Abstract
The polyamines (PA) putrescine, spermidine, and spermine have numerous roles in the growth of both prokaryotic and eukaryotic cells. For example, it is well known that putrescine and spermidine are strongly involved in proliferation and viability of Escherichia coli cells. Studies of polyamine functions and distributions in E. coli cells have revealed that polyamines mainly exist as an RNA–polyamine complex. Polyamines stimulate the assembly of 30S ribosomal subunits and thereby increase general protein synthesis 1.5- to 2.0-fold. Moreover, these studies have shown that polyamines stimulate synthesis of 20 different proteins at the level of translation, which are strongly involved in cell growth and viability. The genes encoding these 20 different proteins were termed as the “polyamine modulon.” We here review the mechanism of activation of 30S ribosomal subunits and stimulation of specific proteins. Other functions of polyamines in E. coli are also described.
Keywords: ribosome, antioxidant, polyamine, translation, translation regulation, ribosome assembly, RNA, initiation codon, polyamine modulon, ribosome activity, Shine-Dalgarno sequence
Introduction
The polyamines, consisting of putrescine (NH2(CH2)4NH2), spermidine (NH2(CH2)3NH(CH2)4NH2), and spermine (NH2(CH2)3NH(CH2)4NH(CH2)3NH2), have been implicated in numerous growth processes in Escherichia coli and mammalian cells (1–6). E. coli is useful for studying the functions of polyamines, because various mutants are available, and cloning of genes is relatively easy. Polyamine content in E. coli consisting of putrescine and spermidine is adjusted by biosynthesis (7, 8), uptake (9), and excretion (9). Dr. Tabor's lab isolated a polyamine-deficient E. coli mutant HT283, in which the rate of cell growth is about 30% that of cells cultured with polyamine addition (7, 10). They reported that polyamines are involved in some aspects of protein synthesis (7, 10) and that polyamines are necessary for efficient translation of amber codons in cells containing bacteriophage T7 carrying an amber mutation in gene 1 (11). Our group also studied the effect of polyamines on protein synthesis in a cell-free system or using a polyamine-requiring mutant MA261 (speB, agmatine ureohydrolase, and speC, ornithine decarboxylase-deficient mutant) (8), and the results are summarized below.
Effect of polyamines on protein synthesis in E. coli cell-free systems and on the assembly of ribosomal subunits
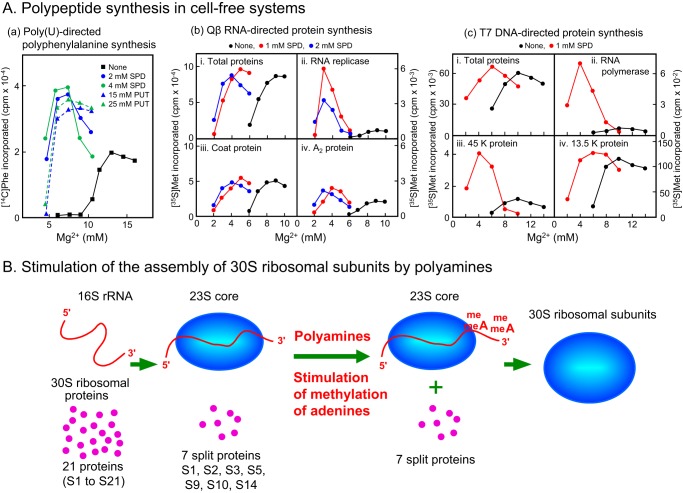
The effects of polyamines were studied using polyamine-free dialyzed ribosomes and Sephadex G-50–treated supernatant at various Mg2+ concentrations (12). As shown in Fig. 1A, addition of 2–4 mm spermidine or 15–25 mm putrescine with various concentrations of Mg2+ increased poly(U)-directed polyphenylalanine synthesis up to twice that synthesized at an optimal Mg2+ concentration (13 mm) in the absence of polyamines. Spermine exists together with putrescine and spermidine in mammalian cells (13). The effective concentration of spermine for stimulation of protein synthesis was approximately one-fifth that of spermidine (14). Thus, the effects of polyamines on protein synthesis have a rank order of potency spermine > spermidine > putrescine. These results show that polyamines not only have a sparing effect on the Mg2+ requirement for polyphenylalanine synthesis but also a stimulatory effect, which cannot be fulfilled by Mg2+ in the absence of polyamines. Then, the degree of stimulation of polypeptide synthesis by spermidine was examined using various synthetic mRNAs, and it was found that stimulation of polypeptide synthesis by spermidine depends on the uracil content in mRNAs (15). Polyphenylalanine synthesis was also stimulated when [14C]Phe-tRNA was used instead of [14C]Phe, indicating that stimulation by polyamines is based on stimulation at the level of ribosomal polypeptide synthesis. Next, the effect of polyamines on Qβ RNA-directed synthesis of proteins was tested. It was found that only Qβ RNA-directed RNA replicase synthesis was stimulated by polyamines (16). In the case of T7-DNA–directed protein synthesis, synthesis of T7 RNA polymerase and the 42-kDa protein, but not the 13.5-kDa protein, were stimulated by spermidine mainly at the level of translation (17). The results confirm that specific types of protein synthesis are stimulated by polyamines.
Figure 1.
Effect of polyamines on polypeptide synthesis in cell-free systems (A) and stimulation of the assembly of 30S ribosomal subunits (B). A, poly(U)-directed (panel a), Qβ RNA-directed (panel b), and T7 DNA (panel c)-directed protein synthesis was measured by changing Mg2+ concentrations. PUT, putrescine; SPD, spermidine. B, methylation of adenines at 1,518 and 1,519 in 16S rRNA on 23S core particles was stimulated by polyamines.
It also became clear that the assembly of 30S ribosomal subunits in E. coli was stimulated by polyamines (18, 19). Stimulation of the assembly of 30S ribosomal subunits, consisting of 16S rRNA and 21 ribosomal proteins (S1 to S21), by polyamines occurred by polyamine stimulation of enzymatic methylation of two adjacent adenine residues near the 3′-end of 16S rRNA (Fig. 1B) (20). Translation efficiency increased 1.5- to 2.0-fold due to stimulation of the assembly of the 30S ribosomal subunit by polyamines. These results support the idea that polyamines function at the level of translation through their interaction with RNA.
Polyamine distribution in E. coli
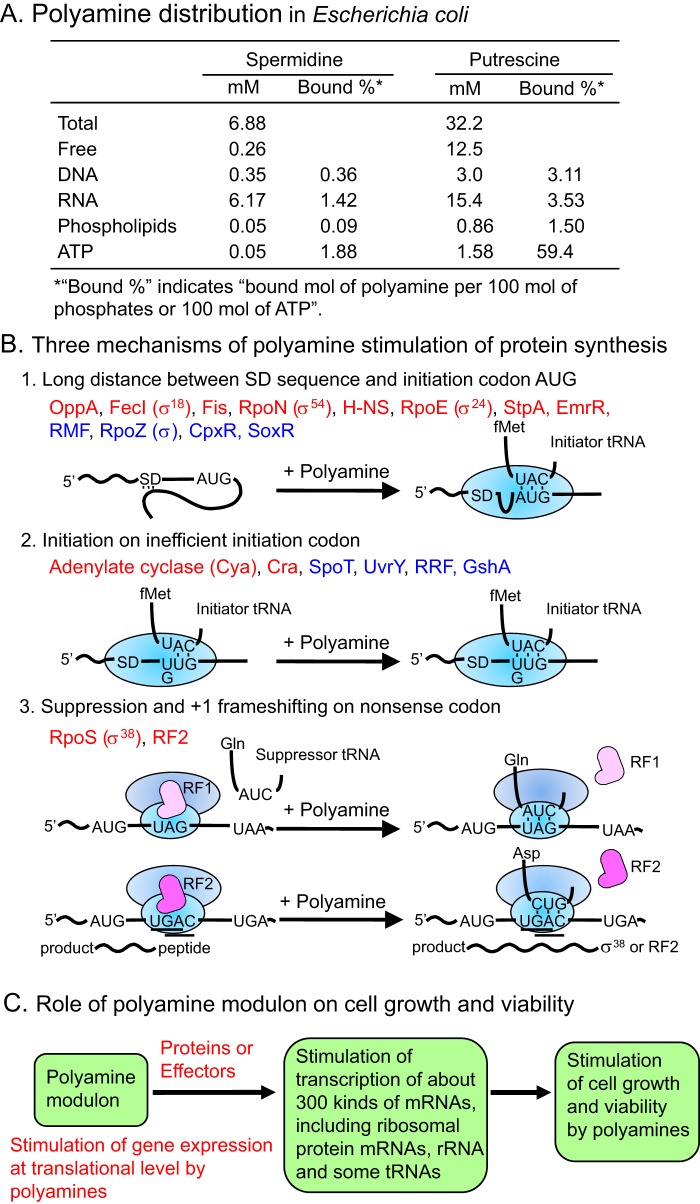
To confirm that polyamines also function at the level of translation in vivo, polyamine distribution in E. coli was estimated by measuring the binding constants of polyamines for the target molecules and the concentration of the target molecules in E. coli W3110 harvested at logarithmic phase (21). Intracellular water space of E. coli was estimated to be 2.9 μl of cell volume/mg of protein (22). As shown in Fig. 2A, the total spermidine and putrescine concentrations in E. coli were 6.88 and 32 mm, respectively, each mainly existing as a polyamine–RNA complex, supporting the idea that major polyamines function at the level of translation. It has been reported that the yeast tRNAPhe crystal structure includes two molecules of spermine (23), which is close to our estimation for polyamine binding to RNA (24).
Figure 2.
Polyamine distribution in E. coli (A), three mechanisms of polyamine stimulation of protein synthesis (B), and role of the polyamine modulon on cell growth and viability (C). A, polyamine distribution was estimated as described previously (21). B, proteins encoded by the polyamine modulon shown in red are involved in cell growth, and those in blue are involved in cell viability.
Polyamine stimulation of synthesis of specific proteins at the level of translation in E. coli
It has been examined as to what kinds of protein synthesis are stimulated by polyamines using a polyamine-requiring mutant of E. coli MA261 (8). Cell growth of E. coli MA261 slows down in the absence of polyamines. When putrescine is added to the culture medium, it is taken up into the cells by polyamine transporters consisting of PotA–D proteins (spermidine-preferential uptake system) and PotF–I proteins (putrescine-specific uptake system) (9), and spermidine is synthesized from putrescine. Accordingly, cell growth of E. coli MA261 was stimulated ∼3-fold by the addition of putrescine (0.6 mm) to the medium.
A set of genes whose expression is enhanced by polyamines at the level of translation was classified as a “polyamine modulon” (25, 26). In E. coli, we have thus far identified 20 kinds of genes as components of the polyamine modulon (5, 27). There are several mechanisms underlying polyamine stimulation of the synthesis of various members of the polyamine modulon (Fig. 2B). First, polyamine stimulation of protein synthesis can occur when a Shine-Dalgarno (SD)2 sequence (28) in the mRNA is obscure or is distant from the initiation codon AUG. Polyamines cause structural changes of a region of the SD sequence and the initiation codon AUG facilitating formation of the initiation complex. This is the case for oppA, fecI (σ18), fis, rpoN (σ54), hns, rpoE (σ24), stpA, emrR, rmf, rpoZ (ω), cpxR, and soxR. Proteins annotated in red in Fig. 2B are involved in cell growth during the logarithmic phase, and those shown in blue are involved in cell viability at the stationary phase. In a second mechanism, polyamines enhance the inefficient initiation codon UUG- or GUG-dependent fMet-tRNA binding to cya, cra, spoT, uvrY, frr (RRF), or gshA mRNA–ribosome complex. In a third mechanism, polyamines stimulate read-through of the amber codon UAG by Gln-tRNASupE on ribosome-associated rpoS mRNA or stimulate a +1 frameshifting at the 26th UGA codon of prfB mRNA encoding RF2 (translation releasing factor 2).
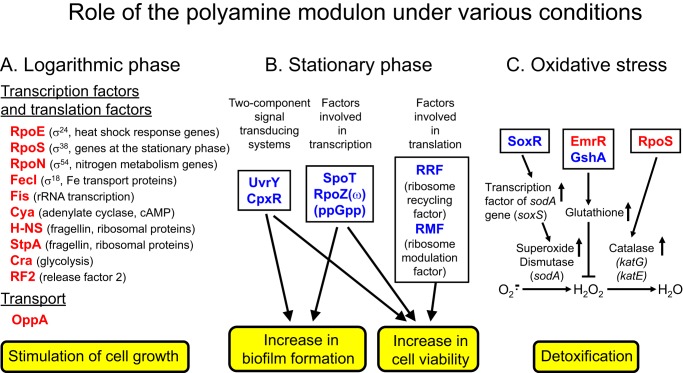
Because the synthesis of many transcription factors was stimulated by polyamines, transcription profiles of the logarithmic phase culture of E. coli MA261 with or without putrescine were determined by a two-color (Cy3 and Cy5) cDNA microarray analysis (29, 30). Expression of 2,742 genes was detected in cells cultured with or without putrescine. Among these, 309 genes were up-regulated more than 2-fold by polyamines (Fig. 2C). In 309 up-regulated genes, 240 kinds of mRNA were under the control of nine transcription factors mainly existing in logarithmic phase and two transcription factors mainly existing in stationary phase (see Fig. 4) (25).
Figure 4.
Role of the polyamine modulon under various conditions. Twenty kinds of proteins encoded by polyamine modulon are shown in red and blue as indicated in the legend of Fig. 2. Proteins encoded by polyamine modulon are involved at logarithmic phase (A), at stationary phase (B), and under oxidative stress conditions (C). RpoS (σ38) are indicated in both A and C. Proteins encoded by the polyamine modulon shown in red are involved in cell growth, and those in blue are involved in cell viability.
Molecular mechanism of polyamine stimulation of EmrR, SoxR, and GshA synthesis
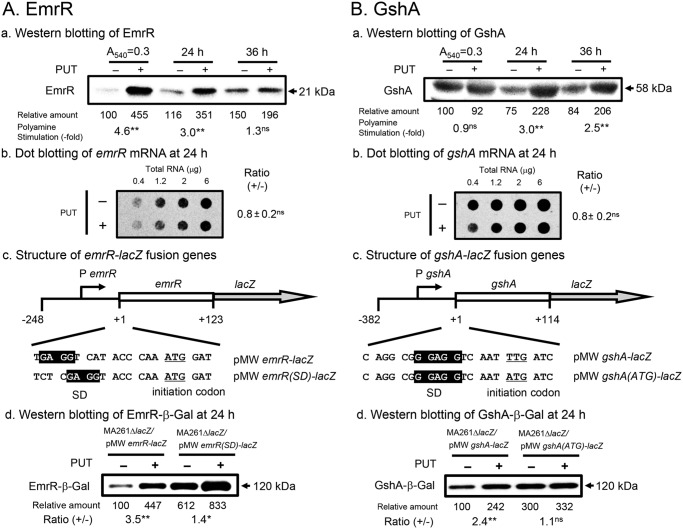
Under oxidative stress conditions caused by 0.6 μm K2TeO3, an inducer of oxidative stress (31, 32), synthesis of SoxR, EmrR, GshA, and RpoS was stimulated by polyamines (27). Because RpoS has been already identified as a polyamine modulon protein through polyamine stimulation of suppression of an amber termination codon in the ORF (33), the mechanism of polyamine stimulation of EmrR, SoxR, and GshA was studied by measuring protein and mRNA levels by Western blotting (34) and dot blotting (35), respectively. As shown in Fig. 3, stimulation of the synthesis of EmrR and GshA proteins occurred at the level of translation. The nucleotide sequence of emrR and gshA mRNAs was then determined. The SD sequence of emrR mRNA was 11 nucleotides upstream of the initiation codon AUG, whereas the initiation codon of gshA mRNA was UUG instead AUG. When the position of the SD sequence of emrR mRNA was shifted to a normal position (8 nucleotides upstream of the initiation codon AUG), the synthesis of an EmrR–β-gal fusion protein measured in the absence of polyamines increased, and the degree of polyamine stimulation was reduced (Fig. 3A). In soxR mRNA, the SD sequence is also distant from the initiation codon AUG. So, SoxR synthesis was stimulated by polyamines. Stimulation of EmrR and SoxR syntheses by polyamines was due to a selective structural change of the bulged-out region of dsRNA in the initiation region of mRNA by spermidine, similar to stimulation of OppA synthesis by polyamines (36, 37). When the initiation codon UUG of gshA mRNA was changed to AUG, synthesis of GshA–β-gal fusion protein in the absence of polyamines increased, and the degree of polyamine stimulation was reduced (Fig. 3B). These results indicate that EmrR and SoxR syntheses are enhanced by polyamines due to the unusual position of the SD sequence and that GshA synthesis is due to the presence of the inefficient initiation codon UUG instead of AUG. The results indicate that polyamines stimulate protein synthesis encoded by inefficient mRNAs.
Figure 3.
Mechanism of polyamine stimulation of EmrR (A) and GshA (B) synthesis. E. coli MA261 was cultured in the presence of 0.6 μm K2TeO3, with or without 0.6 mm putrescine, and harvested at A540 = 0.3, for 24 and 36 h. Protein and mRNA levels of EmrR and GshA were measured (panels a and b). Wild and mutated mRNAs of emrR and gshA fused to lacZ were constructed, and the effect of polyamines on the synthesis of fusion proteins was examined (panels c and d). *, p < 0.05; **, p < 0.01.
Role of polyamines at various cell growth conditions
Members of the polyamine modulon were classified according to the growth conditions (Fig. 4). At the logarithmic phase, 10 members of the polyamine modulon were identified as transcriptional and translational factors. Another member of the polyamine modulon is OppA, which is involved in the transport of oligopeptides (38). Thus, polyamines greatly contribute to cell growth at the logarithmic phase. At the stationary phase, six kinds of protein synthesis were stimulated by polyamines. UvrY and CpxR are response regulators in two-component signal-transducing systems, which are involved in biofilm formation (39, 40). SpoT and RpoZ are proteins involved in the function of ppGpp, a stringent response factor, i.e. a regulating factor of transcription (41, 42). RRF and ribosome modulation factor (RMF) increased cell viability through adjusting protein synthesis (39, 43). Under conditions of oxidative stress, synthesis of two transcription factors (SoxR and EmrR) and a GSH synthetic enzyme (GshA) was stimulated by polyamines (27). Because SoxR is a transcription factor for expression of the superoxide response regulon, it stimulated the transcription of the sodA gene encoding a superoxide dismutase (Fig. 4C). Furthermore, the level of glutathione (GSH) was increased through polyamine stimulation of the synthesis of EmrR and GshA (Fig. 4C). GshA directly increases GSH synthesis. Stimulation of EmrR synthesis probably causes the inhibition of the efflux of many metabolites with small molecular weight, which includes GSH. Thus, hydrogen peroxide is detoxified effectively by GSH peroxidase through the increase in GSH. It was also shown that transcription of genes for katG and katE encoding catalases is enhanced through polyamine stimulation of RpoS (σ38) synthesis (27). It has been reported that RpoS also induced the stimulation of gadE (a regulator of GDAR (glutamate-dependent acid resistance) system at acidic pH (44)), which is important for survival under acidic conditions. The results confirm that polyamines strongly contribute to cell growth and viability of E. coli under various conditions.
Factors involved in the decrease in toxicity of spermidine accumulation
Although it is normally difficult for spermidine to overaccumulate in E. coli due to the existence of spermidine acetyltransferase (45) as well as spermidine excretion protein complex (MdtJI) (46), the mechanism of detoxification of excessive spermidine levels was studied (47). E. coli CAG2242 cells are deficient in the speG gene encoding spermidine acetyltransferase (48). When these cells were cultured in the presence of 0.5 to 4 mm spermidine, their viability was greatly decreased through inhibition of protein synthesis by overaccumulation of spermidine. When these CAG2242 cells were cultured with a high concentration of spermidine (4 mm), a revertant strain was obtained. We found that a 55-kDa protein, glycerol kinase, was overexpressed in the revertant and that synthesis of RMF and the RNA polymerase σ38 subunit (RpoS), important factors for cell viability, were increased in the revertant. Levels of l-glycerol 3-phosphate were also increased in the revertant. Transformation of glpFK, which encodes a glycerol diffusion facilitator (glpF) and glycerol kinase (glpK), to E. coli CAG2242 partially prevented the cell death caused by accumulation of spermidine. It was also found that l-glycerol 3-phosphate inhibited spermidine binding to ribosomes and attenuated the inhibition of protein synthesis caused by high concentrations of spermidine. These results indicate that l-glycerol 3-phosphate reduces the binding of excess amounts of spermidine to ribosomes so that protein synthesis is recovered.
Role of EF-P and eIF5A in protein synthesis
Eukaryotic initiation factor 5A (eIF5A) is the only protein containing hypusine (Nϵ-(4-amino-2-hydroxybutyl)-lysine) derived from spermidine at Lys-50 in eukaryotic cells (49) and is essential for cell growth through stimulation of protein synthesis containing polyproline peptides (50). EF-P in E. coli is post-translationally modified by a hydroxylated β-lysine attached to Lys-34 and is essential for the synthesis of a subset of proteins containing proline stretches (51). So, EF-P functions similarly to eukaryotic eIF5A in E. coli, although spermidine is not involved in the activation of EF-P.
Other functions of polyamines in E. coli
In addition to polyamine effects at the level of translation, polyamines function in the following aspects.
1) Polyamines protect E. coli cells from the toxic effect of oxygen, i.e. when E. coli cell growth was inhibited in the presence of 95% O2, 5% CO2, cell growth was recovered by the addition of 0.1 mm putrescine, spermidine, or cadaverine (52).
2) Polyamines contribute to pH adjustment in cells by PotE (putrescine-ornithine antiporter) and CadB (cadaverine-lysine antiporter) when cells were cultured at acidic pH (53, 54).
3) Polyamines stabilize bacterial spheroplasts and protoplasts from osmotic shock (55).
4) Polyamines may stimulate B to Z conversion of DNA (56, 57), although the interaction between polyamines and DNA is weak (see Fig. 2).
5) Polyamine content in E. coli is partially regulated by the transporter systems (9), and spermidine uptake is catalyzed by PotA–D proteins (58). Among the four proteins, PotD protein is a substrate-binding protein existing in the periplasm. When spermidine is present in excess in cells, the PotD precursor protein binds to the transcriptional initiation site of the potABCD operon, and the transcription of the operon was inhibited (59). This contributes to maintenance of optimal spermidine concentration in E. coli.
Conclusions
Based on the idea that “there is no function without interaction with targeted molecules,” the physiological functions of polyamines were studied at the level of RNA, because polyamines mainly exist as a polyamine–RNA complex. In E. coli, we found that assembly of 30S ribosomal subunits is stimulated by polyamines and that 20 kinds of protein syntheses encoded by members of the polyamine modulon are stimulated by polyamines at the level of translation. These polyamine functions in E. coli strongly contribute to the stimulation of cell growth and the increase in cell viability by polyamines. The concept of the “polyamine modulon” is applicable to eukaryotic cells (5, 60). Other polyamine functions except protein synthesis were also mentioned.
Acknowledgments
This review was written in celebration of Dr. Herb Tabor's 100th birthday, and we greatly appreciate Dr. Tabor's kind support for our polyamine research. We are grateful to Drs. A. E. Pegg, A. J. Michael, and K. Williams for their help in preparing this manuscript.
This work was supported in part by a Grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan. This article is part of a series on “Polyamines,” written in honor of Dr. Herbert Tabor's 100th birthday. The authors declare that they have no conflicts of interest with the contents of this article.
- SD
- Shine-Dalgarno
- RMF
- ribosome modulation factor
- RRF
- ribosome recycling factor.
References
- 1. Tabor H., and Tabor C. W. (1964) Spermidine, spermine, and related amines. Pharmacol. Rev. 16, 245–300 [PubMed] [Google Scholar]
- 2. Tabor C. W., and Tabor H. (1984) Polyamines. Annu. Rev. Biochem. 53, 749–790 10.1146/annurev.bi.53.070184.003533 [DOI] [PubMed] [Google Scholar]
- 3. Cohen S. S. (1998) A Guide to Polyamines, Oxford University Press, New York [Google Scholar]
- 4. Igarashi K., and Kashiwagi K. (2000) Polyamines: mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 271, 559–564 10.1006/bbrc.2000.2601 [DOI] [PubMed] [Google Scholar]
- 5. Igarashi K., and Kashiwagi K. (2015) Modulation of protein synthesis by polyamines. IUBMB Life 67, 160–169 10.1002/iub.1363 [DOI] [PubMed] [Google Scholar]
- 6. Pegg A. E. (2016) Functions of polyamines in mammals. J. Biol. Chem. 291, 14904–14912 10.1074/jbc.R116.731661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hafner E. W., Tabor C. W., and Tabor H. (1979) Mutants of Escherichia coli that do not contain 1,4-diaminobutane (putrescine) or spermidine. J. Biol. Chem. 254, 12419–12426 [PubMed] [Google Scholar]
- 8. Cunningham-Rundles S., and Maas W. K. (1975) Isolation, characterization, and mapping of Escherichia coli mutants blocked in the synthesis of ornithine decarboxylase. J. Bacteriol. 124, 791–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Igarashi K., and Kashiwagi K. (2010) Characteristics of cellular polyamine transport in prokaryotes and eukaryotes. Plant Physiol. Biochem. 48, 506–512 10.1016/j.plaphy.2010.01.017 [DOI] [PubMed] [Google Scholar]
- 10. Tabor H., Hafner E. W., and Tabor C. W. (1980) Construction of an Escherichia coli strain unable to synthesize putrescine, spermidine, or cadaverine: characterization of two genes controlling lysine decarboxylase. J. Bacteriol. 144, 952–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tabor H., and Tabor C. W. (1982) Polyamine requirement for efficient translation of amber codons in vivo. Proc. Natl. Acad. Sci. U.S.A. 79, 7087–7091 10.1073/pnas.79.23.7087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Igarashi K., Sugawara K., Izumi I., Nagayama C., and Hirose S. (1974) Effect of polyamines of polyphenylalanine synthesis by Escherichia coli and rat-liver ribosomes. Eur. J. Biochem. 48, 495–502 10.1111/j.1432-1033.1974.tb03790.x [DOI] [PubMed] [Google Scholar]
- 13. Michael A. J. (2016) Polyamines in eukaryotes, bacteria, and archaea. J. Biol. Chem. 291, 14896–14903 10.1074/jbc.R116.734780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ogasawara T., Ito K., and Igarashi K. (1989) Effect of polyamines on globin synthesis in a rabbit reticulocyte polyamine-free protein synthetic system. J. Biochem. 105, 164–167 10.1093/oxfordjournals.jbchem.a122633 [DOI] [PubMed] [Google Scholar]
- 15. Igarashi K., Watanabe Y., and Hirose S. (1975) Dependency of spermidine stimulation of polypeptide synthesis on the uracil content of messenger ribonucleic acid. Biochem. Biophys. Res. Commun. 67, 407–413 10.1016/0006-291X(75)90330-7 [DOI] [PubMed] [Google Scholar]
- 16. Watanabe Y., Igarashi K., and Hirose S. (1981) Differential stimulation by polyamines of phage RNA-directed synthesis of proteins. Biochim. Biophys. Acta 656, 134–139 10.1016/0005-2787(81)90078-2 [DOI] [PubMed] [Google Scholar]
- 17. Watanabe Y., Igarashi K., Mitsui K., and Hirose S. (1983) Differential stimulation by polyamines of phage DNA-directed in vitro synthesis of proteins. Biochim. Biophys. Acta 740, 362–368 10.1016/0167-4781(83)90083-0 [DOI] [PubMed] [Google Scholar]
- 18. Echandi G., and Algranati I. D. (1975) Defective 30S ribosomal particles in a polyamine auxotroph of Escherichia coli. Biochem. Biophys. Res. Commun. 67, 1185–1191 10.1016/0006-291X(75)90798-6 [DOI] [PubMed] [Google Scholar]
- 19. Igarashi K., Kishida K., and Hirose S. (1980) Stimulation by polyamines of enzymatic methylation of two adjacent adenines near the 3′ and end of 16S ribosomal RNA of Escherichia coli. Biochem. Biophys. Res. Commun. 96, 678–684 10.1016/0006-291X(80)91408-4 [DOI] [PubMed] [Google Scholar]
- 20. Igarashi K., Kishida K., Kashiwagi K., Tatokoro I., Kakegawa T., and Hirose S. (1981) Relationship between methylation of adenine near the 3′ end of 16-S ribosomal RNA and the activity of 30-S ribosomal subunits. Eur. J. Biochem. 113, 587–593 10.1111/j.1432-1033.1981.tb05103.x [DOI] [PubMed] [Google Scholar]
- 21. Miyamoto S., Kashiwagi K., Ito K., Watanabe S., and Igarashi K. (1993) Estimation of polyamine distribution and polyamine stimulation of protein synthesis in Escherichia coli. Arch. Biochem. Biophys. 300, 63–68 10.1006/abbi.1993.1009 [DOI] [PubMed] [Google Scholar]
- 22. Bakker E. P., and Mangerich W. E. (1981) Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J. Bacteriol. 147, 820–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quigley G. J., Teeter M. M., and Rich A. (1978) Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Proc. Natl. Acad. Sci. U.S.A. 75, 64–68 10.1073/pnas.75.1.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watanabe S., Kusama-Eguchi K., Kobayashi H., and Igarashi K. (1991) Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J. Biol. Chem. 266, 20803–20809 [PubMed] [Google Scholar]
- 25. Yoshida M., Kashiwagi K., Shigemasa A., Taniguchi S., Yamamoto K., Makinoshima H., Ishihama A., and Igarashi K. (2004) A unifying model for the role of polyamines in bacterial cell growth, the polyamine modulon. J. Biol. Chem. 279, 46008–46013 10.1074/jbc.M404393200 [DOI] [PubMed] [Google Scholar]
- 26. Igarashi K., and Kashiwagi K. (2006) Polyamine modulon in Escherichia coli: genes involved in the stimulation of cell growth by polyamines. J. Biochem. 139, 11–16 10.1093/jb/mvj020 [DOI] [PubMed] [Google Scholar]
- 27. Sakamoto A., Terui Y., Yoshida T., Yamamoto T., Suzuki H., Yamamoto K., Ishihama A., Igarashi K., and Kashiwagi K. (2015) Three members of polyamine modulon under oxidative stress conditions: two transcription factors (SoxR and EmrR) and a glutathione synthetic enzyme (GshA). PLoS ONE 10, e0124883 10.1371/journal.pone.0124883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shine J., and Dalgarno L. (1974) The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc. Natl. Acad. Sci. U.S.A. 71, 1342–1346 10.1073/pnas.71.4.1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schena M., Shalon D., Davis R. W., and Brown P. O. (1995) Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270, 467–470 10.1126/science.270.5235.467 [DOI] [PubMed] [Google Scholar]
- 30. Oshima T., Wada C., Kawagoe Y., Ara T., Maeda M., Masuda Y., Hiraga S., and Mori H. (2002) Genome-wide analysis of deoxyadenosine methyltransferase-mediated control of gene expression in Escherichia coli. Mol. Microbiol. 45, 673–695 10.1046/j.1365-2958.2002.03037.x [DOI] [PubMed] [Google Scholar]
- 31. Chasteen T. G., Fuentes D. E., Tantaleán J. C., and Vásquez C. C. (2009) Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiol. Rev. 33, 820–832 10.1111/j.1574-6976.2009.00177.x [DOI] [PubMed] [Google Scholar]
- 32. Arenas F. A., Díaz W. A., Leal C. A., Pérez-Donoso J. M., Imlay J. A., and Vásquez C. C. (2010) The Escherichia coli btuE gene, encodes a glutathione peroxidase that is induced under oxidative stress conditions. Biochem. Biophys. Res. Commun. 398, 690–694 10.1016/j.bbrc.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoshida M., Kashiwagi K., Kawai G., Ishihama A., and Igarashi K. (2002) Polyamines enhance synthesis of the RNA polymerase σ38 subunit by suppression of an amber termination codon in the open reading frame. J. Biol. Chem. 277, 37139–37146 10.1074/jbc.M206668200 [DOI] [PubMed] [Google Scholar]
- 34. Nielsen P. J., Manchester K. L., Towbin H., Gordon J., and Thomas G. (1982) The phosphorylation of ribosomal protein S6 in rat tissues following cycloheximide injection, in diabetes, and after denervation of diaphragm. A simple immunological determination of the extent of S6 phosphorylation on protein blots. J. Biol. Chem. 257, 12316–12321 [PubMed] [Google Scholar]
- 35. Sambrook J., Fritsch E. F., and Maniatis T. (2001) in Molecular Cloning: A Laboratory Manual (Sambrook J., and Russell D. W., eds) 3rd Ed, pp. 7.46–47.50, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 36. Yoshida M., Meksuriyen D., Kashiwagi K., Kawai G., and Igarashi K. (1999) Polyamine stimulation of the synthesis of oligopeptide-binding protein (OppA). Involvement of a structural change of the Shine-Dalgarno sequence and the initiation codon AUG in oppA mRNA. J. Biol. Chem. 274, 22723–22728 10.1074/jbc.274.32.22723 [DOI] [PubMed] [Google Scholar]
- 37. Higashi K., Terui Y., Suganami A., Tamura Y., Nishimura K., Kashiwagi K., and Igarashi K. (2008) Selective structural change by spermidine in the bulged-out region of double-stranded RNA and its effect on RNA function. J. Biol. Chem. 283, 32989–32994 10.1074/jbc.M806027200 [DOI] [PubMed] [Google Scholar]
- 38. Kashiwagi K., Yamaguchi Y., Sakai Y., Kobayashi H., and Igarashi K. (1990) Identification of the polyamine-induced protein as a periplasmic oligopeptide binding protein. J. Biol. Chem. 265, 8387–8391 [PubMed] [Google Scholar]
- 39. Yamamoto K., and Ishihama A. (2005) Transcriptional response of Escherichia coli to external copper. Mol. Microbiol. 56, 215–227 10.1111/j.1365-2958.2005.04532.x [DOI] [PubMed] [Google Scholar]
- 40. Sakamoto A., Terui Y., Yamamoto T., Kasahara T., Nakamura M., Tomitori H., Yamamoto K., Ishihama A., Michael A. J., Igarashi K., and Kashiwagi K. (2012) Enhanced biofilm formation and/or cell viability by polyamines through stimulation of response regulators UvrY and CpxR in the two-component signal transducing systems, and ribosome recycling factor. Int. J. Biochem. Cell Biol. 44, 1877–1886 10.1016/j.biocel.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 41. Cashel M. (1975) Regulation of bacterial ppGpp and pppGpp. Annu. Rev. Microbiol. 29, 301–318 10.1146/annurev.mi.29.100175.001505 [DOI] [PubMed] [Google Scholar]
- 42. Terui Y., Akiyama M., Sakamoto A., Tomitori H., Yamamoto K., Ishihama A., Igarashi K., and Kashiwagi K. (2012) Increase in cell viability by polyamines through stimulation of the synthesis of ppGpp regulatory protein and ω protein of RNA polymerase in Escherichia coli. Int. J. Biochem. Cell Biol. 44, 412–422 10.1016/j.biocel.2011.11.017 [DOI] [PubMed] [Google Scholar]
- 43. Hirokawa G., Nijman R. M., Raj V. S., Kaji H., Igarashi K., and Kaji A. (2005) The role of ribosome recycling factor in dissociation of 70S ribosomes into subunits. RNA 11, 1317–1328 10.1261/rna.2520405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chattopadhyay M. K., Keembiyehetty C. N., Chen W., and Tabor H. (2015) Polyamines stimulate the level of the σ38 subunit (RpoS) of Escherichia coli RNA polymerase, resulting in the induction of the glutamate decarboxylase-dependent acid response system via the gadE regulon. J. Biol. Chem. 290, 17809–17821 10.1074/jbc.M115.655688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sugiyama S., Ishikawa S., Tomitori H., Niiyama M., Hirose M., Miyazaki Y., Higashi K., Murata M., Adachi H., Takano K., Murakami S., Inoue T., Mori Y., Kashiwagi K., Igarashi K., and Matsumura H. (2016) Molecular mechanism underlying promiscuous polyamine recognition by spermidine acetyltransferase. Int. J. Biochem. Cell Biol. 76, 87–97 10.1016/j.biocel.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 46. Higashi K., Ishigure H., Demizu R., Uemura T., Nishino K., Yamaguchi A., Kashiwagi K., and Igarashi K. (2008) Identification of a spermidine excretion protein complex (MdtJI) in Escherichia coli. J. Bacteriol. 190, 872–878 10.1128/JB.01505-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raj V. S., Tomitori H., Yoshida M., Apirakaramwong A., Kashiwagi K., Takio K., Ishihama A., and Igarashi K. (2001) Properties of a revertant of Escherichia coli viable in the presence of spermidine accumulation: increase in l-glycerol 3-phosphate. J. Bacteriol. 183, 4493–4498 10.1128/JB.183.15.4493-4498.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carper S. W., Willis D. G., Manning K. A., and Gerner E. W. (1991) Spermidine acetylation in response to a variety of stresses in Escherichia coli. J. Biol. Chem. 266, 12439–12441 [PubMed] [Google Scholar]
- 49. Park M. H. (2006) The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J. Biochem. 139, 161–169 10.1093/jb/mvj034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park M. H., Lee Y. B., and Joe Y. A. (1997) Hypusine is essential for eukaryotic cell proliferation. Biol. Signals 6, 115–123 10.1159/000109117 [DOI] [PubMed] [Google Scholar]
- 51. Doerfel L. K., Wohlgemuth I., Kothe C., Peske F., Urlaub H., and Rodnina M. V. (2013) EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science 339, 85–88 10.1126/science.1229017 [DOI] [PubMed] [Google Scholar]
- 52. Chattopadhyay M. K., Tabor C. W., and Tabor H. (2003) Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc. Natl. Acad. Sci. U.S.A. 100, 2261–2265 10.1073/pnas.2627990100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kashiwagi K., Miyamoto S., Suzuki F., Kobayashi H., and Igarashi K. (1992) Excretion of putrescine by the putrescine-ornithine antiporter encoded by the potE gene of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 89, 4529–4533 10.1073/pnas.89.10.4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Soksawatmaekhin W., Kuraishi A., Sakata K., Kashiwagi K., and Igarashi K. (2004) Excretion and uptake of cadaverine by CadB and its physiological functions in Escherichia coli. Mol. Microbiol. 51, 1401–1412 10.1046/j.1365-2958.2003.03913.x [DOI] [PubMed] [Google Scholar]
- 55. Souzu H. (1986) Fluorescence polarization studies on Escherichia coli membrane stability and its relation to the resistance of the cell to freeze-thawing. II. Stabilization of the membranes by polyamines. Biochim. Biophys. Acta 861, 361–367 10.1016/0005-2736(86)90439-6 [DOI] [PubMed] [Google Scholar]
- 56. Thomas T. J., and Thomas T. (1994) Polyamine-induced Z-DNA conformation in plasmids containing (dA-dC)n. (dG-dT)n inserts and increased binding of lupus autoantibodies to the Z-DNA form of plasmids. Biochem. J. 298, 485–491 10.1042/bj2980485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thomas T., Gallo M. A., Klinge C. M., and Thomas T. J. (1995) Polyamine-mediated conformational perturbations in DNA alter the binding of estrogen receptor to poly(dG-m5dC)·poly(dG-m5dC) and a plasmid containing the estrogen response element. J. Steroid Biochem. Mol. Biol. 54, 89–99 10.1016/0960-0760(95)00126-K [DOI] [PubMed] [Google Scholar]
- 58. Furuchi T., Kashiwagi K., Kobayashi H., and Igarashi K. (1991) Characteristics of the gene for a spermidine and putrescine transport system that maps at 15 min on the Escherichia coli chromosome. J. Biol. Chem. 266, 20928–20933 [PubMed] [Google Scholar]
- 59. Antognoni F., Del Duca S., Kuraishi A., Kawabe E., Fukuchi-Shimogori T., Kashiwagi K., and Igarashi K. (1999) Transcriptional inhibition of the operon for the spermidine uptake system by the substrate-binding protein PotD. J. Biol. Chem. 274, 1942–1948 10.1074/jbc.274.4.1942 [DOI] [PubMed] [Google Scholar]
- 60. Kashiwagi K., Terui Y., and Igarashi K. (2018) Modulation of protein synthesis by polyamines in mammalian cells. Methods Mol. Biol. 1694, 325–336 10.1007/978-1-4939-7398-9_27 [DOI] [PubMed] [Google Scholar]