Abstract
Piericidin A1, a member of ɑ-pyridone antibiotic, exhibits various biological activities such as antimicrobial, antifungal, and antitumor properties and possesses potent respiration-inhibitory activity against insects due to its competitive binding capacity to mitochondrial complex I. The biosynthetic pathway of piericidin A1 has been reported in Streptomyces piomogeues var. Hangzhouwanensis, while the regulatory mechanism remains poorly understood. In this study, a Streptomyces antibiotic regulatory protein (SARP) family transcriptional regulator PieR was characterized. Genetic disruption and complementation manipulations revealed that PieR positively regulated the production of piericidin A1. Moreover, the overexpression of pieR contributed to the improvement of piericidin A1 productivity. The real-time quantitative PCR (RT-qPCR) was carried out and the data showed that pieR stimulated the transcription of all the biosynthesis-related genes for piericidin A1. In order to explore the regulatory mechanism, electrophoresis mobility shift assays (EMSA) and DNase I footprinting experiments have been conducted. A protected region covering 50 nucleotides within the upstream region of pieR was identified and two 5-nt direct repeat sequences (5′-CCGGA-3′) in the protected region were found. These findings, taken together, set stage for transcriptional control engineering in the view of optimizing piericidin A1 production and thus provide a viable potent route for the construction of strains with high productivity.
Keywords: ɑ-pyridone antibiotic, Transcriptional regulation, SARP family regulator, Secondary metabolic, Streptomyces
1. Introduction
Microbial natural products have been studied for hundreds of years because of the multiple biological activities and potent pharmaceutical potential that can be used in agriculture, graziery and chemical industry [1,2]. Streptomyces are well-known producers of tremendous biological active compounds, and serve as powerful and potent source of important pharmaceutical candidates [3]. ɑ-pyridone antibiotic was first discovered at 19th century by Tuson from castor bean, named ricinine [4]. Subsequently, a series of ɑ-pyridone natural products have been discovered with various biological activities including pesticidal, antifungal, antimalarial and antineoplastic [5,6]. The biosynthesis of these products often assembles with other large biosynthetic machineries, such as polyketide synthase (PKS) and nonribosomal peptide synthase (NRPS) [7]. However, biosynthetic mechanisms of pyridone-based natural products have not been fully revealed.
Piericidins are a family of α-pyridone antibiotics that are isolated mainly from various Streptomyces species of terrestrial, marine, and symbiotic origins [[8], [9], [10]]. Structurally, piericidins feature a pyridone core attached with variable polyene side chains. Piericidin A1, the prototypical member of piericidins, was firstly isolated from Streptomyces mobaraensis in the late 1950s, and then was also isolated from other Streptomyces [9]. Because of the structural similarity to ubiquinone, piericidin A1 exhibits potent inhibitory activity toward mitochondrial NADH dehydrogenase [11]. Meanwhile, it shows diverse antibacterial and antifungal activities and can selectively kill some insects [12]. Recently, it has been identified as a highly selective antitumor agent in animal model [13]. During the screening for antitumor agents, piericidin A1 has been shown to reduce the resistance of tumor cell against intracellular toxicity and anti-tumor therapeutic agents by inhibiting stress protein GRP78 [13], and inhibit the growth of filopodia protrusion of human epidermal carcinoma A431 cells combined with glucopiericidin A [14]. Moreover, piericidin A is proved to be associated with the quorum sensing of Chromobacterium violaceum CV026 strain related to potato soft rot [15].
Since the first isolation, the total complex chemical synthesis of it has been achieved successfully [16], nevertheless, alternative more efficient green approaches are needed. Previous study has contributed to the identification of the biosynthetic gene cluster (pie cluster) of piericidin A1 in Streptomyces piomogeues var. Hangzhouwanensis and the reveal of its biosynthesis pathway [17]. The α-pyridone ring formation is dependent on hydrolysis of the linear β,δ-diketo carboxylic acid synthesized by type I polyketide synthases, followed by amidation and cyclization. This was strikingly different from the previously characterized hybrid polyketide synthase-nonribosomal peptide synthetase (PKS-NRPS) involved in α-pyridone pathways. Three post-modification proteins encoded in the gene cluster were proved responsible for the hydroxylation and methylation in piericidin A1 [18]. The production of antibiotics in Streptomycetes is usually controlled by multiple regulatory proteins that respond to internal physiological and environmental conditions. Typically, Streptomyces have more than 8000 protein-coding genes, and >10% of the coding genes are predicted to be transcription factors, exhibiting remarkable regulatory capacity and flexibility [19]. Regulation is critical for optimizing protein levels and the subsequent cellular levels of metabolites [20,21]. Genetic manipulation of regulatory genes has emerged as an important tool for construction of high-yield strains [[22], [23], [24], [25]], while transcriptional control engineering has been proved to be a valuable tool for titrating protein (and therefore activity) levels for titratability of metabolites of interest, and engineering a plethora of gene circuits [21] in synthetic biology.
In order to explore the biosynthetic regulatory mechanism and provide insight into future molecular synthetic engineering construction of piericidin A1 and its derivates, the regulatory role of PieR from Streptomyces piomogeues var. Hangzhouwanensis was characterized in this study. The bioinformatic analysis of PieR has revealed that it is a possible member of Streptomyces antibiotic regulatory protein (SARP) family of transcriptional regulators. In this study, the genetic manipulation of pieR proved its positive stimulation on piericidin A1 biosynthesis and the overexpression of pieR resulted in a 2.3-fold improvement of piericidin A1 productivity than wild type (WT) strain. Meanwhile, the target gene of PieR was identified by EMSA and the PieR-binding sequence was determined by DNase I footprinting. The findings reported here showed that PieR appeared to promote the expression of the pie cluster and in turn contributed to further accumulation of piericidin A1.
2. Materials and methods
2.1. Strains and general techniques
Strains and plasmids used in this study were listed in Table 1. S. piomogeues var. Hangzhouwanensis, the wild type producer of piericidin A1, was used as original strain for construction of pieR interruption and over-expression mutants. Escherichia coli strain BW25113 was used for the construction of pieR mutant, which was manipulated according to the previous protocol [26]. E. coli strain DH10B was used for general cloning [27]. E. coli strain ET12567 carrying plasmid pUZ8002 was used for conjugation with Streptomyces [28]. E. coli BL21 (DE3)/pLysE was used as host for protein expression. pET28a (Novagen) was used as protein expression vectors. pIJ778 was used as template for the amplification of aadA+ oriT cassette for the disruption of pieR. The integrative plasmid pSET152 was used for gene complementation. General genetic manipulation of E. coli or Streptomyces were carried out according to the reported procedure [29,30].
Table 1.
Strains and plasmids used in this study.
| Strain or plasmid | Description | Source |
|---|---|---|
| Strains | ||
| Streptomyces | ||
| S. piomogeues var. Hangzhouwanensis | Wild-type strain producing piericidin A1 | Zhejiang Academy of Agricultural Sciences |
| ΔpieR | a disruption of pieR by PCR targeting | This study |
| ΔpieR-C | ΔpieR::pSET152-pieR, complemental strain | This study |
| ΔpieR::pSET152 | control strain of ΔpieR-C | This study |
| OpieR | WT::pSET152-pieR, over-expression strain | This study |
| WT::pSET152 | control strain of OpieR | This study |
| E.coli strains | ||
| BW25113 | Containing λ RED recombination plasmid | [26,27] |
| BW25113/fosmid 7D9 | Containing fosmid 7D9 | This study |
| ET12567/pUZ8002 | Mediating intergenic conjugation | [28] |
| DH10B | Host for cloning | [27] |
| BL21(DE3) | Host for protein expression | Stratagene |
| Plasmids | ||
| pOJ446 | aac (3)IV, SCP2, reppMB1*, attФC31, oriT | [47] |
| Fosmid7D9 | pOJ446 derivative carrying pieR-A1 gene | Current study |
| pLY-1 | Fosmid7D9 derivative which pieR was partially replaced by spectinomycin resistance gene | This study |
| pIJ778 | Containing spectinomycin resistance marker cat | [26] |
| pSET152 | Integrative plasmid used for complementation | [47] |
| pLY-2 | pSET152 derivative carrying pieR gene | This study |
| pET28a | Protein expression vector | Novagen |
| pLY-3 | pET28a derivative carrying pieR gene | This study |
2.2. Construction of gene disruption, complementation and over-expression mutants
The disruption of pieR was conducted using PCR targeting system based on homologous recombination [26]. Plasmid pIJ778 was used as template for PCR amplification of the 1.4 kb gene disruption aadA+oriT cassette. The resultant fragment was electroporated into E. coli BW25113/fosmid 7D9 (containing the whole pie gene cluster) for the replacement of 462 bp of pieR. The positive mutant was verified by PCR and then, was introduced into S. piomogeues var. Hangzhouwanensis by intergeneric conjugation. The double-crossover strain ΔpieR was obtained from antibiotic selection (spectinomycin) on YMS solid medium and was further confirmed by PCR. The pSET152 derivate plasmid containing the intact pieR was introduced into the ΔpieR and wide type strain for the construction of complementary and overexpression strain, respectively. All the primers used here were listed in Table 2.
Table 2.
Primers used in this study.
| Primer name | Sequence (5′-3′) |
|---|---|
| VLY-1R | GGCGAACCTGCACAGCTACGT |
| VLY-1F | CTCGGCTTTCCTCTTCGTCTCG |
| 16S-R | CATGCAAGTCGAACGGTGAA |
| 16S-F | GCCGTGTCTCAGTCCCAGTG |
| PieE-R | CCGCTGCTCGCCAACCACAT |
| PieE-F | ACCAGCAGCGGCTCCATCAG |
| RA1-R | GGCCGTCCTACGTCCATTCG |
| RA1-F | AGCGGTGGGTTCCCTCCTTC |
| A1A2-R | AGGAGGAGGAGCACGGAAGC |
| A1A2-F | GCGGTTCTGCTGCCGCAGCA |
| A2A3-R | CGACGAGCTGCTCGACATCG |
| A2A3-F | ATGGTCAGCCGCTTGAGGGT |
| A3A4-R | AGCAGTTCCTGACGCCCTAC |
| A3A4-F | TTCTTGTGCCACTTGAGCCT |
| A4A5-R | CGTCAGCTGGTGGTTCTGCT |
| A4A5-F | GGACGACCAACTGGACGAGA |
| A6B1-R | GGCGACCACTTCTCCCTGAC |
| A6B1-F | TGGTGGTCGTTGTCGATGCT |
| B1C-R | GCCCCACCCCTGAAGACCCC |
| B1C-F | TGAGCTGACGCATGCCCTTG |
| CD-R | CCTGGACCGTTTCTTCTTCG |
| CD-F | GTGTCGGTCATGGCGCTCAG |
| DB2-R | GACCTCTACCGCCCGACCAT |
| DB2-F | TTGGTCAGCACGTCGTGGAA |
| B2E-R | GGTGCGCTGGTTCAGCAGGT |
| B2E-F | TCGGCGTTCACAAGGACACG |
| pieR-R | AAAGAATTCTCAGGCGGACCCGGCGAGGCG |
| pieR-F | AAACATATGGTGATTTTCAGTGTTCTTGGCCC |
2.3. Fermentation and detection of piericidin A1
Spores were inoculated into YEME medium (yeast extract, 3 g/l; tryptone, 5 g/l; maltose, 3 g/l; glucose, 10 g/l; sucrose, 103 g/l) in the proportion of 0.1% and cultivated at 30 °C for 3 days. Then, 5 ml seed broth was inoculated into 100 ml fermentation medium and cultivated at 30 °C for another 3 days [17]. After fermentation, add 100 ml acetone into fermentation broth and shake for 12 h to disrupt cell. Next, centrifuge fermentation broth and evaporate the supernatant. After acetone is evaporated, add equally volume of ethyl acetate to extract piericidin A1 twice. Finally, evaporate ethyl acetate to dryness and dissolve the extract in 1 ml methanol. The process of extract and evaporate was conducted in the dark environment in case of the degradation of piericidin A1 [7]. Before detection, the extract was diluted 10 times with methanol and filtrated with 0.22 μm membrane. The resultant methanol extract was analyzed by Agilent HPLC series 1100 with an Agilent ZORBAX SB-C18 column (5 μm, 4.6 × 250 mm). The column was equilibrated with 80% solvent A (H2O) and 20% solvent B (acetonitrile) and developed with a linear gradient (5–35 min, from 20% B to 80% B, 35–40 min, from 80% B to 100% B) and then kept 100% B for 5 min at a flow rate of 0.6 ml/min and UV detection at 232 nm. LC-MS analysis was conducted with Agilent 1100 series LC/MSD Trap system with drying gas flow 10 ml/min, nebulizer 30 psi and drying gas temperature 350 °C. Pure piericidin A1 standard was used as control.
2.4. Growth measurement
Spores were inoculated as described above. 1 ml culture was collected at different time point (0, 3, 6, 9, 12, 24, 36, 48 h) to monitor the OD600 for the depiction of growth curve and another 1 ml culture was centrifuged and dried at 65 °C for biomass measurement.
2.5. RNA isolation, co-transcription analysis and real-time quantitative PCR (RT-qPCR)
1 ml fermentation culture was collected and washed by 1 ml water. After treated with lysozyme for 2 h, cells were disrupted by magnetic beads with 1 ml Redzol (sbs). Then, RNA was extracted according to the procedure provided by manufacturer. The quality of RNA was detected by NanoDrop 2000C (Thermo, USA). Reverse transcription was conducted before co-transcription analysis. Extracted RNA was firstly treated with DNase I for 4 h to eliminate gDNA. Then, reverse transcription experiment was carried out using Revert Aid Minus Fist Strand cDNA Synthesis Kit (Thermo Scientific). 12 pairs of primer listed in Table 2 were synthesized and PCR amplification was used to amplify the intergenic region. Genomic DNA of wild type strain was used as a control. 16s rRNA gene and pieE gene was used as internal reference. The results were analyzed by agarose gel electrophoresis.
RT-qPCR experiment was conducted by 7500 Fast Real-Time PCR System using SYBR Green Master Mix (YEASEN). The transcription level of gene was analyzed according to comparative CT (ΔΔCT).
2.6. Expression and purification of PieR
The primers for cloning pieR were listed in Table 2. After amplified by PCR and digested with NdeI and EcoR I, pieR was cloned into pET-28a. The recombinant plasmid pLY-3 was verified by sequencing. Then, pLY-3 was transformed into E. coli strain BL21 (DE3) for PieR expression. The resultant E. coli BL21 cell was cultured at 37 °C and 220 rpm in LB medium supplemented with kanamycin (final concentration is 50 g/ml) to OD600 = 0.6. Isopropylthio-β-d-galactoside (IPTG) with final concentration 0.4 mM was added into the culture after cooling at 4 °C for 30 min to induce protein expression. The cells were further cultured at 30 °C for 6 h, and then the cells were harvested by centrifugation (×750 g, 25 min, 4 °C) and resuspended in Buffer A (50 mM HEPES, 500 mM NaCl, 10% glycerol, pH 7.0) and lysed by high pressure cracker at 600 bar. Cellular debris was removed by centrifugation (×750 g, 60 min, 4 °C), and the supernatant was used to purify the protein by nickel-affinity chromatography using standard protocols. The protein was eluted with increasing gradient of buffer B (1M imidazole in buffer A). Purified protein was concentrated and exchanged into buffer A with the centrifugal filters (Amicon). The protein was stored in buffer A at −80 °C. Protein concentration was determined with the Bradford assay using bovine serum albumin as a standard.
2.7. Electrophoretic mobility shift assay (EMSA)
For preparation of fluorescence (FAM) labeled probes, FAM-labeled oligos of the promoter regions were PCR amplified with 2x TOLO HIFI DNA polymerase premix (TOLO Biotech, shanghai) using primers of Probe1-F-M13F-47 (FAM) and Probe1-R listed in Table 3. The FAM-labeled probes were purified by the Wizard® SV Gel and PCR Clean-Up System (Promega, USA) and were quantified with NanoDrop 2000C (Thermo, USA). EMSA was performed in a reaction buffer of 50 mM Tris-HCl (pH 8.0), 100 mM KCl, 2.5 mM MgCl2, 0.2 mM DTT, 10% glycerol with 40 ng probes. PieR proteins with final concentration of 0, 2, 5, 10 μg was added, respectively. Meanwhile, 2 μg salmon sperm DNA was also included in the reaction system. After incubation for 30 min at 25 °C, the reaction system was loaded into 2% TBE gel buffered with 0.5×TBE.
Table 3.
Probes used in this study.
| Probe name | Sequence (5′-3′) |
|---|---|
| Probe1-F | CGCCAGGGTTTTCCCAGTCACGACGGCCCGCACCAGAGGAATTC |
| Probe1-R | GGTTTGGTATGGCACGCCCAC |
| M13F-47 | CGCCAGGGTTTTCCCAGTCACGAC |
2.8. DNase I footprinting assay
For preparation of fluorescent FAM labeled probes, PCR amplified the promoter region with 2x TOLO HIFI DNA polymerase premix (TOLO Biotech, Shanghai) from the fosmid 7D9 using primers of M13F-47 (FAM) and probe1-R. The FAM-labeled probes were firstly purified by the Wizard® SV Gel and PCR Clean-Up System (Promega, USA), and then were quantified with NanoDrop 2000C (Thermo, USA). DNase I footprinting assays were performed according to the procedures described before Wang et al. [31]. For each assay, 350 ng probes were incubated with different amounts of proteins (0, 0.6 μg) in a total volume of 40 μl. After incubation at 30 °C for 20 min, 0.015 unit DNase I (Promega) and 100 nmol freshly prepared CaCl2 prepared in another 10 μl solution was added and further incubated at 37 °C for 1 min. The reaction was quenched by adding 140 μl DNase I stop solution (200 mM unbuffered sodium acetate, 30 mM EDTA and 0.15% SDS). Samples were firstly extracted with phenol/chloroform, then precipitated with ethanol. Pellets were dissolved in 30 μl MiniQ water. Methods for preparation of the DNA ladder, electrophoresis and data analysis were the same as described before [31], except that the GeneScan-LIZ600 size standard (Applied Biosystems) was used.
2.9. Multiple sequence alignment and secondary structure prediction
Multiple sequence alignment was conducted using BioEdit software and the referred homologous proteins were listed in Table 4. The prediction of secondary structure of PieR is conducted by PSIPRED v3.3.
Table 4.
Proteins used in the alignment analysis.
| Protein | Strains | Identity |
|---|---|---|
| ARV85759.1PieR | Streptomyces philanthi bv. triangulum | 71% |
| ACE02599.1 AurD | Streptomyces thioluteus | 54% |
| AGG68812.1 TylT | Streptomyces fradiae | 41% |
| BAA14186.1 AfsR | Streptomyces coelicolor | 39% |
| AAV31783.1 SanG | Streptomyces ansochromogenes | 36% |
| ACR48345.1 NosP | Streptomyces actuosus | 33% |
2.10. Phylogenetic analysis
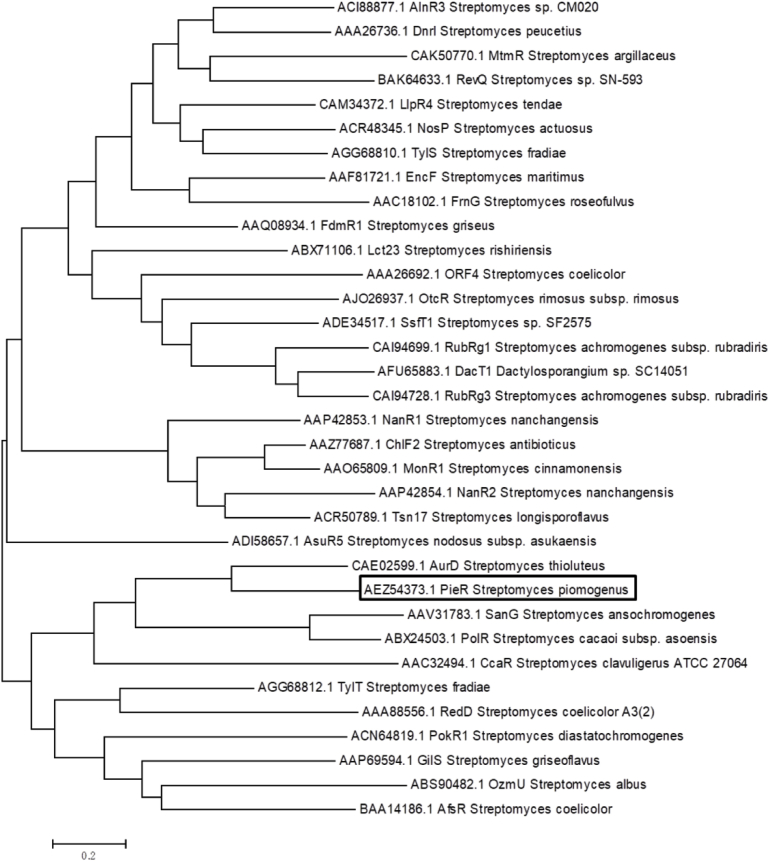
Multiple sequences were firstly aligned using ClustalW and the phylogenetic tree of PieR with other SARPs was generated by MEGA (Version 5.10) using neighbor-joining with Poisson correction and 500 replicate bootstrap analysis [32,33]. The detailed information about the selected SARPs listed as bellow. PieR, from Streptomyces philanthi bv. triangulum (ARV85759.1); AurD, from Streptomyces thioluteus (ACE02599.1); TylT, from Streptomyces fradiae (AGG68812.1); AfsR, from Streptomyces coelicolor (BAA14186.1); SanG, from Streptomyces ansochromogenes (AAV31783.1); NosP, from Streptomyces actuosus (ACR48345.1).
3. Results
3.1. pieR encodes a putative SARP family transcriptional regulator
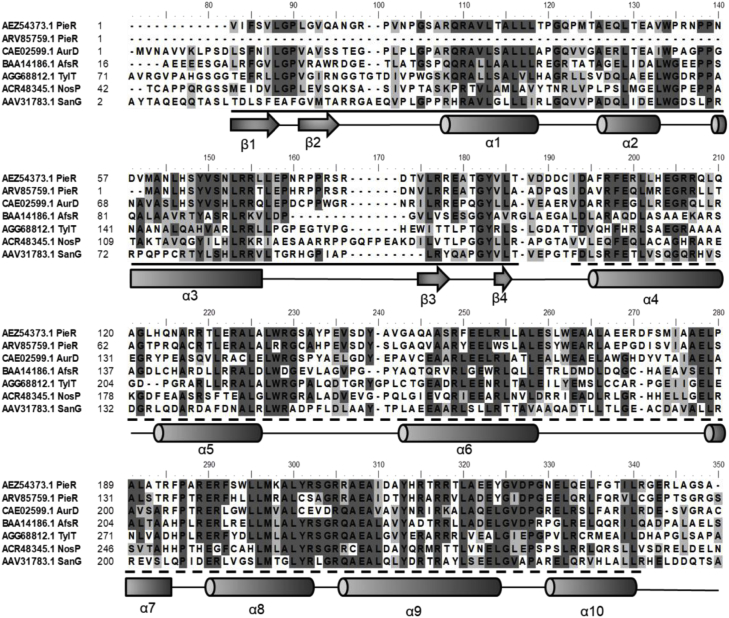
Bioinformatics analysis of PieR revealed that it possessed the conserved DNA binding domain in N terminal that resembles to that of OmpR and bacterial transcription activation domain in C terminal (BTAD domain), which are characteristic of SARP family proteins (Fig. 1). SARPs usually act as pathway-specific regulators directly affecting the transcription of specific gene cluster. Many SARP proteins have been characterized as activators for antibiotics synthesis, such as NosP [34] and PolR [35]. Meanwhile, PieR shows 71% identity with another PieR from another piericidin-producing strain Streptomyces philanthi and 54% identity with AurD from Streptomyces thioluteus (Table 4). It is located upstream of pieA1, which is the first structural gene encoding a putative type I PKS. The secondary structure of PieR was analyzed by PSIPRED. As is shown in Fig. 1, the possible DNA binding domain of PieR covers three α-helixes packed against two antiparallel β-sheets forming the characterized winged helix-turn-helix (HTH) domain [36]. The BTAD domain was proposed to compose seven α-helixes (α4-α10) in accordance with a previous finding [37]. All these data contributed to the assignment of PieR as a typical SARP family transcriptional regulator.
Fig. 1.
Sequence alignment of PieR with other homologous SARP proteins. The HTH domain was underlined with solid line and BTAD domain was underlined with dotted line. The secondary structure element α-helix and β-sheet were indicated by cylinders and arrows, respectively.
3.2. PieR positively activates the production of piericidin A1
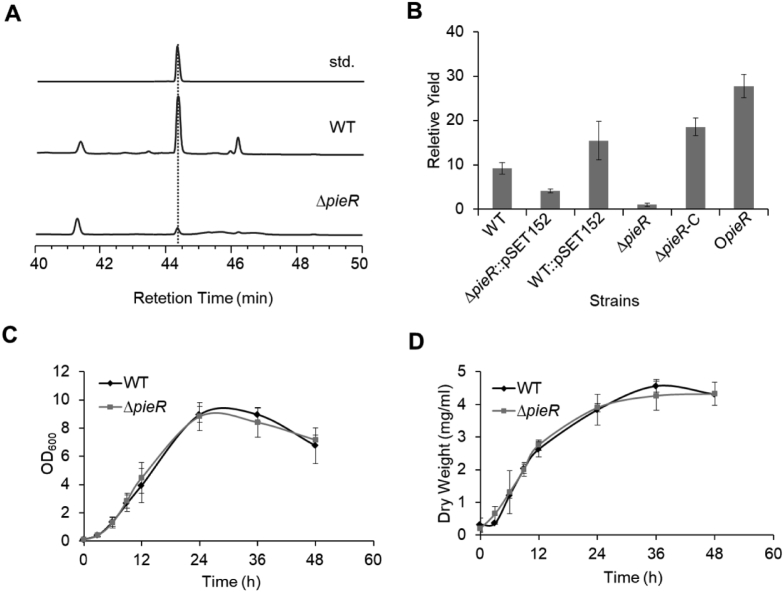
To determine the role of pieR in piericidin biosynthesis, pieR was disrupted via PCR-targeting system, which has been widely applied to gene disruption in Streptomyces (Fig. S1A) and the disruption of pieR was verified by PCR (Fig. S1B). To inspect the effect of the pieR disruption on the production of piericidin A1, the ΔpieR mutant strain and WT strain were cultured in fermentation medium for 3 days and the fermentation product of ΔpieR mutant was firstly qualitatively analyzed by ESI-MS (Fig. S2) and the mass/charge (m/z) signal is consistent with previous study [17]. Then, the production was quantitatively compared to that of WT strain by high-performance liquid chromatography (HPLC). As can be seen from Fig. 2A, the yield of piericidin A1 in ΔpieR was only 11% of the WT strain, suggesting the positive role of pieR in piericidin A1 biosynthesis. To verify that the decreased production of piericidin A1 was directly resulted from the interruption of pieR, a single copy of pieR on integrative plasmid pSET152 was then transferred into ΔpieR strain for the construction of the complementary strain (ΔpieR-C). The production of piericidin A1 in ΔpieR-C strain nearly restored to that of WT strain, deducting the effect of empty plasmid (Fig. 2B). As the overexpression of positive regulatory genes have been proved as an important method for increasing the production of target natural product, to further consolidate the positive role, a copy of pieR was also integrated into the WT strain, resulting in the overexpressing strain (OpieR). After excluding the productivity change exerted by empty plasmid, the piericidin A1 production in OpieR is up to 2.3 times of WT strain (Fig. 2B). To validate that the productivity changes were only induced by the regulatory role of pieR, both of the growth curve and biomass were characterized in the WT and ΔpieR strains (Fig. 2C and D). Consistently, the WT and ΔpieR strains shared similar characters and exhibited negligible differences. Taken together, these results obviously provided sufficient support for the positive regulatory role of pieR in piericidin A1 biosynthesis.
Fig. 2.
Effects of deletion and overexpression of pieR on the production of piericidin A1. (A) HPLC analysis of the production of piericidin A1 in WT and ΔpieR strains. (B) Quantitative analysis of piericidin A1 in WT, ΔpieR, ΔpieR-C, ΔpieR:pSET152, WT:pSET152-pieR and OpieR strains. To facilitate the comparison, the productivity of piericidin A1 in ΔpieR is determined as 1. (C) Characterization of the growth curve of WT and ΔpieR in YEME medium. (D) Biomass assay of WT and ΔpieR in YEME medium.
3.3. PieR activates the transcription of the pie cluster
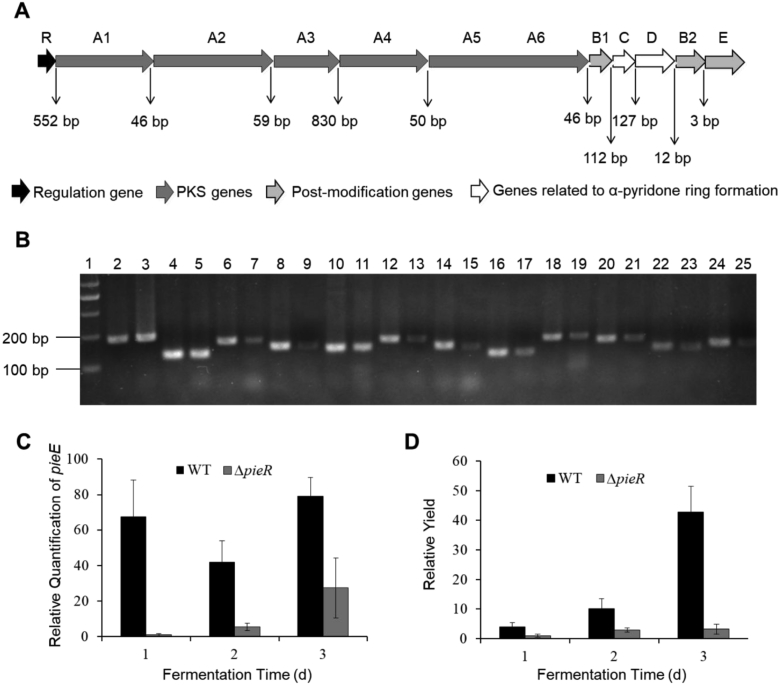
Previous study has revealed that the pie cluster contained 12 genes in the same direction (Fig. 3A). In order to verify the number of operons within this cluster and facilitate the transcriptional analysis of all the genes, one-step RT-PCR was performed using primers listed in Table 2 to detect mRNA spanning different ORFs. All the intergenic gaps between neighboring genes with the same orientation were tested (Fig. 3A), excepting the two genes pieA5 and pieA6 that overlapped 88 bp. The result showed that all the intergenic gaps are positive to RT-PCR amplification. Therefore, all of the genes were organized into one operon and co-transcribed from the same promoter upstream of pieR, forming the pie operon (Fig. 3B). To validate that pieR affected the production of piericidin A1 by regulating the transcription of pie gene cluster, the reverse transcription polymerase chain reaction (RT-PCR) was conducted. The used RNAs were isolated from the ΔpieR and WT strains grown in fermentation medium for every 24 h, respectively. The structural gene pieE was selected as the representative in the transcription of the pie cluster. From the data depicted in Fig. 3C, the transcription level of pie operon of ΔpieR mutant was much lower than that of the WT strain. The transcriptions of pie cluster reached the maximum at the third day in both strains (Fig. 3C). Correspondingly, the time-course analysis of piericidin A1 productivity was also carried out. From Fig. 3D, the yield of piericidin A1 was negligible compared with that of WT strain, and the productivity also reached the highest point at the third day (Fig. 3D). All these data strongly supported that pieR, a potent regulatory gene, activated the piericidin A1 biosynthesis at the transcriptional level.
Fig. 3.
Transcription analysis of the biosynthetic gene cluster of piericidin A1 and productivity analysis in ΔpieR strains. (A) Organization of the genes encoded by pie cluster. Genes are assigned in different colors, regulatory gene is marked in black, PKS genes are in dark gray, genes related to α-pyridone ring formation are in white, while post-modification genes are marked in light gray. The vertical solid arrows showed the position of primers used for RT-qPCR, the numbers represent the lengths (bp) of the intergenic regions between adjacent genes. (B) PCR confirmation of the amplicons fragments on an ethidium bromide-dyed agarose gel. The amplicons were designed to cover the adjacent genes, genomic DNA was used as the positive control. Line 1, Marker; Line 2, genomic DNA used as templet to amplify 16s rRNA; Line 3, cDNA used as templet to amplify 16s rRNA; Line 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, genomic DNA used as template for the amplification of pieE (partial) and the intergenic regions between pieR-A1, pieA1-A2, pieA2-A3, pieA3-A4, pieA4-A5, pieA6-B1, pieB1-C, pieC-D, pieD-B2, pieB2-E, respectively. While line 5, 7, 9, 11, 13, 15, 17,19, 21, 23, 25, cDNA used for the amplification of pieE (partial) and the intergenic regions between pieR-A1, pieA1-A2, pieA2-A3, pieA3-A4, pieA4-A5, pieA6-B1, pieB1-C, pieC-D, pieD-B2, pieB2-E, respectively. (C) The transcription analysis in ΔpieR during fermentation. pieE was selected as representative, 16s rRNA gene as reference and the WT strain was used as control. (D) Time-course analysis of piericidin A1 production in ΔpieR strains. The yield of it in the WT strain was used as control.
3.4. PieR binds specifically to the upstream region of pieR
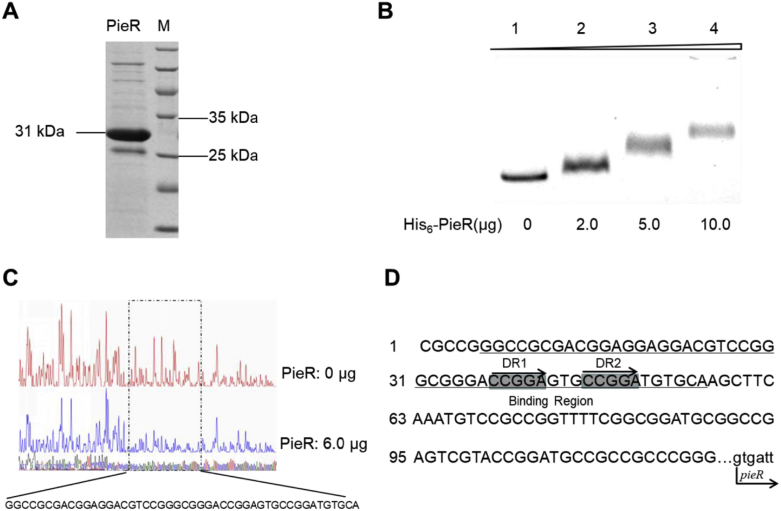
Given the above-revealed positive regulatory role of pieR, to explore the possible regulatory mechanism, pieR was next expressed for the identification of target binding region in vitro. When the previously annotated pieR gene (Genebank HQ840721.1) was expressed in E. coli BL21 (DE3), the protein existed in the form of insoluble inclusion body. The subsequent optimization of culture condition and expressing vectors with different tag failed as well. However, we tried to clone a longer fragment covering the upstream 174 bp and the previously annotated pieR gene. This newly cloned fragment (774 bp) began with GTG and was then cloned into the pET28a plasmid for the construction of pLY3. Fortunately, when this recombinant plasmid was expressed in E. coli BL21 (DE3), an obvious overexpressed protein band (31 kDa) could be detected in the soluble component. Finally, the expressed product could be purified as a His6-PieR recombinant protein (the whole sequence analyzed in Fig. 1). The purity of purified protein was analyzed by SDS-PAGE (Fig. 4A) and the concentration was determined by Bradford assay using bovine serum albumin as a standard. Using the purified PieR, the EMSA experiment was performed according to the protocol described before [31]. The fragment of 326 bp upstream of pie cluster was amplified by PCR with primers probe1-R(5′-GTTTGGTATGGCACGCCCAC-3′) and probe1-F(5′-CGCCAGGGTTTTCCCAGTCACGACGGCCCGCACCAGAGGAATTCG-3′), the resultant fragment was then used as template for the preparation of fluorescent probe with primers M13F-47(5′- CGCCAGGGTTTTCCCAGTCACGAC-3′) and probe1-R(5′-GTTTGGTATGGCACGCCCAC-3′). The resultant fluorescent probe was incubated with purified PieR of gradient concentrations in a typical reaction system, which was composed of 100 mM KCl, 2.5 mM MgCl2, 0.2 mM DTT supplied with 2 μg salmon sperm DNA under room temperature for 30 min. After that, the reaction product was transferred to gel analysis. As was shown in Fig. 4B, the PieR binding to the upstream of pieR and generated significantly shifted bands. DNase I footprinting assay with FAM-labeled primers uncovered one protected region (Fig. 4C) and the binding site is 5′-GGCCGCGACGGAGGAGGACGTCCGGGCGGGACCGGAGTGCCGGATG-TGCA-3' (Fig. 4D). The long protected sequence suggested that DNA secondary structure is important for PieR binding. The sequence analysis revealed two direct repeats 5′-CCGGA-3′ within this binding site (Fig. 4D). According to the typical regulatory mechanisms [23], it was proposed that PieR might directly up-regulate the transcription of the pie operon by acting on the identified binding sites in this region. Therefore, it can conclude that PieR binds to the upstream region of pie cluster and activates the whole gene cluster including itself, resulting in the translation of structural gene and post-modification gene simultaneously. Direct repeats within the target binding region seem to be a common characteristic of SARPs [38]. To explore the relationship of PieR with other SARPs, phylogenetic analysis was conducted. Based on the analysis it can be seen that PieR formed into the same clade with SARP protein AurD and was also close related to other two SARP positive regulators PolR [35] and SanG (Fig. 5).
Fig. 4.
Binding characters of PieR for controlling of the transcription of pie cluster. (A) Purified PieR analyzed by SDS-PAGE. (B) EMSAs for binding of PieR to the upstream region of pieR. The 350 bp FAM-labeled DNA fragment (40 ng) of the upstream region was incubated with increasing concentrations of PieR protein (lanes 2–4; lanes contain 2, 5, 10 μg PieR, respectively). Lane 1, negative control without PieR. The shifted bands are indicated by arrows. (C) Characterization of the direct binding site of PieR by DNase I footprinting. Protected region was indicated. (D) Nucleotide sequence of the PieR-binding sites. The PieR-binding sites are underlined and the direct repeats are marked with gray rectangles. The bent arrows indicate the transcription start points and transcription orientation of pieR.
Fig. 5.
Phylogenetic analysis of PieR with other SARP family proteins.
4. Discussion
Streptomyces are well-known producers of tremendous biological active compounds, and the production of these antibiotics is usually controlled by multiple regulatory proteins, strictly coordinating with their growth and environmental conditions [39]. Transcriptional control that posits the genetic information flow commences with transcription, and accordingly, regulatory tools targeting transcription have received the most attention in terms of tool development and engineering applications. Typically, regulatory proteins act either through pleiotropic or via pathway specific mechanism to control the expression of individual antibiotic gene clusters [40]. Many pathway-specific regulatory proteins belong to SARPs family [34,35], and these proteins were proposed to bind to a direct repeat sequence overlapping with the −35 region of the target promoter [40]. The representative structure of SARPs contains the N-terminal OmpR-like DNA-binding domain, which is in charge of binding with repeated motif and a C-terminal transcriptional activation domain responsible for actuating the transcription of structural gene [41], while some proteins also contain a central ATPase domain with a potential ATP-binding motif [42,43].
Recently, structural analysis has contributed to the revealing of the regulatory mechanism of SARPs [41,44]. Especially, the study of AfsR in Streptomyces coelicolor A3 (2) provided a model for transcriptional activation by SARPs [44]. The AfsR protein acts with DNA and RNA polymerase in a dimer formation to form a complex binding to the −10 promoter region. The ATPase activity is essential for the isomerization of the closed complex between AfsR and RNA polymerase to a transcriptionally competent open complex. However, the regulatory mechanism of other SARP without ATPase, such as the recruitment of RNA polymerase for transcriptional activation still remains elusive.
The biosynthetic gene cluster of piericidin A1 only contains one possible regulatory gene pieR. The bioinformatics analysis of PieR suggested that it is a possible SARP family regulatory protein, containing N terminal conserved DNA binding HTH domain and C terminal BTAD domain, which are characteristic of SARP family proteins. Furthermore, PieR can be grouped into a clade designed “small SARPs” according to the number of amino acids (less than 300 amino acids long) [41]. In this study, the annotated SARP protein-encoding gene was manipulated genetically firstly, and was proved to play potent positive regulatory role in piericidin A1 biosynthesis. Consistently, the over-expression of pieR contributed to the higher yield of piericidin A1. Furthermore, the RT-PCR analysis showed that pieR directly regulated the transcription of all the genes that are organized into one operon. This regulation is concise and facilitates the coordinated expression of the biosynthetic genes to form effective biosynthetic machinery. The identified direct repeats in the possible binding region of PieR through EMSA and DNase I footprinting assay is consistent with the reported binding characteristic of SARP. The phylogenetic analysis of PieR with other SARPs showed that it formed into the same clade with other homologous “small SARP” AurD, while other homologous proteins SanG, NosP, TylT, AfsR spread on different clades. Nevertheless, it was also close related to two “large SARPs” PolR and SanG (Fig. 5). To date, both of pathway-specific and pleiotropic global SARPs have been reported to regulate the biosynthesis of natural products. Most of the SARPs act as activators to positively regulate the transcription of antibiotic gene clusters by binding specific sequence of target promoter, few has also been reported negatively control the biosynthesis, such as FarR4 [45]. Various regulatory modes have been reported, and the regulatory mechanism is strictly dependent on the domain arrangement. PolR and SanG have been reported to positively regulated the biosynthesis of polyoxin in Streptomyces cacaoi subsp. Asoensis [35] and nikkomycin in Streptomyces ansochromogenes [46], respectively. The two SARP proteins SanG and PolR possess three major functional domains: an OmpR-like DNA-binding domain, a central ATP-binding motif and a C-terminal half homologous to the guanylate cyclase domain of the LuxR family. The binded ATP was proposed to stabilize the confirmation of SanG, meanwhile, the ATP hydrolysis activity might account for the inactivation of the target gene. While AfsR, containing a motif similar to the characterized Walker-box ATPases and a C-terminal tetratricopeptide repeat domain, was proved to bind to the promoter region of regulatory gene. However, something is always exceptional. Recently, the “small SARP” NosP has been characterized to activate the nosiheptide production responding to both peptidyl and small-molecule ligands, which is unprecedent in Streptomyces. Considering the diverse regulatory mode, further studies focusing on the mechanism of PieR would facilitate the understanding of SARPs and on this basis set stage for developing potent regulatory elements toward effective synthetic route for valuable intermediates in response to pharmaceutical development.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31630002, 31700029, 31770038, 31470183, 21661140002 and 31170085); the Ministry of Science and Technology; Shanghai Pujiang Program from the Shanghai Municipal Council of Science and Technology (12PJD021); and China Postdoctoral Science Foundation (2017M620151).
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.synbio.2018.12.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 2.Cantrell C.L., Dayan F.E., Duke S.O. Natural products as sources for new pesticides. J Nat Prod. 2012;75:1231–1242. doi: 10.1021/np300024u. [DOI] [PubMed] [Google Scholar]
- 3.Hwang K.S., Kim H.U., Charusanti P., Palsson B.O., Lee S.Y. Systems biology and biotechnology of Streptomyces species for the production of secondary metabolites. Biotechnol Adv. 2014;32:255–268. doi: 10.1016/j.biotechadv.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Tuson R.V. XXII.—note on an alkaloïd contained in the seeds of the Ricinus communis, or castor-oil plant. J Chem Soc. 1864;17:195–197. [Google Scholar]
- 5.Isaka M., Tanticharoen M., Kongsaeree P., Thebtaranonth Y. Structures of cordypyridones A-D, antimalarial N-hydroxy- and N-methoxy-2-pyridones from the insect pathogenic fungus Cordyceps nipponica. J Org Chem. 2001;66:4803–4808. doi: 10.1021/jo0100906. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson J.M., Hanson J.R., Hitchcock P.B., Claydon N. Structure and biosynthesis of harzianopyridone, an antifungal metabolite of Trichoderma harzianum. J Chem Soc Perkin Trans. 1989;1:1885–1887. [Google Scholar]
- 7.Gui C., Li Q., Mo X., Qin X., Ma J., Ju J. Discovery of a new family of Dieckmann cyclases essential to tetramic acid and pyridone-based natural products biosynthesis. Org Lett. 2015;17:628–631. doi: 10.1021/ol5036497. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi N., Suzuki A., Tamura S. Structure of piericidin A. J Am Chem Soc. 1965;87:2066–2068. doi: 10.1021/ja01087a050. [DOI] [PubMed] [Google Scholar]
- 9.Kroiss J., Kaltenpoth M., Schneider B., Schwinger M., Hertweck C., Maddula R. Symbiotic Streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat Chem Biol. 2010;6:261–263. doi: 10.1038/nchembio.331. [DOI] [PubMed] [Google Scholar]
- 10.Shaaban K.A., Helmke E., Kelter G., Fiebig H.H., Laatsch H. Glucopiericidin C: a cytotoxic piericidin glucoside antibiotic produced by a marine Streptomyces isolate. J Antibiot. 2010;64:205–209. doi: 10.1038/ja.2010.125. [DOI] [PubMed] [Google Scholar]
- 11.Gutman M., Singer T., Beinert H., Casida J. Reaction sites of rotenone, piericidin A, and amytal in relation to the nonheme iron components of NADH dehydrogenase. Proc Natl Acad Sci U S A. 1970;65:763–770. doi: 10.1073/pnas.65.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall C., Wu M., Crane F.L., Takahashi H., Tamura S., Folkers K., Piericidin A. A new inhibitor of mitochondrial electron transport. Biochem Biophys Res Commun. 1966;25:373–377. doi: 10.1016/0006-291x(66)90214-2. [DOI] [PubMed] [Google Scholar]
- 13.Hwang J.H., Kim J.Y., Cha M.R., Ryoo I.J., Choo S.J., Cho S.M. Etoposide-resistant HT-29 human colon carcinoma cells during glucose deprivation are sensitive to piericidin A, a GRP78 down-regulator. J Cell Physiol. 2008;215:243–250. doi: 10.1002/jcp.21308. [DOI] [PubMed] [Google Scholar]
- 14.Kitagawa M., Ikeda S., Tashiro E., Soga T., Imoto M. Metabolomic identification of the target of the filopodia protrusion inhibitor glucopiericidin A. Chem Biol. 2010;17:989–998. doi: 10.1016/j.chembiol.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Kang J.E., Han J.W., Jeon B.J., Kim B.S. Efficacies of quorum sensing inhibitors, piericidin A and glucopiericidin A, produced by Streptomyces xanthocidicus KPP01532 for the control of potato soft rot caused by Erwinia carotovora subsp. atroseptica. Microbiol Res. 2016;184:32–41. doi: 10.1016/j.micres.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Lipshutz B.H., Amorelli B. Total synthesis of piericidin A1. Application of a modified negishi carboalumination-nickel-catalyzed cross-coupling. J Am Chem Soc. 2009;131:1396–1397. doi: 10.1021/ja809542r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q., Yao F., Chooi Y.H., Kang Q., Xu W., Li Y. Elucidation of Piericidin A1 biosynthetic locus revealed a thioesterase-dependent mechanism of alpha-pyridone ring formation. Chem Biol. 2012;19:243–253. doi: 10.1016/j.chembiol.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y., Zhang W., Zhu Y., Zhang Q., Tian X., Zhang S. Elucidating hydroxylation and methylation steps tailoring piericidin A1 biosynthesis. Org Lett. 2014;16:736–739. doi: 10.1021/ol4034176. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Rodriguez A., Robledo-Casados I., Sanchez S. An overview on transcriptional regulators in Streptomyces. Biochim Biophys Acta. 2015;1849:1017–1039. doi: 10.1016/j.bbagrm.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Chen J., Liu M., Liu X., Miao J., Fu C., Gao H. Interrogation of Streptomyces avermitilis for efficient production of avermectins. Synth Syst Biotechnol. 2016;1:7–16. doi: 10.1016/j.synbio.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engstrom M.D., Pfleger B.F. Transcription control engineering and applications in synthetic biology. Synth Syst Biotechnol. 2017;2:176–191. doi: 10.1016/j.synbio.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W., Zhang Q., Guo J., Chen Z., Li J., Wen Y. Increasing avermectin production in Streptomyces avermitilis by manipulating the expression of a novel TetR-family regulator and its target gene product. Appl Environ Microbiol. 2015;81:5157–5173. doi: 10.1128/AEM.00868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin J.F., Liras P. Engineering of regulatory cascades and networks controlling antibiotic biosynthesis in Streptomyces. Curr Opin Microbiol. 2010;13:263–273. doi: 10.1016/j.mib.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Wu H., Wang Y., Yuan L., Mao Y., Wang W., Zhu L. Inactivation of SACE_3446, a TetR family transcriptional regulator, stimulates erythromycin production in Saccharopolyspora erythraea. Synth Syst Biotechnol. 2016;1:39–46. doi: 10.1016/j.synbio.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei J., He L., Niu G. Regulation of antibiotic biosynthesis in actinomycetes: perspectives and challenges. Synth Syst Biotechnol. 2018;3:229–235. doi: 10.1016/j.synbio.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gust B., Challis G.L., Fowler K., Kieser T., Chater K.F. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci U S A. 2003;100:1541–1546. doi: 10.1073/pnas.0337542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Datsenko K.A., Wanner B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paget M.S., Chamberlin L., Atrih A., Foster S.J., Buttner M.J. Evidence that the extracytoplasmic function sigma factor sigmaE is required for normal cell wall structure in Streptomyces coelicolor A3(2) J Bacteriol. 1999;181:204–211. doi: 10.1128/jb.181.1.204-211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrock J., Russel D.W. Cold Spring Harbor Laboratory Press; cold spring harbor, NY: 2001. Molecular cloning: a laboratory manual. [Google Scholar]
- 30.Kieser T., Bibb M.J., Buttner M.J., Chater K.F., Hopwood D.A. John Innes Foundation; Norwich, UK: 2000. Practical Streptomyces genetics. [Google Scholar]
- 31.Wang Y., Cen X.F., Zhao G.P., Wang J. Characterization of a new GlnR binding box in the promoter of amtB in Streptomyces coelicolor inferred a PhoP/GlnR competitive binding mechanism for transcriptional regulation of amtB. J Bacteriol. 2012;194:5237–5244. doi: 10.1128/JB.00989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson J.D., Higgins D., Gibson T.J., CLUSTAL W. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J., Li Y., Niu G., Guo H., Qiu Y., Lin Z. NosP-regulated nosiheptide production responds to both peptidyl and small-molecule ligands derived from the precursor peptide. Cell Chem Biol. 2018;25:143–153 e4. doi: 10.1016/j.chembiol.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Li R., Xie Z., Tian Y., Yang H., Chen W., You D. polR, a pathway-specific transcriptional regulatory gene, positively controls polyoxin biosynthesis in Streptomyces cacaoi subsp. asoensis. Microbiology. 2009;155:1819–1831. doi: 10.1099/mic.0.028639-0. [DOI] [PubMed] [Google Scholar]
- 36.Maris A.E., Walthers D., Mattison K., Byers N., Kenney L.J. The response regulator OmpR oligomerizes via beta-sheets to form head-to-head dimers. J Mol Biol. 2005;350:843–856. doi: 10.1016/j.jmb.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 37.Alderwick L.J., Molle V., Kremer L., Cozzone A., Dafforn T., Besra G. Molecular structure of EmbR, a response element of Ser/Thr kinase signaling in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2006;103:2558–2563. doi: 10.1073/pnas.0507766103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu G., Chater K.F., Chandra G., Niu G., Tan H. Molecular regulation of antibiotic biosynthesis in Streptomyces. Microbiol Mol Biol Rev. 2013;77:112–143. doi: 10.1128/MMBR.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bibb M.J. Regulation of secondary metabolism in streptomycetes. Curr Opin Microbiol. 2005;8:208–215. doi: 10.1016/j.mib.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 40.Takano E. Gamma-butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Curr Opin Microbiol. 2006;9:287–294. doi: 10.1016/j.mib.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Rhee J.E., Sheng W., Morgan L.K., Nolet R., Liao X., Kenney L.J. Amino acids important for DNA recognition by the response regulator OmpR. J Biol Chem. 2008;283:8664–8677. doi: 10.1074/jbc.M705550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li R., Liu G., Xie Z., He X., Chen W., Deng Z. PolY, a transcriptional regulator with ATPase activity, directly activates transcription of polR in polyoxin biosynthesis in Streptomyces cacaoi. Mol Microbiol. 2010;75:349–364. doi: 10.1111/j.1365-2958.2009.06968.x. [DOI] [PubMed] [Google Scholar]
- 43.Anton N., Mendes M., Martin J., Aparicio J.F. Identification of PimR as a positive regulator of pimaricin biosynthesis in Streptomyces natalensis. J Bacteriol. 2004;186:2567–2575. doi: 10.1128/JB.186.9.2567-2575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka A., Takano Y., Ohnishi Y., Horinouchi S. AfsR recruits RNA polymerase to the afsS promoter: a model for transcriptional activation by SARPs. J Mol Biol. 2007;369:322–333. doi: 10.1016/j.jmb.2007.02.096. [DOI] [PubMed] [Google Scholar]
- 45.Kurniawan Y.N., Kitani S., Maeda A., Nihira T. Differential contributions of two SARP family regulatory genes to indigoidine biosynthesis in Streptomyces lavendulae FRI-5. Appl Microbiol Biotechnol. 2014;98:9713–9721. doi: 10.1007/s00253-014-5988-9. [DOI] [PubMed] [Google Scholar]
- 46.He X., Li R., Pan Y., Liu G., Tan H. SanG, A transcriptional activator, controls nikkomycin biosynthesis through binding to the sanN-sanO intergenic region in Streptomyces ansochromogenes. Microbiology. 2010;156:828–837. doi: 10.1099/mic.0.033605-0. [DOI] [PubMed] [Google Scholar]
- 47.Bierman M., Logan R., O'Brien K., Seno E.T., Rao R.N., Schoner B.E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.