Abstract
Background
Although HIV self-testing strategies have been recommended by the World Health Organization, HIV self-tests are not yet approved in Canada. Currently approved HIV self-tests offer toll-free lines that are insufficient for initiating expedited linkages to counseling and care, accurate interpretation, and support during HIV self-testing. We developed an innovative, multilingual software app called HIVSmart! to plug these gaps.
Objective
This study aimed to test our app-optimized oral HIV self-testing strategy for feasibility in men who have sex with men (MSM) who presented to test at a large sexual health clinic (Clinique Médicale L’Actuel) in Montreal.
Methods
Between July 2016 and February 2017, we offered a strategy consisting of the OraQuick In-Home HIV Test (an investigational device) and a tablet installed with the HIVSmart! app to study participants, who presented at a private office in the clinic, mimicking an unsupervised home environment. We evaluated the strategy for its feasibility, acceptability, and preference. Using the HIVSmart! app, participants were guided through the self-testing process. We determined feasibility with a metric defined as the completion rate, which consisted of the following 3 steps: (1) self-test conduct; (2) self-test interpretation; and (3) linkages to care. Participants independently performed, interpreted, recorded their self-test and result, engaged in pre- and posttest counseling, and sought linkages to care. Laboratory tests (p24, Western Blot, and RNA), as per country algorithms, were expedited, and linkages based on the rapid test status were arranged.
Results
Mean age of the 451 participants enrolled was 34 (range, 18-73) years. Of all participants, 97.1% (438/451) completed and submitted the survey through the HIVSmart! app. In total, 84.7% (371/438) of the participants were well educated (beyond high school) and 52.5% (230/438) had been tested within the past 6 months. Of the 451, 11.5% (52/451) were on pre-exposure prophylaxis. Feasibility (completion rate), an average proportion of the 3 steps, was computed to be 96.6% (419/451). The acceptability of the strategy was high at 98.5% (451/458). A majority of the participants (448/451, 99.3%) were found to be self-tested and lab-confirmed negative and were counseled after self- and rapid tests. In total, 0.7% (3/451) of the participants who self-tested positive and were lab-confirmed positive were linked to a physician within the same day. Furthermore, 98.8% (417/422) of the participants found the app to be useful and 94.0% (424/451) were willing to recommend it to a friend or partner.
Conclusions
The HIVSmart! app-optimized strategy was feasible, accepted, and preferred by an educated, urban MSM population of Montreal. With the app, participants were able to perform, interpret, store results, and get rapidly linked to care. The HIVSmart!-optimized, self-testing strategy could be adapted and contextualized to many at-risk populations within Canada and worldwide, thereby maximizing its public health impact.
Keywords: feasibility, HIV, impact, mobile phone, MSM, self-testing
Introduction
In 2014, the Joint United Nations Programme on HIV/AIDS released its 90-90-90 targets, calling for 90% of those living with HIV to be tested, 90% of those tested positive to be on treatment, and 90% on those on treatment to remain virologically suppressed [1]. To reach the first 90 by 2020, it is imperative that we identify those who are living unaware of their HIV status. One such strategy that has the potential to reach the undiagnosed is HIV self-testing. In 2016, the World Health Organization recommended the scale-up of self-testing as an additional alternative to the conventional HIV testing services [2,3]. HIV self-tests are now available for use, sale, and distribution in many countries. More than 59 countries have HIV self-testing policies in place [4-7]. The benefits of self-testing include privacy, confidentiality, convenience, engagement in self-care, empowerment, and proactivity in seeking health [8]. The potential concerns with HIV self-testing are the rapid establishment of linkages for self-testers to counseling and care, rapid reporting of false-negative results in the acute window period, and affordability of self-tests. Nonetheless, benefits of HIV self-testing are generally understood to outweigh the associated risks [8,9].
Evidence and data on self-testing in Canada are sparse [10-12]. Neither oral nor blood-based tests are approved yet by Health Canada [4]. Canadian policies on HIV self-testing are in development. Recently, we surveyed Canadian stakeholders involved in HIV testing initiatives across the country and found that many were in favor of self-testing but were concerned about linkages to care and accuracy of self-tests. Furthermore, HIV self-testing strategies were perceived to pose an economic threat to the prevailing HIV testing models in Canada [13]. While global research on the implementation of HIV self-testing has increased exponentially, few studies have explored self-testing in the Canadian context. To date, a hypothetical self-testing study used focus groups or surveys of attitudes and acceptability, while another evaluated a self-testing strategy in a low-risk student population [11,12]. Thus, implementation research evidence on HIV self-testing is needed for Canada.
In any setting, understanding the context in which self-testing should be implemented is critical to its success. In Canada, the HIV epidemic is disproportionally represented in key populations, such as men who have sex with men (MSM), injection drug users (IDUs), Aboriginal populations, and immigrants from HIV endemic countries. Approximately 18%-25% of Canadian MSM populations are unaware of their HIV-positive status [14], and the number may be proportionally higher for IDUs, Aboriginals, and immigrants, which underscores the need for accessible HIV self-testing services.
Methods
HIVSmart!
HIVSmart! (Figure 1), a Canadian innovation funded by Grand Challenges Canada [15], (which is funded by the Government of Canada), is a multiplatform smartphone-, tablet-, or Web-based (Android, iPhone, and iPad) confidential software app. This study was funded by the Canadian Institutes of Health Research [16]. HIVSmart! plugs gaps in the self-testing process, works with any approved HIV self-test, engages, and proactively informs any intended user of an HIV self-test. It interprets and stores data confidentially, links users to counseling or care within a rapid turnaround time, and encourages them to stay in care. For this study, we adapted the HIVSmart! app-based program for Canadians. Reverse innovation entailed language adaptations (French-Canadian) and customizations to the US Food and Drug Administration-approved oral self-test products and obtaining Health Canada’s Investigational Testing Authorization for research.
Figure 1.
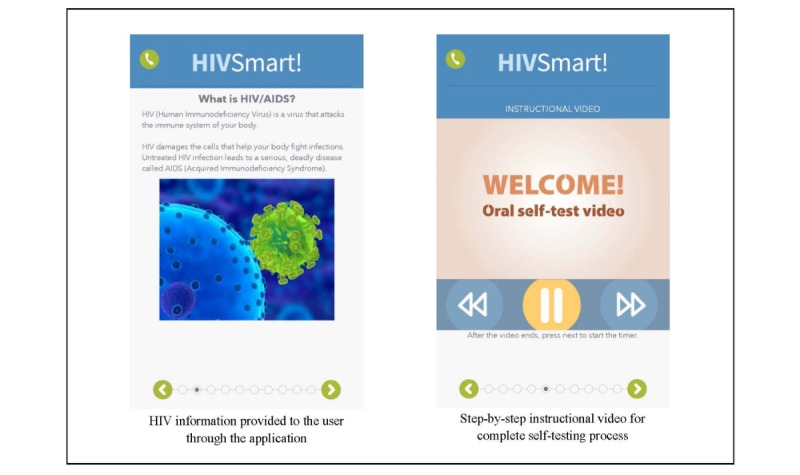
Screenshots of the HIVSmart! app.
HIVSmart! is unique in that it is a complete app-based solution. It is an improvement from the Web prototype of HIVSmart!, which was initially evaluated in health care professionals of South Africa and students of McGill University [12,17]. This novel app-based solution is currently being tested at scale in South Africa [18].
Study Participants and Design
Between July 2016 and February 2017, we conducted a cross-sectional feasibility study at Clinique Médicale L’Actuel, a private, sexual health clinic specializing in testing and treatment of HIV and sexually transmitted infections, located in urban Montreal. Ethical approvals were obtained from Clinique L’Actuel’s independent review board (Veritas Institutional Review Board), and the McGill University Health Centre Research Ethics Board. Study procedures were duly followed and complied with the regulations stipulated by the Institutional Review Boards of McGill University Health Centre and Veritas.
In high risk, MSM populations presenting to the clinic, we set out to achieve the following objectives. We aimed to evaluate the feasibility, acceptability, and preference for an unsupervised HIV self-testing strategy that involved a self-test (OraQuick In-Home HIV Test) and an accompanying optimized app (HIVSmart!) in an unsupervised setting that mimicked a home environment.
Participants were recruited by convenience sampling; clinic staff recruited participants during routine and drop-in clinic visits. The study was advertised via flyers, social media, and through e-newsletters.
Participants were deemed eligible to join the study if they were 18 years or older, self-identified as an MSM, of unknown HIV status, and comfortable using smartphones or tablets. Participants receiving pre- or postexposure prophylaxis (PrEP or PEP) antiretrovirals for prevention (ARVs) were also invited to participate so as to not exclude these high-risk groups. Participants were excluded if they self-reported a previously confirmed positive HIV diagnosis.
A flow chart outlining the study flow is represented in Figure 2. Once deemed eligible to participate, the study procedure was explained to participants. Informed consent (written) was obtained from all participants before beginning the study. Participants could withdraw consent at any point throughout the study. At this time, participants were informed about the strategy that involved deidentified data collection on a confidential, compliant secure server.
Figure 2.
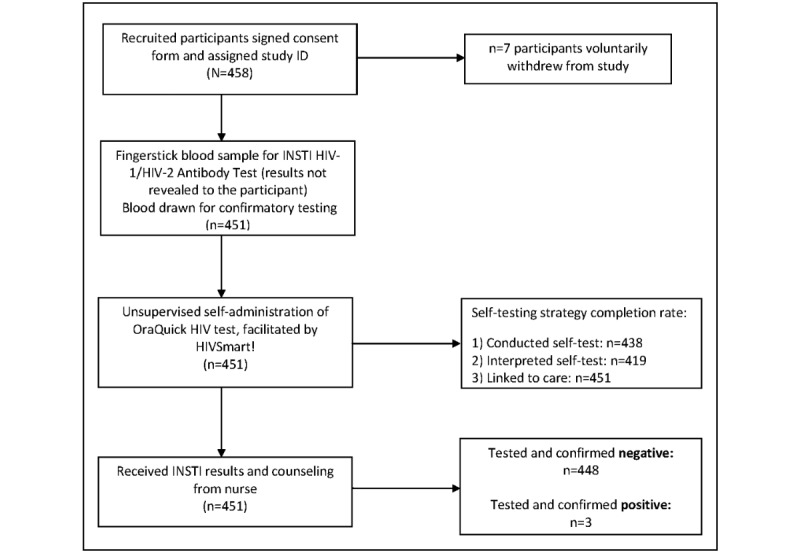
Flow of study participants. ID: identifier.
Study Procedure
As per Health Canada’s product monographs and Investigational Testing Authorization recommendations, the usual clinic procedures were followed in the study. Following participants’ informed consent and agreement to participate, participants were explained about data storage and confidentiality. Following this, a rapid test (INSTI HIV-1/HIV-2 Antibody Test; bioLytical Laboratories) was performed by the research nurse. However, the rapid test result was not revealed to participants at this time. Blood was drawn for laboratory-based confirmatory testing, following the clinic’s protocol (Centre hospitalier de l’Université de Montréal): p24 antigen testing and anti-HIV-1/2 testing for all samples, confirmed by Western Blot if abnormal. Samples underwent RNA testing in case of suspected acute HIV infection. Lab testing conformed to the national testing algorithms.
Participants were then brought to a private office in the clinic and provided the OraQuick Test kit along with a tablet installed with HIVSmart!. The process of self-testing went thus: participants were left alone (unsupervised) to navigate the app on the tablet, stage their personal risk for HIV, perform the self-test as per instructions on HIVSmart!, and conduct the test unsupervised, mimicking a home environment. All participants followed instructions on pretest counseling, staging, conducting results, and storing their results on screen. Participants were asked to wait for their test result using a timer built into the app. They were encouraged to use the phone number provided in the app to call for counseling or assistance at any time and respond to a research questionnaire within the app. Upon completion of the self-testing process, participants were offered the choice of calling the provided phone number to receive posttest counseling or receiving face-to-face posttest counseling with a research nurse. Phone-based counseling was rapidly followed by face-to-face counseling with the research nurse. Upon meeting with the research nurse, who interpreted and recorded the self-test results in real-time separately, the INSTI rapid tests results were provided to participants and they were offered posttest counseling based on their INSTI results. Participants with positive HIV results were seen by a physician immediately for follow-up care. Participants with negative self-tests were encouraged to return for retesting in 3 months. As per the Institutional Review Board recommendations, all participants were offered a modest compensation for their time (Can $20).
Outcomes and Metrics
The primary outcome of this study was the feasibility of the HIVSmart! self-testing strategy. Feasibility was documented by the metric completion rate, defined as the number of participants who successfully completed all steps of the self-testing procedure, over the total number of consenting participants. Steps were defined as follows: (1) self-test conduct; (2) self-test interpretation; and (3) seeking linkage to care. It was computed as an average proportion of the completion for each step in the procedure.
Secondary outcomes were patient-centered outcomes (acceptability of the strategy, preference for counseling, and costs) that were collected using the metrics listed below:
Acceptability: the number of participants who consented to evaluate the strategy, over the total number of people approached for recruitment. Refusals were documented by staff at the clinic.
Preference for (counseling): participants were asked to respond to a questionnaire, regarding preferences for suitable posttest counseling options, in a hypothetical scenario where self-tests were available for purchase in pharmacies.
Preference for (cost): participants were asked in a questionnaire to select a price point at which self-tests should be made available in Canada (<Can $10, Can $10-20, Can $20-40, and >Can $40).
Usefulness of the app: participants were asked if the app was found to be useful in assisting them in the self-testing process.
Recommendations to friends or partners: participants were asked if the app would be recommended by them to their friends or partners.
Data Analyses and Sample Size Estimations
Our sample size was estimated using the completion rate as the main outcome. Estimating a conservative completion rate of 70% with a CI width of 10% (SD 0.0565%), we calculated a target sample size of 400.
Basic proportions for demographics and outcomes obtained through the HIVSmart! app were computed with their 95% CIs. A stratified analysis comparing participants receiving ARVs (PrEP or PEP) versus no ARVs was conducted for the main feasibility outcome variables with Fisher’s exact test, which determines significant differences between groups (StataCorp, 2013, Stata Statistical Software: Release 13, StataCorp LP).
Results
Participant Characteristics
A total of 451 participants met the eligibility criteria and accepted to evaluate the HIVSmart! self-testing strategy (Table 1); 97.1% (438/451) of the participants submitted the HIVSmart! survey (Table 2).
Table 1.
Descriptive characteristics of study participants.
| Characteristics | Value (N=451) | |
| Age (years), mean (range) | 33.6 (32.6-34.7) | |
| Antiretrovirals for prevention status, n (%) |
|
|
|
|
None | 394 (87.4) |
|
|
Postexposure prophylaxis | 5 (1.1) |
|
|
Pre-exposure prophylaxis | 52 (11.5) |
Table 2.
Participant information collected through the HIVSmart! survey (n=438).
| Information | Value, n (%) | |
| Gender (self-identified) | ||
|
|
Male | 432 (98.6) |
|
|
Transgender | 2 (0.5) |
|
|
I do not wish to answer | 3 (0.7) |
|
|
Other | 1 (0.2) |
| Sexual orientation | ||
|
|
Homosexual | 400 (91.3) |
|
|
Bisexual | 32 (7.3) |
|
|
Heterosexual | 4 (0.9) |
|
|
I do not wish to answer | 1 (0.2) |
|
|
Other | 1 (0.2) |
| Highest level of education | ||
|
|
High school degree not completed | 13 (3.0) |
|
|
High school | 51 (11.6) |
|
|
College or technical school | 125 (28.5) |
|
|
Undergraduate degree | 169 (38.6) |
|
|
Graduate degree (master’s or PhD) | 77 (17.6) |
|
|
Other | 1 (0.2) |
|
|
I do not wish to answer | 2 (0.5) |
| Employment status | ||
|
|
Unemployed | 46 (10.5) |
|
|
Employed | 348 (79.5) |
|
|
Other | 36 (8.2) |
|
|
I do not wish to answer | 8 (1.8) |
| Tested for HIV in the past 6 months | ||
|
|
No, I’ve never been tested | 25 (5.7) |
|
|
Yes, in the last 6 months | 230 (52.5) |
|
|
Yes, more than 6 months ago | 178 (40.6) |
|
|
I have been tested, but did not want to receive my result | 1 (0.2) |
|
|
I do not know if I have ever been tested | 3 (0.7) |
|
|
I do not wish to answer | 1 (0.2) |
| Sexually active | ||
|
|
No | 16 (3.7) |
|
|
Yes | 421 (96.1) |
|
|
I do not wish to answer | 1 (0.2) |
| Number of different sexual partners in the past 6 months | ||
|
|
0 | 20 (4.6) |
|
|
1 | 38 (8.7) |
|
|
2-5 | 174 (39.7) |
|
|
6-10 | 110 (25.1) |
|
|
≥11 | 96 (21.9) |
| In the past 6 months: | ||
|
|
Had sex without a condom | 307 (70.1) |
|
|
Had sex with an HIV-infected partner | 74 (16.9) |
|
|
Had sex with a sex worker | 18 (4.1) |
|
|
Had sex under the influence of alcohol | 195 (44.5) |
|
|
Had sex under the influence of drugs | 83 (18.9) |
|
|
Had sex with multiple partners | 219 (50.5) |
|
|
Injected drugs (excluding medicine) | 5 (1.1) |
| Exposure to HIV (eg, needles) in the workplace in the past 6 months | ||
|
|
No | 426 (97.3) |
|
|
Yes | 10 (2.3) |
|
|
I do not wish to answer | 2 (0.5) |
Participants were well educated, with 84.7% (371/438) educated beyond high school; 79.5% (348/438) were employed, and 52.5% (230/438) had been tested in the past 6 months. Participants self-identified themselves as male (98.6%, 432/438) and homosexual (91.3%, 400/438); 96.1% (421/438) of participants were sexually active, with 25.1% (110/438) stating 6-10 partners in the past 6 months; 21.9% (96/438) stating ≥11; and 70.1% (307/438) engaged in condom-less sex. In total, 11.5% (52/451) participants were currently taking PrEP and 1.1% (5/451) were taking PEP.
Acceptability was high at 98.5% (451/458); 7 participants refused to participate or withdrew themselves from the study.
Feasibility, as documented by the completion rate of the HIVSmart! self-testing strategy, was computed by taking an average of the 3 steps of the self-testing strategy—(1) self-test conduct; (2) self-test interpretation; and (3) linkages to care.
Self-test conduct: 97.1% (438/451) of the participants conducted the self-test successfully.
Self-test interpretation: 92.9% (419/451) of participants interpreted their self-test successfully.
Linkages to care: 100% (451/451) of the participants sought linkages to care successfully.
To compute feasibility, we took an average proportion of the 3 steps highlighted above, resulting in the overall feasibility of 96.7%.
Regarding Test Interpretations
Incomplete test conduct occurred in the initial set-up stages of the study when participants were unable to submit their results through the app because of Wi-Fi connectivity issues. These were later resolved by resolving incompatibility issues of the app and the clinic’s Wi-Fi server.
Regarding test interpretations, a few participants mistakenly interpreted their negative result (the control line) as their positive result, despite instructions. An invalid result was also recorded that was truly negative.
Regarding test results, 3 participants tested positive for HIV (0.7% seropositivity) with both the self-test and rapid test, which were rapidly confirmed by laboratory results, and the participants were linked to a physician within the same day (linkage: 3/3, 100%) and returned for a follow-up appointment. All negative rapid and self-test results (448/451, 99.3%) were confirmed negative through laboratory testing, and all were linked to counseling (448/448, 100%). Lab testing conformed to the national testing algorithms.
Regarding linkages, all participants (451/451, 100%) were linked to in-person counseling following the self-testing procedure. All participants used the phone line and later met the research nurse. The average turnaround time to linkage to counseling ranged from 2 to 6 hours.
Regarding the preference for counseling, participants were in favor of counseling over the phone, followed by face-to-face counseling in a clinic. Some favored counseling over the internet (chat or website; 132/421, 31.4%) or in a pharmacy (121/421, 28.7%), both followed by face-to-face counseling in clinic and counseling in clinics only (132/421, 31.4%). Participants were generally not in favor of no face-to-face counseling (28/421, 6.7%) or no counseling at all (3/421, 0.7%).
Regarding cost preferences, half of the participants (206/421, 48.8%) selected Can $10-20 and 27.4% (115/421) selected <Can $10. In terms of the usefulness of the app, 98.8% (417/422) of the participants found the app helpful in guiding them through the self-testing process. Finally, 94.3% (395/419) of the participants said that they would recommend this self-testing strategy to their partner.
Discussion
Principal Findings
In this Canadian Institutes of Health Research funded innovative Canadian study, we investigated the feasibility of implementing an app-optimized unsupervised HIV self-testing strategy in a clinical setting.
Our unsupervised self-testing strategy was found to be feasible to operationalize (419/451, 96.6%), was well accepted (451/458, 98.5%), and preferred by participants. HIV was detected, and all participants were linked to care within a working day. All of our self-testers were linked to counseling or directed to a physician within hours, an essential service to offer with self-testing. Participants found the app-based approach to be a useful (417/422, 98.8%) in completing the HIV self-testing procedure, and a majority (395/419, 94.3%) wanted to recommend it to their friend.
This app-optimized, self-testing strategy was aimed to plug gaps in the self-testing process that are associated with an accurate detection, self-test interpretation, and rapid initiation of linkages to counseling and care. We were limited by the lack of approved HIV self-tests in Canada, which restricted our evaluation to a clinic environment [19-21] instead of homes. To stimulate a home environment, we set up kiosks in clinics where participants could test unsupervised, yet a nurse was always available to offer counseling and support [22]. Regarding costs, we found that a majority of participants believed that an acceptable price to pay for an HIV self-test in Canada was between Can $10 and $20. This finding is in line with 3 studies from New York City, where participants felt that an affordable and accessible price point was US $15-$25 (compared with the prevailing US $40) [23-25].
This is the first Canadian study to report data on the use of an app-based strategy. Many digital innovations (ie, Web-based programs, kiosk-based tablets, and short message service [SMS] text messaging services) are available, yet a complete, portable, and patient-friendly app-based solution for self-testing from engagement to linkage to counseling and care is novel [26].
In 5 studies that have evaluated some digital innovations, we observed a few limitations in their offer of services that impacted the process. A Dutch study evaluated a Web-based strategy, where participants could purchase self-tests and access self-test instructions and counseling, but they did not provide data or information on linkages [27]. Of 4 US studies, 2 reported positive findings on the feasibility of use of a kiosk tablet for HIV self-testing in emergency rooms but reported poor engagement (50%) of participants and limited data on detection and linkages [28,29]. Another US study evaluating the use of SMS text messaging self-test results only by participants found that it was preferred by participants [30]. The fourth study provided written and Web-based video instructions to participants to choose a picture that resembled their self-test result and reported comparable results to ours, with 100% of positive OraQuick results and 98% of negative results being interpreted correctly [31]. None of these studies evaluated linkages to care.
The HIVSmart! app is an integrated innovation. It offers a personalized experience of self-testing from access to linkage, which is a step up from a website-, tablet-, or SMS text messaging-only service and is housed in a secure Health Insurance Portability and Accountability Act-compliant cloud-based platform. Integrated innovations [32], like HIVSmart!, are a new trend in the digital innovations space, as reported in a recent systematic review [33]. In 2011-2013, we evaluated a Web-based HIVSmart! strategy successfully in South African health care professionals [17]. We are currently testing the strategy at scale in South African townships in a project funded by both the Governments of South Africa and Canada [18]. A prototype of this strategy was evaluated in students way back in 2009 [12].
With the increasing availability of PrEP in Canada, and the desire to self-test frequently expressed by those on PrEP, we included a subsample of participants on PrEP in this study. However, neither our main outcomes nor HIV status differed by the PrEP status. Our study was underpowered to detect subgroup differences (small number of PrEP participants). Studies suggest that PrEP increases the window period for seroconversion, taking longer to get a positive test result [34,35]. Yet, many of our self-test results were consistent with the rapid test results. Concordant with a study in PrEP users from Kenya, participants were in favor of self-testing [36].
Limitation
A limitation of this study included convenience sampling that raises a concern of selection bias.
Conclusions
In Canada, future research with HIV self-tests from provinces with high rates of undocumented HIV infection (Saskatchewan) and marginalized populations with undiagnosed HIV infection is warranted. Future research that incorporates digital strategies to plug service delivery gaps important for HIV self-testing will make it easier to offer and document self-testing.
Rapid approvals by Food and Drug Administration, Conformité Européenne, and World Health Organization Prequalification of self-tests, both oral and blood-based HIV self-tests, will help expand options to self-test in Canada. It will also increase the visibility of HIV self-tests in pharmacies, clinics, and outreach settings and democratize the process of HIV self-testing. Finally, the adoption of proven digital solutions will help improve engagement and expedite rapid linkages to care. Doing so will help address the last mile problem of detecting undiagnosed HIV infection in marginalized Canadians, thereby accelerating progress toward Joint United Nations Programme on HIV/AIDS 90-90-90 targets in Canada.
We conclude that the HIVSmart! app-optimized strategy is feasible, accepted, and preferred by an educated, urban MSM population of Montreal. With the app, participants were able to perform, interpret, store results, and rapidly link to care. The HIVSmart!-optimized, self-testing strategy could be adapted and contextualized to many at-risk populations within Canada and worldwide, thereby maximizing its public health impact.
Acknowledgments
The authors are grateful to all the staff at Clinique Médicale L’Actuel Montreal. We would also like to acknowledge Dr Marc Steben, Dr Bertrand Lebouché, and Dr Jean Pierre Routy for supporting the study. This work was funded by an operating grant from the Canadian Institutes of Health Research (grant #HHP-137872) and the Fonds de recherche du Québec - Santé Research-Scholar Junior 2 and Senior, awarded to NPP.
Abbreviations
- ITA
Investigational Testing Authorization
- MSM
men who have sex with men
- PEP
postexposure prophylaxis
- PrEP
pre-exposure prophylaxis
- SMS
short service message
Footnotes
Authors' Contributions: NPP was involved in the concept, execution, write-up, critique, and overall responsibility of data for the project; MS was involved in execution, data analyses, write-up, and critique; LD was involved in execution, data collection, write-up, and critique; AG was involved in execution, data collection, write-up, and critique; KB was involved in execution, data collection, and write-up; AFV was involved in execution, data collection, write-up, and critique; LJ was involved in data analyses, write-up, and critique; and RT was involved in execution, data collection, write-up, critique, and responsibility for the project implementation.
Conflicts of Interest: None declared.
References
- 1.Joint United Nations Programme on HIV/AIDS . UNAIDS.org. Geneva: UNAIDS; 2014. [2017-08-16]. 90-90-90: An ambitious treatment target to help end the AIDS epidemic http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. [PubMed] [Google Scholar]
- 2.Consolidated Guidelines on HIV Testing Services: 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection. Geneva: World Health Organization; 2016. Nov 29, [2018-11-07]. https://www.who.int/hiv/pub/self-testing/hiv-self-testing-guidelines/en/ [PubMed] [Google Scholar]
- 3.Consolidated Guidelines on HIV Testing Services: 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection. Geneva: World Health Organization; 2015. Jul 15, [2018-11-07]. https://www.who.int/hiv/pub/guidelines/hiv-testing-services/en/ [PubMed] [Google Scholar]
- 4.Epstein Jay S. July 3 Approval Letter, OraQuick In-Home HIV Test. Silver Spring: U.S. Food and Drug Administration,; 2012. Jul 03, [2018-10-29]. https://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/ucm310592.htm . [Google Scholar]
- 5.Pebody Roger. First HIV home test approved for sale in UK. London: NAM Publications; 2015. Apr 24, [2018-10-25]. http://www.aidsmap.com/First-HIV-home-test-approved-for-sale-in-UK/page/2964537/ [Google Scholar]
- 6.HIVST HIVST.org: HIV self-testing research and policy hub. 2017. [2017-08-15]. http://www.hivst.org/
- 7.Unitaid, World Health Organization . Market and technology landscape: HIV rapid diagnostic tests for self-testing, 4th edition. Geneva: Unitaid; 2018. Jul, [2018-09-20]. https://unitaid.org/assets/HIVST-landscape-report.pdf . [Google Scholar]
- 8.Wood BR, Ballenger C, Stekler JD. Arguments for and against HIV self-testing. HIV AIDS (Auckl) 2014;6:117–26. doi: 10.2147/HIV.S49083. doi: 10.2147/HIV.S49083.hiv-6-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown AN, Djimeu EW, Cameron DB. A review of the evidence of harm from self-tests. AIDS Behav. 2014 Jul;18 Suppl 4:S445–9. doi: 10.1007/s10461-014-0831-y. http://europepmc.org/abstract/MED/24989129 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Health Canada Draft Guidance Document on HIV Simple/Rapid Test Kits. 2017. Sep 14, Consultation on HIV Simple/Rapid Test Kits https://tinyurl.com/yc487qw2.
- 11.Haig TA, Otis J, Veillette-Bourbeau L, Caruso J, Jolimore J, Ferlatte O, Maxwell J, Rousseau R. HIV self-testing for MSM: acceptability among community members and service providers in Vancouver, Toronto, and Montreal. Canadian Journal of Infectious Diseases & Medical Microbiology (Supplement B) 2015;26:13B. [Google Scholar]
- 12.Pant Pai Nitika, Bhargava M, Joseph L, Sharma J, Pillay S, Balram B, Tellier P. Will an unsupervised self-testing strategy be feasible to operationalize in Canada? Results from a pilot study in students of a large canadian university. AIDS Res Treat. 2014;2014:747619. doi: 10.1155/2014/747619. doi: 10.1155/2014/747619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pai NP, Smallwood M, Gulati D, Lapczak N, Musten A, Gaydos C, Johnston C, Steben M, Wong T, Engel N, Kim J. What do Key Stakeholders Think About HIV Self-Testing in Canada? Results from a Cross-Sectional Survey. AIDS Behav. 2018 Feb;22(2):606–615. doi: 10.1007/s10461-017-1764-z. http://europepmc.org/abstract/MED/28439755 .10.1007/s10461-017-1764-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Public Health Agency of Canada . Summary: Etimates of HIV incidence, prevalence and proportion undiagnosed in Canada, 2014. Ottawa: 2015. Nov 27, https://www.canada.ca/content/dam/canada/health-canada/migration/healthy-canadians/publications/diseases-conditions-maladies-affections/hiv-aids-estimates-2014-vih-sida-estimations/alt/hiv-aids-estimates-2014-vih-sida-estimations-eng.pdf . [Google Scholar]
- 15.Pai Nitika. Grand Challenges Canada. 2011. To develop a synergsitic, innovative, implementation strategy for self-testing for HIV in South Africa http://www.grandchallenges.ca/grantee-stars/0027-01/
- 16.Pai Nitika. Candian Institutes of Health Research. 2017. Feasibility of an innovative AideSmart! app-based multiplexed, point-of-care screening/counselling strategy for HIV-associated co-infections (HCV, Syphilis, HBV) in key Canadian populations https://tinyurl.com/y9v4ep8x.
- 17.Pant Pai Nitika, Behlim T, Abrahams L, Vadnais C, Shivkumar S, Pillay S, Binder A, Deli-Houssein R, Engel N, Joseph L, Dheda K. Will an unsupervised self-testing strategy for HIV work in health care workers of South Africa? A cross sectional pilot feasibility study. PLoS One. 2013;8(11):e79772. doi: 10.1371/journal.pone.0079772. http://dx.plos.org/10.1371/journal.pone.0079772 .PONE-D-13-29602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pai Nitika. Grand Challenges Canada. 2015. HIVSmart! A smartphone app-based HIV self-testing strategy to identify undiagnosed cases of HIV http://www.grandchallenges.ca/grantee-stars/0710-05/
- 19.Choko AT, MacPherson P, Webb EL, Willey BA, Feasy H, Sambakunsi R, Mdolo A, Makombe SD, Desmond N, Hayes R, Maheswaran H, Corbett EL. Uptake, Accuracy, Safety, and Linkage into Care over Two Years of Promoting Annual Self-Testing for HIV in Blantyre, Malawi: A Community-Based Prospective Study. PLoS Med. 2015 Sep;12(9):e1001873. doi: 10.1371/journal.pmed.1001873. http://dx.plos.org/10.1371/journal.pmed.1001873 .PMEDICINE-D-15-00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thirumurthy H, Masters SH, Mavedzenge SN, Maman S, Omanga E, Agot K. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: a cohort study. Lancet HIV. 2016 Dec;3(6):e266–74. doi: 10.1016/S2352-3018(16)00041-2. http://europepmc.org/abstract/MED/27240789 .S2352-3018(16)00041-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maheswaran H, Petrou S, MacPherson P, Choko AT, Kumwenda F, Lalloo DG, Clarke A, Corbett EL. Cost and quality of life analysis of HIV self-testing and facility-based HIV testing and counselling in Blantyre, Malawi. BMC Med. 2016 Feb 19;14:34. doi: 10.1186/s12916-016-0577-7. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-016-0577-7 .10.1186/s12916-016-0577-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wray T, Chan PA, Simpanen E, Operario D. eTEST: Developing a Smart Home HIV Testing Kit that Enables Active, Real-Time Follow-Up and Referral After Testing. JMIR Mhealth Uhealth. 2017 May 08;5(5):e62. doi: 10.2196/mhealth.6491. http://mhealth.jmir.org/2017/5/e62/ v5i5e62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frye V, Wilton L, Hirshfied S, Chiasson MA, Usher D, Lucy D, McCrossin J, Greene E, Koblin B, Kobin B, All About Me Study Team “Just Because It's Out There, People Aren't Going to Use It.” HIV Self-Testing Among Young, Black MSM, and Transgender Women. AIDS Patient Care STDS. 2015 Nov;29(11):617–24. doi: 10.1089/apc.2015.0100. http://europepmc.org/abstract/MED/26376029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippman SA, Moran L, Sevelius J, Castillo LS, Ventura A, Treves-Kagan S, Buchbinder S. Acceptability and Feasibility of HIV Self-Testing Among Transgender Women in San Francisco: A Mixed Methods Pilot Study. AIDS Behav. 2016 Apr;20(4):928–38. doi: 10.1007/s10461-015-1236-2. http://europepmc.org/abstract/MED/26511864 .10.1007/s10461-015-1236-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers JE, El-Sadr Davis Olivia Y, Weinstein ER, Remch M, Edelstein A, Khawja A, Schillinger JA. Availability, Accessibility, and Price of Rapid HIV Self-Tests, New York City Pharmacies, Summer 2013. AIDS Behav. 2017 Feb;21(2):515–524. doi: 10.1007/s10461-016-1594-4.10.1007/s10461-016-1594-4 [DOI] [PubMed] [Google Scholar]
- 26.LeGrand S, Muessig KE, Horvath KJ, Rosengren AL, Hightow-Weidman LB. Using technology to support HIV self-testing among MSM. Curr Opin HIV AIDS. 2017 Sep;12(5):425–431. doi: 10.1097/COH.0000000000000400. http://europepmc.org/abstract/MED/28617712 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartelsman M, Joore IK, van Bergen JE, Hogewoning AA, Zuure FR, van Veen MG, HIV Transmission Elimination AMsterdam (H-TEAM) initiative HIV testing week 2015: lowering barriers for HIV testing among high-risk groups in Amsterdam. BMC Infect Dis. 2017 Dec 01;17(1):529. doi: 10.1186/s12879-017-2617-0. https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-017-2617-0 .10.1186/s12879-017-2617-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaydos CA, Solis M, Hsieh Y, Jett-Goheen M, Nour S, Rothman RE. Use of tablet-based kiosks in the emergency department to guide patient HIV self-testing with a point-of-care oral fluid test. Int J STD AIDS. 2013 Sep;24(9):716–21. doi: 10.1177/0956462413487321. http://europepmc.org/abstract/MED/23970610 .0956462413487321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haukoos JS, Hopkins E, Bender B, Al-Tayyib A, Long J, Harvey J, Irby J, Bakes K, Denver Emergency Department HIV Testing Research Consortium Use of kiosks and patient understanding of opt-out and opt-in consent for routine rapid human immunodeficiency virus screening in the emergency department. Acad Emerg Med. 2012 Mar;19(3):287–93. doi: 10.1111/j.1553-2712.2012.01290.x. doi: 10.1111/j.1553-2712.2012.01290.x. [DOI] [PubMed] [Google Scholar]
- 30.Daniels J, Rosengren L, Young S, Klausner JD. Will Men Who Have Sex With Men Use Short-Messaging Services to Send Photos of Completed HIV Self-Tests to Researchers? J Assoc Nurses AIDS Care. 2016;27(5):722–6. doi: 10.1016/j.jana.2016.05.002.S1055-3290(16)30041-3 [DOI] [PubMed] [Google Scholar]
- 31.Chavez P, Wesolowski L, Owen M, Gravens L, Sullivan P, MacGowan R. Perceptions and performance of self-administered rapid HIV tests conducted by untrained users in real world settings. 2016 HIV Diagnostics Conference; 21-24 March, 2016; Atlanta, Georgia, USA. 2016. http://hivtestingconference.org/wp-content/uploads/2016/03/2016-HIV-DX-Program-Book_WEB_FINAL.pdf . [Google Scholar]
- 32.El-Noush H, Silver KL, Pamba AO, Singer PA. Innovating for women's, children's, and adolescents' health. BMJ. 2015 Sep 14;351:h4151. doi: 10.1136/bmj.h4151. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=26371217 . [DOI] [PubMed] [Google Scholar]
- 33.Daher J, Vijh R, Linthwaite B, Dave S, Kim J, Dheda K, Peter T, Pai NP. Do digital innovations for HIV and sexually transmitted infections work? Results from a systematic review (1996-2017) BMJ Open. 2017 Nov 03;7(11):e017604. doi: 10.1136/bmjopen-2017-017604. http://bmjopen.bmj.com/cgi/pmidlookup?view=long&pmid=29101138 .bmjopen-2017-017604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donnell D, Ramos E, Celum C, Baeten J, Dragavon J, Tappero J, Lingappa JR, Ronald A, Fife K, Coombs RW, Partners PrEP Study Team The effect of oral preexposure prophylaxis on the progression of HIV-1 seroconversion. AIDS. 2017 Dec 10;31(14):2007–2016. doi: 10.1097/QAD.0000000000001577. http://europepmc.org/abstract/MED/28692542 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suntharasamai P, Martin M, Choopanya K, Vanichseni S, Sangkum U, Tararut P, Leelawiwat W, Anekvorapong R, Mock PA, Cherdtrakulkiat T, Leethochawalit M, Chiamwongpaet S, Gvetadze RJ, McNicholl JM, Paxton LA, Kittimunkong S, Curlin ME. Assessment of Oral Fluid HIV Test Performance in an HIV Pre-Exposure Prophylaxis Trial in Bangkok, Thailand. PLoS One. 2015;10(12):e0145859. doi: 10.1371/journal.pone.0145859. http://dx.plos.org/10.1371/journal.pone.0145859 .PONE-D-15-37783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ngure K, Heffron R, Mugo N, Thomson KA, Irungu E, Njuguna N, Mwaniki L, Celum C, Baeten JM. Feasibility and acceptability of HIV self-testing among pre-exposure prophylaxis users in Kenya. J Int AIDS Soc. 2017 Dec 10;20(1):21234. doi: 10.7448/IAS.20.1.21234. http://europepmc.org/abstract/MED/28362073 . [DOI] [PMC free article] [PubMed] [Google Scholar]


