39400.x [DOI] [Google Scholar]
- 66. Gaugas J. M., and Dewey D. L. (1979) Evidence for serum binding of oxidized spermine and its potent G1-phase inhibition of cell-proliferation. Br. J. Cancer 39, 548–557 10.1038/bjc.1979.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Averill-Bates D. A., Agostinelli E., Przybytkowski E., Mateescu M. A., and Mondovi B. (1993) Cytotoxicity and kinetic analysis of purified bovine serum amine oxidase in the presence of spermine in Chinese hamster ovary cells. Arch. Biochem. Biophys. 300, 75–79 10.1006/abbi.1993.1011 [DOI] [PubMed] [Google Scholar]
- 68. Agostinelli E., Przybytkowski E., and Averill-Bates D. A. (1996) Glucose, glutathione, and cellular response to spermine oxidation products. Free Radic. Biol. Med. 20, 649–656 10.1016/0891-5849(95)02149-3 [DOI] [PubMed] [Google Scholar]
- 69. Wang L., Liu Y., Qi C., Shen L., Wang J., Liu X., Zhang N., Bing T., and Shangguan D. (2018) Oxidative degradation of polyamines by serum supplement causes cytotoxicity on cultured cells. Sci. Rep. 8, 10384 10.1038/s41598-018-28648-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Averill-Bates D. A., Agostinelli E., Przybytkowski E., and Mondovi B. (1994) Aldehyde dehydrogenase and cytotoxicity of purified bovine serum amine oxidase and spermine in Chinese hamster ovary cells. Biochem. Cell Biol. 72, 36–42 10.1139/o94-006 [DOI] [PubMed] [Google Scholar]
- 71. Averill-Bates D. A., Ke Q., Tanel A., Roy J., Fortier G., and Agostinelli E. (2008) Mechanism of cell death induced by spermine and amine oxidase in mouse melanoma cells. Int. J. Oncol 32, 79–88 [PubMed] [Google Scholar]
- 72. Madeo F., Eisenberg T., Pietrocola F., and Kroemer G. (2018) Spermidine in health and disease. Science 359, eaan2788 10.1126/science.aan2788 [DOI] [PubMed] [Google Scholar]
- 73. Tomitori H., Usui T., Saeki N., Ueda S., Kase H., Nishimura K., Kashiwagi K., and Igarashi K. (2005) Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke 36, 2609–2613 10.1161/01.STR.0000190004.36793.2d [DOI] [PubMed] [Google Scholar]
- 74. Sakata K., Kashiwagi K., Sharmin S., Ueda S., Irie Y., Murotani N., and Igarashi K. (2003) Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem. Biophys. Res. Commun. 305, 143–149 10.1016/S0006-291X(03)00716-2 [DOI] [PubMed] [Google Scholar]
- 75. Murata M., Noda K., Kawasaki A., Yoshida S., Dong Y., Saito M., Dong Z., Ando R., Mori S., Saito W., Kanda A., and Ishida S. (2017) Soluble vascular adhesion protein-1 mediates spermine oxidation as semicarbazide-sensitive amine oxidase: possible role in proliferative diabetic retinopathy. Curr. Eye Res. 42, 1674–1683 10.1080/02713683.2017.1359847 [DOI] [PubMed] [Google Scholar]
- 76. Smith D. J., Salmi M., Bono P., Hellman J., Leu T., and Jalkanen S. (1998) Cloning of vascular adhesion protein 1 reveals a novel multifunctional adhesion molecule. J. Exp. Med. 188, 17–27 10.1084/jem.188.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nicoletti R., Venza I., Ceci G., Visalli M., Teti D., and Reibaldi A. (2003) Vitreous polyamines spermidine, putrescine, and spermine in human proliferative disorders of the retina. Br. J. Ophthalmol. 87, 1038–1042 10.1136/bjo.87.8.1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Babbar N., and Casero R. A. Jr. (2006) Tumor necrosis factor-α increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res. 66, 11125–11130 10.1158/0008-5472.CAN-06-3174 [DOI] [PubMed] [Google Scholar]
- 79. Smirnova O. A., Keinanen T. A., Ivanova O. N., Hyvonen M. T., Khomutov A. R., Kochetkov S. N., Bartosch B., and Ivanov A. V. (2017) Hepatitis C virus alters metabolism of biogenic polyamines by affecting expression of key enzymes of their metabolism. Biochem. Biophys. Res. Commun. 483, 904–909 10.1016/j.bbrc.2017.01.032 [DOI] [PubMed] [Google Scholar]
- 80. Goodwin A. C., Destefano Shields C. E., Wu S., Huso D. L., Wu X., Murray-Stewart T. R., Hacker-Prietz A., Rabizadeh S., Woster P. M., Sears C. L., and Casero R. A. Jr. (2011) Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 108, 15354–15359 10.1073/pnas.1010203108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Goodwin A. C., Jadallah S., Toubaji A., Lecksell K., Hicks J. L., Kowalski J., Bova G. S., De Marzo A. M., Netto G. J., and Casero R. A. Jr. (2008) Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate 68, 766–772 10.1002/pros.20735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chaturvedi R., Asim M., Romero-Gallo J., Barry D. P., Hoge S., de Sablet T., Delgado A. G., Wroblewski L. E., Piazuelo M. B., Yan F., Israel D. A., Casero R. A. Jr., Correa P., Gobert A. P., Polk D. B., et al. (2011) Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 141, 1696-1708.e1-2 10.1053/j.gastro.2011.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. O'Hagan H. M., Wang W., Sen S., Destefano Shields C., Lee S. S., Zhang Y. W., Clements E. G., Cai Y., Van Neste L., Easwaran H., Casero R. A., Sears C. L., and Baylin S. B. (2011) Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell 20, 606–619 10.1016/j.ccr.2011.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chaturvedi R., Asim M., Piazuelo M. B., Yan F., Barry D. P., Sierra J. C., Delgado A. G., Hill S., Casero R. A. Jr., Bravo L. E., Dominguez R. L., Correa P., Polk D. B., Washington M. K., Rose K. L., et al. (2014) Activation of EGFR and ERBB2 by Helicobacter pylori results in survival of gastric epithelial cells with DNA damage. Gastroenterology 146, 1739–1751.e14 10.1053/j.gastro.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chaturvedi R., de Sablet T., Asim M., Piazuelo M. B., Barry D. P., Verriere T. G., Sierra J. C., Hardbower D. M., Delgado A. G., Schneider B. G., Israel D. A., Romero-Gallo J., Nagy T. A., Morgan D. R., Murray-Stewart T., et al. (2015) Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene 34, 3429–3440 10.1038/onc.2014.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Murray-Stewart T., Sierra J. C., Piazuelo M. B., Mera R. M., Chaturvedi R., Bravo L. E., Correa P., Schneider B. G., Wilson K. T., and Casero R. A. (2016) Epigenetic silencing of miR-124 prevents spermine oxidase regulation: implications for Helicobacter pylori-induced gastric cancer. Oncogene 35, 5480–5488 10.1038/onc.2016.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Purcell R. V., Pearson J., Aitchison A., Dixon L., Frizelle F. A., and Keenan J. I. (2017) Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS ONE 12, e0171602 10.1371/journal.pone.0171602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sears C. L., and Pardoll D. M. (2011) Perspective: α-bugs, their microbial partners, and the link to colon cancer. J. Infect. Dis. 203, 306–311 10.1093/jinfdis/jiq061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tjalsma H., Boleij A., Marchesi J. R., and Dutilh B. E. (2012) A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat. Rev. Microbiol. 10, 575–582 10.1038/nrmicro2819 [DOI] [PubMed] [Google Scholar]
- 90. Sears C. L., Geis A. L., and Housseau F. (2014) Bacteroides fragilis subverts mucosal biology: from symbiont to colon carcinogenesis. J. Clin. Invest. 124, 4166–4172 10.1172/JCI72334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xie Y., Murray-Stewart T., Wang Y., Yu F., Li J., Marton L. J., Casero R. A. Jr., and Oupický D. (2017) Self-immolative nanoparticles for simultaneous delivery of microRNA and targeting of polyamine metabolism in combination cancer therapy. J. Control Release 246, 110–119 10.1016/j.jconrel.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Snezhkina A. V., Krasnov G. S., Lipatova A. V., Sadritdinova A. F., Kardymon O. L., Fedorova M. S., Melnikova N. V., Stepanov O. A., Zaretsky A. R., Kaprin A. D., Alekseev B. Y., Dmitriev A. A., and Kudryavtseva A. V. (2016) The dysregulation of polyamine metabolism in colorectal cancer is associated with overexpression of c-Myc and C/EBPβ rather than enterotoxigenic Bacteroides fragilis infection. Oxid. Med. Cell. Longev. 2016, 2353560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hu T., Sun D., Zhang J., Xue R., Janssen H. L. A., Tang W., and Dong L. (2018) Spermine oxidase is upregulated and promotes tumor growth in hepatocellular carcinoma. Hepatol. Res. 10.1111/hepr.13206 [DOI] [PubMed] [Google Scholar]
- 94. Bussière F. I., Chaturvedi R., Cheng Y., Gobert A. P., Asim M., Blumberg D. R., Xu H., Kim P. Y., Hacker A., Casero R. A. Jr., and Wilson K. T. (2005) Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric-oxide synthase translation. J. Biol. Chem. 280, 2409–2412 10.1074/jbc.C400498200 [DOI] [PubMed] [Google Scholar]
- 95. Hong S. K., Chaturvedi R., Piazuelo M. B., Coburn L. A., Williams C. S., Delgado A. G., Casero R. A. Jr, Schwartz D. A., and Wilson K. T. (2010) Increased expression and cellular localization of spermine oxidase in ulcerative colitis and relationship to disease activity. Inflamm. Bowel Dis. 16, 1557–1566 10.1002/ibd.21224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gobert A. P., Al-Greene N. T., Singh K., Coburn L. A., Sierra J. C., Verriere T. G., Luis P. B., Schneider C., Asim M., Allaman M. M., Barry D. P., Cleveland J. L., Destefano Shields C. E., Casero R. A. Jr., et al. (2018) Distinct immunomodulatory effects of spermine oxidase in colitis induced by epithelial injury or infection. Front. Immunol. 9, 1242 10.3389/fimmu.2018.01242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nagesh Babu G., Sailor K. A., Sun D., and Dempsey R. J. (2001) Spermidine/spermine N1-acetyl transferase activity in rat brain following transient focal cerebral ischemia and reperfusion. Neurosci. Lett. 300, 17–20 10.1016/S0304-3940(01)01538-5 [DOI] [PubMed] [Google Scholar]
- 98. Zahedi K., Bissler J. J., Wang Z., Josyula A., Lu L., Diegelman P., Kisiel N., Porter C. W., and Soleimani M. (2007) Spermidine/spermine N1-acetyltransferase overexpression in kidney epithelial cells disrupts polyamine homeostasis, leads to DNA damage, and causes G2 arrest. Am. J. Physiol. Cell Physiol. 292, C1204–C1215 10.1152/ajpcell.00451.2006 [DOI] [PubMed] [Google Scholar]
- 99. Zahedi K., Barone S., Kramer D. L., Amlal H., Alhonen L., Jänne J., Porter C. W., and Soleimani M. (2010) The role of spermidine/spermine N1-acetyltransferase in endotoxin-induced acute kidney injury. Am. J. Physiol. Cell Physiol. 299, C164–C174 10.1152/ajpcell.00512.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zahedi K., Barone S. L., Xu J., Steinbergs N., Schuster R., Lentsch A. B., Amlal H., Wang J., Casero R. A. Jr., and Soleimani M. (2012) Hepatocyte-specific ablation of spermine/spermidine-N1-acetyltransferase gene reduces the severity of CCl4-induced acute liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G546–G560 10.1152/ajpgi.00431.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zahedi K., Huttinger F., Morrison R., Murray-Stewart T., Casero R. A., and Strauss K. I. (2010) Polyamine catabolism is enhanced after traumatic brain injury. J. Neurotrauma 27, 515–525 10.1089/neu.2009.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zahedi K., Lentsch A. B., Okaya T., Barone S., Sakai N., Witte D. P., Arend L. J., Alhonen L., Jell J., Jänne J., Porter C. W., and Soleimani M. (2009) Spermidine/spermine-N1-acetyltransferase ablation protects against liver and kidney ischemia-reperfusion injury in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G899–G909 10.1152/ajpgi.90507.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zahedi K., Barone S., Wang Y., Murray-Stewart T., Roy-Chaudhury P., Smith R. D., Casero R. A. Jr., and Soleimani M. (2014) Proximal tubule epithelial cell specific ablation of the spermidine/spermine N1-acetyltransferase gene reduces the severity of renal ischemia/reperfusion injury. PLoS ONE 9, e110161 10.1371/journal.pone.0110161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schwartz C. E., Wang X., Stevenson R. E., and Pegg A. E. (2011) Spermine synthase deficiency resulting in X-linked intellectual disability (Snyder-Robinson syndrome). Methods Mol. Biol. 720, 437–445 10.1007/978-1-61779-034-8_28 [DOI] [PubMed] [Google Scholar]
- 105. Li C., Brazill J. M., Liu S., Bello C., Zhu Y., Morimoto M., Cascio L., Pauly R., Diaz-Perez Z., Malicdan M. C. V., Wang H., Boccuto L., Schwartz C. E., Gahl W. A., Boerkoel C. F., and Zhai R. G. (2017) Spermine synthase deficiency causes lysosomal dysfunction and oxidative stress in models of Snyder-Robinson syndrome. Nat. Commun. 8, 1257 10.1038/s41467-017-01289-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shi Y., Lan F., Matson C., Mulligan P., Whetstine J. R., Cole P. A., Casero R. A., and Shi Y. (2004) Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941–953 10.1016/j.cell.2004.12.012 [DOI] [PubMed] [Google Scholar]
- 107. Zahedi K., Barone S., Destefano-Shields C., Brooks M., Murray-Stewart T., Dunworth M., Li W., Doherty J. R., Hall M. A., Smith R. D., Cleveland J. L., Casero R. A. Jr., and Soleimani M. (2017) Activation of endoplasmic reticulum stress response by enhanced polyamine catabolism is important in the mediation of cisplatin-induced acute kidney injury. PLoS ONE 12, e0184570 10.1371/journal.pone.0184570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nakamura M., Uemura T., Saiki R., Sakamoto A., Park H., Nishimura K., Terui Y., Toida T., Kashiwagi K., and Igarashi K. (2016) Toxic acrolein production due to Ca2+ influx by the NMDA receptor during stroke. Atherosclerosis 244, 131–137 10.1016/j.atherosclerosis.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 109. Uemura T., Watanabe K., Ishibashi M., Saiki R., Kuni K., Nishimura K., Toida T., Kashiwagi K., and Igarashi K. (2016) Aggravation of brain infarction through an increase in acrolein production and a decrease in glutathione with aging. Biochem. Biophys. Res. Commun. 473, 630–635 10.1016/j.bbrc.2016.03.137 [DOI] [PubMed] [Google Scholar]
- 110. Saiki R., Park H., Ishii I., Yoshida M., Nishimura K., Toida T., Tatsukawa H., Kojima S., Ikeguchi Y., Pegg A. E., Kashiwagi K., and Igarashi K. (2011) Brain infarction correlates more closely with acrolein than with reactive oxygen species. Biochem. Biophys. Res. Commun. 404, 1044–1049 10.1016/j.bbrc.2010.12.107 [DOI] [PubMed] [Google Scholar]
- 111. Masuko T., Takao K., Samejima K., Shirahata A., Igarashi K., Casero R. A. Jr., Kizawa Y., and Sugita Y. (2018) N1-Nonyl-1,4-diaminobutane ameliorates brain infarction size in photochemically induced thrombosis model mice. Neurosci. Lett. 672, 118–122 10.1016/j.neulet.2018.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Averill-Bates D. A., Chérif A., Agostinelli E., Tanel A., and Fortier G. (2005) Anti-tumoral effect of native and immobilized bovine serum amine oxidase in a mouse melanoma model. Biochem. Pharmacol. 69, 1693–1704 10.1016/j.bcp.2005.02.025 [DOI] [PubMed] [Google Scholar]
- 113. Lord-Fontaine S., Agostinelli E., Przybytkowski E., and Averill-Bates D. A. (2001) Amine oxidase, spermine, and hyperthermia induce cytotoxicity in P-glycoprotein overexpressing multidrug resistant Chinese hamster ovary cells. Biochem. Cell Biol. 79, 165–175 10.1139/o00-097 [DOI] [PubMed] [Google Scholar]
- 114. Arancia G., Calcabrini A., Marra M., Crateri P., Artico M., Martone A., Martelli F., and Agostinelli E. (2004) Mitochondrial alterations induced by serum amine oxidase and spermine on human multidrug resistant tumor cells. Amino Acids 26, 273–282 [DOI] [PubMed] [Google Scholar]
- 115. Calcabrini A., Arancia G., Marra M., Crateri P., Befani O., Martone A., and Agostinelli E. (2002) Enzymatic oxidation products of spermine induce greater cytotoxic effects on human multidrug-resistant colon carcinoma cells (LoVo) than on their wild-type counterparts. Int. J. Cancer 99, 43–52 10.1002/ijc.10310 [DOI] [PubMed] [Google Scholar]
- 116. Agostinelli E., Condello M., Tempera G., Macone A., Bozzuto G., Ohkubo S., Calcabrini A., Arancia G., and Molinari A. (2014) The combined treatment with chloroquine and the enzymatic oxidation products of spermine overcomes multidrug resistance of melanoma M14 ADR2 cells: a new therapeutic approach. Int. J. Oncol. 45, 1109–1122 10.3892/ijo.2014.2502 [DOI] [PubMed] [Google Scholar]
- 117. Sinigaglia G., Magro M., Miotto G., Cardillo S., Agostinelli E., Zboril R., Bidollari E., and Vianello F. (2012) Catalytically active bovine serum amine oxidase bound to fluorescent and magnetically drivable nanoparticles. Int. J. Nanomedicine 7, 2249–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Venditti I., Hassanein T. F., Fratoddi I., Fontana L., Battocchio C., Rinaldi F., Carafa M., Marianecci C., Diociaiuti M., Agostinelli E., Cametti C., and Russo M. V. (2015) Bioconjugation of gold-polymer core-shell nanoparticles with bovine serum amine oxidase for biomedical applications. Colloids Surf. B Biointerfaces 134, 314–321 10.1016/j.colsurfb.2015.06.052 [DOI] [PubMed] [Google Scholar]
- 119. Agostinelli E., Vianello F., Magliulo G., Thomas T., and Thomas T. J. (2015) Nanoparticle strategies for cancer therapeutics: nucleic acids, polyamines, bovine serum amine oxidase and iron oxide nanoparticles (Review). Int. J. Oncol. 46, 5–16 10.3892/ijo.2014.2706 [DOI] [PubMed] [Google Scholar]
- 120. Routhu N. K., Xie Y., Dunworth M., Casero R. A. Jr., Oupicky D., and Byrareddy S. N. (2018) Polymeric prodrugs targeting polyamine metabolism inhibit Zika virus replication. Mol. Pharm. 15, 4284–4295 10.1021/acs.molpharmaceut.8b00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pledgie A., Huang Y., Hacker A., Zhang Z., Woster P. M., Davidson N. E., and Casero R. A. Jr. (2005) Spermine oxidase SMO(PAOh1), not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J. Biol. Chem. 280, 39843–39851 10.1074/jbc.M508177200 [DOI] [PubMed] [Google Scholar]
- 122. Pledgie-Tracy A., Billam M., Hacker A., Sobolewski M. D., Woster P. M., Zhang Z., Casero R. A., and Davidson N. E. (2010) The role of the polyamine catabolic enzymes SSAT and SMO in the synergistic effects of standard chemotherapeutic agents with a polyamine analogue in human breast cancer cell lines. Cancer Chemother. Pharmacol. 65, 1067–1081 10.1007/s00280-009-1112-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Murray-Stewart T., Dunworth M., Lui Y., Giardiello F. M., Woster P. M., and Casero R. A. Jr. (2018) Curcumin mediates polyamine metabolism and sensitizes gastrointestinal cancer cells to antitumor polyamine-targeted therapies. PLoS ONE 13, e0202677 10.1371/journal.pone.0202677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Murray-Stewart T., and Casero R. A. (2017) Regulation of polyamine metabolism by curcumin for cancer prevention and therapy. Med. Sci. 5, E38 10.3390/medsci5040038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Murray-Stewart T., Ferrari E., Xie Y., Yu F., Marton L. J., Oupicky D., and Casero R. A. Jr. (2017) Biochemical evaluation of the anticancer potential of the polyamine-based nanocarrier Nano11047. PLoS ONE 12, e0175917 10.1371/journal.pone.0175917 [DOI] [PMC free article] [PubMed] [Google Scholar]


